Abstract
Aims
Cardiac stem cells (CSCs) show potential as a cellular therapeutic approach to blunt tissue damage and facilitate reparative and regenerative processes after myocardial infarction. Despite multiple published reports of improvement, functional benefits remain modest using normal stem cells delivered by adoptive transfer into damaged myocardium. The goal of this study is to enhance survival and proliferation of CSCs that have undergone lineage commitment in early phases as evidenced by expression of proteins driven by the α-myosin heavy chain (αMHC) promoter. The early increased expression of survival kinases augments expansion of the cardiogenic CSC pool and subsequent daughter progeny.
Materials & methods
Normal CSCs engineered with fluorescent reporter protein constructs under control of the αMHC promoter show transgene protein expression, confirming activity of the promoter in CSCs. Cultured CSCs from both nontransgenic and cardiac-specific transgenic mice expressing survival kinases driven by the αMHC promoter were analyzed to characterize transgene expression following treatments to promote differentiation in culture.
Results & conclusion
Therapeutic genes controlled by the αMHC promoter can be engineered into and expressed in CSCs and cardiomyocyte progeny with the goal of improving the efficacy of cardiac stem cell therapy.
Keywords: AKT, cardiac regeneration, cardiac stem cell, heart, Pim-1, survival kinase, transgene
The heart is an attractive candidate for regenerative tissue therapy owing to the endogenous reservoir of stem cells that maintain the myocardium and replace myocytes over the lifetime of the organism [1]. However, these endogenous stem cells do not have the capacity to repair the extensive damage caused by myocardial infarction or chronic cardiovascular disease, leading to deterioration of hemodynamic output and eventual cardiac insufficiency. Cardiac stem cells (CSCs) are small, undifferentiated c-kit+ and/or Sca-1+ cells resident within the myocardium, which may coexpress cardiac-specific markers such as Gata4, Nkx2.5 or Mef2c [2]. CSCs harvested from myocardial tissue and grown in culture improve cardiac function after injection following a myocardial infarction in mouse [3,4], rat [5,6] and large animal [7] experimental models. Adoptively transferred CSCs improve myocardial remodeling following injury by a combination of blunting cell death, recruitment of endogenous reparative mechanisms and contribution of new cells to the damaged myocardium. Collectively, these salutary effects improve cardiac performance and ameliorate scar formation but usually the benefit is relatively modest and recovery of function still falls well below that of the healthy heart. One issue is the relatively short-lived nature of the adoptively transferred cell population, which results in limited engraftment and persistence after delivery [8,9]. Thus, molecular intervention strategies to enhance CSC proliferation, persistence and engraftment upon delivery would be a valuable and important advance in myocardial regenerative therapy. One such strategy involves the delivery of survival genes to the cells ex vivo prior to reintroduction into the myocardium, which improves and augments the beneficial effects of CSC therapy [10,11]. Appropriate regulatory elements to control these survival genes and their deployment in engineered stem cells remains an issue, since previous studies involve use of high-level viral promoters with unrestricted tissue expression. A more desirable approach would involve the focused use of cardiac-specific promoters to augment cardiogenic cell survival and commitment as part of an integrated stem cell-engineering strategy.
Assessment of the cardioprotective effects mediated by multiple survival and angiogenic proteins has been facilitated through use of cardiac-specific transgenesis in genetically engineered mice. Dozens of transgenic mouse lines have been reported that overexpress a gene of interest specifically in cardiomyocytes by controlling transgene expression with a portion of the mouse α-myosin heavy chain (αMHC) promoter. Several mouse models have been used to confirm that pro-survival and anti-apoptotic genes protect the myocardium in αMHC-driven transgenic mice [12]. Subramaniam et al. developed and characterized the αMHC transgenic promoter system, which is comprised of a 5.5-kB region between the murine α- and β-myosin heavy chain genes [13]. αMHC-driven transgene expression in the adult mouse was initially described as restricted to the myocardium and the large pulmonary veins of the lung [13]. The adjacent relationship between the α- and βMHC gene structure is conserved between human and mouse, and the αMHC (MHC6) promoter regions have a 60% sequence identity using Smith–Waterman local alignment.
Recent studies from our group have demonstrated that AKT (also known as protein kinase B) targeted to the nucleus protects the heart from ischemic injury without causing hypertrophy [14,15] and expands the CSC population in vivo [16] when expressed using the αMHC promoter. Another pro-survival kinase downstream of nuclear AKT known as Pim-1 is also highly protective in the myocardium in both infarction and pressure overload studies [17,18] when expressed using the αMHC promoter. Therefore, the capacity of the αMHC promoter to express cardioprotective genes within CSCs and cardiomyocyte could be advantageous in a genetic-engineering approach to enhance the survival, persistence, proliferation and engraftment of adoptively transferred CSCs into the myocardium. This study characterizes αMHC promoter expression in cultured cardiac CSCs to assess transgene expression and cellular phenotype.
Materials & methods
Animal models
All transgenic mice were derived in FVB strain (nontransgenic [NTG]) mouse line. Animals are housed in approved facilities and all surgeries were performed under anesthesia according to Institutional Animal Care and Use Committee-approved protocols. The MHCGFP mouse line expresses enhanced green fluorescent protein (GFP) under the control of the murine αMHC promoter, which is a 5.5-Kb section of the endogenous mouse αMHC promoter commonly used for cardiomyocyte-specific transgenic expression [13]. The Pim-1 wild-type (Pim-wt) mouse line overexpresses the 33-kDA human Pim-1 protein isoform as well as GFP driven by the αMHC promoter. The Akt-nuc mouse line expresses nucleartargeted AKT/PKB that is myc-tagged on the C-terminus driven by the αMHC promoter. Neonatal rat cardiomyocytes (NRCMs) were harvested from 2–3-day-old Sprague-Dawley rats, as previously described [19], and cultured in M199 media supplemented with 10% fetal bovine serum (FBS).
CSC isolation & culture
Cardiac stem cells were isolated and cultured as previously described [5]. Briefly, two mice per preparation are anesthetized using ketaminexylazine solution and the heart was cannulated through the aortic arch and perfused at 37°C in oxygenated basic buffer (Joklik’s Modification of MEME, 0.7 g/l HEPES, 1.25 g/l taurine, 20 U/l insulin, penicillin/streptomycin/glutamine, amphotericin, gentamicin, pH 7.3) on a Radnotti apparatus. The heart was then digested for 12 min at 37°C in 320 units/ml of collagenase II in oxygenated basic buffer. Afterwards, the heart was then minced in basic buffer plus 0.5% bovine serum albumin and the cardiomyocytes pelleted for 1 min at 100 g and discarded. Remaining cells in the supernatant were passed through a 25 μM filter and pelleted. The cell pellet was resuspended and incubated with anti-c-kit (CD117) Miltenyi beads in phosphate-buffered saline (PBS) plus 0.5% bovine serum albumin; washed and then isolated on a magnetic column to extract c-kit+ CSCs according to manufacturer’s instructions. CSCs are cultured according to standard tissue culture protocols in CSC media (DMEM/F12, 10% embryonic stem cell-grade FBS, penicillin/ streptomycin/glutamine, insulin–transferring– selenium, 1000 U/ml leukemia inhibitory factor, 40 ng/ml EGF and 20 ng/ml basic FGF).
Trypan blue assay
Cell cultures were counted after passage using 50% trypan blue solution by hemocytometer determination. For trypan blue assay (Figure 1E), 20,000 cells per well were seeded in a six-well plate and counted at 2 or 4 days postplating.
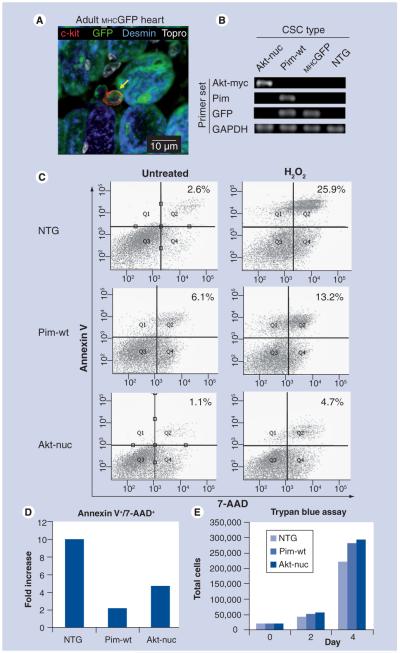
Figure 1. Cardiac stem cells from transgenic mice express αMHC driven transgenes in vivo and in culture.
(A) Immunolabeling of adult MHCGFP mouse heart section shows colocalization of c-kit (red) and GFP (green) staining. Desmin (blue) also colocalizes with GFP in mature myocytes. Scale bar is 10 μM. (B) CSCs isolated from MHCGFP, MHCAkt-nuc and MHCPim-wt were cultured in growth media and harvested for RNA isolation and cDNA synthesis. cDNA was amplified using primers specific for the transgene inserted into each CSC line. (C) Flow cytometric analysis of NTG, MHCPim-wt and MHCAkt-nuc CSCs either untreated (left) or hydrogen peroxide treated (right) stained for annexin V and 7-AAD apoptosis markers (representative of n = 2 independent experiments). (d) Fold-increase quantitation of annexin V+/7-AAD+ CSCs after hydrogen peroxide treatment in (C). (e) Trypan blue exclusion hemocytometer counts of NTG, MHCPim-wt and MHCAkt-nuc CSCs seeded at equal amounts on day 0 and counted 2 or 4 days later (representative of n = 3 independent experiments).
7-AAD: 7-amino-actinomycin D; CSC: Cardiac stem cell; GFP: Enhanced green fluorescent protein; MHC: Myosin heavy chain; NTG: Nontransgenic.
Flow cytometric analysis & treatments
Cardiac stem cells were seeded into six-well plates in CSC media for at least 24 h before treatment. Treated cells received 400 μM hydrogen peroxide (H2O2) for 4 h. After peroxide incubation, treated cells and untreated controls were labeled with BD Pharmingen annexin V-phycoerythrin Apoptosis Detection Kit (559763) according to manufacturer instructions. Briefly, cells were suspended at 100,000 cells per 100 μl in annexin V-binding buffer. Each set of treated and untreated cells was incubated for 20 min at room temperature in the dark with 0.75 μl annexin V and 2 μl 7-amino-actinomycin D (7-AAD) per 100,000 cells. A total of 300 μl of binding buffer was added postincubation and the cells were analyzed by flow cytometry with a BD FACSAria instrument. Unstained and single stain controls were used to establish baseline fluorescence levels for the samples.
RNA isolation & PCR
Cardiac stem cells were cultured according to standard procedure in CSC media and then harvested, counted and pelleted. One to two million cells from each CSC cell line were used to extract RNA using a Qiagen RNeasy RNA Isolation kit according to manufacturer’s protocols. cDNA was synthesized using 2 μg of RNA from each CSC line, random hexamer primers and the Applied Bioscience High Capacity Reverse Transcription kit according to the manufacturer’s instructions. cDNA was amplified using primers specific for each transgene as a well as GAPDH as a control.
Immunocytochemistry
Cardiac stem cells were plated on Permanox® chamber slides for at least 24 h. NRCMs were cultured on laminin-coated glass slides. After incubation, the slides were then washed once with PBS and fixed in 4% paraformaldehye (PFA) for at least 20 min at 4°C. After fixation, the slides were washed twice with PBS, permeablized with PBS plus 0.2% Triton-X 100; washed again and blocked with blocking buffer (PBS plus 10% horse serum) for 30 min. Primary antibodies were diluted in blocking buffer and incubated on the slides overnight at 4°C and then washed three-times in PBS. Fluorescently conjugated secondary antibodies were diluted in blocking buffer, incubated at room temperature for 2 h and then washed three-times in PBS. A table of antibodies and dilution ratios is available in Supplementary Table 1 (see online www.futuremedicine.com/toc/rme/4/6). Topro-3-Iodide (Topro) was included in the final wash to fluorescently label the DNA/nucleus. The coverslip was mounted with Vectashield® to preserve fluorescent signal. All slides were imaged using a Leica TCS SP2 confocal microscope.
Immunohistochemistry
The hearts were fixed by retroperfusion of 10% neutral buffered formalin and paraffin embedded as previously described [20]. Paraffin sections of mouse hearts cut at 4 μm were deparaffinized in xylene and rehydrated in a series of graded alcohols to distilled water. Antigen retrieval was performed in 10 mmol/l citrate pH 6.0 using 1100-W microwave oven for 3 min at high power and 12 min at 50% power. The slides were allowed to cool to room temperature, washed three-times in TN buffer (NaCl 150 mmol/l, Tris 100 mmol/l, pH 7.5) and quenched with 3% hydrogen peroxide in TN buffer for 20 min to block endogenous peroxidase activity. Slides were then washed in TN buffer and blocked for 1 h in TNB (1X TN buffer containing 0.5% blocking buffer, proprietary formula from Perkin Elmer™ kit). Primary antibodies were diluted in TNB and incubated overnight at 4°C. Slides were then washed three-times in TN and incubated for 2 h at room temperature in the dark with species-specific secondary antibodies conjugated to a fluorophore in TNB. Nuclei were stained for 20 min with Topro. Detection of some antigens required signal amplification using horseradish peroxidase-conjugated antibodies or strepavidin followed by reaction with a fluorescent tyramide substrate. Coverslips were mounted with Vectashield and imaged by confocal microscopy.
Treatments to promote differentiation
For the purposes of these studies, the term ‘differentiation’ refers to the cellular phenotypic responses to culture conditions involving inductive stimuli to promote lineage commitment. Induction of cardiomyogenic differentiation for imaging was performed by culturing CSCs on a layer of fixed NRCMs. Briefly, NRCMs were cultured for 3 days on laminin-coated glass chamber slides. Conditioned media was removed and stored for later use at 4°C. The NRCMs were washed twice with PBS and fixed in 4% PFA for exactly 20 min then washed three more times with PBS. The PFA was neutralized by a 30 min incubation of 0.1 M glycine followed a PBS wash and an overnight incubation with 10% FBS media. CSCs were passaged according to standard procedure, pelleted, resuspended in NRCM conditioned M199 plus 10% FBS media and added to the layer of fixed NRCMs for 7 days. Fresh media M199 plus 10% FBS media was supplemented to the slides on day 4. On day 7 the slides containing CSCs differentiated on NRCMs were fixed and stained for immuno-cytochemistry. Nonspecific control differentiation treatment involved CSC culture on Permanox slides for at least 24 h in CSC media and then replacing the media with differentiation media (α-MEM, 10% FBS, penicillin/streptomycin/glutamine, 10 nM dexamethasone and sodium bicarbonate pH 7.3). The CSCs were incubated with the differentiation media for 7 days and then fixed and stained for immunocytochemistry.
Cardiomyogenic differentiation for qPCR analysis
Nontransgenic CSCs were transduced with a lentiviral vector encoding GFP and subseuqently cocultured with pre-plated NRCMs in M199 plus 10% FBS for 7 days. Cocultured cells were trypsinized and cells with high-level GFP expression were separated from NRCMs by fluorescence-activated cell sorting. RNA was isolated from the GFP+ fraction using Trizol®/ chloroform extraction. cDNA was obtained with the Applied Bioscience High Capacity cDNA Reverse Transcription Kit. Taqman qPCR assays (Applied Biosystems) were performed using an MJ Research Opticon instrument for the following genes: troponin T (Mm00441922_m1), tropomyosin (Mm00600378_m1) and α-smooth muscle actin (Mm01546133_m1). β-actin served as an internal control. Data were analyzed using the Opticon instrument software with calculated Ct values and the ΔΔCt method for determining relative RNA/cDNA quantities.
Plasmid constructs & transfection
Plasmids were constructed using a pEGFP-C1 backbone. The cytomegalovirus (CMV)-td-Tomato-Flag construct expresses the Tomato fluorescent protein fused to a C-terminal Flag-tag under the control of the CMV promoter. The αMHC-GFP construct expresses EGFP under the same 5.5 kb αMHC promoter used to create transgenic mice. The αMHC-YFP-HA construct expresses yellow fluorescent protein fused to a C-terminal HA-tag under the αMHC promoter. Transfections were accomplished using 0.25–20.5 μG of purified plasmid per slide chamber in 1 ml of CSC media using the FuGENE® 6 transfection reagent according to manufacturer’s instructions.
RT-PCR primer sets & sequences
All primers were designed using Vector NTI 10 from Invitrogen. Transgene-specific primers were designed for the sequence of DNA that was used to create the respective transgenic animals. Primer sequences, annealing temperatures and expected product lengths are available in Supplementary Table 2.
Results
CSCs isolated from mice created by αMHC-specific transgenesis show reporter gene expression as well as the pro-proliferative & anti-apoptotic effects of these genes
Cardiac stem cells were previously reported to be expanded owing to αMHC-driven Akt-nuc expression in vivo [16]. Consistent with this finding, immunohistochemical staining of myocardial sections obtained from a transgenic mouse expressing GFP under control of the αMHC promoter (MHCGFP) heart section show colocalization of c-kit and GFP (Figure 1A). GFP was also expressed in mature myocytes as shown by colocalization of GFP with desmin staining (Figure 1A). Next, CSCs were isolated from MHCGFP as well as two additional cardiac-specific transgenic lines expressing survival kinases driven by the αMHC promoter: MHCAkt-nuc and MHCPim-wt [14,21]. The Akt-nuc construct possesses three C-terminal nuclear localization sequence repeats followed by an C-terminal myc-tag that allows for specific primers designed for the nuclear localization/Myc-tag sequence (‘Akt-myc’, Figure 1B). GFP mRNA expression was detected in cultured CSCs derived from MHCGFP, MHCAkt-nuc and MHCPim-wt myocardium by RT-PCR (Figure 1B) together with GAPDH expression as a quality control. Protective effects of both Pim-1 and Akt-nuc overexpression are evident after hydrogen peroxide treatment for 4 h. NTG CSCs show significant induction of annexin V/7-AAD staining (tenfold) indicative of apoptosis, whereas MHCPim-wt (twofold) and MHCAkt-nuc (fivefold) CSCs show a lesser induction (Figure 1C & D). MHCPim-wt and MHCAkt-nuc CSCs exhibit faster proliferation rates during in vitro culture by trypan blue exclusion assay (Figure 1E).
CSCs express fluorescent markers & tags when transfected with plasmid constructs under the control of the αMHC promoter
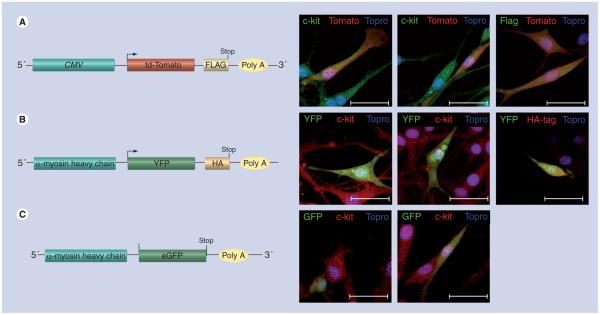
Nontransgenic CSCs were transfected with plasmids encoding fluorescent/tagged proteins driven by either the αMHC or CMV promoter to assess transgene expression. CSCs were transfected with a plasmid encoding the red/orange-fluorescent protein, td-Tomato, with a C-terminal Flag-tag under control of the constitutively active CMV viral promoter. CSCs expressing td-Tomato protein were readily evident within 24–48 h posttransfection. Immunocytochemistry colocalized td-Tomato-positive CSCs with both c-kit and Flag-tag markers (Figure 2A). Transfection of CSCs with a construct encoding fluorescent reporters under control of the αMHC promoter also resulted in expression for GFP (Figure 2B) or yellow fluorescent protein (Figure 2C). Transgene expression regulated by the αMHC promoter is specific to CSCs relative to either c-kit+ bone marrow stem cells (Supplementary Figure 1) or HEK-293 cells (data not shown) where fluorescent reporter protein expression was not observed.
Figure 2. Nontransgenic cardiac stem cells express tagged fluorescent marker proteins upon transfection with plasmid constructs under the control of the αMHC promoter.
Nontransgenic cardiac stem cells were cultured in growth media, transfected with plasmid constructs, allowed to express for 48 h and fixed. (A) CMV-Td-Tomato-Flag construct (left). Cells were transfected with a Tomato-Flag construct under the viral CMV promoter as a control and stained with c-kit or Flag-tag (green) and Topro (blue), td-Tomato protein fluorescence (red) (right). (B) αMHC-YFP-HA construct (left). Cells were transfected with a YFP-HA construct under the αMHC promoter and stained with c-kit or HA (red), Topro (blue) and YFP fluorescence (green) (right). (C) Diagram of αMHC-GFP construct (left). Cells were transfected with a GFP construct under control of the αMHC promoter and stained with c-kit (red), Topro (blue) and GFP protein fluorescence (green) (right). Scale bar is 40 μm.
CMV: Cytomegalovirus; eGFP: Enhanced Green fluorescent protein; MHC: Myosin heavy chain; YFP: Yellow fluorescent protein.
CSCs express transgenes controlled by the αMHC promoter in the appropriate subcellular localization in culture by confocal microscopy
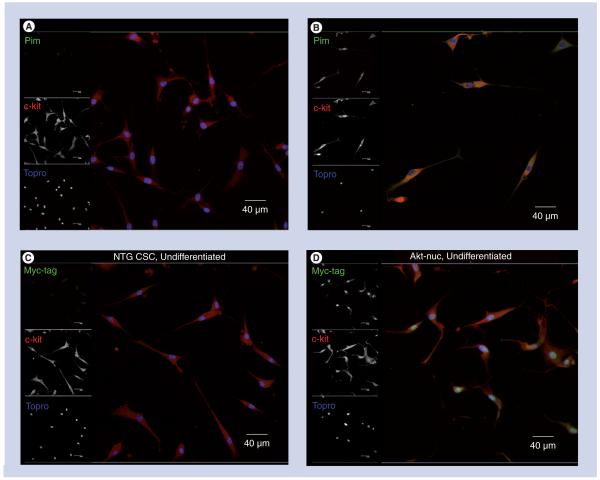
Cardiac stem cells derived from transgenic mouse lines express fluorescent reporter constructs, as confirmed by confocal microscopy. CSCs obtained from NTG, MHCAkt-nuc and MHCPim-wt mouse lines show immunoreactivity for appropriate expressed exogenous proteins. A representative scan shows increased Pim staining in the cytoplasm of the MHCPim-wt CSCs (Figure 3A & B). Similarly, CSCs from MHCAkt-nuc mice show nuclear localization of the exogenous protein by immunolabeling for the myc-tag relative to the NTG sample (Figure 3C & D). Bone marrow cells isolated from MHCPim-wt and MHCGFP transgenic mouse lines do not express GFP by flow cytometric analysis (Supplementary Figure 2).
Figure 3. Cardiac stem cells express transgenes controlled by the αMHC promoter in the appropriate subcellular localization in culture by confocal microscopy.
(A) NTG and (B) MHCPim-wt CSCs cultured in growth media, fixed and immunolabeled with antibodies to Pim (green), c-kit (red) and Topro (blue). MHCPim-wt CSCs show increased Pim expression localized to the cytoplasm. (C) NTG and (d) MHCAkt-nuc CSCs cultured in growth media, fixed and immunolabeled with Myc-tag (green), c-kit (red) and Topro (blue). MHCAkt-nuc CSCs show nuclear-localized myc-tag expression. Scale bar is 40 μm.
CSC: Cardiac stem cell; MHC: Myosin heavy chain; NTG: Nontransgenic.
Cardiomyogenic differentiation of CSCs results in persistent αMHC-driven transgene expression
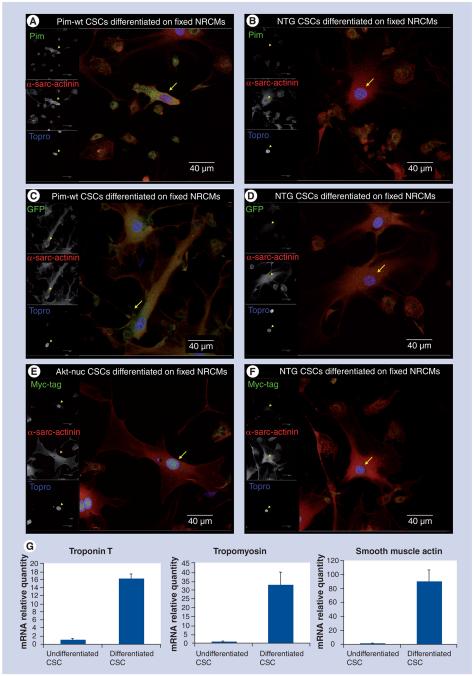
Cardiomyogenic induction of lineage commitment for CSCs can be promoted by coculture with NRCMs [22]. Monolayers of NRCM used in coculture studies are fixed prior to addition of CSCs to eliminate potentially confounding results caused by cell fusion. Conditioned media from the NRCM cultures is added to provide for cell–cell as well as soluble differentiation signals. The fixed layer of NRCMs lose Topro-dependent nuclear staining after 7 days of incubation (Supplementary Figure 3), allowing for visualization of nuclei present only in CSCs. Cultured CSCs derived from NTG, MHCAkt-nuc and MHCPim-wt myocardial tissue were subjected to coculture with fixed NRCMs for 7 days and then immunolabeled for nuclei (Topro) and α-sarcomericactinin (α-sarc-actinin) to identify myocytes as well as either Pim and GFP (MHCPim-wt) or myc-tag (MHCAkt-nuc). CSCs derived from MHCPim-wt mice display increased levels of Pim (Figure 4A) and GFP (Figure 4C) immunoreactivity relative to NTG controls (Figure 4B & D). CSCs derived from MHCAkt-nuc mice show nuclearlocalized myc-tag (Figure 4E), whereas the myc-tag is absent in the NTG control group (Figure 4F). Further characterization of CSC differentiation was performed using NTG CSCs lentivirally transduced to express high levels of GFP and cocultured with NRCMs. Cocultured CSCs were isolated after 7 days based on GFP expression, and mRNA levels of cardiac-specific and smooth muscle-specific genes were assessed by RT-qPCR. CSCs plated in these coculture studies express approximately 16-fold more troponin T, 33-fold more tropomyosin and 90-fold more α-smooth muscle actin mRNA levels relative to control samples (Figure 4G).
Figure 4. Cardiomyogenic differentiation allows for persistent αMHC driven transgene expression.
(A) MHCPim-wt and (B) NTG CSCs differentiated on fixed NRCMs for 7 days, fixed and immunolabeled for Pim (green), α-sarcomeric actinin (red) and Topro (blue). MHCPim-wt differentiated CSCs express more Pim protein. (C) MHCPim-wt and (D) NTG CSCs differentiated on fixed NRCMs for 7 days, fixed and immunolabeled for GFP (green), α-sarcomeric actinin (red) and Topro (blue). MHCPim-wt differentiated CSCs express GFP. (E) MHCAkt-nuc and (F) NTG CSCs differentiated on fixed NRCMs for 7 days, fixed and immunolabeled for myc-tag (green), α-sarcomeric actinin (red) and Topro (blue). MHCAkt-nuc differentiated CSCs express increased nuclear-localized myc-tag. Scale bar is 40 μm. (G) RT-qPCR analysis of NTG CSCs postdifferentiation on live NRCMs shows an increase in relative mRNA levels of troponin T (left), tropomyosin (middle) and α-smooth muscle actin (right) compared with undifferentiated CSCs.
CSC: Cardiac stem cell; GFP: Green fluorescent protein; MHC: Myosin heavy chain; NRCM: Neonatal rat cardiomyocyte; NTG: Nontransgenic.
Noncardiogenic differentiation leads to loss of transgene expression in cultured CSCs
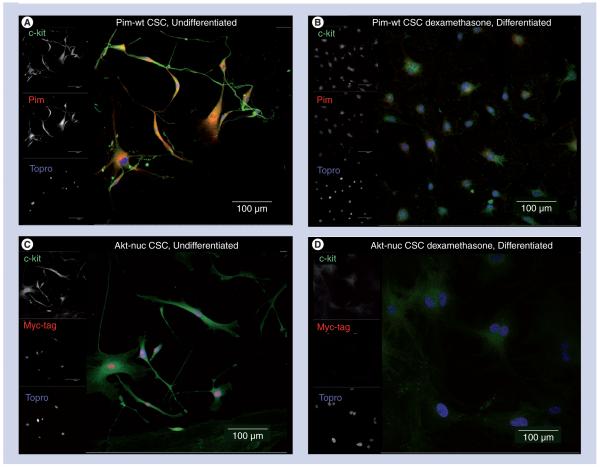
Dexamethasone steroid treatment of CSCs has been reported to promote cardiomyocytelike, endothelial cell [5], smooth muscle [5] or adipocyte-like cellular phenotypes (Supplementary Figure 4). Treatment of cultured CSCs derived from MHCPim-wt mice with 10 nM dexamethasone results in loss of immunoreactivity for both c-kit and Pim protein (Figures 5A & B). Identical treatment of cultured CSCs derived from MHCAkt-nuc mice (Figure 5C) results in loss of nuclear myc-tag immunoreactivty after 7 days of exposure to dexamethasone (Figure 5D).
Figure 5. Differentiation of cardiac stem cells by exposure to dexamethasone results in loss of transgene expression.
(A) MHCPim-wt CSCs cultured in growth media, fixed and immunolabeled for c-kit (green), Pim (red) and Topro (blue). (B) MHCPim-wt CSCs treated with dexamethasone containing media for 7 days, fixed and immunolabeled for c-kit (green), Pim (red) and Topro (blue) show decreased c-kit as well as Pim expression. (C) MHCAkt-nuc CSCs cultured in growth media, fixed and immunolabeled for c-kit (green), myc-tag (red) and Topro (blue). (D) MHCAkt-nuc CSCs treated with dexamethasone containing media for 7 days, fixed and immunolabeled for c-kit (green), Myc-tag (red) and Topro (blue) show decreased c-kit as well as myc-tag expression. Scale bar is 100 μm.
CSC: Cardiac stem cell.
Discussion
Since heart disease remains a major cause of morbidity and mortality, the treatment of this intractable disease by regeneration of damaged tissues or weakened cells remains a novel and exciting therapeutic approach. Initial steps toward implementation of regenerative therapy have already been accomplished: reliable isolation and culture conditions exist for CSCs [2,3,5]; animal studies have indicated that injection of syngeneic or autologous CSCs regenerate myocardium, decrease infarct size and improve function relative to controls [5,13,23,24]; and some stem cell types are adept at avoiding allogeneic rejection even without immunosuppressive drugs [23]. Studies following CSC injection in several models have failed to demonstrate any evidence of oncogenic transformation [7,8,10,11]. However, the persistence, long-term engraftment and replicative capacity of adoptively transferred CSCs remains a weak aspect of their use in the clinical setting. Increased efficacy of CSCs for clinical treatment of heart failure may rest upon genetic modification using signaling molecules with demonstrated propensity for enhancing survival and proliferation without inhibiting cardio genic commitment or leading to oncogenic transformation. These questions can be studied using CSCs in vitro with well-known signaling molecules to optimize gene expression, characterize the phenotypic properties of genetically engineered cells and assess safety issues prior to use in experimental animal models.
The focal point of this report is the demonstration of transgene expression in CSCs driven by the αMHC promoter in cell lines obtained from both NTG as well as three genetically engineered transgenic lines. Although αMHC promoter expression is generally considered to primarily occur in cardiomyocytes, the expression observed in CSCs may represent initial expression levels occurring at a very early stage of cardiogenic lineage commitment. Transgenic mRNA expression is maintained during ex vivo culture and expansion of CSCs (Figure 1) and appropriate protein localization was confirmed by immunocytochemistry (Figure 3). These data indicate that cultured CSCs maintain these essential in vivo phenotypic characteristics even through stressful isolation procedures and subsequent artificial in vitro culture conditions.
Cardiac stem cells exhibiting cardiogenic commitment should retain αMHC-driven gene expression, whereas those moving toward nonmyocyte lineages stop expressing αMHC-driven genes. Indeed, CSCs in differentiating coculture with fixed NRCMs persist in αMHC-driven transgene expression (Figure 4), whereas those subjected to dexamethasone treatment lose expression mediated by the αMHC promoter (Figure 5). Cardiogenic-associated transgene expression could be valuable in a therapeutic setting to augment persistence and proliferation of only those CSCs destined for myocardial fates. Cells committing to adipocyte or fibroblast types would lose the growth- and survival-promoting effects mediated by the transgene, leaving the cell without a persistent advantage. Thus, use of cardiac-specific promoters that are activated early in the CSC lineage program would provide a distinct advantage for the expansion of that progenitor cell population as well as their engraftment and retention in the myocardium, presumably leading to increased reparative and regenerative processes.
The feasibility of this ex vivo engineering strategy depends upon the capacity to modify isolated CSCs under in vitro culture conditions to express transgenes driven by the αMHC promoter. Clearly, CSCs express fluorescent proteins driven off the αMHC promoter after simple plasmid transfection (Figure 2). There may be advantages to the use of plasmid-based strategies to circumvent issues of long-term gene expression and potential for oncogenic transformation. If desired, greater efficiency and permanent transgene expression could be obtained by lentiviral or retroviral transduction instead of plasmid transfection and we are assessing the utility of those genetic-engineering approaches with ongoing studies.
The genetic modifications most likely to provide salutary effects in CSCs are those associated with increased cellular proliferation, survival and engraftment in a regulated fashion that would not result in loss of capabilities for differentiation as seen in oncogenic cell lines. Fortunately, as far as a target tissue, the myocardium is notoriously resistant to oncogenic transformation even when tumorigenic genes are expressed at high levels in cardiomyocytes by transgenesis. The inherent inert quality of the adult heart as a predominantly postmitotic organ may prove a saving feature for cells delivered for regenerative therapy, as this characteristic would reduce the potential for oncogenic genes to promote uncontrolled cell growth in the cardiomyocyte context. As previous studies have already pointed toward a number of intriguing genes encoding cardioprotective proteins such as Akt-nuc, Pim-1, IGF-1 and hGF, future studies will need to determine the feasibility of creating genetically engineered CSCs that possess enhanced survival and proliferation for the efficacious treatment of heart disease.
Conclusion
Cardiac stem cell-mediated regenerative cell therapy is a promising new treatment for heart disease, but the outcome of treatment could be markedly improved by increased survival and proliferation of cardiogenic cells. Genetic modification of CSCs with cardiac-specific promoters analogous to the murine αMHC promoter that is expressed early in cardiogenic lineage commitment could provide an important advance in our efforts to implement regenerative medicine with increased benefit and lowered safety risks.
Executive summary.
-
■
Cardiac stem cells (CSCs) express α-myosin heavy chain (αMHC)-driven transgenes in vivo and in vitro, demonstrating both early commitment to cardiomyocyte lineage in vivo and persistence of precardiac cell phenotype in vitro.
-
■
Cardiac-specific expression of survival and proliferative signaling will be advantageous for selective repopulation of myocardial tissue.
-
■
Nontransgenic CSCs express exogenously created gene constructs under control of the αMHC promoter when transfected into the cultured cells.
-
■
CSCs exposed to cardiogenic differentiation signals continue to express αMHC-driven transgenes.
-
■
Future CSC therapies will potentially benefit from engineering the donor cells with genes encoding cardioprotective proteins such as Akt-nuc, Pim-1 or other pro-survival proteins.
-
■
CSC engineering could be accomplished safely and effectively by putting the cardioprotective genes under the control of the αMHC promoter.
Supplementary Material
Acknowledgements
The authors wish to thank all members of the Sussman laboratory for helpful discussion and comments. The αMHC promoter sequence was kindly received from J Robbins at Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA. The td-Tomato red fluorescent protein construct, pRSETB-tdTomato, was a generous gift from R Tsien at University of California, San Diego, CA, USA. All transgenic mice were developed by the Transgenic Core Facility at San Diego State University, San Diego, CA, USA.
MA Sussman is supported by NIH grants 5R01HL067245, 1R01HL091102, 1P01HL085577 and 1P01AG023071 (Anversa P.I.).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investi gations involving human subjects, informed consent has been obtained from the participants involved.
Bibliography
- 1.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urbanek K, Cesselli D, Rota M, et al. Stem cell niches in the adult mouse heart. Proc. Natl Acad. Sci. USA. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc. Natl Acad. Sci. USA. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 5.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 6.Rota M, Padin-Iruegas ME, Misao Y, et al. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ. Res. 2008;103:107–116. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linke A, Muller P, Nurzynska D, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc. Natl Acad. Sci. USA. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Lee A, Huang M, et al. Imaging survival and function of transplanted cardiac resident stem cells. J. Am. Coll. Cardiol. 2009;53:1229–12240. doi: 10.1016/j.jacc.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 10.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 11.Haider H, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1α/CXCR4 signaling to promote myocardial repair. Circ. Res. 2008;103:1300–1308. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 12.Molkentin JD, Robbins J. With great power comes great responsibility: using mouse genetics to study cardiac hypertrophy and failure. J. Mol. Cell. Cardiol. 2009;46:130–136. doi: 10.1016/j.yjmcc.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramaniam A, Jones WK, Gulick J, Wert S, Neumann J, Robbins J. Tissue-specific regulation of the alpha-myosin heavy chain gene promoter in transgenic mice. J. Biol. Chem. 1991;266:24613–24620. [PubMed] [Google Scholar]
- 14.Rota M, Boni A, Urbanek K, et al. Nuclear targeting of Akt enhances ventricular function and myocyte contractility. Circ. Res. 2005;97:1332–1341. doi: 10.1161/01.RES.0000196568.11624.ae. [DOI] [PubMed] [Google Scholar]
- 15.Tsujita Y, Muraski J, Shiraishi I, et al. Nuclear targeting of Akt antagonizes aspects of cardiomyocyte hypertrophy. Proc. Natl Acad. Sci. USA. 2006;103:11946–11951. doi: 10.1073/pnas.0510138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gude N, Muraski J, Rubio M, et al. Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population. Circ. Res. 2006;99:381–388. doi: 10.1161/01.RES.0000236754.21499.1c. [DOI] [PubMed] [Google Scholar]
- 17.Muraski JA, Rota M, Misao Y, et al. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat. Med. 2007;13:1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 18.Muraski JA, Fischer KM, Wu W, et al. Pim-1 kinase antagonizes aspects of myocardial hypertrophy and compensation to pathological pressure overload. Proc. Natl Acad. Sci. USA. 2008;105:13889–13894. doi: 10.1073/pnas.0709135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato T, Muraski J, Chen Y, et al. Atrial natriuretic peptide promotes cardiomyocyte survival by cGMP-dependent nuclear accumulation of zyxin and Akt. J. Clin. Invest. 2005;115:2716–2730. doi: 10.1172/JCI24280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fransioli J, Bailey B, Gude NA, et al. Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem Cells. 2008;26:1315–1324. doi: 10.1634/stemcells.2007-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiraishi I, Melendez J, Ahn Y, et al. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ. Res. 2004;94:884–891. doi: 10.1161/01.RES.0000124394.01180.BE. [DOI] [PubMed] [Google Scholar]
- 22.Rota M, Kajstura J, Hosoda T, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc. Natl Acad. Sci. USA. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl Acad. Sci. USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawn B, Tiwari S, Kucia MJ, et al. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646–1655. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.