Abstract
Theoretical calculations suggest that small daily reductions in energy intake can cumulatively lead to substantial weight loss, but experimental data to support these calculations are lacking. We conducted a 1-year randomized controlled pilot study of low (10%) or moderate (30%) energy restriction (ER) with diets differing in glycemic load in 38 overweight adults (mean ± s.d., age 35 ± 6 years; BMI 27.6 ± 1.4 kg/m2). Food was provided for 6 months and self-selected for 6 additional months. Measurements included body weight, resting metabolic rate (RMR), adherence to the ER prescription assessed using 2H2 18O, satiety, and eating behavior variables. The 10%ER group consumed significantly less energy (by 2H2 18O) than prescribed over 12 months (18.1 ± 9.8%ER, P = 0.04), while the 30%ER group consumed significantly more (23.1 ± 8.7%ER, P < 0.001). Changes in body weight, satiety, and other variables were not significantly different between groups. However, during self-selected eating (6–12 months) variability in % weight change was significantly greater in the 10%ER group (P < 0.001) and poorer weight outcome on 10%ER was predicted by higher baseline BMI and greater disinhibition (P < 0.0001; adj R2 = 0.71). Weight loss at 12 months was not significantly different between groups prescribed 10 or 30%ER, supporting the efficacy of low ER recommendations. However, long-term weight change was more variable on 10%ER and weight change in this group was predicted by body size and eating behavior. These preliminary results indicate beneficial effects of low-level ER for some but not all individuals in a weight control program, and suggest testable approaches for optimizing dieting success based on individualizing prescribed level of ER.
INTRODUCTION
Dietary energy restriction (ER) remains a cornerstone of most approaches to long-term weight loss, and current recommendations promote the use of moderate energy deficits of 500–1,000 kcal/day below the amount required for weight maintenance (1). This deficit translates to a reduction in energy intake of typically 20–40% for an individual with an energy requirement of 2,500 kcal/day and is estimated to result in weight loss of 1–2 lb/week (1). Moderate levels of ER are recommended on the grounds that greater degrees of ER do not achieve better long-term weight loss (1) and may result in a greater loss of fat-free mass (FFM) (2). It has also been suggested that much smaller deficits, of just ≤100 kcal/day, may produce sustainable weight loss benefits over time (3). However, experimental data to support this suggestion are lacking.
Although some studies have examined the effects of moderate vs. severe levels of ER on weight loss (4,5), and others have compared different behavioral programs that had secondary effects on energy intake (4,6,7), to our knowledge only one study has directly compared two levels of ER within the currently recommended range (8). That study examined two moderate (500 or 1,000 kcal/day) levels of ER in combination with the weight control drug orlistat and reported no significant effect of level of prescribed energy on weight loss over 1 year. The reasons for the similar weight loss despite different energy prescriptions were not examined in detail beyond the reported food intake, but might potentially include noncompliance in response to hunger (9), desire to eat favorite foods (10), metabolic adaptation to different degrees of ER (11), or perhaps psychological factors such as the impact of setting attainable vs. unattainable goals (12).
We describe here an analysis of data from the first phase of the Comprehensive Assessment of the Long-term Effects of Restricting Intake of Energy study at Tufts (13,14), testing the hypothesis that individuals randomized to very low (10%) ER lose less body weight and fat over 1 year than individuals randomized to moderate (30%) ER. To our knowledge, this is the first study comparing the efficacy of low vs. moderate ER prescriptions to facilitate long-term weight loss. We also measured adherence to the two prescribed levels of ER using the doubly labeled water (DLW) technique that allowed assessments independent of subject reporting, and investigated predictors of variability in individual weight loss success on the two energy levels.
METHODS AND PROCEDURES
Study population
Subjects were overweight but otherwise healthy men and women aged 24–42 years who completed the first phase of the Comprehensive Assessment of the Long-term Effects of Restricting Intake of Energy study at Tufts University (13,14). A total of 46 subjects were enrolled in this study and those who dropped out (n = 7) mainly did so because of scheduling conflicts and unplanned life changes unrelated to group randomization. The study was conducted with approval by the Institutional Review Board of Tufts Medical Center. All subjects gave written, informed consent prior to participating and were provided a stipend (clinical trial # NCT00099099).
Study protocol
This year-long intervention study included a 7-week baseline period (phase 1) when subjects were requested to maintain a stable weight and continue eating their usual diet. Baseline weight maintenance energy requirements (assumed to be equal to total energy expenditure, TEE, as measured by DLW (15)) as well as outcome variables were assessed. Following phase 1, subjects were randomized to either 10 or 30%ER relative to baseline energy requirements and to either a high glycemic (HG) or low glycemic (LG) load dietary regimen (described below). By design, 12 subjects were randomized to 10%ER and 34 subjects to 30%ER because the primary purpose of the 10% group was to obtain experience in recruiting and randomizing a low-level ER group in preparation for a subsequent study. A block randomization stratified on BMI, gender, and diet group (10%HG, 10%LG, 30%HG, 30%LG) was employed. Phase 2 was a 24-week (“6 month”) ER phase following baseline when subjects were provided with all their food, and phase 3 was the next consecutive 24 weeks when subjects were instructed to continue their dietary regimen on their own. Visits to the research center were scheduled weekly throughout the study for a variety of activities including behavioral support groups, individual meetings with the study dietitian, safety monitoring, and outcome testing, all of which were identical for both the ER groups. All outcome assessments were performed by staff blinded to participant randomization. We wanted to ensure that subjects on both prescriptions were equally motivated to adhere to their intervention and so subjects were not informed of their randomization until month 3 of ER. By deferring the announcement of their randomization we hoped to divert their focus away from the prescription to the actual intervention.
Study diets
The HG and LG diets were used in this protocol to examine the role of glycemic load in facilitating long-term adherence to ER prescriptions and in achieving and maintaining weight loss. The details of the two diets have been described previously (13,14). Both diets met dietary reference intakes for dietary fiber, limited inclusion of high energy density foods, and liquid calories, were matched for palatability and dietary variety, and had a relatively high variety of low energy–dense foods. The diets differed in the ratio of macronutrients (HG: 60% carbohydrate, 20% fat, 20% protein vs. LG: 40% carbohydrate, 30% fat, 30% protein) and additionally the carbohydrate sources in the LG diet were lower in glycemic index based on published glycemic indexes of different carbohydrate sources (16). Subjects were also provided with daily multivitamin and calcium (500 mg/day) supplements.
During phase 2, all food was provided to subjects and they were asked to consume only this food, which was collected from the research center 2–3 times/week. During phase 3, subjects were instructed to self-select and prepare their own food at home to maintain their randomization. To prepare for this phase, subjects worked with the study dietitian to develop an individualized plan including menus, recipes, portion sizes, and food lists consistent with their randomization. Food scales were provided to help with appropriate portioning.
Body weight, height, and composition
Height was measured at the beginning of the study to ±0.1 cm, and fasting weight was measured on a calibrated scale at weekly intervals to ±0.1 kg. Air displacement plethysmography (BOD POD; Life Measurement, Concord, CA) was used to assess body fatness in duplicate at baseline and at 3, 6, and 12 months (refs. 13,14,17).
Calculated energy intake and adherence to ER
TEE was measured over 28 days at baseline and over 14 days at 1, 3, 6, and 12 months of ER using duplicate consecutive DLW assessments (15,18). Details of the protocol, analytical methods, and calculations have been described previously (13,19). Single measurements during ER were used to calculate actual energy intake, as TEE determined from VCO2 using an assumed respiratory quotient of 0.86 plus estimated change in body energy stores based on weight change (20). Please note that assumptions in respiratory quotient have very little effect on values for TEE in DLW studies (21). Values for weight change in this study were calculated from regression of weekly fasting weights made during the period 7 days before to 7 days after each TEE measurement and the energy content of weight change was taken as the mean for the population (8.45 kcal/g, computed from the body composition assessments; although this value is somewhat higher than older published data (22), the older values are derived from much shorter periods of weight loss). Mean ER for 0–6, 6–12, and 0–12 months ER were calculated as time weighted averages of the individual estimates at individual time points.
In addition to DLW, we used daily meal checklists for the entire 6-month food-provided phase where subjects were required to check each provided meal that was consumed and record any additional foods consumed and any left over food. After 6 months, subjects completed 7-day food records from which dietary intake was assessed.
Resting metabolic rate and physical activity level
Resting metabolic rate (RMR) was measured for 30 min on two mornings at baseline, 6, and 12 months of ER, after the subject slept overnight in the research center and fasted for 12 h according to our usual procedures (23). The ratio of TEE to RMR was calculated as an index of physical activity level (PAL).
Self-reported hunger, desire to eat, dietary satisfaction, and weight self-efficacy
At the end of each day subjects were asked to complete 100-mm visual analog scales (24) on level of hunger for the day, desire to eat nonstudy foods, and satisfaction with the amount of consumed foods. The scales were five-point anchored scales with descriptors such as “Not at all hungry” to “Extremely hungry” at opposite ends and intermediate evenly spaced descriptors such as “Slightly hungry,” “Moderately hungry,” and “Very hungry.” Subjects were asked to draw a vertical line along this five-point anchored scale for their subjective rating of the above variables and the reading was measured in millimeters. Daily values were averaged for analyses for the baseline period, and intervals of ER. On average, subjects completed >70% of their daily logs. Subjects also completed the Eating Inventory at screening (which served as the baseline measure) and at months 6 and 12, and restraint, disinhibition, and hunger were calculated using this questionnaire (25).
Data analysis
Statistical analyses were performed using SAS for Windows (version 8.2; SAS Institute, Cary, NC). Analyses were performed for subjects with complete data and for all enrolled subjects. Subject characteristics were compared using t-tests for independent samples. For changes in body composition and other variables between baseline and at time points during ER mixed model analyses with repeated measures were performed to determine the effects of prescribed ER (10%/30%), diet composition (HG/LG), and their interactions over time. A general linear model was used to examine the changes in TEE and RMR over time and between the two ER groups after adjusting for the changes in FFM and fat mass. One sample t-tests were performed to determine whether %ER achieved by each group was different from prescribed %ER. Multiple regression models (backward stepwise) were developed to examine the best predictors of weight change for both 0–6 months and 6–12 months while controlling for diet group (LG vs. HG). Values are expressed as mean ± s.d. unless otherwise specified. All P values were two-sided and a P value of ≤0.05 was taken to indicate statistical significance.
The results from the analyses with all enrolled subjects (N = 46) were statistically similar to the results for those with complete data (N = 38, data presented in this article). There was no significant difference in weight loss at 6 or 12 months between the two diets at 30%ER (ref. 13) and therefore diet groups were combined in the presentation of data. Some results from the 30%ER group (TEE, RMR, %fat, and FFM), but not the key variables in this paper (weight loss in diet groups combined, %ER, variability in weight change) were published elsewhere (13,14) in the context of examining differences between the HG and LG diet groups.
RESULTS
Baseline characteristics of the subjects are shown in Table 1. There were no statistically significant differences among the ER groups for any baseline variable (P = 0.12–0.92).
Table 1.
Baseline subject characteristics (mean ± s.d.)
| 10%ER | 30%ER | |
|---|---|---|
| (n = 9) | (n = 29) | |
| Age (years) | 36 ± 3 | 35 ± 5 |
| Gender (men, women) | 3, 6 | 6, 23 |
| Height (cm) | 171.4 ± 9.7 | 168.5 ± 10.6 |
| Weight (kg) | 84.9 ± 9.8 | 78.3 ± 10.8 |
| BMI (kg/m2) | 28.8 ± 1.3 | 27.5 ± 1.5 |
| % Body fat | 37.3 ± 4.4 | 35.1 ± 7.8 |
| Fat-free mass (kg) | 53.1 ± 6.9 | 50.9 ± 10.7 |
| Fat mass (kg) | 31.6 ± 5.9 | 27.4 ± 6.7 |
Independent sample t-tests were used to examine differences in baseline variables between the 10 and 30%ER groups and no statistically significant differences were observed (P = 0.12–0.92).
ER, energy restriction.
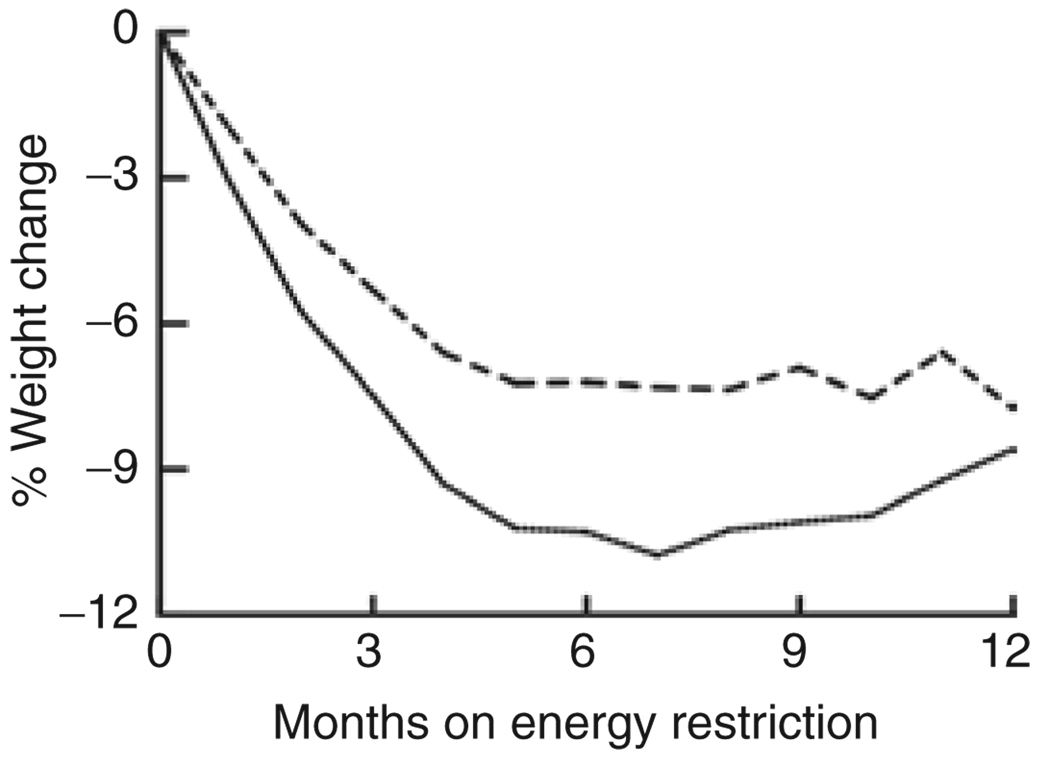
Table 2 shows data on body weight and composition, %ER, and energy expenditure, and Figure 1 illustrates mean values for percent weight change. The mean percentage weight lost during ER was not significantly different between 10 and 30%ER groups during 0–6 months when food was provided (P = 0.08; mean percentage weight change ± s.d. and 95% lower and upper confidence intervals (CI) −6.97 ± 6.4, 95% CI, −11.9 to −2.1 in the 10%ER group, −10.20 ± 3.9, 95% CI, −11.7 to −8.7 in the 30%ER group) or during 6–12 months of ER when food was self-selected (P = 0.38; 0.92 ± 5.1, 95% CI, −3.0 to 4.9 in the 10%ER group, 2.03 ± 2.6, 95% CI, 1.1–3.0 in the 30%ER group). Variability in percentage weight change was not significantly different between 10 and 30%ER groups for 0–6 months but was significantly higher in the 10%ER group for 6–12 months (P < 0.01).
Table 2.
Body composition, energy expenditure, and %ER (mean ± s.d.)
| 10%ER | 30%ER | |
|---|---|---|
| (n = 9) | (n = 29) | |
| Body weight (kg) | ||
| Baseline | 84.8 ± 10.8 | 78.6 ± 10.7 |
| % Δ 6 months | −7.0 ± 6.4 | −10.2 ± 3.9 |
| % Δ 6–12 months | 0.9 ± 5.1 | 2.0 ± 2.6 |
| % Fat | ||
| Baseline | 37.3 ± 4.4 | 35.1 ± 7.8 |
| Δ 6 months | −15.1 ± 10.6 | −20.1 ± 14.3 |
| Δ 6–12 months | 0.9 ± 3.8 | 1.0 ± 2.0 |
| %ER | ||
| 0–6 monthsa | 19.9 ± 15.5 | 30.7 ± 10.9 |
| 6–12 months | 16.4 ± 12.3 | 15.4 ± 11.8 |
| 0–12 months | 18.1 ± 9.8 | 23.1 ± 8.7 |
| TEE (kcal/d) | ||
| Baseline | 2,949 ± 362 | 2,842 ± 454 |
| Δ 6 months | −347 ± 261 | −348 ± 268 |
| Δ 6–12 months | −99 ± 361 | −47 ± 257 |
| RMR (kcal/d) | ||
| Baseline | 1,690 ± 233 | 1,593 ± 219 |
| Δ 6 months | −58 ± 93 | −105 ± 95 |
| Δ 6–12 months | −18 ± 81 | −54 ± 66 |
| PAL | ||
| Baseline | 1.75 ± 0.15 | 1.78 ± 0.18 |
| Δ 6 months | −0.14 ± 0.1 | −0.11 ± 0.2 |
| Δ 6–12 months | −0.08 ± 0.2 | −0.09 ± 0.2 |
A mixed model analysis of repeated measures was used to examine changes over time, between the ER groups and for interactions effects of ER time and diet group. Statistically significant changes over time (P < 0.001) but not between the ER groups were observed for TEE, RMR, PAL, and % body fat.
ER, energy restriction; PAL, physical activity level; RMR, resting metabolic rate; TEE, total energy expenditure.
%ER significantly different between the groups (0–6 months, P = 0.02). No significant difference in %ER between 6 and 12 months or between 0 and 12 months.
Figure 1.
Mean percentage weight change in the 10% (dotted line) and 30% (solid line) energy-restricted (ER) groups over 12 months. Significant change in percent weight over time (P < 0.001), no significant difference in change in percent weight overtime between the 10 and 30%ER (P = 0.87).
Table 2 also summarizes measured %ER determined with DLW measurements (isotopic measurements of energy intake during the intervention divided by TEE at baseline). On average there was no significant difference in measured %ER between the two ER groups over 12 months of ER (18.1 ± 9.8 in the 10%ER group vs. 23.1 ± 8.7 in the 30%ER group; P = 0.16) and the 10%ER group restricted themselves significantly more than their prescription (P = 0.04), whereas the 30%ER group restricted themselves significantly less (P < 0.0001). In addition, although %ER was significantly different between the ER groups during the 0- to 6-month period, (P = 0.02), %ER decreased significantly between 6 and 12 months in the 30%ER group (P < 0.001) but not in the 10%ER group (P = 0.16). However, there were no significant diet by %ER by time interactions (P = 0.31).
As also shown in Table 2, there was a significant change from baseline in all energy expenditure and body composition variables but no significant difference in changes over time between 10 and 30%ER groups (P = 0.44–0.87). There were no statistically significant diet by %ER by time interactions for any of these variables (P = 0.43 for %body fat, P = 0.20 for TEE; P = 0.49 for RMR, and P = 0.29 for PAL). The overall change in TEE adjusted for both FFM and fat mass changes were significant at 6 months (P = 0.008) and at 12 months (P < 0.001) of ER, but these changes were not significant between the two ER groups (P = 0.78 at 6 months and P = 0.49 at 12 months). RMR changes adjusted for both FFM and fat mass changes were not significantly different over time (P = 0.14 at 6 months and P = 0.51 at 12 months) or between the ER groups at 6 months (P = 0.08) or at 12 months (P = 0.15) (data not shown).
Self-reported hunger, desire to eat nonstudy foods, and satisfaction with the amount of provided food, assessed using daily visual analog scale, are shown in Table 3. There was a small but significant increase in hunger (P = 0.03) and the desire to eat nonstudy foods (P = 0.003) over the 12-month intervention. Although changes in the 10%ER group tended to be smaller, changes over time were not significantly different between the two ER groups (P = 0.77 for hunger and P = 0.49 for the desire to eat nonstudy foods). There was no significant change over time or between the two ER groups in self-reported satisfaction with the amount of food (P = 0.56 for time; P = 0.16 for group) and no diet by ER by time interaction effects for any of the visual analog scale variables (P = 0.39 for hunger; P = 0.66 for satisfaction with the amount of prescribed food; P = 0.31 for the desire to eat nonstudy foods).
Table 3.
Hunger and satiety (mean ± s.d.)
| 10%ER | 30%ER | |
|---|---|---|
| (n = 9) | (n = 29) | |
| “Level of hunger for the day” | ||
| Baseline | 26.5 ± 14.8 | 35.2 ± 16.6 |
| Δ 3 months | 0.9 ± 6.6 | 2.9 ± 10.4 |
| Δ 6 months | −0.7 ± 8.5 | 5.5 ± 10.6 |
| Δ 12 months | 6.5 ± 7.4 | 6.2 ± 8.4 |
| “Desire to eat something not in study foods” |
||
| Baseline | 24.9 ± 17.7 | 25.7 ± 17.7 |
| Δ 3 months | 5.7 ± 10.0 | 11.2 ± 16.6 |
| Δ 6 months | 8.7 ± 19.9 | 16.6 ± 16.6 |
| Δ 12 months | 8.8 ± 19.7 | 13.2 ± 14.6 |
| “Satisfaction with the amount of food given” |
||
| Baseline | 67.2 ± 13.6 | 66.9 ± 16.3 |
| Δ 3 months | 4.5 ± 5.4 | −4.9 ± 10.9 |
| Δ 6 months | 2.1 ± 11.2 | −7.3 ± 12.5 |
| Δ 12 months | 0.4 ± 3.3 | −3.9 ± 8.1 |
Data collected using a 100-mm visual analog scale with a five-point anchors. A mixed model analysis of repeated measures was used to examine changes over time, between the ER groups and for interactions effects. Level of hunger (P = 0.03) for the day and desire to eat something not in study foods (P = 0.003) increased over time, but the change over time was not significantly different between the ER groups. No significant ER by time by diet group interactions were observed.
ER, energy restriction.
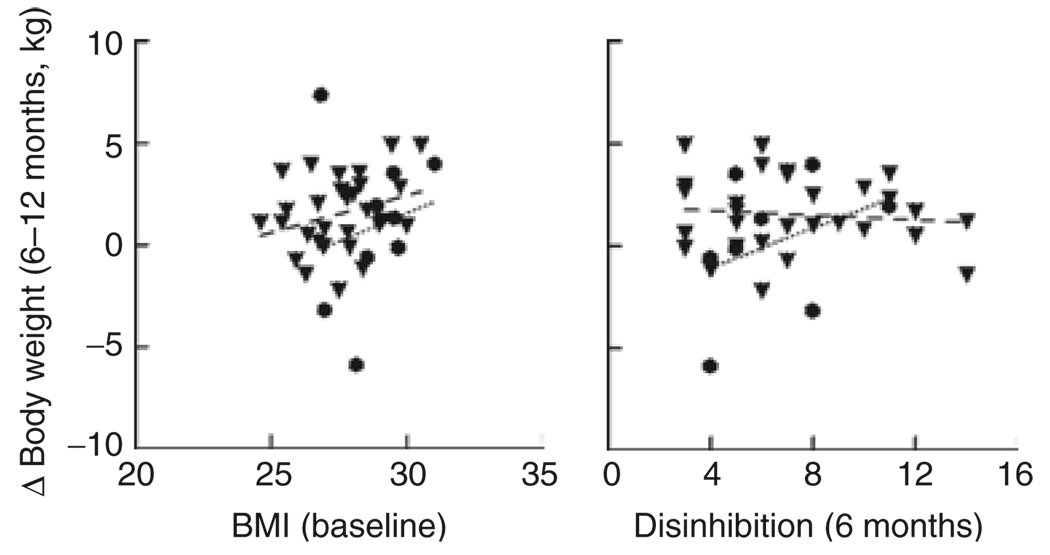
Energy expenditure and eating behavior variables were examined as predictors of weight change. There were no significant predictors identified for 0–6 months of ER when all food was provided. However, during 6–12 months of ER when food was self-selected, higher baseline BMI and greater 6-month disinhibition scores predicted poorer weight outcome (i.e., weight gain) in the 10%ER group but not in the 30%ER group (P < 0.0001; adj R2 = 0.71) (Figure 2). In a separate model (not including baseline BMI) that had a slightly lower R2, lower baseline PAL also predicted weight change during 6–12 months in the 10%ER group but not in the 30%ER group (P < 0.0001; adj R2 = 0.69).
Figure 2.
Predictors of weight loss success during 6–12 months of energy restriction (ER). A higher baseline BMI and greater disinhibition at 6 months of ER (P < 0.0001; adj R2 = 0.71) predicted weight gain in the 10% ER group (•) but not in the 30% ER group (▼).
DISCUSSION
Many studies have examined the role of dietary composition in successful weight control (13,26–32) and recognize that weight loss on a particular regimen depends primarily on the extent to which energy intake is reduced (4). However, there has been very little research on the effect of the prescribed level of ER on actual energy intakes and consequent weight change. The results of this long-term pilot study suggest that prescribing a low-level (10%) ER may result in a mean actual ER and weight loss over 1 year comparable to values obtained from prescribing a moderate (30%) ER. Our findings extend and are consistent with both the results of Toplak et al. (8) who compared two levels of restriction approximating 20 and 40%ER, and with the theoretical prediction of Hill et al. (3) that small reductions in energy intake should cumulate in substantial weight loss over time. We also observed for the first time that individual success with a low ER prescription was significantly more variable than that with a moderate ER prescription during the self-selected foods phase, and that reduced success on the low ER group during this time was predicted by higher BMI at baseline and higher disinhibition at 6 months.
This study was a pilot for a larger investigation and by design randomized relatively few subjects to 10%ER. The finding may thus be influenced by small sample size. Nevertheless, the study is the first to compare low and moderate levels of ER for effects on long-term weight change, and also the first to measure adherence to different levels of ER using an objective method not dependent on reporting of food intake by subjects (15,20). This latter feature of the design was particularly important, given the well-known tendency for food intake to be underreported with different biases between different groups (20,33).
Using DLW to assess actual energy intake we showed that, in analyses controlling for diet group randomization, 10%ER restricted their energy intake by a significantly greater amount than prescribed on average, whereas 30%ER subjects restricted energy intake initially to prescribed levels but adherence fell off over time and on average was not significantly different between the two groups over 12 months (18% vs. 23%, respectively). The underlying reasons for the different levels of adherence to different ER prescriptions are not known; however, the provided meals were more satiating than foods the subjects were used to and some subjects on the 10%ER reported themselves unable to finish all their provided food. They returned leftover foods so the under-eating was seen both in the DLW assessments and the food logs. In addition, given that visual analog scale assessments of hunger and satiety were not significantly different between groups at the levels of ER measured, we speculate that randomization to low-level ER prescription may have created empowerment by setting a more easily attainable goal (12) that in turn resulted in subjects exceeding their targets. These results and considerations, together with the study of Toplak et al. (8) which reported no difference in weight loss between prescriptions approximating 20 and 40%ER, imply that ER in the range 10–20% may approximate the maximum level consistent with a higher long-term adherence in groups of individuals. Additional data are needed to examine the relationship between prescribed and achieved levels of ER from adequately powered studies.
The other main observation in this study was that, during 6–12 months of ER when subjects self-selected their own food and on average were maintaining weight, variability in weight change was significantly greater in the 10%ER group than in the 30%ER group. We predicted and confirmed that relative failure of weight control was associated with a higher BMI, a higher score for disinhibited eating behavior, and a lower PAL, consistent with previous studies that have shown these variables are linked to weight gain (34–37). The reason for why these variables predicted poor outcome in the 10%ER group but not the 30%ER group is not known. It is possible that the lower degree of restriction in the 10%ER group led to an increased exposure to stimuli for disinhibition such as discretionary foods. Another possibility is that there was lower intrinsic signaling of negative energy balance in the 10%ER group which reduced awareness of ongoing adherence from metabolic signaling in a way that allowed other factors to influence adherence in positive or negative ways. One implication of these findings, if confirmed, is that some individuals can sustain more weight loss on low-level ER and others on moderate ER, and that it may be possible to identify groups who will respond better to one or the other type of regimen.
These findings combined with previous related research suggest that 10–20% reductions in energy intake may on average be as effective for achieving long-term weight loss as higher levels of restriction in some individuals but not others, and suggest avenues for future research that may lead to optimization of weight loss programs based on individualizing the prescribed degree of ER.
ACKNOWLEDGMENTS
We thank the subjects for their committed participation in this study, and the staff of the Metabolic Research Unit for their expert assistance. This project work was supported by National Institutes of Health grants: AG20480 and H150001, and by the U.S. Department of Agriculture under agreement no. 58-1950-4-401. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.NHLBI. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. National Institutes of Health, National Heart, Lung and Blood Institute; The Evidence Report. 2002 [PubMed]
- 2.Forbes GB. Body fat content influences the body composition response to nutrition and exercise. Ann N Y Acad Sci. 2000;904:359–365. doi: 10.1111/j.1749-6632.2000.tb06482.x. [DOI] [PubMed] [Google Scholar]
- 3.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JW, Luan J, Høie LH. Structured weight-loss programs: meta-analysis of weight loss at 24 weeks and assessment of effects of intervention intensity. Adv Ther. 2004;21:61–75. doi: 10.1007/BF02850334. [DOI] [PubMed] [Google Scholar]
- 5.Frost G, Masters K, King C, et al. A new method of energy prescription to improve weight loss. J Hum Nutr Diet. 2007;20:152–156. doi: 10.1111/j.1365-277X.2007.00775.x. [DOI] [PubMed] [Google Scholar]
- 6.Wadden TA, Foster GD, Letizia KA. One-year behavioral treatment of obesity: comparison of moderate and severe caloric restriction and the effects of weight maintenance therapy. J Consult Clin Psychol. 1994;62:165–171. doi: 10.1037//0022-006x.62.1.165. [DOI] [PubMed] [Google Scholar]
- 7.Shah M, McGovern P, French S, Baxter J. Comparison of a low-fat, ad libitum complex-carbohydrate diet with a low-energy diet in moderately obese women. Am J Clin Nutr. 1994;59:980–984. doi: 10.1093/ajcn/59.5.980. [DOI] [PubMed] [Google Scholar]
- 8.Toplak H, Ziegler O, Keller U, et al. X-PERT: weight reduction with orlistat in obese subjects receiving a mildly or moderately reduced-energy diet: early response to treatment predicts weight maintenance. Diabetes Obes Metab. 2005;7:699–708. doi: 10.1111/j.1463-1326.2005.00483.x. [DOI] [PubMed] [Google Scholar]
- 9.LaPorte DJ, Stunkard AJ. Predicting attrition and adherence to a very low calorie diet: a prospective investigation of the eating inventory. Int J Obes. 1990;14:197–206. [PubMed] [Google Scholar]
- 10.Kearney MH, Rosal MC, Ockene JK, Churchill LC. Influences on older women’s adherence to a low-fat diet in the Women’s Health Initiative. Psychosom Med. 2002;64:450–457. doi: 10.1097/00006842-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 12.Cervone D, Jiwani N, Wood R. Goal setting and the differential influence of self-regulatory processes on complex decision-making performance. J Pers Soc Psychol. 1991;61:257–266. doi: 10.1037//0022-3514.61.2.257. [DOI] [PubMed] [Google Scholar]
- 13.Das SK, Gilhooly CH, Golden JK, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;85:1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 14.Das SK, Gilhooly CH, Golden JK, et al. Long-term effects of energy-restricted diets differing in glycemic load on metabolic adaptation and body composition. Open Nutr J. 2008;2:76–85. [PMC free article] [PubMed] [Google Scholar]
- 15.Schoeller DA. Measurement of energy expenditure in free-living humans by using doubly labeled water. J Nutr. 1988;118:1278–1289. doi: 10.1093/jn/118.11.1278. [DOI] [PubMed] [Google Scholar]
- 16.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 17.McCrory MA, Gomez TD, Bernauer EM, Molé PA. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med Sci Sports Exerc. 1995;27:1686–1691. [PubMed] [Google Scholar]
- 18.Roberts SB, Young VR, Fuss P, et al. Energy expenditure and subsequent nutrient intakes in overfed young men. Am J Physiol. 1990;259:R461–R469. doi: 10.1152/ajpregu.1990.259.3.R461. [DOI] [PubMed] [Google Scholar]
- 19.DeLany JP, Schoeller DA, Hoyt RW, Askew EW, Sharp MA. Field use of D218O to measure energy expenditure of soldiers at different energy intakes. J Appl Physiol. 1989;67:1922–1929. doi: 10.1152/jappl.1989.67.5.1922. [DOI] [PubMed] [Google Scholar]
- 20.Bathalon GP, Tucker KL, Hays NP, et al. Psychological measures of eating behavior and the accuracy of 3 common dietary assessment methods in healthy postmenopausal women. Am J Clin Nutr. 2000;71:739–745. doi: 10.1093/ajcn/71.3.739. [DOI] [PubMed] [Google Scholar]
- 21.Surrao J, Sawaya AL, Dallal GE, Tsay R, Roberts SB. Use of food quotients in human doubly labeled water studies: comparable results obtained with 4 widely used food intake methods. J Am Diet Assoc. 1998;98:1015–1020. doi: 10.1016/S0002-8223(98)00232-6. [DOI] [PubMed] [Google Scholar]
- 22.Saltzman E, Roberts SB. The role of energy expenditure in energy regulation: findings from a decade of research. Nutr Rev. 1995;53:209–220. doi: 10.1111/j.1753-4887.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- 23.Das SK, Roberts SB, McCrory MA, et al. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr. 2003;78:22–30. doi: 10.1093/ajcn/78.1.22. [DOI] [PubMed] [Google Scholar]
- 24.De Graaf C. The validity of appetite ratings. Appetite. 1993;21:156–160. doi: 10.1016/0195-6663(93)90008-8. [DOI] [PubMed] [Google Scholar]
- 25.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 26.Moriguti JC, Das SK, Saltzman E, et al. Effects of a 6-week hypocaloric diet on changes in body composition, hunger, and subsequent weight regain in healthy young and older adults. J Gerontol A Biol Sci Med Sci. 2000;55:B580–B587. doi: 10.1093/gerona/55.12.b580. [DOI] [PubMed] [Google Scholar]
- 27.McManus K, Antinoro L, Sacks F. A randomized controlled trial of a moderate-fat, low-energy diet compared with a low fat, low-energy diet for weight loss in overweight adults. Int J Obes Relat Metab Disord. 2001;25:1503–1511. doi: 10.1038/sj.ijo.0801796. [DOI] [PubMed] [Google Scholar]
- 28.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 29.Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348:2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 30.Brehm BJ, Seeley RJ, Daniels SR, D’Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88:1617–1623. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- 31.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 32.Schoeller DA, Buchholz AC. Energetics of obesity and weight control: does diet composition matter? J Am Diet Assoc. 2005;105:S24–S28. doi: 10.1016/j.jada.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 33.Schoeller DA. Limitations in the assessment of dietary energy intake by self-report. Metab Clin Exp. 1995;44:18–22. doi: 10.1016/0026-0495(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 34.Lawson OJ, Williamson DA, Champagne CM, et al. The association of body weight, dietary intake, and energy expenditure with dietary restraint and disinhibition. Obes Res. 1995;3:153–161. doi: 10.1002/j.1550-8528.1995.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 35.Williamson DA, Lawson OJ, Brooks ER, et al. Association of body mass with dietary restraint and disinhibition. Appetite. 1995;25:31–41. doi: 10.1006/appe.1995.0039. [DOI] [PubMed] [Google Scholar]
- 36.Hays NP, Roberts SB. Aspects of eating behaviors “disinhibition” and “restraint” are related to weight gain and BMI in women. Obesity (Silver Spring) 2008;16:52–58. doi: 10.1038/oby.2007.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catenacci VA, Ogden LG, Stuht J, et al. Physical activity patterns in the National Weight Control Registry. Obesity (Silver Spring) 2008;16:153–161. doi: 10.1038/oby.2007.6. [DOI] [PMC free article] [PubMed] [Google Scholar]