SUMMARY
1. AMP-activated protein kinase (AMPK) is a serine/threonine protein kinase involved in the regulation of cellular and organismal metabolism. AMPK has a heterotrimeric structure, consisting of a catalytic α-subunit and regulatory β- and γ-subunits, each of which has two or more isoforms that are differentially expressed in various tissues and that arise from distinct genes. The AMPK system acts as a sensor of cellular energy status that is conserved in all eukaryotic cells. In addition, AMPK is activated by physiological stimuli and oxidants.
2. The importance of AMPK in cardiovascular functions is best demonstrated by recent studies showing that widely used drugs, including statins, metformin and rosiglitazone, execute cardiovascular protective effects at least partly through the activation of AMPK. As a consequence, AMPK has been proposed as a candidate target for therapeutic intervention in the treatment of both Type 2 diabetes and metabolic syndrome owing to its central role in the regulation of energy balance; it may also have a role in weight control.
3. In the present brief review, we summarize the recent progress of AMPK signalling and regulation focusing on vascular endothelial cells. We further hypothesize that AMPK is a dual sensor for energy and redox status within a cell and AMPK may be a therapeutic target for protecting vascular endothelial function.
Keywords: AMP-activated kinase, atherosclerosis, diabetes mellitus, endothelium, energy metabolism, hypertension, nitric oxide, oxidative stress, peroxynitrite, superoxide anions
OVERALL REVIEW OF AMP-ACTIVATED KINASE
The AMP-activated protein kinase (AMPK) is a serine/threonine kinase and a member of the Snf1/AMPK protein kinase family that is found in all eukaryotes.1,2 AMPK has been proposed to act as a cellular energy sensor, which switches on catabolic pathways that produce ATP and switches off anabolic pathways that consume ATP. AMPK is a heterotrimer, containing α-, β- and γ-subunits, each of which has at least two isoforms. The α-subunit contains the catalytic site; however, all subunits are necessary for full activity. Increases in the ratio of AMP to ATP activate AMPK by a number of mechanisms, including direct allosteric activation and covalent modification due to activation by an AMP-dependent AMPK kinase (AMPKK), which phosphorylates the α-subunit on Thr172. Until recently, it was believed that this occurred as a result of activation of AMPKK by an increase in the AMP/ATP ratio. However, recent studies from Woods et al.3 and Carlson et al.4 failed to demonstrate such an increase in AMPKK activity in response to various AMPK activators. Rather, their results indicate that AMP binds to AMPK and that this makes it more susceptible to phosphorylation by AMPKK. Interestingly, the first AMPKK that has been identified is LKB1, a tumour suppressor that is mutated in humans with Peutz–Jegher syndrome,5 a disorder associated with an increased risk of developing carcinomas of the colon, stomach and pancreas. A second AMPKK has been identified as calcium calmodulin-dependent kinase kinase (CaMKK; see below).6
AMPK is ubiquitous in distribution and its activity increases in a variety of cells in response to stressors such as hypoxia, oxidant stress, hyperosmolarity and, in muscle, exercise. As noted first by Hardie et al.,7 AMPK activation appears to be a fundamental component of the response of many cells to stresses that threaten their viability. AMPK can be phosphorylated and activated in various tissues by hormones that act through Gq receptors8 and by adiponectin,9 leptin,10 α- and β-adrenoreceptor agonists,11,12 pharmacological agents such as metformin13,14 and the thiazolidinediones,15 and oxidants such as ONOO−16,17 and H2O2.18 In addition, AMPK may be regulated by cellular glycogen content, possibly as a consequence of AMPK binding to glycogen via its β-subunit.19 Activation of AMPK leads to the phosphorylation of a number of target molecules that result in, among other things, increases in fatty acid oxidation and muscle glucose transport (to generate more ATP), as well as inhibition of various synthetic processes (to conserve ATP). Acetyl CoA carboxylase (ACC) and 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase20 were the first molecules shown to be AMPK targets; however, other molecules are being identified at a rapid rate. For example, AMPK phosphorylates and activates malonyl CoA decarboxylase,21 an enzyme involved in malonyl CoA turnover in many tissues, and it phosphorylates and inhibits glycerophosphate acyltransferase,22 the first committed enzyme in glycerolipid synthesis. Likewise, both endothelial and neuronal nitric oxide synthase (eNOS and nNOS, respectively) have been shown to be targets for AMPK in endothelium and muscle by Chen et al.23 and Winder et al.24 have shown that treatment of intact rats with 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) increases the expression of a variety of proteins in muscle, including the glucose transporter GLUT-4 and several mitochondrial oxidative enzymes. In addition, AMPK activation has been shown to alter the expression of a variety of genes, including those for uncoupling protein (UCP)-3 and GLUT-4 in muscle,25 and fatty acid synthase (FAS)26 and phosphoenolpyruvate carboxykinase27 in hepatocytes. In addition, one recent study from our group13 showed that nicotine, via peroxynitrite (ONOO−), activates AMPK, resulting in enhanced threonine phosphorylation and consequent inhibition of FAS in 3T3L1 adipocytes.
Since its discovery, investigations into the regulation and the effects of AMPK have expanded very rapidly. The AMPK pathway and modulation of energy balance through the AMPK system presents an attractive therapeutic target for intervention in many conditions of disordered energy balance, including obesity, Type 2 diabetes, stroke and atherosclerosis. Among these, research on the protective actions of and the regulation of energy metabolism by AMPK in the endothelium is becoming an important area. Due to page limitation, the scope of the present review will be limited to new developments related to AMPK as a protector and a metabolic sensor in the endothelium. We focus on the effect of AMPK activation on cellular factors contributing to endothelial cell damage and dysfunction and discuss the possibility that AMPK may be a therapeutic target for atherosclerosis.
ENDOTHELIUM: BARRIER AND ATHEROGENESIS INITIATIVE FACTOR
The endothelium is a single layer of cells that covers the inner surface of all vessels in the body, including conduit vessels, resistance vessels, precapillary arterioles and capillaries. The endothelial layer forms a barrier between the elements of the blood and the tissues by virtue of its direct contact with the circulating blood. The intact function and structure of the endothelium are prerequisite for maintaining normal vascular function and anti-atherogenesis. The arterial wall endothelium has various important physiological functions, including adjusting vascular tone and permeability, antithrombosis and the secretion of diverse active materials. Arterial wall endothelium damage is an initiating factor for atherogenesis. The endothelium is susceptible to changes in blood elements, such as increases in blood glucose or free fatty acids (FFAs), angiotensin II, adrenaline, noradrenaline, bradykinin, as well as hypertension and hypercholesterolaemia. All these factors can cause vascular endothelium damage.
Endothelial dysfunction is characterized by increased superoxide production, impaired nitric oxide (NO) activity, increased endothelial apoptosis and a reduction in endothelium-dependent vasodilation.28,29 Chronic dysfunction of the endothelium is implicated in the pathophysiology of several cardiovascular disorders, including atherosclerosis, hypertension, diabetic vasculopathy and heart failure.30 There are many factors that contribute to endothelial damage and dysfunction. The most important factors are hyperglycaemia and FFAs induced by insulin resistance syndrome/diabetes. Hyperglycaemia and FFAs can result in a serial of pathophysiological changes at the molecular level, including inflammation,31 endothelial cell apoptosis,29,32 increases in cell Ca2+, reactive oxygen species (ROS)33,34 and nuclear factor (NF)-κB activation,35 and decreases in eNOS activity and NO bioavailability, which contribute to or accelerate endothelial damage and dysfunction.28
AMPK EXPRESSION AND FUNCTION IN ENDOTHELIAL CELLS
AMPK is a heterotrimeric protein consisting of three subunits, α, β and γ, each of which has at least two isoforms that can lead to the formation of 12 different complexes. These combinations render AMPK complexes with different properties and relative tissue specificity.36 The α-subunit contains the catalytic site, whereas the β- and γ-regulatory subunits are important in maintaining the stability of the heterotrimer complex.37 However, all subunits are necessary for full activity.38 Different tissues express distinct α-catalytic subunits that account for the major part of AMPK activity. Muscle cells mainly express AMPK complexes containing the α2-subunit39 and adipocytes express the α1 isoform.12,40 The liver expresses both α1 and α2 isoforms.41 AMPK is expressed in both endothelial cells and smooth muscle cells. Both α1- and α2-catalytic subunits are expressed in arterial smooth muscle cells, although their relative proportion differs between different arteries.42 We found that both α1- and α2-catalytic subunits are expressed in endothelial cells43 and the predominant isoform is α1.16,43 Although the AMPKα2 isoform is barely detectable in endothelial cells, it still has important physiological functions; for example, it is essential for angiogenesis in response to hypoxic stress.44 The functional significance of these different combinations remains unclear; however, it is noted that AMPK complexes containing the α1 isoform are less sensitive to AMP.45 To date, there are no data concerning the respective expression of other AMPK subunits in endothelial cells.
Several studies have suggested that endothelial AMPK may play important physiological functions, such as modulation of endothelial cell energy supply,46 protection from apoptosis,32 mediation of endothelial NOS activation in response to shear stress47 and regulation of inflammation, angiogenesis and maintenance of perfusion.44,48
REGULATION OF AMPK IN ENDOTHELIAL CELLS
AMPK kinase
The control of AMPK activity is complex and the classic view is that AMPK is activated allosterically by an increase in the intracellular AMP/ATP ratio and/or by the phosphorylation of Thr172 within the α-subunit. Several protein kinases responsible for this phosphorylation have been identified, including Peutz-Jeghers syndrome kinase LKB1 (LKB1),5 a tumour suppressor kinase in complex with two accessory subunits termed STE20-related adaptor (STRAD) and MO25 and the Ca2+/calmodulin-dependent protein kinase kinase (CaMKK).6 The major breakthrough in identifying the first AMPKK came from research on the regulation of the AMPK orthologue Snf-1 in Saccharomyces cerevisiae.7 The T-loop residue of Snf-1 was phosphorylated by a group of three related protein kinases bearing homology to mammalian LKB1, which was subsequently identified by several laboratories as being the major upstream kinase for AMPK.5,49 Subsequent studies suggested that AMPK could not be activated in mammalian cells that lacked LKB1 expression or in cells that were treated with heat shock protein (Hsp) 90 inhibitors, which decrease LKB1 expression.50 In addition, LKB1 turned out to phosphorylate the T-loop of all 12 human kinases that are phylogenetically related to AMPK (AMPK subfamily).51
Several cellular stress conditions, such as glucose deprivation, hypoxia, ischaemia and heat shock, are responsible for the depletion of cellular ATP and elevation of AMP levels that are associated with an increased AMP/ATP ratio.1 The γ-subunit of AMPK has been identified as the protein that binds AMP. When the γ-subunit binds AMP, AMPK is allosterically activated and becomes a better substrate of AMPKK LKB1.52 Then, the AMPK α-subunit undergoes phosphorylation by LKB1 on Thr172 and its activity increases significantly.52 In vitro studies have suggested that binding of AMP to AMPK is likely to be the principal regulatory mechanism stimulating phosphorylation of AMPK by LKB1.5 However, several lines of evidence point to the presence of non-LKB1 AMPKKs.6 Under some conditions (e.g. hyperosmotic stress or antidiabetic drug metformin), AMPK is activated without a detectable change in the AMP/ATP ratio.53 In yeast, three protein kinases, namely Elm1, Pak1 and Tos3, have been identified to phosphorylate and activate SNF1, the yeast homolog of AMPK.54,55 The most closely related mammalian protein kinases to these yeast kinases are members of the CaMKK family.54,55 The finding that CaMKKβ acts upstream of AMPK suggests that, in addition to changes in the AMP/ATP ratio, an increase in intracellular Ca2+ may act as a second pathway to activate AMPK.56
In endothelial cells, AMPK is activated by two AMPKK pathways, LKB1 and CaMKKβ.56 Several direct and indirect arguments suggest that LKB1 is involved in AMPK activation in endothelial cells. Treatment of endothelial cells with AICAR, a drug that is transformed in the cell into 5-amino-4-imidazolecarboxamide (AICA)–riboside (also termed ‘ZMP’), an analogue of AMP, activates AMPK in endothelial cells.16,57 Studies by our group indicate that ONOO− and hypoxia–reoxygenation activate AMPK by stimulating LKB1 phosphorylation at Ser428.17 Furthermore, direct mutation of Ser428 of LKB1 into alanine and the kinase-inactive LKB1 mutant abolished ONOO−-induced AMPK activation.17 A study of human endothelial cells by Stahmann et al.56 showed that AMPK is activated by thrombin through a Ca2+-dependent mechanism. Inhibition of CaMKK with STO-609 or downregulation of CaMKKβ using RNA interference decreased thrombin-induced AMPK activation significantly, indicating that CaMKKβ was the responsible AMPK kinase. Endothelial AMPK represents an important protective and adaptive mechanism in responsible to various cellular stresses, including of increased FFA,57 hypoxic stress44 and peroxynitrite,58,59 and mediates the actions of hormones, such as adiponectin62 and oestradiol,61 metformin16 and vascular mediators, such as bradykinin64 and histamine or thrombin.63
AMPK kinase kinase
As mentioned above, LKB1 is an important upstream kinase for AMPK. However, paradoxically, neither the activity of LKB1 itself nor that of AMPK-related kinases was regulated directly by the stimuli known to activate AMPK, such as AMP, AICAR, muscle contraction, hypoxia–reoxygenation and metformin.43,58,64 Thus, how these stimuli lead to LKB1-dependent AMPK activation and whether an upstream kinase for LKB1 exists remain unclear.
Our recent studies in endothelial cells17 found that atypical protein kinase Cξ (PKCξ), a protein kinase of the AGC family, regulates AMPKK LKB1 acting as AMPK kinase kinase (AMPKKK). The human LKB1 is a serine–threonine kinase of 433 amino acids, which contains both a kinase domain and a nuclear localization signal in its N-terminal region.50 The C-terminal region of LKB1 consists of 124 residues and contains several post-translational modifications. Five phosphorylation sites have been identified; two residues are autophosphorylation sites (Thr336 and Thr402) and three others (Ser325, Thr363 and Ser428) are phosphorylated by upstream kinases.50 Possibly, the LKB1 C-terminal region serves as a regulatory domain mediating dynamic interactions with several classes of proteins and promotes subcellular targeting.65 We found that stimulation of bovine aortic endothelial cells (BAEC) with phorbol myristate acetate activated AMPK in parallel with increased LKB1 phosphorylation at Ser428.17 Mutation of Ser428 into alanine abolished ONOO−-enhanced AMPK activation. These results suggest that Ser428 located in the C-terminus of LKB1 may play a crucial role in regulating AMPK activation. Indeed, a recent study by Forcet et al.66 suggests that naturally occurring C-terminal mutations, which neither disrupt LKB1 kinase activity nor interfere with LKB1-induced growth arrest, reduce LKB1-mediated activation of AMPK and impair downstream signalling. We have provided evidence that PKCξ regulates AMPK activation by phosphorylating LKB1, which results in increased association of LKB1 with AMPK.17 The key evidence stems from studies in endothelial cells that show that ONOO−, a potent oxidant formed by the combination of superoxide anions (·O2−) and NO at a diffusion-controlled rate, activated AMPK independent of cellular AMP/ATP ratios.16,43,58 This AMPK activation pathway was LKB1 dependent, involved LKB1 phosphorylation at Ser428 within the C-terminal part of LKB1 and led to the association of LKB1 with AMPK. AMPK is not activated by ONOO− in cells lacking LKB1 (HeLa-S3 cells) but expressing normal levels of CaMKK.6 In addition, ONOO− increased the coimmunoprecipitation of LKB1 with AMPK, which was sensitive to inhibition of PKCξ. Furthermore, inhibition of PKCξ attenuated ONOO−-enhanced LKB1-Ser428 phosphorylation, as well as the association of LKB1 with AMPK. More importantly, recombinant PKCξ, in a concentration-dependent manner, caused the phosphorylation of LKB1-Ser428 in vitro. Inhibition of PKCξ abolished ONOO−-enhanced association of LKB1 with AMPK, but not with microtubule-associated protein/microtubule affinity-regulating kinase-3 (MARK-3), another member of the AMPK family, suggesting that PKCξ–LKB1 signalling is exclusively active towards the AMPK pathway.17 These results are in line with previous findings43,64 that AMPK stimuli, such as AICAR or exercise, activate AMPK without altering the activities of LKB1 and other members of the AMPK family. This selective effect of PKCξ–LKB1 on AMPK may involve the PKCξ-dependent phosphorylation of LKB1-Ser428 within the C terminus of LKB1. Because phosphorylation of none of the other known regulatory sites on LKB1 was affected by PKCξ and a low level of LKB1/MARK-3 interaction was independent of PKCξ inhibition, LKB1-Ser428 phosphorylation by PKCξ may be the specific step leading to LKB1-dependent AMPK activation.
We have used endothelial cells to establish a novel PKCξ–LKB1–AMPK signalling axis. In addition, we have also replicated these findings in a variety of tissues, including human retinal pericytes, rat smooth muscle cells and mouse 3T3-L1 preadipocytes (M-H Zou and Y Wu, unpubl. obs., 2007).17 Furthermore, PKCξ inhibition attenuates AMPK activation by a variety of known AMPK stimuli, including oxidant (ONOO−), pharmacological reagent (metformin) and hypoxia–reoxygenation, implying that the PKCξ–LKB1–AMPK pathway is not unique to the endothelium and may be a common pathway shared by other physiological stimuli in a variety of tissues. Thus, we can expect broader implications of this signalling pathway in cell biology. A number of reports has shown that PKCξ plays critical roles in signalling pathways that control cell growth, differentiation and survival.50,67 Thus, the interactions of PKCξ with LKB1 and AMPK that we have described above may affect our understanding of not only vascular biology, but also cancer development. LKB1 is an upstream activator of AMPK and regulation of the AMPK pathway is believed to be directly involved in LKB1 tumour suppressor function.68 Consistent with this model, LKB1 has recently been identified as a negative regulator of mammalian targets of rapamycin (mTOR) signalling through the sequential stimulation of AMPK and of the tuberous sclerosis (TSC) 1/TSC2 tumour suppressor complex.69 Furthermore, ACC, a substrate of AMPK, controls fatty acid synthetic metabolism, which is frequently dysregulated in tumours.70 We found that phosphorylation of LKB1-Ser428 in its C-terminal part increased association of LKB1 with AMPK, as well as activation of its downstream pathways, thus reinforcing the idea that AMPK plays a role in the control of cell transformation. Indeed, C-terminal mutations in LKB1, which decrease LKB1-mediated AMPK activation, compromise the ability of LKB1 to establish and maintain polarity of both intestinal epithelial cells and migrating astrocytes. Our findings further support the notion that LKB1 tumour suppressor activity depends, at least in part, on the regulation of AMPK signalling and downstream effects on cell polarization.
Serine/threonine phosphatases
Reversible protein phosphorylation is a major mechanism that is used in eukaryotic organisms to regulate all kinds of cellular functions, from metabolic pathways to cell death. At least one-third of human proteins contain covalently bound phosphate. More than 98% of protein phosphorylation occurs on serine and threonine residues. The opposing actions of protein kinases and protein phosphatases control the degree of protein phosphorylation within the cell. Similarly, the delicate AMPK phosphorylation is maintained by upstream protein kinase AMPKK and serine/threonine phosphatase. Two serine/threonine phosphatases, namely protein phosphatase 2A (PP2A) and protein phosphatase 2C (PP2C), were identified to inactivate the active and phosphorylated form of AMPK in cell-free assays.71
It has been shown that PP2C is responsible for dephosphorylation of AMPK.72 Wang and Unger75 have demonstrated that AMPK phosphorylation was significantly reduced in both Zucker diabetic rat fa/fa rats and ob/ob mouse hearts compared with lean, wild-type controls and the reduction in active phosphorylated AMPKα is associated with an increase in PP2C. The PP2C are characterized by the requirement of Mn2+ or Mg2+ for activity.74 Two isoforms of PP2C, α and β, are known from a variety of mammalian tissues.75 They are considered monomeric (43–48 kDa), share 75% sequence identity and have the same enzymatic characteristics.76 Both isozymes are ubiquitously expressed. PP2Cα was expressed mainly in different epithelial cell types in organs and tissues including the skin, lungs, kidney, breast, digestive tract, endocrine glands, testis, prostate, ovary, uterus, brain, lymph nodes and bone marrow. Human endothelial cells, smooth muscle cells and extracellular matrix contained no or very little PP2Cα.77 In contrast with the other Ser/Thr protein phosphatases, how the activity, localization and substrate specificity of PP2C are governed is not clear.76 An alignment of the amino acid sequence of AMPK with that of other serine/threonine protein kinases around the regulatory phosphorylation site (subdomains VII–VIII) revealed a high degree of conservation.78 Phosphopeptides derived from this region of AMPK and containing the almost invariant threonine (Thr172 in AMPK) were found to be good substrates for PP2Cα.78 PP2C plays an important role in regulating AMPK phosphorylation in physiological conditions and the basal AMPK phosphorylation is largely dependent on PP2C activity. Using the inactivation assay as the criterion, Davies et al.71 found that dephosphorylation of AMPK by PP2Cα is inhibited by 5′-AMP. Therefore, the AMP activation of AMPK has two aspects: (i) binding of AMP to the AMPK γ-subunit leads to allosterical changes in AMPK and renders AMPK a better substrate for AMPKK LKB1; and (ii) binding of AMP to the phosphoform shields the phosphorylation site against the action of protein phosphatase, thus protecting the phosphorylation status.
It is known that PP2A is a major protein phosphatase in all eukaryotic cells that has a wide range of biological functions, including control of the cell cycle, organization of the cytoskeleton, transcription of immediate early genes, and cholesterol and protein biosynthesis. PP2A is a multimeric ubiquitously expressed serine/threonine phosphatase consisting of scaffolding A, regulatory B and catalytic C subunits. PP2A has been shown to be ceramide responsive in vitro and in vivo59,79 and is also termed ‘ceramide-activated protein phosphatase’ (CAPP).79 In contrast with PP2C, endothelial cells have abundant PP2A expression.57,80 Increasing evidence indicates that PP2A plays important roles in maintaining endothelial cell physiological functions, including regulation of endothelial cell cytoskeletal structure, protection of the endothelial cell barrier,81 maintenance of endothelial cells in a resting state and limiting the motility that is needed for the morphogenic process of angiogenesis.80 Moreover, PP2A can regulate eNOS phosphorylation status directly by dephosphorylating serine 1177/1179.82 However, excessive PP2A activation in pathological conditions results in endothelial cell damage or dysfunction by inhibition of AMPK activity. Our recent studies59 have demonstrated that prolonged exposure of endothelial cells to palmitate significantly suppresses the phosphorylation of AMPK and its downstream enzyme ACC. We have provided compelling evidence obtained in vitro and in vivo that palmitate inhibits both AMPK and eNOS phosphorylation by ceramide-dependent PP2A activation. This observation provides further support for the concept that PP2A is an important component for the dephosphorylation and inactivaton of AMPK and may directly modulate AMPK function. In support of this idea, it has been reported that the PP2A complex is involved in regulating the interaction between AMPK α2 and γ185 and inactivation of AMPK in pancreatic β-cells,84 and that the active phosphorylated form of AMPK can be inactivated in cell-free assays by PP2A.71 Thus, PP2A may be a physiologically relevant AMPK phosphatase.
AMPK AS A SENSOR FOR REACTIVE OXYGEN AND NITROGEN SPECIES IN ENDOTHELIAL CELLS
Reactive nitrogen species (RNS) are derived from NO. Of these, peroxynitrite (ONOO−) is the best characterized and appears to have the most biological activity. Peroxynitrite is formed by the biradical reaction of NO and O2−. Many oxidation and nitration products are produced from the reaction of ONOO− with cellular macromolecules. Reactive nitrogen species comprise a class of products formed by cells in response to various stress stimuli. There is evidence that cells exposed to oxidants such as ONOO−16,17 and H2O218 activate AMPK, although the mechanism remains to be determined. In our exciting preliminary studies, we found that ONOO− activated AMPK in endothelial cells and increased the phosphorylation of its downstream enzymes eNOS-Ser13,79 and ACC-Ser.79 In order to determine whether activation of AMPK by ONOO− was due to AMP accumulation, we examined cellular adenosine nucleotide levels in BAEC exposed to bolus additions of 50 μmol/L ONOO−. We found that bolus addition of ONOO− did not result in a drop in the ATP/AMP ratio.58 Using pharmacological and molecular biological approaches, we identified that ONOO− may activate AMPK via an AMP-independent process and involves c-Src/phosphatidylinositol 3-kinase (PI3-K)/3-phosphoinositide-dependent kinase 1 leading to PKCξ phosphorylation and activation, which activates LKB1 and increases LKB1–AMPK association. This activation is LKB1 dependent. In the early phase (less than 5 min) of hypoxic endothelial cells, inhibition of ONOO− formation by overexpression of superoxide dismutase (SOD; to scavenge O2−) or NOS inhibition with NG-nitro-L-arginine methyl ester (L-NAME; to prevent the formation of NO) attenuated hypoxia–reoxygenation-enhanced phosphorylation of both AMPK and ACC. Because · O2− alone has no effect on AMPK activation and the addition of authentic ONOO− did not affect the AMP/ATP ratio, the attenuation of AMPK and ACC phosphorylation by L-NAME and SOD in cells exposed to hypoxia–reoxygenation suggests the involvement of ONOO− and cannot be explained solely by the AMP/ATP ratio. Inhibition of c-Src activity or PI3-K with a dominant negative mutant also blocked both AMPK and ACC phosphorylation. Thus, our results suggest that a signalling pathway initiated by ONOO− is capable of activating AMPK independent of changes in the AMP/ATP ratio.
Interestingly, our recent studies indicate NO can also activate AMPK (M-H Zou and J-H Zhang, unpub. obs., 2007). However, NO-induced AMPK activation is LKB1 independet. Nitric oxide can increase intracellular Ca2+ content and activate CaMKKβ, an AMPKK. It is noted that the structure-similar isogenous derivatives (NO and ONOO−) activate AMPK through an entirely distinct pathway. The probable explanation is that ONOO−-induced AMPK activation is a response to cellular stress under non-physiological conditions, such as oxidative stress or inhibition of the mitochondrial respiration chain, whereas NO, which exists in normal endothelial cells, activates AMPK and then regulates endothelial metabolism and function under physiological conditions.
NITRIC OXIDE, AMPK AND ENDOTHELIAL FUNCTION
There are roughly 1014 endothelial cells in our vasculature to protect us against atherosclerosis and thrombosis.85 A major weapon of endothelial cells to fight vascular diseases is eNOS, an enzyme that generates the vasoprotective molecule NO·,85 which requires Ca2+/calmodulin, flavin adenine dinucleotide, flavin mononucleotide and tetrahydrobiopterin (BH4) as cofactors.86 Vascular NO· has a variety of functions, but its most important action is to dilate all types of blood vessels and maintain vascular homeostasis by stimulating soluble guanylyl cyclase and increasing cGMP in smooth muscle cells. Impairment of endothelium-dependent relaxations (EDR) represents reduced eNOS-derived NO bioavailability and is present in atherosclerotic vessels even before vascular structural changes occur.87 Endothelial dysfunction, as characterized by an impairment of EDR and reduced NO bioactivity, is the critical step for atherogenesis. In addition, vascular NO· is a potent inhibitor of platelet aggregation and adhesion and can also inhibit leucocyte adhesion to the vessel wall. White cell adherence is an early event in the development of atherosclerosis; therefore, NO· may protect against the onset of atherogenesis.85 Furthermore, NO· has been shown to inhibit DNA synthesis, mitogenesis and proliferation of vascular smooth muscle cells, which are important components of vessel wall remodelling during atherosclerosis formation.88 In addition, NO· suppresses smooth muscle exposure to platelet-derived growth factor(s) by inhibiting platelet aggregation and adhesion. Thus, NO· also prevents the formation of fibrous plaque, a later step in atherogenesis. Based on these effects, endothelial NO· probably represents the most important anti-atherogenic defence in the vasculature.85 It is therefore not surprising that loss of endothelial NO function is associated with several cardiovascular disorders, including atherosclerosis, which is due either to decreased production or to increased degradation of NO.
AMPK is an important activator of eNOS and also the focus of considerable interest as a therapeutic target for the treatment of Type 2 diabetes and the metabolic syndrome, which are both associated with endothelial dysfunction and cardiovascular disease, yet the role of AMPK in the endothelium is poorly characterized. Ongoing studies within our group aim at understanding the role of AMPK in the endothelium. We61 and others23 have demonstrated that AMPK phosphorylates and activates eNOS in cultured endothelial cells. Recent studies in our laboratory have indicated that AMPK mediates the stimulation of NO synthesis in response to several agonists (M-H Zou and Y Wu, accepted for publication, 2008). In addition, we have studied the functional effects of AMPK activation in the endothelium and have demonstrated that AMPK activation increases endothelium-dependent vasodilatation in animal model (M-H Zou and Y Wu, accepted for publication, 2008). Such data indicate an anti-atherogenic role for the AMPK cascade.
OXIDATIVE STRESS, AMPK AND ENDOTHELIAL FUNCTION
Oxidative stress in the vascular wall is an important feature of vascular disease. Reactive oxygen species (ROS) are increasingly recognized as the major cause of compromised endothelial cell function by oxidative modification of cellular components, such as proteins, lipids, carbohydrates and DNA, but also by interfering with signal transduction pathways and the action of endothelial products necessary for the regulation of vascular homeostasis.89 In addition, ROS influence endothelial barrier function and leucocyte adhesion and transmigration.90 Many in vitro and in vivo studies suggest that oxidative stress is increased in patients or animal models with diabetes or dyslipidaemia.91,92 Oxidative stress may be crucial for the pathogenesis of diabetic mellitus and its complications. Mitochondria have been reported to be the major source of ROS. Therefore, mitochondrial ROS production seems to be one of the important targets to prevent a progression of diabetic complications. The most universal and critical mitochondrial function is oxidative phosphorylation. The overall system of oxidative phosphorylation includes five large multi-enzyme complexes, designated complexes I, II, III, IV and ATP synthase. This mitochondrial respiratory system is thought to be the major source of ROS under normal physiological conditions. Two main sites of superoxide generation exist in this system: NADH dehydrogenase at complex I and the interface between coenzyme Q (CoQ) and complex III. It has been shown that hyperglycaemia could increase the production of ROS from the mitochondrial electron transport chain.33
The endothelium is the first organ that comes in contact with and perceives high glucose or high lipid concentrations and, therefore, is very susceptible to these changes. Although endothelial dysfunction encompasses a broad range of abnormalities, a reduced bioavailability of endothelium-derived NO is the most widely studied aspect. A reduction in NO bioavailability in the vessel wall impairs endothelium-dependent vasorelaxation and reduces other beneficial effects of NO, such as its inhibition of platelet and leucocyte adhesion and its antiproliferative effects.30 A decline in NO bioavailability may be caused by: (i) reduced activity or expression of eNOS; (ii) a deficiency of eNOS substrate (L-arginine) or cofactors (BH4);93,94 and/or (iii) increased inactivation of NO by O2−. The latter is now recognized as a fundamentally important underlying mechanism in most settings. In addition, recent studies suggest that ROS or ONOO−, the reaction product of NO and · O2−, oxidize and degrade BH4, leading to eNOS uncoupling and the promotion of endothelial dysfunction.93 Our studies also suggest that high glucose via the mitochondrial respiration chain leads to ROS overproduction, which decreases NO production either directly by reacting with NO and then converting into ONOO− or ROS or indirectly via ONOO−-induced degradation of BH4, which leads to eNOS uncoupling and then decreases in NO levels.
Our previous investigations have suggested that increased ROS production promotes AMPK activation by the LKB1 complex in cultured BAEC.16 Thus, AMPK activation in response to oxidative stress may be implicated in an anti-oxidative or protective role of AMPK in endothelial cells. Studies from Ido et al.32 have shown that incubation of human umbilical vein endothelial cells (HUVEC) with 30 compared with 5 mmol/L glucose for 72 h causes a significant increase in apoptosis related to an increase in oxidative stress. However, incubation with the AMPK activator AICAR increased AMPK activity and completely prevented this change, suggesting that AMPK could play an important role in protecting the endothelial cell against the adverse effects of sustained hyperglycaemia. Interestingly, Ouslimani et al. reported that metformin, an AMPK activator, decreased intracellular production of ROS in aortic endothelial cells mainly by NAD(P)H oxidase and also, to a lesser extent, by the respiratory mitochondrial chain.95 Kukidome et al. provided more direct evidence that activation of AMPK reduces hyperglycaemia-induced mitochondrial ROS production by induction of Mn-SOD and promotion of mitochondrial biogenesis through activation of the AMPK–peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) pathway in HUVEC.96 These studies support the notion that AMPK has a beneficial effect on endothelial function by suppressing oxidative stress in endothelial cells.
AMPK AND LIPOTOXICITY IN ENDOTHELIAL CELLS
Fatty acids can be a major fuel of the endothelium and, over a brief period of time, they can be derived predominantly from intracellular stores.97 In fact, high rates of fatty acid oxidation are demonstrable in HUVEC when the cells are provided with carnitine.97 Endothelial cells can maintain their energy state when fatty acids are essentially their sole fuel.98 The AMPK–ACC–malonyl CoA–carnitine palmitoyl-transferase 1 mechanism for regulating long-chain fatty acid oxidation, similar to that of muscle, operates in the endothelial cell and is regulated by AMPK under physiological conditions.97,98 Endothelial cells also have the capacity to store triglycerides (TG), which are hydrolysed continuously to provide an endogenous source of FFAs.97
However, excessive exposure of vascular endothelium to FFAs leads to endothelial dysfunction. This overexposure is associated with insulin resistance and diabetes, in which the ability of insulin-resistant adipocytes to store and retain FFAs is compromised, leading to a sustained elevation in serum FFAs. In a similar manner, diets rich in fat and/or calories are considered a risk factor for the development of atherosclerosis. Certain diet-derived lipids and their derivatives can disrupt normal endothelial integrity, thus reducing the ability of the endothelium to act as a selectively permeable barrier to blood components. An excess of FFAs or suppressed fat oxidation in endothelial cells leads to increased de novo synthesis of diacylglycerol (DAG),99 which activates certain isoforms of protein kinase C (PKC).34 Overactivity of PKC contributes to endothelial damage and vasculopathy by stimulating endothelial superoxide production via NAD(P)H oxidase,34 inhibiting NOS activity,100 impairing endothelial responsiveness to insulin100 and promoting activation of NF-κB.101 High levels of FFAs also lead to endothelial cell lipid accumulation. Lipid accumulation per se in vascular endothelial cells may play an important role in the pathogenesis of atherosclerosis in obese subjects. Selective lipid accumulation and fatty acid changes in endothelial cells can modulate membrane fluidity, proteoglycan metabolism and signal transduction mechanisms. Reduced AMPK activation may play an important role in the lipid accumulation and genesis of endothelial dysfunction in obese rats.102
Recent studies105,104 in heart and liver have revealed that AMPK may be sensitive to the ‘lipid status’ of a cell and activation may be influenced by intracellular fatty acid availability independent of cellular AMP levels. Indeed, a reduced fat oxidative capacity has been reported in Type 2 diabetic patients.105 In addition, feeding of a high-fat diet significantly decreases p-AMPK in the liver and muscles of rodents.106,107 AMPK activity is reduced in aortic endothelium or skeletal muscle of obese rats compared with lean animals.102 These investigations raise the possibility that chronic exposure of cells to fatty acids inhibits AMPK activation. Recently, our studies59 indicated that long-term high levels of FFAs trigger a ceramide–PP2A-dependent inhibition of both AMPK and eNOS, which may contribute to vascular endothelial injury and dysfunction. Taken together, impaired endothelial AMPK activation may contribute to lipid accumulation, lipotoxicity and endothelial dysfunction.
One feasible way to alleviate the endothelial lipotoxicity associated with insulin resistance syndrome/diabetes is to lessen the excessive flux of FFAs into the endothelium, which can be achieved by eating very low-fat meals, improving the insulin sensitivity of insulin-resistant adipocytes, achieving appropriate weight loss and/or by administering insulin-sensitizing agents, such as thiazolidinediones or bioactive chromium, which improve adipocyte insulin responsiveness.108 The other alternative way to deal with endothelial lipotoxicity is to promote FFA oxidation and ameliorate lipid accumulation in endothelial cells, which can be achieved by activating endothelial AMPK.46,97 As in muscle cells, AMPK has been shown to phosphorylate and inhibit ACC activity; this leads to a reduction in malonyl-CoA, which, in turn, disinhibits carnitine palmitoyltransferase and accelerates mitochondrial FFA oxidation.108 Accordingly, Ruderman’s group has reported that treatment of endothelial cells with AICAR, a natural activator of AMPK, largely offsets the adverse effects of palmitate on endothelial superoxide production and NF-κB activation.98 The recently characterized hormone adiponectin, which can decrease lipid accumulation in non-adipose tissues, is now known to activate endothelial AMPK via a membrane receptor.109 The study of Lee et al.102 indicated that reduced AMPK activation may play an important role in lipid accumulation and the genesis of endothelial dysfunction in obese rats and that α-lipoic acid improves vascular dysfunction by normalizing lipid metabolism and activating AMPK in endothelial cells.
OTHER FUNCTIONS OF AMPK IN ENDOTHELIAL CELLS
Apart from activating eNOS and suppressing oxidative stress, AMPK has other potential benefits to endothelial cells. Activated AMPK increases fatty acid oxidation by phosphorylating and inhibiting ACC, leading to a decrease in the concentration of malonyl-CoA.98 In addition, AMPK decreases fatty acid incorporation into glycerolipids, either secondary to its effect on fatty acid oxidation or by virtue of the fact that, in some tissues, it phosphorylates and inhibits sn-glycerophosphate acyltransferase, the first committed enzyme in DAG and TG synthesis.110 AMPK is involved in eNOS activation and subsequent NO production in response to shear stress. Hence, AMPK, in addition to serving as an energy sensor, also plays an important role in regulating vascular tone.47 An additional benefit of endothelial AMPK activity is that it may inhibit glycerol-3-phosphate acyltransferase, required for de novo synthesis of DAG.110 That is, AMPK may lessen endothelial DAG production (and, thus, PKC activation) both by diminishing the availability of the FFA substrate required for synthesis and by directly inhibiting the enzyme that catalyses it. Endothelial AMPK has the further advantage of activating NOS via phosphorylation on Ser1177,23 thus opposing the adverse impact of PKC on this enzyme. AMPK could play an important role in protecting the endothelial cell against hyperglycaemia-induced alterations in fatty acid metabolism, impaired Akt activation by insulin, increased caspase 3 activity and apoptosis.32 In addition, AMPK mediates the inhibitory effect of the high molecular weight form of adiponectin on endothelial cell apoptosis.111 Furthermore, AMPK activation inhibits fatty acid-induced increases in NF-κB transactivation114 and cytokine-induced NF-κB activation in vascular endothelial cells,113 suggesting that it has beneficial effects on endothelial inflammation induced by deleterious stimuli.
In this regard, therapeutic strategies that target the endothelium to stimulate AMPK and fatty acid oxidation may have positive effects on endothelial energy metabolism and may improve endothelial function.
CONCLUSIONS AND PERSPECTIVES
Although the AMPK pathway is traditionally thought of as a sensor of cellular energy status and a regulator of metabolism, recent discoveries have now provided novel evidence that it may function as a protector of endothelial function. AMPK has pleiotropic effects that may be beneficial to endothelial function and anti-atherogenesis, including: (i) induction of the eNOS/NO pathway resulting in increased NO bioavailability; (ii) suppression of endothelial ROS production induced by deleterious stimuli, such as hyperglycaemia or high FFAs; (iii) improving endothelial FFA oxidation, leading to suppression of lipid accumulation; and (iv) anti-apoptotic and anti-inflammatory effects, as well as adjustments of vascular tone (Fig. 1). Together, additional insights into AMPK physiology in the endothelium and the consequences of its modification may suggest novel therapeutic targets for cardiovascular disease. Indeed, two major classes of insulin-sensitizing drugs, namely metformin and thiazolidinediones, may have great potential for promoting vascular health and lessening diabetes risk in insulin-resistance syndromes mainly by activating the AMPK system. However, both these drug classes appear to activate AMPK indirectly, either by inhibiting the respiratory chain or by stimulating the release of adiponectin. Moreover, gastrointestinal side-effects, weight gain, renal and liver toxicity and a slight risk of lactic acidosis would make these drugs logistically difficult to use widely for the many millions of individuals who are susceptible to insulin-resistant and cardiovascular diseases. Therefore, a better-tolerated, safer compound that directly and effectively activates AMPK in endothelial cells, yielding the same beneficial therapeutic effects of existing drugs while avoiding unwanted side-effects, may prove to have substantial practical value for promoting vascular health.
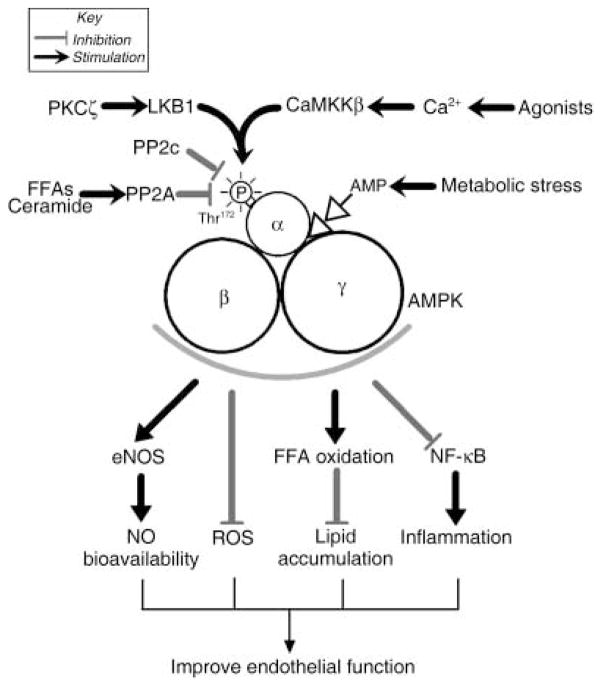
Fig. 1.
Pathways that regulate P-activated protein kinase (AMPK) activation and AMPK-regulated pathways in endothelial cells. When the γ-subunit binds AMP, AMPK is allosterically activated and becomes a better substrate for AMPK kinase LKB1. AMPK is also regulated by upstream kinase calcium calmodulin-dependent kinase kinase β (CaMKKβ) and protein phosphatase 2A and 2C (PP2C and PP2A, respectively). Available studies suggest that AMPK activation improves endothelial function through multiple pathways, as indicated. See text for details. PKCξ, protein kinase Cξ; FFAs, free fatty acids; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; NF-kB, nuclear factor-κB; ROS, reactive oxygen species.
Acknowledgments
We sincerely apologize to the authors of those papers we have had to omit from the reference list owing to page limitations. We thank all members of Dr Zou’s laboratory for useful discussion. The authors’ work reported herein was supported by National Institutes of Health grants (HL079584, HL07439 and HL080499), a research award from the American Diabetes Association, a research award from the Juvenile Diabetes Research Foundation, a research award from the Oklahoma Center for Advancement of Science and Technology and funds from the Travis Endowed Chair in Endocrinology, University of Oklahoma Health Sciences Center.
List of abbreviations
- ACC
Acetyl CoA carboxylase
- AICAR
5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside
- AMPK
AMP-activated protein kinase
- AMPKK
AMPK kinase
- BH4
Tetrahydrobiopterin
- CaMKK
Calcium calmodulin-dependent kinase kinase
- eNOS
Endothelial nitric oxide synthase
- FFAs
Free fatty acids
- NF-κB
Nuclear factor-κB
- NO
Nitric oxide
- PKC
Protein kinase C
- PP2A
Protein phosphatase 2A
- PP2C
Protein phosphatase 2C
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
References
- 1.Kemp BE, Stapleton D, Campbell DJ, et al. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans. 2003;31:162–8. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- 2.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: Possible roles in type 2 diabetes. Am J Physiol. 1999;277:E1–10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 3.Woods A, Johnstone SR, Dickerson K, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: Effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–17. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- 5.Hawley SA, Boudeau J, Reid JL, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280(29):060–6. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 7.Hardie DG, Carling D, Halford N. Roles of the Snf1/Rkin1/AMP-activated protein kinase family in the response to environmental and nutritional stress. Semin Cell Biol. 1994;5:409–16. doi: 10.1006/scel.1994.1048. [DOI] [PubMed] [Google Scholar]
- 8.Soltoff SP. Evidence that tyrphostins AG10 and AG18 are mitochondrial uncouplers that alter phosphorylation-dependent cell signaling. J Biol Chem. 2004;279(10):910–18. doi: 10.1074/jbc.M305396200. [DOI] [PubMed] [Google Scholar]
- 9.Kubota N, Yano W, Kubota T, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Uotani S, Abe T, Yamaguchi Y. Leptin activates AMP-activated protein kinase in hepatic cells via a JAK2-dependent pathway. Biochem Biophys Res Commun. 2006;351:171–5. doi: 10.1016/j.bbrc.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson DS, Chernogubova E, Dallner OS, Cannon B, Bengtsson T. Beta-adrenoceptors, but not alpha-adrenoceptors, stimulate AMP-activated protein kinase in brown adipocytes independently of uncoupling protein-1. Diabetologia. 2005;48:2386–95. doi: 10.1007/s00125-005-1936-7. [DOI] [PubMed] [Google Scholar]
- 12.Daval M, Diot-Dupuy F, Bazin R, et al. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem. 2005;280(25):250–7. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- 13.An Z, Wang H, Song P, Zhang M, Gong X, Zou MH. Nicotine-induced activation of AMP-activated protein kinase Inhibits fatty acid synthase in 3T3-L1 adipocytes: A role for oxidant stress. J Biol Chem. 2007;282(26):793–801. doi: 10.1074/jbc.M703701200. [DOI] [PubMed] [Google Scholar]
- 14.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–6. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeBrasseur NK, Kelly M, Tsao TS, et al. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab. 2006;291:E175–81. doi: 10.1152/ajpendo.00453.2005. [DOI] [PubMed] [Google Scholar]
- 16.Zou MH, Kirkpatrick SS, Davis BJ, et al. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279(43):940–51. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- 17.Xie Z, Dong Y, Zhang M, et al. Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem. 2006;281:6366–75. doi: 10.1074/jbc.M511178200. [DOI] [PubMed] [Google Scholar]
- 18.Choi SL, Kim SJ, Lee KT, et al. The regulation of AMP-activated protein kinase by H2O2. Biochem Biophys Res Commun. 2001;287:92–7. doi: 10.1006/bbrc.2001.5544. [DOI] [PubMed] [Google Scholar]
- 19.Iseli TJ, Walter M, van Denderen BJ, et al. AMP-activated protein kinase beta subunit tethers alpha and gamma subunits via its C-terminal sequence (186–270) J Biol Chem. 2005;280(13):395–400. doi: 10.1074/jbc.M412993200. [DOI] [PubMed] [Google Scholar]
- 20.Gao G, Widmer J, Stapleton D, et al. Catalytic subunits of the porcine and rat 5′-AMP-activated protein kinase are members of the SNF1 protein kinase family. Biochim Biophys Acta. 1995;1266:73–82. doi: 10.1016/0167-4889(94)00222-z. [DOI] [PubMed] [Google Scholar]
- 21.Hopkins TA, Dyck JR, Lopaschuk GD. AMP-activated protein kinase regulation of fatty acid oxidation in the ischaemic heart. Biochem Soc Trans. 2003;31:207–12. doi: 10.1042/bst0310207. [DOI] [PubMed] [Google Scholar]
- 22.Ruderman NB, Park H, Kaushik VK, et al. AMPK as a metabolic switch in rat muscle, liver and adipose tissue after exercise. Acta Physiol Scand. 2003;178:435–42. doi: 10.1046/j.1365-201X.2003.01164.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen ZP, Mitchelhill KI, Michell BJ, et al. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–9. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 24.Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–26. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- 25.Brauner P, Kopecky P, Flachs P, et al. Expression of uncoupling protein 3 and GLUT4 gene in skeletal muscle of preterm newborns: Possible control by AMP-activated protein kinase. Pediatr Res. 2006;60:569–75. doi: 10.1203/01.PDR.0000242301.64555.e2. [DOI] [PubMed] [Google Scholar]
- 26.Woods A, Azzout-Marniche D, Foretz M, et al. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol. 2000;20:6704–11. doi: 10.1128/mcb.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubert A, Husson A, Chedeville A, Lavoinne A. AMP-activated protein kinase counteracted the inhibitory effect of glucose on the phosphoenolpyruvate carboxykinase gene expression in rat hepatocytes. FEBS Lett. 2000;481:209–12. doi: 10.1016/s0014-5793(00)02006-8. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000;49:1231–8. doi: 10.2337/diabetes.49.7.1231. [DOI] [PubMed] [Google Scholar]
- 29.Artwohl M, Roden M, Waldhausl W, Freudenthaler A, Baumgartner-Parzer SM. Free fatty acids trigger apoptosis and inhibit cell cycle progression in human vascular endothelial cells. FASEB J. 2004;18:146–8. doi: 10.1096/fj.03-0301fje. [DOI] [PubMed] [Google Scholar]
- 30.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:A7–11. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 31.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–72. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 32.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: Inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–67. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 33.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 34.Inoguchi T, Li P, Umeda F, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–45. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 35.Pieper GM, Riaz Ul H. Activation of nuclear factor-kappaB in cultured endothelial cells by increased glucose concentration: Prevention by calphostin C. J Cardiovasc Pharmacol. 1997;30:528–32. doi: 10.1097/00005344-199710000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J. 2000;346:659–69. [PMC free article] [PubMed] [Google Scholar]
- 37.Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Dealing with energy demand: The AMP-activated protein kinase. Trends Biochem Sci. 1999;24:22–5. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 38.Dyck JR, Gao G, Widmer J, et al. Regulation of 5′-AMP-activated protein kinase activity by the noncatalytic beta and gamma subunits. J Biol Chem. 1996;271(17):798–803. doi: 10.1074/jbc.271.30.17798. [DOI] [PubMed] [Google Scholar]
- 39.Stapleton D, Mitchelhill KI, Gao G, et al. Mammalian AMP-activated protein kinase subfamily. J Biol Chem. 1996;271:611–14. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- 40.Lihn AS, Jessen N, Pedersen SB, Lund S, Richelson B. AICAR stimulates adiponectin and inhibits cytokines in adipose tissue. Biochem Biophys Res Commun. 2004;316:853–8. doi: 10.1016/j.bbrc.2004.02.139. [DOI] [PubMed] [Google Scholar]
- 41.Woods A, Salt I, Scott J, Hardie DG, Carling D. The alpha1 and alpha2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett. 1996;397:347–51. doi: 10.1016/s0014-5793(96)01209-4. [DOI] [PubMed] [Google Scholar]
- 42.Evans AM, Hardie DG, Galione A, Peers C, Kumar P, Wyatt CN. AMP-activated protein kinase couples mitochondrial inhibition by hypoxia to cell-specific Ca2+ signalling mechanisms in oxygen-sensing cells. Novartis Found Symp. 2006;272:234–52. [PubMed] [Google Scholar]
- 43.Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 44.Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278(31):000–6. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- 45.Salt I, Celler JW, Hawley SA, et al. AMP-activated protein kinase: Greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J. 1998;334:177–87. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dagher Z, Ruderman N, Tornheim K, Ido Y. The effect of AMP-activated protein kinase and its activator AICAR on the metabolism of human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1999;265:112–15. doi: 10.1006/bbrc.1999.1635. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Lee TS, Kolb EM, et al. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol. 2006;26:1281–7. doi: 10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]
- 48.Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005;96:838–46. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- 49.Shaw RJ, Kosmatka M, Bardeesy N, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–35. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boudeau J, Sapkota G, Alessi DR. LKB1, a protein kinase regulating cell proliferation and polarity. FEBS Lett. 2003;546:159–65. doi: 10.1016/s0014-5793(03)00642-2. [DOI] [PubMed] [Google Scholar]
- 51.Lizcano JM, Goransson O, Toth R, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–43. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG. 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem. 1995;270(27):186–91. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- 53.Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277(25):226–32. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 54.Hong SP, Leiper FC, Woods A, Carling D, Carlson M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc Natl Acad Sci USA. 2003;100:8839–43. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutherland CM, Hawley SA, McCartney RR, et al. Elm1p is one of three upstream kinases for the Saccharomyces cerevisiae SNF1 complex. Curr Biol. 2003;13:1299–305. doi: 10.1016/s0960-9822(03)00459-7. [DOI] [PubMed] [Google Scholar]
- 56.Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta. Mol Cell Biol. 2006;26:5933–45. doi: 10.1128/MCB.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem. 2007;282:9777–88. doi: 10.1074/jbc.M608310200. [DOI] [PubMed] [Google Scholar]
- 58.Zou MH, Hou XY, Shi CM, et al. Activation of 5′-AMP-activated kinase is mediated through c-Src and phosphoinositide 3-kinase activity during hypoxia–reoxygenation of bovine aortic endothelial cells. Role of peroxynitrite. J Biol Chem. 2003;278(34):003–10. doi: 10.1074/jbc.M300215200. [DOI] [PubMed] [Google Scholar]
- 59.Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA. Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem. 2002;277(32):552–7. doi: 10.1074/jbc.M204512200. [DOI] [PubMed] [Google Scholar]
- 60.Ouchi N, Kobayashi H, Kihara S, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–9. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schulz E, Anter E, Zou MH, Keaney JF., Jr Estradiol-mediated endothelial nitric oxide synthase association with heat shock protein 90 requires adenosine monophosphate-dependent protein kinase. Circulation. 2005;111:3473–80. doi: 10.1161/CIRCULATIONAHA.105.546812. [DOI] [PubMed] [Google Scholar]
- 62.Mount PF, Hill RE, Fraser SA, et al. Acute renal ischemia rapidly activates the energy sensor AMPK but does not increase phosphorylation of eNOS-Ser1177. Am J Physiol Renal Physiol. 2005;289:F1103–15. doi: 10.1152/ajprenal.00458.2004. [DOI] [PubMed] [Google Scholar]
- 63.Thors B, Halldorsson H, Thorgeirsson G. Thrombin and histamine stimulate endothelial nitric-oxide synthase phosphorylation at Ser1177 via an AMPK mediated pathway independent of PI3K-Akt. FEBS Lett. 2004;573:175–80. doi: 10.1016/j.febslet.2004.07.078. [DOI] [PubMed] [Google Scholar]
- 64.Sakamoto K, McCarthy A, Smith D, et al. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–20. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puceat M, Vassort G. Signalling by protein kinase C isoforms in the heart. Mol Cell Biochem. 1996;157:65–72. doi: 10.1007/BF00227882. [DOI] [PubMed] [Google Scholar]
- 66.Forcet C, Etienne-Manneville S, Gaude H, et al. Functional analysis of Peutz–Jeghers mutations reveals that the LKB1 C-terminal region exerts a crucial role in regulating both the AMPK pathway and the cell polarity. Hum Mol Genet. 2005;14:1283–92. doi: 10.1093/hmg/ddi139. [DOI] [PubMed] [Google Scholar]
- 67.Farese RV. Function and dysfunction of aPKC isoforms for glucose transport in insulin-sensitive and insulin-resistant states. Am J Physiol Endocrinol Metab. 2002;283:E1–11. doi: 10.1152/ajpendo.00045.2002. [DOI] [PubMed] [Google Scholar]
- 68.Carling D. AMP-activated protein kinase: Balancing the scales. Biochimie. 2005;87:87–91. doi: 10.1016/j.biochi.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 69.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–9. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 70.Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 71.Davies SP, Helps NR, Cohen PT, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–5. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 72.Ingebritsen TS, Stewart AA, Cohen P. The protein phosphatases involved in cellular regulation. 6. Measurement of type-1 and type-2 protein phosphatases in extracts of mammalian tissues: An assessment of their physiological roles. Eur J Biochem. 1983;132:297–307. doi: 10.1111/j.1432-1033.1983.tb07362.x. [DOI] [PubMed] [Google Scholar]
- 73.Wang MY, Unger RH. Role of PP2C in cardiac lipid accumulation in obese rodents and its prevention by troglitazone. Am J Physiol Endocrinol Metab. 2005;288:E216–21. doi: 10.1152/ajpendo.00004.2004. [DOI] [PubMed] [Google Scholar]
- 74.McGowan CH, Cohen P. Protein phosphatase-2C from rabbit skeletal muscle and liver: An Mg2+-dependent enzyme. Methods Enzymol. 1988;159:416–26. doi: 10.1016/0076-6879(88)59041-9. [DOI] [PubMed] [Google Scholar]
- 75.McGowan CH, Cohen P. Identification of two isoenzymes of protein phosphatase 2C in both rabbit skeletal muscle and liver. Eur J Biochem. 1987;166:713–21. doi: 10.1111/j.1432-1033.1987.tb13570.x. [DOI] [PubMed] [Google Scholar]
- 76.Klumpp S, Selke D, Hermesmeier J. Protein phosphatase type 2C active at physiological Mg2+: Stimulation by unsaturated fatty acids. FEBS Lett. 1998;437:229–32. doi: 10.1016/s0014-5793(98)01237-x. [DOI] [PubMed] [Google Scholar]
- 77.Lifschitz-Mercer B, Sheinin Y, Ben-Meir D, et al. Protein phosphatase 2Calpha expression in normal human tissues: An immunohistochemical study. Histochem Cell Biol. 2001;116:31–9. doi: 10.1007/s004180100291. [DOI] [PubMed] [Google Scholar]
- 78.Marley AE, Sullivan JE, Carling D, et al. Biochemical characterization and deletion analysis of recombinant human protein phosphatase 2C alpha. Biochem J. 1996;320:801–6. doi: 10.1042/bj3200801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268(15):523–30. [PubMed] [Google Scholar]
- 80.Gabel S, Benefield J, Meisinger J, Petruzzelli GJ, Young M. Protein phosphatases 1 and 2A maintain endothelial cells in a resting state, limiting the motility that is needed for the morphogenic process of angiogenesis. Otolaryngol Head Neck Surg. 1999;121:463–8. doi: 10.1016/S0194-5998(99)70238-X. [DOI] [PubMed] [Google Scholar]
- 81.Tar K, Csortos C, Czikora I, et al. Role of protein phosphatase 2A in the regulation of endothelial cell cytoskeleton structure. J Cell Biochem. 2006;98:931–53. doi: 10.1002/jcb.20829. [DOI] [PubMed] [Google Scholar]
- 82.Urbich C, Reissner A, Chavakis E, et al. Dephosphorylation of endothelial nitric oxide synthase contributes to the anti-angiogenic effects of endostatin. FASEB J. 2002;16:706–8. doi: 10.1096/fj.01-0637fje. [DOI] [PubMed] [Google Scholar]
- 83.Gimeno-Alcaniz JV, Sanz P. Glucose and type 2A protein phosphatase regulate the interaction between catalytic and regulatory subunits of AMP-activated protein kinase. J Mol Biol. 2003;333:201–9. doi: 10.1016/j.jmb.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 84.Ravnskjaer K, Boergesen M, Dalgaard LT, Mandrup S. Glucose-induced repression of PPARalpha gene expression in pancreatic beta-cells involves PP2A activation and AMPK inactivation. J Mol Endocrinol. 2006;36:289–99. doi: 10.1677/jme.1.01965. [DOI] [PubMed] [Google Scholar]
- 85.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation. 2006;113:1708–14. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 86.Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217–30. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 87.Kawashima S, Yokoyama M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:998–1005. doi: 10.1161/01.ATV.0000125114.88079.96. [DOI] [PubMed] [Google Scholar]
- 88.Jeremy JY, Rowe D, Emsley AM, Newby AC. Nitric oxide and the proliferation of vascular smooth muscle cells. Cardiovasc Res. 1999;43:580–94. doi: 10.1016/s0008-6363(99)00171-6. [DOI] [PubMed] [Google Scholar]
- 89.Napoli C, de Nigris F, Palinski W. Multiple role of reactive oxygen species in the arterial wall. J Cell Biochem. 2001;82:674–82. doi: 10.1002/jcb.1198. [DOI] [PubMed] [Google Scholar]
- 90.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–41. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 91.Ugochukwu NH, Figgers CL. Attenuation of plasma dyslipidemia and oxidative damage by dietary caloric restriction in streptozotocin-induced diabetic rats. Chem Biol Interact. 2007;169:32–41. doi: 10.1016/j.cbi.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 92.Kitayama J, Faraci FM, Lentz SR, Heistad DD. Cerebral vascular dysfunction during hypercholesterolemia. Stroke. 2007;38:2136–41. doi: 10.1161/STROKEAHA.107.481879. [DOI] [PubMed] [Google Scholar]
- 93.Xu J, Xie Z, Reece R, Pimental D, Zou MH. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: Role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler Thromb Vasc Biol. 2006;26:2688–95. doi: 10.1161/01.ATV.0000249394.94588.82. [DOI] [PubMed] [Google Scholar]
- 94.Xu J, Wu Y, Song P, Zhang M, Wang S, Zou MH. Proteasome-dependent degradation of guanosine 5′-triphosphate cyclohydrolase I causes tetrahydrobiopterin deficiency in diabetes mellitus. Circulation. 2007;116:944–53. doi: 10.1161/CIRCULATIONAHA.106.684795. [DOI] [PubMed] [Google Scholar]
- 95.Ouslimani N, Peynet J, Bonnefont-Rousselot D, Therond P, Legrand A, Beaudeux JL. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism. 2005;54:829–34. doi: 10.1016/j.metabol.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 96.Kukidome D, Nishikawa T, Sonoda K, et al. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55:120–7. [PubMed] [Google Scholar]
- 97.Dagher Z, Ruderman N, Tornheim K, Ido Y. Acute regulation of fatty acid oxidation and AMP-activated protein kinase in human umbilical vein endothelial cells. Circ Res. 2001;88:1276–82. doi: 10.1161/hh1201.092998. [DOI] [PubMed] [Google Scholar]
- 98.Ruderman NB, Cacicedo JM, Itani S, et al. Malonyl-CoA and AMP-activated protein kinase (AMPK): Possible links between insulin resistance in muscle and early endothelial cell damage in diabetes. Biochem Soc Trans. 2003;31:202–6. doi: 10.1042/bst0310202. [DOI] [PubMed] [Google Scholar]
- 99.Lee TS, Saltsman KA, Ohashi H, King GL. Activation of protein kinase C by elevation of glucose concentration: Proposal for a mechanism in the development of diabetic vascular complications. Proc Natl Acad Sci USA. 1989;86:5141–5. doi: 10.1073/pnas.86.13.5141. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 100.Michell BJ, Chen Z, Tiganis T, et al. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276(17):625–8. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 101.Park JY, Kim YM, Song HS, et al. Oleic acid induces endothelin-1 expression through activation of protein kinase C and NF-kappa B. Biochem Biophys Res Commun. 2003;303:891–5. doi: 10.1016/s0006-291x(03)00436-4. [DOI] [PubMed] [Google Scholar]
- 102.Lee WJ, Lee IK, Kim HS, et al. Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:2488–94. doi: 10.1161/01.ATV.0000190667.33224.4c. [DOI] [PubMed] [Google Scholar]
- 103.Clark H, Carling D, Saggerson D. Covalent activation of heart AMP-activated protein kinase in response to physiological concentrations of long-chain fatty acids. Eur J Biochem. 2004;271:2215–24. doi: 10.1111/j.1432-1033.2004.04151.x. [DOI] [PubMed] [Google Scholar]
- 104.Suchankova G, Tekle M, Saha AK, Ruderman NB, Clarke SD, Gettys TW. Dietary polyunsaturated fatty acids enhance hepatic AMP-activated protein kinase activity in rats. Biochem Biophys Res Commun. 2005;326:851–8. doi: 10.1016/j.bbrc.2004.11.114. [DOI] [PubMed] [Google Scholar]
- 105.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94:2349–56. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muse ED, Obici S, Bhanot S, et al. Role of resistin in diet-induced hepatic insulin resistance. J Clin Invest. 2004;114:232–9. doi: 10.1172/JCI21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wilkes JJ, Nguyen MT, Bandyopadhyay GK, Nelson E, Olefsky JM. Topiramate treatment causes skeletal muscle insulin sensitization and increased Acrp30 secretion in high-fat-fed male Wistar rats. Am J Physiol Endocrinol Metab. 2005;289:E1015–22. doi: 10.1152/ajpendo.00169.2005. [DOI] [PubMed] [Google Scholar]
- 108.McCarty MF. AMPK activation as a strategy for reversing the endothelial lipotoxicity underlying the increased vascular risk associated with insulin resistance syndrome. Med Hypotheses. 2005;64:1211–15. doi: 10.1016/j.mehy.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 109.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278(45):021–6. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 110.Park H, Kaushik VK, Constant S, et al. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem. 2002;277(32):571–7. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- 111.Kobayashi H, Ouchi N, Kihara S, Ruderman NB, Ido Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:E27–31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cacicedo JM, Yagihashi N, Keaney JF, Jr, Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;324:1204–9. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- 113.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–8. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]