Abstract
In the course of efforts to establish quantitative MRI-based norms for healthy brain development (Brain Development Cooperative Group, 2006), previously unreported associations of parental education and temporal and frontal lobe volumes with full scale IQ and its verbal and performance subscales were discovered. Our findings were derived from the largest, most representative MRI sample to date of healthy children and adolescents, ages 4 years 10 months to 18 years 4 months. We first find that parental education has a strong association with IQ in children that is not mediated by total or regional brain volumes. Second, we find that our observed associations between temporal gray matter, temporal white matter and frontal white matter volumes with full scale IQ, between 0.14 to 0.27 in children and adolescents, are due in large part to their correlations with performance IQ and not verbal IQ. The volumes of other lobar gray and white matter, subcortical gray matter (thalamus, caudate nucleus, putamen and globus pallidus), cerebellum and brainstem do not contribute significantly to IQ variation. Third, we find that head circumference is an insufficient index of cerebral volume in typically developing older children and adolescents. The relations between total and regional brain volumes and IQ can best be discerned when additional variables known to be associated with IQ, especially parental education and other demographic measures, are considered concurrently.
INTRODUCTION
Developmental brain mechanisms that mediate the association of parental demographic factors with IQ remain poorly understood. IQ is a composite measure of diverse cognitive functions that is associated with brain development and environmental factors. Since the work of Alfred Binet in the late 1800s, adult IQ to brain size correlation estimates have been inconsistent, ranging from 0 to ~0.6 with central tendency ~0.3 to 0.4 (Andreasen, et al., 1993; McDaniel, 2005; Vernon, Wickett, Bazana, & Stelmark, 2000; Witelson, Beresh, & Kigar, 2006). Full scale IQ and its performance (PIQ) and verbal (VIQ) subscales have been associated with assessments of brain development and demographic factors (Fisch, Bilek, Horrobin, & Chang, 1976; Gale, O'Callaghan, Bredow, & Martyn, 2006; Gale, O'Callaghan, Godfrey, Law, & Martyn, 2004; Ounsted, Moar, & Scott, 1988; Reiss, Abrams, Singer, Ross, & Denckla, 1996; Shaw, et al., 2006; Weinberg, Dietz, Penick, & McAlister, 1974). Brain structure volumes change significantly across childhood and adolescence with cross-sectional and longitudinal age (Giedd, et al., 1999; Lenroot & Giedd, 2006) and these changes may be associated with variations in IQ test performance. It is also known that the volumetric variances of human brain structure sizes change markedly throughout life, differ within and between individuals (Sowell, et al., 2003), increase within and between males and females during puberty (Sowell, et al., 2003) and may have far reaching behavioral, pathological and social significance (Rutter, Caspi, & Moffitt, 2003; Shaw, et al., 2006). Maternal education likely affects brain development of their offspring and VIQ by predominantly genetic, but also modest shared environmental influences (Neiss M & Rowe, 2000). Developmental brain research has yet to reliably quantify the associations between parental demographic factors and regional gray and white matter brain volumes mediated by variations in total brain volume, structural connectivity and functional connectivity .
Brain growth can be measured accurately by in vivo magnetic resonance imaging (MRI), allowing investigation of the influence of brain growth on IQ and the influences of demographic, hormonal, cellular and genetic factors on healthy brain development from early childhood through adolescence and into adulthood (Hulshoff Pol, et al., 2006; Juraska & Markham, 2004; Zinkstok, et al., 2006). Due to the lack of any previous MRI study in which sampling was planned and completed to create a normatively representative sample of healthy children and adolescents, there are deficiencies in our understanding of how IQ in children may be associated with brain development and parental and offspring demographic factors. At present, our knowledge of key associations between these variables in neurodevelopmental disorders is limited because pathology and its correlates can be discerned only in relation to typical development. In this paper, we employ MRI and clinical measurements from the largest, most representative sample of typically developing children collected to date to further determine how brain structure sizes and demographics are associated with IQ.
METHODS
Participants
Through application of strict inclusion and exclusion criteria (Brain Development Cooperative Group, 2006), the Brain Development Cooperative Group acquired a sample of children and adolescents from across the United States using an epidemiologic sampling plan that combined cross-sectional and accelerated longitudinal study design principles (Brain Development Cooperative Group, 2006; Harezlak, Ryan, Giedd, & Lange, 2005). Family income levels and race/ethnicity proportions within each income level were matched to their joint distribution as determined by the US Census 2000 in each cell of a 3 × 7 table of income levels by race/ethnicity groupings. Because there is a plethora of co-factors that could be associated with assessments of brain structure, the Brain Development Cooperative Group decided to balance the sample with respect to age, sex, income and race/ethnicity, shown previously to relate to brain structure in the preceding order of importance. Siblings were excluded from the sample. Handedness was determined by observing hand preferences for writing and responses to seven gestural commands (e.g., show me how you use a hammer, throw a ball). Participants were classified as either right-handed (at least 7 out of 8 right-handed responses) or non-right-handed (Waber, de Moor et al., 2007); handedness was not studied in the present analysis. Informed consent/assent was obtained from all participants. Institutional Review Boards approved all protocols at parent institutions.
Family Income
Family income was employed as a surrogate for “socioeconomic status”. For the purposes of this study, due to regional income variability, adjusted family income (AFI) was defined as AFI = ((A / B) / C) * D, where A was the midpoint of each family’s self-reported income bracket, B was the US Housing and Urban Development (HUD) adjustment factor for family size, C was the local median family income for the Metropolitan Statistical Area (MSA) in which the family resided at the time of the interview, and D was the US median family income.
Parental Education
Parental education was defined as the highest level of education completed by either the mother or the father.
IQ Assessment
The Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999) measured intelligence in children 6 years of age and older. For younger children (4 years and 10 months to 5 years and 11 months), only a General Conceptual Ability (GCA) score from the Differential Ability Scale (Elliott, 1990) was available. Verbal IQ (VIQ) was assessed by the Vocabulary and Similarities subtests (VIQ), performance IQ (PIQ) by the Matrix Reasoning and Block Design subtests, and full scale IQ (FSIQ) by the sum of VIQ and PIQ.
Head Circumference
Maximal occipitofrontal head circumference (HC) was measured by paper tape and adequate reliability was established across all sites.
Imaging
Structural magnetic resonance images of brain anatomy were collected at 1.5 Tesla without sedation under a 3D T1-weighted spoiled gradient recalled (SPGR) echo sequence (Brain Development Cooperative Group, 2006). We applied an automated tissue segmentation algorithm to obtain volumes of gray matter (GM), white matter (WM) and cerebrospinal fluid and for regional brain structure extraction (Collins, Holmes, Peters, & Evans, 1995). Careful human interactive quality control was performed for all acquired images; images failing this step were excluded from the analysis. Total brain volume (TBV) was defined as the sum of the lobar GM and WM, subcortical GM, cerebellum, brainstem and cerebrospinal fluid (CSF) volumes. Volumes were estimated for the intracranial cavity (ICC), frontal, temporal, parietal and occipital lobar WM and GM, subcortical GM (thalamus, caudate nucleus, putamen and globus pallidus), cerebellum and brainstem. For more detail, visit www.NIH-PediatricMRI.org.
Biostatistical power
Effect sizes reported previously in the literature (Lange, Giedd, Castellanos, Vaituzis, & Rapoport, 1997) determined minimum sample sizes to detect regional associations with 80% power at a false positive rate of 5% and where coefficients of variation (standard deviation expressed as a percentage of the mean) themselves varied between 9.4 (putamen) to 19.3 (amygdala) in a non-representative sample similar in age range (Giedd, et al., 1996). Coefficient of variation is more useful than standard deviation when comparing an ensemble of measurements having different means and different variability and when mean and variance appear correlated (van Belle, Heagerty, et al. 2004), as is the case for brain structure sizes (Lange, Giedd et al. 1997; Kennedy, Lange et al. 1998; Caviness, Lange et al. 1999). For the cross-sectional baseline scans analyzed in this report, we have a minimum of 80% power to detect age-dependent volumetric differences in all measured structures with the current sample size at all ages.
Biostatistical Analysis
All missing data were treated as missing at random. Two-sample t-tests, proceeded by F-tests for equality of variance, and univariate linear regressions were conducted in our initial data explorations prior to formal model selection. False positive error rate was set at 0.05 for each test. A robust smoothing algorithm (Cleveland, 1981) was applied to scatter-plots of age by HC, TBV and ICC, with kernel width tuning held constant for all plots, to produce model-free summary curves. All regional brain volumes and AFI were expressed as deviations from their sampling means scaled to unit variance. We tested differences in IQ-volumetric correlations in males, females and age groups by use of R. A. Fisher’s z-transform (van Belle, Heagerty, et al. 2004).
Data analysis proceeded in three sequential sections. Section 1 was a series of association analyses between IQ, family income and parental education. Section 2 expanded Section 1 by adding total and regional brain volumes. Section 3 further extended Sections 1 and 2 by adding formal regression analysis.
Analytic stages of regression models (Section 3)
Due to the range of hypotheses of varying complexity currently tested in the field, we conducted our section 3 analysis in three stages. Each stage included a sex-stratified analysis, allowing different residual variation for males and females, and a combined analysis treating sex as an additional covariate with pooled residual variance. In stage 1, we investigated relations between TBV and PIQ, VIQ and FSIQ while covarying for age and sex. In stage 2, we replaced TBV with the volumes of the cortical lobes, subcortical GM and cerebellum by regression models. Relative analyses were also performed, in which we represented regional volumetric proportions of TBV as deviations from their means scaled to unit variance. In stage 3, we applied the same analyses as in stage 2 to lobar GM and lobar WM separately. The main associations of age, sex and AFI were fixed by study design and hence included in all models in accordance with the marginality principle (Venables & Ripley, 2002). In this paper, we occasionally use the term “effect” according to its statistical definition, being the magnitude of the estimated coefficient (parameter) in a regression model. Regression effects are equivalent in significance to the estimated partial correlations from such models. In all three stages, candidate covariates were treated simultaneously in separate models for each brain structure. We employed the Akaike Information Criterion (AIC) (Akaike, 1974) to obtain the best-fitting yet simplest models. The AIC includes or excludes factors by allowing them to compete with each other based on their explanatory power. Simultaneous consideration of the ensemble of covariates that may be associated with IQ and regional brain volumes, including parental education and TBV does not obfuscate the association of each covariate with IQ measurements. Multi-covariate models provide more accurate and precise interpretations of each covariate’s concurrent association in the presence of the others (van Belle, Heagerty, et al. 2004). All data analysis was performed in R version 2.9.0 (04/17/09 build) whose results are equivalent to those produced by SAS.
RESULTS
Participant Demographics and IQ
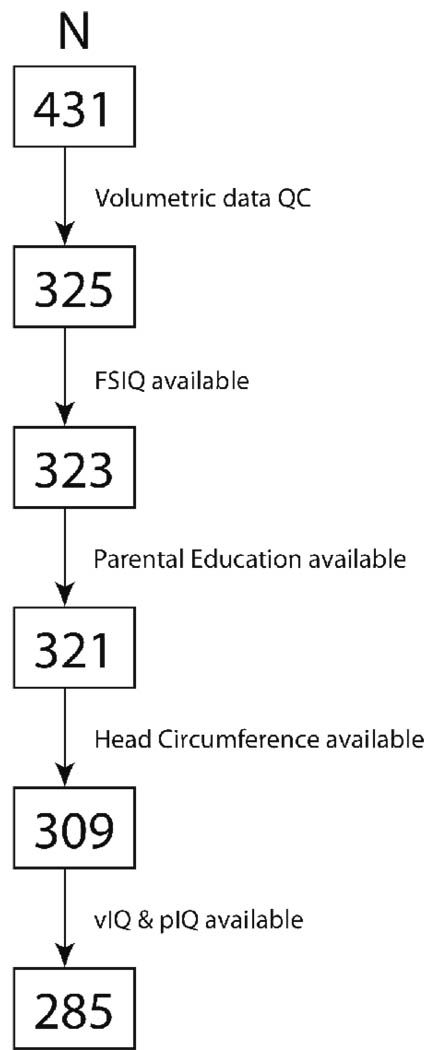
Figure 1 is a record of how we started with our full sample of MRI images to identify our subsample of N = 285 participants for the present study. Table 1 and Table 2 contain participant demographics, means, standard errors and ranges of their IQ scores and discrepancies between their VIQ and PIQ scores.
Figure 1.
Participant accrual and sample inclusion stages.
Table 1.
Participant Demographics and IQ distribution
| Male | Female | Total | |
|---|---|---|---|
| Sample size | 143 | 166 | 309 |
| Age (years)* | 10.95 (0.32) | 10.88 (0.28) | 10.91 (0.21) |
| Age Range | 4:10 to 18:4 | 4:11 to 18:3 | 4:10 to 18:4 |
| Adjusted Family Income† | 70,695 (2,478) | $75,073 (2,619) | 73,047 (1,816) |
| FSIQ | 111 (1.02) | 110 (0.92) | 111 (0.68) |
| pIQ‡ | 111 (1.06) | 108 (1.01) | 110 (0.73) |
| PIQ‡ | 110 (1.20) | 111 (1.04) | 110 (0.79) |
| PIQ – pIQ discrepancy (%) |
|||
| Less than −14 | 13.8 | 11.0 | 12.3 |
| Between −14 and 14 | 73.9 | 70.3 | 71.9 |
| Greater than 14 | 12.3 | 18.7 | 15.8 |
| Right-handed (%) | 85.2 | 89.8 | 87.7 |
| Parental Education** | |||
| High School†† | 19 | 11 | 30 |
| Some College | 29 | 37 | 66 |
| College | 44 | 53 | 97 |
| Some Graduate School | 7 | 12 | 19 |
| Graduate School | 44 | 53 | 97 |
| Total | 143 | 166 | 309 |
| Adjusted Family Income | |||
| Low: Less than $50,000 | 38 | 46 | 84 |
| Medium: $50,000 to $100,000 | 80 | 75 | 155 |
| High: Greater than $100,000 | 25 | 45 | 70 |
| Total | 143 | 166 | 309 |
Mean (SEM).
Derived from tables provided by the US Department of Housing and Urban Development and the US Census 2000.
N = 285, total sample size; n = 155 females, n = 130 males.
Highest level attained by either parent.
Includes one participant neither of whose parents completed high school.
Table 2.
Means, standard errors and ranges of measures of intelligence.
| Male | Female | Total | ||||
|---|---|---|---|---|---|---|
| Intelligence Measure | mean (SEM) | range | mean (SEM) | range | mean (SEM) | range |
| Verbal IQ (Standard Score) | 110 (1.20) | 74–144 | 111 (1.04) | 82–149 | 110 (0.79) | 74–149 |
| Performance IQ (Standard Score) | 111 (1.06) | 80–140 | 108 (1.01) | 72–143 | 110 (0.73) | 72–143 |
| Full Scale IQ (Standard Score) | 111 (1.02) | 74–144 | 110 (0.92) | 79–141 | 111 (0.68) | 74–144 |
Section 1. Associations Between IQ, Family Income and Parental Education
Adjusted Family Income, Verbal IQ, Performance IQ and Full Scale IQ
Verbal IQ
We observed statistically significant increases in mean VIQ between participants whose AFIs were in the low versus higher ranges, of 6.6 points from low to medium AFI (p = 0.0001) and of 5.5 points from low to high AFI (p = 0.01). VIQ was subdivided into three subgroups based on its sampling distribution to arrive at three nearly equi-sized participant groups, low (less than 107), medium (from107 to 114) and high (greater than 114). No significant VIQ group-AFI associations were found.
Performance IQ
We observed that the PIQ-AFI association was stronger than the VIQ-AFI association. Mean PIQ was found to increase by nearly 6 points from low to medium AFI (p = 0.0003) and by over 7 points from low to high AFI (p = 0.0002). We also subdivided PIQ into three subgroups based on its sampling distribution to arrive at three nearly equi-sized participant groups, low (less than 104), medium (from 104 to 115) and high (greater than 115), with PIQs in the lowest AFI range and exhibited the strongest associations.
Full Scale IQ
Mean FSIQ increased significantly with AFI, by about 7 points from low to medium AFI (p < 0.00001) and by about 8 points from low to high AFI (p < 0.0001). FSIQ was also subdivided into three subgroups based on its sampling distribution to arrive at three nearly equi-sized participant groups, low (less than 106), medium (from 106 to 115) and high (greater than 115). We observed a nearly 2 point increase from low to medium AFI in participants with mid-level FSIQ (p = 0.005).
Parental Education, Verbal IQ, Performance IQ and Full Scale IQ
Verbal IQ
We detected a significant increase in mean VIQ between participants whose parental educations were in the lower versus higher ranges. The largest increase in participant VIQ occurred when parental education increased from the high school graduate to the college graduate level. VIQ was observed to increase by 14–15 points if their parents completed college or further higher education compared to those whose parents were only high school graduates from (p < 0.00001). Inspection of the statistical effects of parental education in the participant VIQ subgroups showed significant increases of 7–8 points in VIQ with parental education increases only in participants having VIQs in the lowest range (p = 0.006) and of 5–8 points in the highest range (p = 0.03).
Performance IQ
We observed that PIQ increased significantly with increasing parental education. Mean PIQ was found to increase by 8–10 points (p = 0.004) and mean FSIQ by 12–13 points (p < 0.00001) in participants whose parents completed college or further higher education compared to those whose parents graduated from high school only. Inspection of the statistical effects of parental education in the PIQ subgroups showed a significant 6–8 point change only in participants whose PIQs were in the lowest range (p = 0.01).
Full Scale IQ
The association between parental education and FSIQ was found to be less significant (p < 0.05) than that with PIQ and was seen only in participants whose FSIQs were in the lowest and highest ranges.
Section 2. Associations Between IQ, Total and Regional Brain Volumes, Intracranial Cavity Volume and Head Circumference
Table 3 contains marginal, conditional and joint correlations between FSIQ, VIQ, PIQ, TBV, total lobar GM volume, total lobar WM volume, ICC volume and HC. Each Pearson correlation was computed with and without possible age and sex dependencies. We observed low but statistically significant FSIQ-volumetric correlations, the highest found in TBV and total lobar GM (0.22 – 0.23) and the lowest found in total lobar WM (0.14); Table 3, lower right triangle, first column. The observed brain and head size correlations with FSIQ were due in the main to their significant correlations with PIQ and not VIQ.
Table 3.
Estimated Pearson Correlation Coefficients (N = 309*)
| 4:10 – 11:11 | 12:0 – 18:4 | Total | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intelligence | Brain Volumetrics | Intelligence | Brain Volumetrics | Intelligence | Brain Volumetrics | |||||||||||||||||||
| FSIQ | VIQ | PIQ | TBV | GM | WM | ICC | FSIQ | VIQ | PIQ | TBV | GM | WM | ICC | FSIQ | VIQ | PIQ | TBV | GM | WM | ICC | ||||
| VIQ | 0.86† | VIQ | 0.88 | VIQ | 0.87 | |||||||||||||||||||
| PIQ | 0.81 | 0.40 | PIQ | 0.83 | 0.47 | PIQ | 0.82 | 0.43 | ||||||||||||||||
| TBV | 0.29 | 0.20 | 0.32 | TBV | 0.17 | 0.06 | 0.27 | TBV | 0.23 | 0.14 | 0.28 | |||||||||||||
| Male | GM | 0.24 | 0.16 | 0.27 | 0.88 | GM | 0.14 | 0.04 | 0.23 | 0.89 | GM | 0.20 | 0.13 | 0.23 | 0.88 | |||||||||
| WM | 0.17 | 0.09 | 0.21 | 0.75 | 0.37 | WM | 0.21 | 0.11 | 0.29 | 0.84 | 0.51 | WM | 0.17 | 0.09 | 0.24 | 0.80 | 0.44 | |||||||
| ICC | 0.26 | 0.14 | 0.32 | 0.96 | 0.81 | 0.77 | ICC | 0.19 | 0.07 | 0.30 | 0.95 | 0.85 | 0.81 | ICC | 0.23 | 0.11 | 0.30 | 0.95 | 0.83 | 0.78 | ||||
| HC | 0.20 | 0.11 | 0.23 | 0.79 | 0.68 | 0.61 | 0.82 | HC | 0.13 | 0.14 | 0.09 | 0.26 | 0.26 | 0.24 | 0.35 | HC | 0.17 | 0.13 | 0.16 | 0.46 | 0.43 | 0.38 | 0.55 | |
| FSIQ | VIQ | PIQ | TBV | GM | WM | ICC | FSIQ | VIQ | PIQ | TBV | GM | WM | ICC | FSIQ | VIQ | PIQ | TBV | GM | WM | ICC | ||||
| VIQ | 0.82 | VIQ | 0.87 | VIQ | 0.84 | |||||||||||||||||||
| PIQ | 0.81 | 0.33 | PIQ | 0.84 | 0.47 | PIQ | 0.82 | 0.38 | ||||||||||||||||
| TBV | 0.24 | 0.20 | 0.20 | TBV | 0.20 | 0.13 | 0.21 | TBV | 0.22 | 0.18 | 0.20 | |||||||||||||
| Female | GM | 0.27 | 0.21 | 0.25 | 0.92 | GM | 0.26 | 0.19 | 0.25 | 0.87 | GM | 0.27 | 0.21 | 0.25 | 0.90 | |||||||||
| WM | 0.13 | 0.12 | 0.10 | 0.82 | 0.54 | WM | 0.07 | 0.03 | 0.09 | 0.83 | 0.45 | WM | 0.11 | 0.09 | 0.09 | 0.82 | 0.51 | |||||||
| ICC | 0.20 | 0.19 | 0.15 | 0.94 | 0.84 | 0.81 | ICC | 0.20 | 0.14 | 0.19 | 0.93 | 0.77 | 0.82 | ICC | 0.20 | 0.17 | 0.16 | 0.94 | 0.81 | 0.81 | ||||
| HC | 0.15 | 0.16 | 0.08 | 0.40 | 0.38 | 0.31 | 0.48 | HC | 0.04 | 0.06 | -0.02 | 0.41 | 0.27 | 0.38 | 0.58 | HC | 0.09 | 0.11 | 0.03 | 0.41 | 0.34 | 0.34 | 0.53 | |
| FSIQ | VIQ | PIQ | TBV | GM | WM | ICC | FSIQ | VIQ | PIQ | TBV | GM | WM | ICC | FSIQ | VIQ | PIQ | TBV | GM | WM | ICC | ||||
| VIQ | 0.84 | VIQ | 0.87 | VIQ | 0.85 | |||||||||||||||||||
| PIQ | 0.81 | 0.36 | PIQ | 0.84 | 0.46 | PIQ | 0.82 | 0.40 | ||||||||||||||||
| TBV | 0.25 | 0.17 | 0.26 | TBV | 0.18 | 0.07 | 0.26 | TBV | 0.22 | 0.12 | 0.25 | |||||||||||||
| Total | GM | 0.26 | 0.16 | 0.27 | 0.92 | GM | 0.20 | 0.10 | 0.25 | 0.91 | GM | 0.23 | 0.14 | 0.26 | 0.91 | |||||||||
| WM | 0.15 | 0.09 | 0.17 | 0.82 | 0.56 | WM | 0.15 | 0.05 | 0.21 | 0.87 | 0.60 | WM | 0.14 | 0.07 | 0.18 | 0.85 | 0.58 | |||||||
| ICC | 0.22 | 0.14 | 0.24 | 0.96 | 0.86 | 0.83 | ICC | 0.18 | 0.07 | 0.26 | 0.96 | 0.85 | 0.86 | ICC | 0.20 | 0.10 | 0.24 | 0.96 | 0.86 | 0.84 | ||||
| HC | 0.18 | 0.14 | 0.16 | 0.59 | 0.54 | 0.48 | 0.65 | HC | 0.08 | 0.06 | 0.08 | 0.45 | 0.38 | 0.42 | 0.57 | HC | 0.13 | 0.10 | 0.11 | 0.51 | 0.46 | 0.45 | 0.60 | |
N = 297 for VIQ and PIQ
Entries in bold-face are significant at the 0.05 level
Associations between Total and Regional Brain Volumes, Intracranial Cavity and Head Circumference in Children and Adolescents
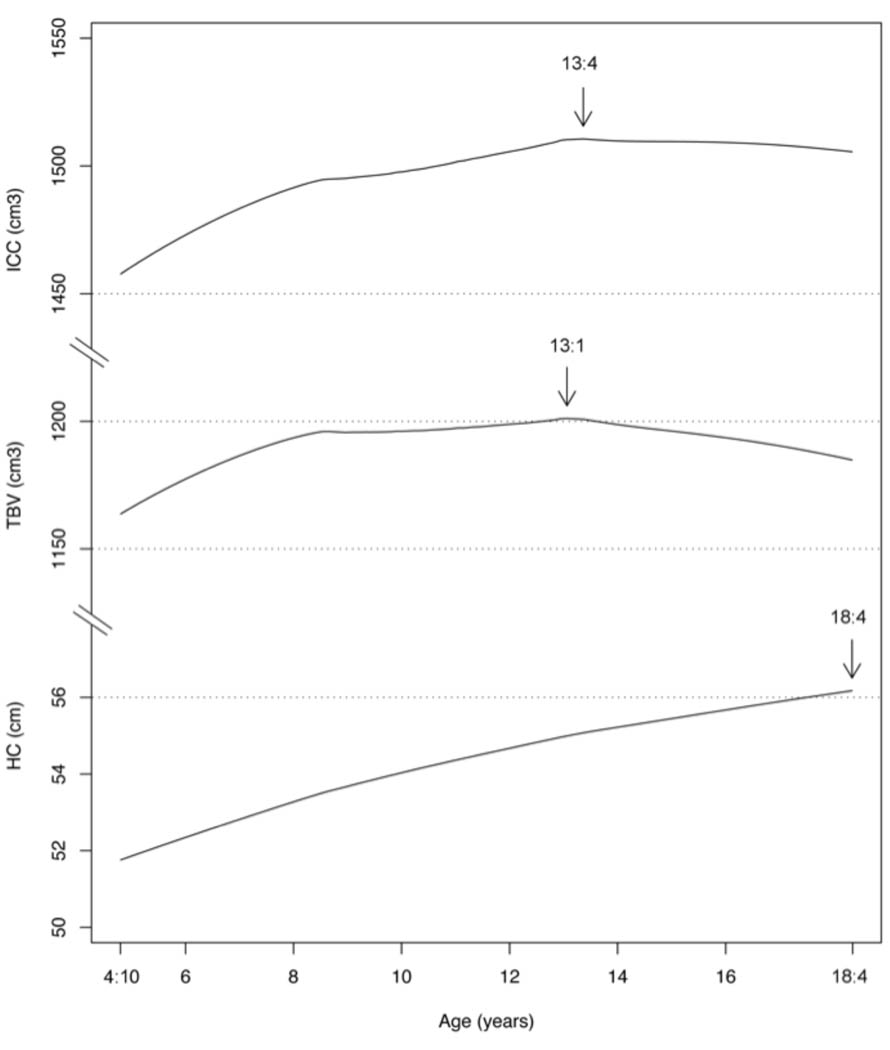
Volumetric correlations in two “younger” and “older” age groups, 4:10–11:1 and 12:0 – 18:4 years of age (Table 3, lower left and center triangles) indicated that the correlations of TBV with HC were highest in the younger children (0.40–0.79) and decreased in the older group (0.26–0.45). In contrast, the correlation between TBV and ICC remained high across the developmental period (0.93–0.96). Age-dependent HC- and ICC-volumetric correlation decreases were anticipated because, as shown in Figure 1, the cross-sectional age trajectories of TBV, ICC, and HC are similar when children are young but diverge during early adolescence when brain and intracranial cavity growth cease but head growth continues.
IQ and Total Brain Volume
We subdivided TBV into three groups, low (less than 2 SDs from the mean), medium (between - 2 SDs and 2 SDs from the mean) and high (greater than 2 SDs from the mean). We observed lower-powered positive VIQ-TBV increases in the subgroup analysis. Mean PIQ was found to increase by 15 points for participants with medium TBV compared to those with low TBV (p < 0.0001). Further subgroup analysis showed that the VIQ-TBV association was significant only in the low PIQ subgroup, by 11 points (p = 0.007). PIQ also showed significant upward shifts with increased TBV. Mean VIQ increased by 13 points for participants with medium compared to low TBV (p = 0.045). No VIQ subgroup exhibited a significant shift. As in PIQ, mean FSIQ was found to increase by 15 points for participants with medium TBV compared to those low TBV (p = 0.003). On average, participants having low VIQ and medium TBV were found to have 7 more VIQ points than those with low VIQ and low TBV (p = 0.041). No other FSIQ subgroup showed a significant shift.
Dissociations of IQ, Total and Regional Brain Volumes, Intracranial Cavity and Head Circumference by Age and Sex
We observed numerous differences between male and female measures of brain size, head size and measures of intelligence.
Dissociations of Total and Regional Brain Volumes and Head Circumference
Analyses of additional conditional correlations by sex (Table 3, lower left and center triangles) and of joint correlations by age and sex together (Table 3, four triangles in its upper left quadrant) identified many significant differences between male and female measures of brain size, head size and intelligence. The most salient dissociation between TBV and HC occurred between younger and older males, a dramatic drop in correlation from 0.79 to 0.26 (p < 0.0000001). Age-dependent changes in GM and WM associations with HC contributed roughly equally to this dissociation in males only (p < 0.001). Additional sexually dimorphic dissociations were evident among age group transitions, showing decreased HC-WM correlation in younger females (0.31) compared to younger males (0.61, p = 0.018).
Dissociations of Intelligence, Total and Regional Brain Volumes, Intracranial Cavity and Head Circumference
The strongest and most consistent associations between IQ measures and brain volumetrics were found in PIQ correlations with TBV, lobar GM and lobar WM. The highest correlation found was between PIQ and TBV for males aged 4:10 – 11:11 years (0.32) and the lowest between PIQ and lobar WM overall (0.18). A major dissociation of IQ and brain volumetrics was observed in the older age group, where only PIQ exhibited correlations with TBV, lobar GM and ICC (0.25–0.26).
Section 3. Comprehensive Linear Regression Models
Stage 1
Table 4 contains results from stage 1 regression analyses. Highly significant effects of parental education, with college graduation as baseline, were evident in IQ for males and females separately and combined. Mean VIQ was observed to decrease by about 18 points in males whose most highly educated parent holds only a high school diploma (p < 0.001) and by about 11 points if that parent attended some college but does not hold a college degree (p = 0.001). In females, corresponding but more moderate VIQ decreases were observed, of 10 and 6 points (p ~ 0.03) and in the sex-combined analysis. Standardized increases in AFI were associated with increased PIQ of 3 points per SD in females (p = 0.001) and of 2 points in the combined sample (p = 0.008).
Table 4.
Linear model summaries, Stage 1. Key: Δ, change on IQ due to statistical effect; p, p-value. College graduation was the baseline for estimating statistical effects of parental education.
| Male | ||||||
|---|---|---|---|---|---|---|
| PIQ | VIQ | FSIQ | ||||
| Estimated effect | Δ | p | Δ | p | Δ | p |
| Income | 3.6 | 0.049 | ||||
| High School | −17.6 | < 0.0001 | −13.6 | < 0.001 | ||
| Some College | −11.3 | < 0.001 | −10.3 | < 0.001 | ||
| Total Brain Volume | 3.1 | 0.002 | 3.0 | 0.002 | ||
| Female | ||||||
|---|---|---|---|---|---|---|
| PIQ | VIQ | FSIQ | ||||
| Estimated effect | Δ | p | Δ | p | Δ | Δ |
| Income | 3.0 | < 0.001 | ||||
| High School | −9.7 | 0.030 | −8.3 | 0.026 | ||
| Some College | −6.4 | 0.033 | −5.8 | 0.019 | ||
| Total Brain Volume | 1.9 | 0.026 | ||||
| Combined | ||||||
|---|---|---|---|---|---|---|
| PIQ | VIQ | FSIQ | ||||
| Estimated effect | Δ | p | Δ | p | Δ | p |
| Income | 2.1 | 0.008 | ||||
| High School | −5.8 | 0.037 | −13.6 | < 0.00001 | −10.8 | < 0.0001 |
| Some College | −4.2 | 0.031 | −8.4 | < 0.0001 | −7.9 | < 0.0001 |
| Total Brain Volume | 2.6 | 0.001 | 2.9 | < 0.001 | ||
The statistical effects of unit increases in TBV on IQ remained significant after adjustments for age-related associations and when all significant demographic associations were considered concurrently. A unit SD increase in TBV was associated with increased PIQ of about 3 points in males (p = 0.002). This increase was not observed in females separately but was reflected in the sex-combined analysis (p = 0.001). FSIQ was observed to increase with unit increases in TBV of about 3 points in males (p = 0.002), of about 2 points in females (p = 0.026), and of about 3 points in the combined sample (p < 0.001).
Stage 2
Table 5 contains findings from our stage 2 regression analysis, in which TBV was partitioned into frontal, temporal, parietal and occipital, subcortical GM and cerebellar volumes. Parental education and AFI associations were found to be similar to those evident in the TBV analysis (Table 4) yet less pronounced in males and females when analyzed separately, as anticipated from results previously reported herein. IQ was associated with frontal and temporal lobe volumes only (Table 5A). In males, PIQ was found to increase by 3 points with each SD unit increase in frontal lobe volume (p = 0.004) and FSIQ increased nearly 3 points with each SD unit variance increase in temporal lobe volume (p = 0.0004). In females, a unit increase in temporal lobe volume was associated with increased PIQ of about 2 points (p = 0.014), increased VIQ of nearly 5 points (p = 0.022) and increased FSIQ of about 5 points (p = 0.004). The full sample exhibited increases in PIQ of nearly 3 points with unit increases in temporal lobe volume (p < 0.001) and in FSIQ of 4 points (p = 0.007). No IQ-volume association persisted in corresponding relative analyses of regional volume to TBV ratios (Table 5B). We also observed increases in PIQ of between 2–3 points associated with AFI in males, females and the full sample (p ~ 0.005).
Table 5.
| Table 5A. Linear model summaries, Stage 2 absolute. Key: Δ, change on IQ due to statistical effect; p, p-value. College graduation was the baseline for estimating statistical effects of parental education. | ||||||
|---|---|---|---|---|---|---|
| Male | ||||||
| PIQ | VIQ | FSIQ | ||||
| Effect | Δ | p | Δ | p | Δ | p |
| High School | −16.9 | < 0.0001 | −13.8 | < 0.001 | ||
| Some College | −10.3 | 0.001 | −10.1 | < 0.001 | ||
| Frontal Lobe | 3.0 | 0.004 | ||||
| Temporal Lobe | 2.8 | 0.004 | ||||
| Female | ||||||
|---|---|---|---|---|---|---|
| PIQ | VIQ | FSIQ | ||||
| Effect | Δ | p | Δ | p | Δ | p |
| Income | 3.0 | 0.001 | 2.2 | 0.024 | ||
| Some College | −5.7 | 0.019 | ||||
| Temporal Lobe | 2.3 | 0.014 | 4.6 | 0.022 | 5.2 | 0.004 |
| Combined | ||||||
|---|---|---|---|---|---|---|
| PIQ | VIQ | FSIQ | ||||
| Effect | Δ | p | Δ | p | Δ | p |
| Income | 2.1 | 0.009 | ||||
| High School | −13.0 | < 0.0001 | −10.3 | < 0.0001 | ||
| Some College | −7.9 | < 0.0001 | −7.4 | < 0.0001 | ||
| Temporal Lobe | 2.7 | <10−3 | 4.0 | 0.007 | ||
| Table 5B. Linear model summaries, Stage 2 absolute. Key: Δ, change on IQ due to statistical effect; p, p-value. College graduation was the baseline for estimating statistical effects of parental education. | ||||||
|---|---|---|---|---|---|---|
| Male | ||||||
| PIQ | VIQ | FSIQ | ||||
| Effect | Δ | p | Δ | p | Δ | p |
| Income | 3.3 | 0.001 | ||||
| High School | −17.3 | < 0.0001 | −13.2 | < 0.001 | ||
| Some College | −10.9 | < 0.001 | −9.5 | 0.001 | ||
| Female | ||||||
|---|---|---|---|---|---|---|
| PIQ | VIQ | FSIQ | ||||
| Effect | Δ | p | Δ | p | Δ | p |
| Income | 3.3 | <10−3 | 2.5 | 0.014 | ||
| Some College | −5.9 | 0.049 | −6.3 | 0.012 | ||
| Combined | ||||||
|---|---|---|---|---|---|---|
| PIQ | VIQ | FSIQ | ||||
| Effect | Δ | p | Δ | p | Δ | p |
| Sex | 3.5 | 0.012 | ||||
| Income | 2.3 | 0.005 | ||||
| High School | −13.5 | < 0.00001 | −10.8 | < 0.0001 | ||
| Some College | −8.4 | < 0.0001 | −7.8 | < 0.0001 | ||
Stage 3
Table 6 contains a summary of our regression analysis from stage 3, which investigated relations between IQ and all tissue-segmented regional brain volumes. Increases in male VIQ were associated with SD unit increases in frontal GM of about 5 points (p = 0.047), and with unit increases of temporal WM of over 5 points (p = 0.036); Table 6A. Increases in PIQ were associated with unit increases in frontal WM of over 3 points in males only (p = 0.002), and with unit increases in temporal GM and WM of about 2 points in the combined sample (p ~ 0.045). FSIQ increases of over 4 points were associated with unit increases in temporal WM in females and the combined sample. Of all brain regions, temporal GM, temporal WM and frontal WM volumes had the most widely distributed associations across the IQ measures in both absolute (Table 6A) and relative (Table 6B) terms. IQ appeared to be unrelated to other regional brain volumes.
Table 6.
| Table 6A. Linear model summaries, Stage 3 absolute, Key: Δ, change on IQ due to statistical effect; p, p-value. College graduation was the baseline for estimating statistical effects of parental education. | ||||||
|---|---|---|---|---|---|---|
| Male | ||||||
| PIQ | VIQ | FSIQ | ||||
| Effect | Δ | p | Δ | p | Δ | p |
| High School | −15.9 | < 0.001 | −13.0 | < 0.001 | ||
| Some College | −10.5 | 0.001 | −9.7 | < 0.001 | ||
| Frontal GM | 4.9 | 0.047 | 2.8 | 0.014 | ||
| Frontal WM | 3.4 | 0.002 | ||||
| Temporal WM | 5.3 | 0.036 | ||||
| Female | ||||||
|---|---|---|---|---|---|---|
| PIQ | VIQ | FSIQ | ||||
| Effect | Δ | p | Δ | p | Δ | p |
| Income | 3.0 | 0.002 | 2.1 | 0.029 | ||
| High School | −7.6 | 0.039 | ||||
| Some College | −5.6 | 0.020 | ||||
| Temporal WM | 4.2 | 0.017 | ||||
| Combined | ||||||
|---|---|---|---|---|---|---|
| PIQ | VIQ | FSIQ | ||||
| Effect | Δ | p | Δ | p | Δ | p |
| Income | 3.0 | <10−4 | ||||
| High School | −12.7 | < 0.0001 | −10.0 | < 0.0001 | ||
| Some College | −8.2 | < 0.001 | −7.5 | < 0.0001 | ||
| Frontal GM | 2.2 | 0.017 | ||||
| Temporal GM | 1.6 | 0.042 | ||||
| Temporal WM | 1.6 | 0.048 | 4.0 | 0.009 | ||
| Table 6B. Linear model summaries, Stage 3 relative. Key: Δ, change on IQ due to statistical effect; p, p-value. College graduation was the baseline for estimating statistical effects of parental education. | ||||||
|---|---|---|---|---|---|---|
| Male | ||||||
| PIQ | VIQ | FSIQ | ||||
| Effect | Δ | p | Δ | p | Δ | p |
| High School | −15.4 | < 0.001 | −12.1 | 0.001 | ||
| Some College | −9.9 | 0.008 | −7.8 | 0.022 | ||
| Frontal Lobe WM | −5.4 | 0.018 | ||||
| Female | ||||||
|---|---|---|---|---|---|---|
| PIQ | VIQ | FSIQ | ||||
| Effect | Δ | p | Δ | p | Δ | p |
| Income | 4.2 | < 0.0001 | 3.8 | 0.001 | 4.5 | < 0.00001 |
| Frontal Lobe WM | −5.4 | 0.005 | ||||
| Temporal Lobe GM | 5.1 | 0.002 | ||||
| Combined | ||||||
|---|---|---|---|---|---|---|
| PIQ | VIQ | FSIQ | ||||
| Effect | Δ | p | Δ | p | Δ | p |
| Income | 2.1 | 0.040 | ||||
| High School | −11.4 | 0.001 | −9.3 | 0.001 | ||
| Some College | −7.6 | 0.002 | −7.0 | 0.001 | ||
| Frontal Lobe WM | −8.7 | 0.001 | −3.3 | 0.008 | ||
| Temporal Lobe WM | 3.5 | 0.024 | ||||
DISCUSSION
Although qualitative relations between child IQ, total brain volume and parental education are well known, the quantitative contributions of child total brain volume and parental demographic factors to variation in child IQ are inconsistent and not known. To our knowledge, ours is the first study to examine simultaneously the associations between IQ and total and regional brain volumes, age, sex, family income and parental education and in a large representative sample of typically developing children and adolescents. First, we find that total and regional brain volumes do not mediate the strong association between parental education and child IQ. Second, we find that child and adolescent performance IQ, not verbal IQ, contributes in page part to our observed correlations, between 0.14 to 0.27, of full scale IQ with temporal gray matter, temporal white matter and frontal white matter volumes. The volumes of other lobar, subcortical and subtentorial structures, when measured at our macroanatomic spatial and tissue imaging resolution, do not contribute significantly to variation in IQ when one acknowledges, concurrently, the association between IQ and parental education levels. We find that associations between VIQ and PIQ and total and regional brain volumes, parental education and family income are not uniform across the brain or the sexes. Third, we find that head circumference is an inadequate index of cerebral volume in typically developing older children and adolescents.
Parental Education
Our results demonstrate the importance of measuring parental education in studies designed to analyze the relations of IQ and total and regional brain volumes. The influence of parental education on IQ has been shown previously for children 4–8 years of age (Gale, et al., 2006; Gale, et al., 2004). The majority of studies examining the relationship of IQ to brain variables have considered full scale IQ only. We found different associations between parent education and brain morphometry on verbal, performance and full scale IQ.
In our analysis, the associations of parental education with childhood IQs are larger than IQ associations with unit increases in TBV and regional brain volumes. The influence of parental education on IQ in children is not mediated by total or regional brain volume measured in our cross-sectional design. Parental education levels are not correlated with any of the brain volumes examined in the children. Parental education may be associated with genetic and non-genetic mechanisms to influence other aspects of brain development that are associated with VIQ and PIQ in children. Genetic mechanisms may influence the correlation between IQ and education in parents and the heritability of IQ (Toga & Thompson, 2005). Non-genetic pathways may include associations between maternal education and prenatal care, nutrition during infancy and early childhood (Isaacs et al., 2008) through their enrichment of the environment and experiences to which a child is exposed during postnatal development. Parental education likely has significant associations with aspects of brain development in children other than total and regional brain volumes. Longitudinal growth trajectories, white matter microstructure, patterns of brain activation and neural network connectivity all appear to be related to IQ (Schmithorst & Holland, 2006; Schmithorst, Wilke, Dardzinski, & Holland, 2005; Shaw, et al., 2006).
IQ and Brain Size in Children, Adolescents and Adults
Our findings replicate past studies that found significant correlations between TBV and IQ and highlighted the importance of frontal and temporal lobes in this relationship. The large NIH sample is, however, the most representative sample of the entire United States population with respect to income and race/ethnicity to date; previous study findings are derived from non-representative samples. Hence, more important than replication, the quantitative findings reported here may be taken as normatively representative and employed for predictive and diagnostic purposes.
The correlation between full scale IQ and cerebral volume in the typically developing children in our study is small but significant (0.20 – 0.27), in general agreement with the correlations reported in the literature in older children and adolescents, and, as expected, lower than in adults. In our study of the NIH sample, the FSIQ-TBV correlation is due to the significant correlation with PIQ and contributions from both lobar gray matter and lobar white matter volumes. Associations between FSIQ and lobar gray matter volume have been reported (Posthuma, et al., 2002; Reiss, et al., 1996). Verbal IQ is correlated to a much lesser degree with TBV, total lobar gray matter and white matter volumes in the children we studied. Our tissue-specific analyses show that the IQ-lobar GM volume associations are most prominent in frontal and temporal lobes. This finding is in agreement with multiple studies, mostly in adults, showing a link between IQ and these volumes (Frangou, Chitins, & Williams, 2004; Reiss, et al., 1996; Shaw, et al., 2006; Thompson, et al., 2001), although some studies find more widely distributed lobar GM involvement (Colom, Jung, & Haier, 2006; Haier, Jung, Yeo, Head, & Alkire, 2004; Wilke, Sohn, Byars, & Holland, 2003). The frontal and temporal lobes develop later than other regions of the brain, are important in complex cognitive processes such as language and executive function, and, as IQ, show increasing heritability with age during development (Lenroot, et al., 2007). No other brain regions appear to be involved in the relationship as directly.
Performance IQ, Temporal GM, Frontal GM and Frontal WM
Our findings suggest that aspects of brain development resulting in increased brain size, particularly increased lobar GM and lobar WM volumes, are more strongly associated with PIQ than VIQ. Non-volumetric aspects of brain development, such as the quality of proximal and distal brain connectivity and neural transmission, may be more important in the development of VIQ. Verbal IQ is more affected than full scale IQ by environmental exposures during fetal and postnatal development (Hibbeln et al., 2007; Horwood et al., 2001; Isaacs et al., 2008). Early nutrition, particularly in pre-term babies and better brain growth with early calorie and nutrient loading, may provide a more complex environment and early stimulation related to income and educational variables combined with better food sources during a critical period of early brain growth that, in turn, relates to intellectual development. It may be that these environmental exposures have relatively greater associations with qualitative aspects of brain development, related to VIQ, than on volumetric aspects of brain development, related to PIQ.
Most studies examining the relation of regional brain volume and IQ have considered gray matter only. Our findings from the large representative sample to date highlight the importance of frontal and particularly temporal WM volume when interpreting IQ variation in children and adolescents. The association between IQ and frontal lobe WM volume was seen in males and in males and females with relative WM volume. The role of white matter in intellectual performance has been suggested by studies that have found an association between voxel-based WM microstructure and measures of cortico-cortical connectivity and IQ (Lerch, et al., 2006; Schmithorst & Holland, 2006; Schmithorst, et al., 2005). Various aspects of intellectual function are known to involve intra- and inter-hemispheric communication between widely distributed cortical regions (Shaw, 2007). Our results suggest that WM connectivity with frontal and temporal cortices may play a critical role in IQ variation in children and adolescents.
Sexual dimorphisms
Consistent with past studies, we find sexual dimorphisms in relations between IQ and brain volume, supporting the evidence that developmental processes linking brain function and IQ may be different in males and females (Schmithorst & Holland, 2006). We also demonstrate the utility of sex-stratified and sex-combined approaches to investigate these dimorphisms. We find multiple, intriguing age-related associations that support the observations of other investigators (Schmithorst & Holland, 2006; Shaw, et al., 2006; Wilke, et al., 2003) and emphasize the need for further developmental approaches for the study of brain-IQ relations (Shaw, 2007).
Head Circumference
Although head circumference is used frequently as a measure of brain size, we find that it is an inadequate surrogate for cerebral volume in older typically developing children. The HC-TBV correlation of 0.59 for the children in our sample is smaller than correlations reported previously (Bartholomeusz, Courchesne, & Karns, 2002; Gale, et al., 2004; Hazlett, et al., 2005; Piven, Arndt, Bailey, & Andreasen, 1996). Very young children aged less than 4 years, 10 months were not included in the analysis presented here. The trajectories of TBV and HC diverge in adolescence when total brain growth ends and HC continues to increase. This differential growth pattern accounts for decreasing correlations of HC and TBV from childhood to adolescence (Lainhart, Lazar, Bigler, & Alexander, 2005). Some samples used to measure the correlation of HC and TBV have included individuals with autism (Hazlett, et al., 2005; Piven, et al., 1996), who as a group are known to have increased HC variance compared to typical individuals (Lainhart, et al., 2006).
The main caution that results from the HC-TBV correlation found in the NIH MRI sample is that head circumference is an insufficient index of TBV in typically developing older children and adolescents. In pediatrics, since the 1970s, it has been well known that the absence of hydrocephalus and gross abnormalities of head shape, head circumference was a practical, low-cost surrogate for brain size particularly during early childhood, where significant disturbances in whole-brain growth are accompanied by abnormal changes in head circumference. As seen in the NIH data (Figure 1), the maxima of TBV, ICC and HC occur, on average, at different ages. Total brain volume is the first to reach its maximum, and plateaus thereafter. Intracranial cavity volume reaches its maximum at a slightly older age, followed by the head circumference maximum which continues to increase (Aylward et al., 2002) due to increased skull and scalp thickness. When total brain volume decreases gradually in adulthood, head circumference and intracranial cavity volume remain relatively constant (Lainhart, et al., 2005) and thus diminshing their value as reliable surrogates of age-related developmental changes in total brain volume in older children and adolescents.
Limitations
Age-related changes found in this study are based on cross-sectional data and need to be confirmed or denied by longitudinal analyses (Shaw, 2007). The sampling distribution of parental education favors college and graduate level education that may limit generalization to less advantaged children and families and may also help explain why IQ scores in this sample are higher than average. Matching parental education proportions to those in the US Census 2000, in addition to AFI and race/ethnicity, would, however, have compromised sampling feasibility and reduced sample size given available resources. Relations between cortical thickness and cognitive ability (Karama, Ad-Dab'bagh et al. 2008) have not been addressed in this paper.
CONCLUSIONS
Our findings contribute to the understanding of neural-environmental associations with IQ in healthy children and may also inform brain-behavior-environment studies of developmental disorders in childhood. Unique relations between IQ, parental education, total brain volume, regional brain volumes, income and sex in children with developmental disabilities, such as autism, need to be investigated and compared to our findings in typically developing healthy children. Causal pathways linking IQ, brain development and parental education need to be understood at genetic and non-genetic levels in both typically developing children and in children with atypical development. In addition to IQ, associations between parental education and brain morphometry with other skills and abilities, such as social cognition, also need to be investigated. These future studies will lead to a better understanding of what can be done to improve cognitive functioning in health, disease and disorder in childhood.
Figure 2.
Empirical model-free curves for head circumference (HC), total brain volume (TBV) and intracranial cavity volume (ICC) by cross-sectional age. Arrows indicate curve maxima.
ACKNOWLEDGEMENTS
This work was supported by NINDS R01 NS34783 and NIMH P50 MH60450 (NL), RO1 HD048946 (EB) and NIMH RO1 MH080826 (JEL). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes Of Mental Health or National Institutes of Health. Data employed in the preparation of this article were obtained from the Pediatric MRI Data Repository created by the NIH MRI Study of Normal Brain Development, Release 1. These data derive from a multi-site, longitudinal study of typically developing children, from ages newborn through young adulthood conducted by the Brain Development Cooperative Group and supported by the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (Contract #s N01-HD02-3343, N01-MH9-0002, and N01-NS-9-2314, -2315, -2316, -2317, -2319 and -2320). A listing of the participating sites and a complete listing of the study investigators can be found at http://www.bic.mni.mcgill.ca/nihpd/info/participating_centers.html. Special thanks to the NIH contract officers for their support. We also acknowledge the important contribution of John Haselgrove, Ph.D. (deceased). We thank all of the parents, children and adolescents who participated in this study.
BRAIN DEVELOPMENT COOPERATIVE GROUP AUTHORSHIP LIST
The MRI Study of Normal Brain Development is a cooperative study performed by six pediatric study centers in collaboration with a Data Coordinating Center (DCC), a Clinical Coordinating Center (CCC), a Diffusion Tensor Processing Center (DPC), and staff of the National Institute of Child Health and Human Development (NICHD), the National Institute of Mental Health (NIMH), the National Institute on Drug Abuse (NIDA), and the National Institute for Neurological Diseases and Stroke (NINDS), Rockville, Maryland.
Key personnel from the six pediatric study centers are as follows: Children’s Hospital Medical Center of Cincinnati, Principal Investigator William S. Ball, M.D., Investigators Anna Weber Byars, Ph.D., Mark Schapiro, M.D., Wendy Bommer, R.N., April Carr, B.S., April German, B.A., Scott Dunn, R.T.; Children’s Hospital Boston, Principal Investigator Michael J. Rivkin, M.D., Investigators Deborah Waber, Ph.D., Robert Mulkern, Ph.D., Sridhar Vajapeyam, Ph.D., Abigail Chiverton, B.A., Peter Davis, B.S., Julie Koo, B.S., Jacki Marmor, M.A., Christine Mrakotsky, Ph.D., M.A., Richard Robertson, M.D., Gloria McAnulty, Ph.D; University of Texas Health Science Center at Houston, Principal Investigators Michael E. Brandt, Ph.D., Jack M. Fletcher, Ph.D., Larry A. Kramer, M.D., Investigators Grace Yang, M.Ed., Cara McCormack, B.S., Kathleen M. Hebert, M.A., Hilda Volero, M.D.; Washington University in St. Louis, Principal Investigators Kelly Botteron, M.D., Robert C. McKinstry, M.D., Ph.D., Investigators William Warren, Tomoyuki Nishino, M.S., C. Robert Almli, Ph.D., Richard Todd, Ph.D., M.D., John Constantino, M.D.; University of California Los Angeles, Principal Investigator James T. McCracken, M.D., Investigators Jennifer Levitt, M.D., Jeffrey Alger, Ph.D., Joseph O’Neil, Ph.D., Arthur Toga, Ph.D., Robert Asarnow, Ph.D., David Fadale, B.A., Laura Heinichen, B.A., Cedric Ireland B.A.; Children’s Hospital of Philadelphia, Principal Investigators Dah-Jyuu Wang, Ph.D. and Edward Moss, Ph.D., Investigators Robert A. Zimmerman, M.D., and Research Staff Brooke Bintliff, B.S., Ruth Bradford, Janice Newman, M.B.A. The Principal Investigator of the data coordinating center at McGill University is Alan C. Evans, Ph.D., Investigators Rozalia Arnaoutelis, B.S., G. Bruce Pike, Ph.D., D. Louis Collins, Ph.D., Gabriel Leonard, Ph.D., Tomas Paus, M.D., Alex Zijdenbos, Ph.D., and Research Staff Samir Das, B.S., Vladimir Fonov, Ph.D., Luke Fu, B.S., Jonathan Harlap, Ilana Leppert, B.E., Denise Milovan, M.A., Dario Vins, B.C., and at Georgetown University, Thomas Zeffiro, M.D., Ph.D. and John Van Meter, Ph.D. Nicholas Lange, Sc.D., Harvard University/McLean Hospital, is a statistical study design and data analysis Investigator to the Data Coordinating Center, assisted by Michael P. Froimowitz, M.S. The Principal Investigator of the Clinical Coordinating Center at Washington University is Kelly Botteron, M.D., Investigators C. Robert Almli Ph.D., Cheryl Rainey, B.S., Stan Henderson M.S., Tomoyuki Nishino, M.S., William Warren, Jennifer L. Edwards M.SW., Diane Dubois R.N., Karla Smith, Tish Singer and Aaron A. Wilber, M.S. The Principal Investigator of the Diffusion Tensor Processing Center at the National Institutes of Health is Carlo Pierpaoli, M.D., Ph.D., Investigators Peter J. Basser, Ph.D., Lin-Ching Chang, Sc.D., Chen Guan Koay, Ph.D. and Lindsay Walker, M.S. The Principal Collaborators at the National Institutes of Health are Lisa Freund, Ph.D. (NICHD), Judith Rumsey, Ph.D. (NIMH), Lauren Baskir, Ph.D. (NIMH), Laurence Stanford, PhD. (NIDA), Karen Sirocco, Ph.D. (NIDA) and from NINDS, Katrina Gwinn-Hardy, M.D., and Giovanna Spinella, M.D. The Principal Investigator of the Spectroscopy Processing Center at the University of California Los Angeles is James T. McCracken, M.D., Investigators Jeffery R. Alger, Ph.D., Jennifer Levitt, M.D., Joseph O’Neill, Ph.D.
REFERENCES
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19(6):716–723. [Google Scholar]
- Andreasen NC, Flaum M, Swayze V, 2nd, O7Leary DS, Alliger R, Cohen G, et al. Intelligence and brain structure in normal individuals. American Journal of Psychiatry. 1993;150(1):130–134. doi: 10.1176/ajp.150.1.130. [DOI] [PubMed] [Google Scholar]
- Aylward EHMN, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59(2):175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Bartholomeusz HH, Courchesne E, Karns CM. Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. Neuropediatrics. 2002;33(5):239–241. doi: 10.1055/s-2002-36735. [DOI] [PubMed] [Google Scholar]
- Brain Development Cooperative Group. The NIH MRI study of normal brain development. NeuroImage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Cleveland WS. LOWESS: A program for smoothing scatterplots by robust locally weighted regression. The American Statistician. 1981;35:54. [Google Scholar]
- Collins DL, Holmes CJ, Peters TM, Evans AC. Automatic 3D model-based neuroanatomical segmentation. Human Brain Mapping. 1995;3:190–208. [Google Scholar]
- Colom R, Jung RE, Haier RJ. Distributed brain sites for the g-factor of intelligence. NeuroImage. 2006;31(3):1359–1365. doi: 10.1016/j.neuroimage.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential Ability Scales. San Antonio, TX: Psychological Corporation, Harcourt Brace and Company; 1990. [Google Scholar]
- Fisch RO, Bilek MK, Horrobin JM, Chang PN. Children with superior intelligence at 7 years of age: a prospective study of the influence of perinatal, medical, and socioeconomic factors. American Journal of Diseases of Children. 1976;130(5):481–487. doi: 10.1001/archpedi.1976.02120060027006. [DOI] [PubMed] [Google Scholar]
- Frangou S, Chitins X, Williams SC. Mapping IQ and gray matter density in healthy young people. NeuroImage. 2004;23(3):800–805. doi: 10.1016/j.neuroimage.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Gale CR, O'Callaghan FJ, Bredow M, Martyn CN. The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics. 2006;118(4):1486–1492. doi: 10.1542/peds.2005-2629. [DOI] [PubMed] [Google Scholar]
- Gale CR, O'Callaghan FJ, Godfrey KM, Law CM, Martyn CN. Critical periods of brain growth and cognitive function in children. Brain. 2004;127(Pt 2):321–329. doi: 10.1093/brain/awh034. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. Structural brain variation and general intelligence. NeuroImage. 2004;23(1):425–433. doi: 10.1016/j.neuroimage.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Harezlak J, Ryan LM, Giedd JN, Lange N. Individual and population penalized regression splines for accelerated longitudinal designs. Biometrics. 2005;61(4):1037–1048. doi: 10.1111/j.1541-0420.2005.00376.x. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Archives of General Psychiatry. 2005;62(12):1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Horwood LJ, Darlow BA, Mogridge N. Breast milk feeding and cognitive ability at 7–8 years. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2001;84:F23–F27. doi: 10.1136/fn.84.1.F23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Posthuma D, Mandl RC, Baare WF, van Oel C, et al. Genetic contributions to human brain morphology and intelligence. The Journal of Neuroscience. 2006;26(40):10235–10242. doi: 10.1523/JNEUROSCI.1312-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs EB, Gadian DG, Sabatini S, Chong WK, Quinn BT, Fischl BR, et al. The effect of early human diet on caudate volumes and IQ. Pediatrics. 2008;63(3):308–314. doi: 10.1203/PDR.0b013e318163a271. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Markham JA. The cellular basis for volume changes in the rat cortex during puberty: white and gray matter. Annals of the New York Academy of Sciences. 2004;1021:431–435. doi: 10.1196/annals.1308.058. [DOI] [PubMed] [Google Scholar]
- Karama S, Ad-Dab'bagh Y, Haier RJ, Deary IJ, Lyttelton OC, Lepage C, et al. Positive association between cognitve ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds. Intelligence. 2008;37(2):145–155. doi: 10.1016/j.intell.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DN, Lange N, Makris N, Bates J, Meyer J, Caviness VS., Jr Gyri of the human neocortex: an MRI-based analysis of volume and variance. Cerebral Cortex. 1998;8(4):372–384. doi: 10.1093/cercor/8.4.372. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, et al. Head circumference and height in autism: a study by the Collaborative Program of Excellence in Autism. American Journal of Medical Genetics Part A. 2006;140(21):2257–2274. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainhart JE, Lazar M, Bigler E, Alexander A. The Brain During Life in Autism: Advances in Neuroimaging Research. In: Casanova M, editor. Recent Developments in Autism Research. New York: NOVA Science Publishers; 2005. [Google Scholar]
- Lange N, Giedd JN, Castellanos FX, Vaituzis AC, Rapoport JL. Variability of human brain structure sizes: ages 4–20 years. Psychiatry Research: Neuroimaging. 1997;74(1):1–12. doi: 10.1016/s0925-4927(96)03054-5. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience & Biobehavioral Reviews. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, et al. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Human Brain Mapping. 2007 doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, et al. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. NeuroImage. 2006;31(3):993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- McDaniel MA. Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence. Inelligence. 2005;33(4):337–346. [Google Scholar]
- Neiss M, Rowe DC. Parental education and child's verbal IQ in adoptive and biological families in the National Longitudinal Study of Adolescent Health. Behavioral Genetics. 30(6):487–495. doi: 10.1023/a:1010254918997. [DOI] [PubMed] [Google Scholar]
- Ounsted M, Moar VA, Scott A. Head circumference and developmental ability at the age of seven years. Acta paediatrica Scandinavica. 1988;77(3):374–379. doi: 10.1111/j.1651-2227.1988.tb10663.x. [DOI] [PubMed] [Google Scholar]
- Piven J, Arndt S, Bailey J, Andreasen N. Regional brain enlargement in autism: a magnetic resonance imaging study. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35(4):530–536. doi: 10.1097/00004583-199604000-00020. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus EJ, Baare WF, Hulshoff Pol HE, Kahn RS, Boomsma DI. The association between brain volume and intelligence is of genetic origin. Nature Neuroscience. 2002;5(2):83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119(Pt 5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. Journal of Child Psychology and Psychiatry. 2003;44(8):1092–1115. doi: 10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Functional MRI evidence for disparate developmental processes underlying intelligence in boys and girls. NeuroImage. 2006;31(3):1366–1379. doi: 10.1016/j.neuroimage.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Human Brain Mapping. 2005;26(2):139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. Intelligence and the developing human brain. Bioessays. 2007;29(10):962–973. doi: 10.1002/bies.20641. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Blumenthal J, Gochman P, Nicolson R, et al. Mapping adolescent brain change reveals dynamic profile of accelerated gray matter loss in childhood-onset schizophrenia. NeuroImage. 2001;13:S1105. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Genetics of brain structure and intelligence. Annual Review of Neuroscience. 2005;28:1–23. doi: 10.1146/annurev.neuro.28.061604.135655. [DOI] [PubMed] [Google Scholar]
- van Belle G, Heagerty PJ, Fisher LJ, Lumley TS. Biostatistics: A Methodology For the Health Sciences. Wiley-Interscience; 2004. [Google Scholar]
- Venables VN, Ripley BD. Modern Applied Statistics with S. 4th ed. Springer-Verlag: 2002. [Google Scholar]
- Vernon PA, Wickett JC, Bazana PG, Stelmark RM. The neuropsychology and psychophysiology of human intelligence. In: Sternberg RJ, editor. Handbook of Intelligence. Cambridge, UK: Cambridge University Press; 2000. pp. 245–264. [Google Scholar]
- Waber DP, De Moor C, Forbes PW, Almli CR, Botteron KN, Leonard G, et al. The NIH MRI study of normal brain development: Performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. Journal of the International Neuropsychological Society. 2007:1–18. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation, Harcourt Brace and Company; 1999. [Google Scholar]
- Weinberg WA, Dietz SG, Penick EC, McAlister WH. Intelligence, reading achievement, physical size and social class. A study of St. Louis Caucasian boys aged 8–0 to 9–6 years, attending regular schools. Journal of Pediatrics. 1974;85(4):482–489. doi: 10.1016/s0022-3476(74)80449-x. [DOI] [PubMed] [Google Scholar]
- Wilke M, Sohn JH, Byars AW, Holland SK. Bright spots: correlations of gray matter volume with IQ in a normal pediatric population. NeuroImage. 2003;20(1):202–215. doi: 10.1016/s1053-8119(03)00199-x. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Beresh H, Kigar DL. Intelligence and brain size in 100 postmortem brains: sex, lateralization and age factors. Brain. 2006;129(Pt 2):386–398. doi: 10.1093/brain/awh696. [DOI] [PubMed] [Google Scholar]
- Zinkstok J, Schmitz N, van Amelsvoort T, de Win M, van den Brink W, Baas F, et al. The COMT val158met polymorphism and brain morphometry in healthy young adults. Neuroscience Letters. 2006;405(1–2):34–39. doi: 10.1016/j.neulet.2006.06.034. [DOI] [PubMed] [Google Scholar]