Abstract
Importance of the field
The high prevalence of colon carcinoma combined with the low compliance of currently recommended screening guidelines explains the continued high mortality rate of colon cancer. Utilizing a strategy of virtual colonoscopy (VC) in asymptomatic patients over 50, with optical colonoscopy (OC) follow-up for removal of detected adenomatous polyps may result in lowering the colon cancer death rate. However, the screening potential of VC has not yet been widely recognized. Debates and doubts of its potential benefits have been frequently seen in the literature since VC was first reported in 1994.
Areas covered in this review
This article reviews the currently available screening options and discuss their advantages and drawbacks.
Take home message
VC has many advantages over the existing screening options and its several drawbacks can be mitigated so that it would become a valuable screening modality. A strategy that utilizes VC for population-based screening over the age of 50 and OC for screening high-risk individuals and those with positive VC findings would result in a significantly reduced rate of colon cancer deaths.
Keywords: Colonic polyp screening, colorectal cancer prevention, early detection, computed tomography, magnetic resonance imaging, virtual colonoscopy, optical colonoscopy
Article highlights
Colon carcinoma has a high prevalence and can be prevented if adequate screening of its precursor, adenomatous polyps, is available to and accepted by the asymptomatic patient population.
Currently available screening options are not effective and sufficient to accomplish the screening task.
Virtual colonoscopy (VC) has been under development to supplement the screening role of optical colonoscopy (OC), which is the current gold standard for evaluation of the entire colon with therapeutic capability and has several drawbacks as a screening tool.
Computed tomography (CT)-based VC, i.e., CT colonography (CTC), has shown a comparable performance to OC in detection of polyps of 8 mm and larger at a less invasive and more cost-effective fashion.
Magnetic resonance imaging (MRI)-based VC, i.e., MR colonography, has some advantages over CTC in terms of differentiating the polyps from other tissues and colonic materials, but its lower spatial resolution and prone to motion artifacts are two examples of drawbacks as compared to CTC.
There are several challenges remaining for CTC to become a screening option. Reducing the associated radiation is one example. With modern CT hardware optimization, statistical image reconstruction of low-dose raw data acquired by low-mAs protocol has shown promise to minimize the radiation.
Improving the detection of small polyps and flat ones is another example. Extracting the whole colon wall, rather than just the inner mucosa surface of the wall, is desired for this task. Partial volume image segmentation has shown great potential to electronically cleanse the colon lumen and accurately extract the colon wall.
Based on the extracted colon wall, both geometric and textural information can be analyzed to detect early sign of abnormalities by either three-dimensional endoscopic views (human observer) or computer-aided detection (computer observer).
This box summaries key points contained in the article
1. Introduction
According to the most recent statistics from American Cancer Society (ACS), (http://www.cancer.org/docroot/home/index.asp), colorectal carcinoma ranks the third most commonly diagnosed cancer and the second leading cause of death from cancer in the United States [1]. It was estimated that more than 150,000 new cases will be diagnosed with more than 50,000 dying from the disease in 2008 [2]. Similar to other cancers, it is often diagnosed at advanced stage, after the patient has developed symptoms. Different from many cancers, colon cancer can be prevented by detection and removal of its precursor lesion, the adenomatous polyp. Colon cancer is amenable to screening because of the long time interval (approximately 10 years) of adenoma-carcinoma sequence. For example, it takes more than two years for an adenomatous polyp to grow to 5 mm size with far less than 1% cancer risk, additional three or more years to 10 mm size with cancer risk approaching to 1%, and another five or more years to 20 mm size and 10% cancer risk [3–19]. Therefore, screening of an asymptomatic patient at an adequate time interval and removal of detected adenomatous polyps of less than 10 mm can effectively reduce the likelihood of developing the cancer [12, 13, 20–26]. There are several options currently available for the screening purpose, each has advantages and drawbacks.
Fecal occult blood testing (FOBT) is probably the easiest method for screening the deadly disease. It can be performed at home by collecting stool samples without bowel preparation and complication and delivering the samples to a service laboratory. Conceptually FOBT is highly sensitive. However, it detects only 30–40% of colorectal cancer and 10% of adenomas at late stage of the malignant transformation with specificity ranging from 88% to 98% [27–30]. This screening option is currently recommended by ACS annually on asymptomatic patients. According to the CPT (Current Procedural Terminology) code, this test costs about five dollars. However, the actual fee may be higher and vary depending on where the laboratory service is performed. Several studies have shown that this test can reduce the cancer mortality of 15% to 33% [30, 31]. Fecal immunochemical or immunoassay testing (FIT) can be more specific than occult blood testing, but shares a similar low sensitivity for the detection and costs more. It is recommended by ACS as an alternative to the FOBT by the time interval, i.e., every year on an asymptomatic patient. Recently stool DNA testing has shown a wide sensitivity range from 52% to 91% and specificity ranging from 93% to 97% also at late stage of the malignant transformation [32, 33]. It costs more than FIT. The time interval for screening an asymptomatic patient by this newer option has not yet been recommended by ACS. While their sensitivities can be improved by adequately taking the stool samples as detailed in [33], the above three screening methods share the same limitation of detection at advanced stage of the malignant transformation. In addition, their detection does not provide the location information where the malignant transformation occurs.
A more sophisticated screening means is double-contrast or air-contrast barium enema (DCBE). It is a procedure of taking X-ray radiographs or projections at various views of the abdomen after the colon mucosal surface is coated with high-density barium and the colon lumen is distended with air introduced through a flexible rectal catheter. Full oral bowel cleansing is needed prior to the examination. It typically takes 20 to 40 minutes to obtain sufficient projections and requires a good deal of patient positioning and cooperation when X-ray projection images are taken. Abnormality is detected by visualizing the projection images. Its sensitivity is less than 70% in detecting polyps of size from 5 to 20 mm diameter and 85% and higher in detecting cancer [34–36]. Complete bowel preparation involves significant invasiveness and is essential for an optimal examination. The risk of bowel perforation is rare, about 1 in 25,000 cases. This screening option is recommended by ACS every five years on an asymptomatic patient if no finding. The CPT code for this test is around $600 and the actual fee may be as high as $1,000.
Different from the FOBT, FIT, stool DNA testing and DCBE, flexible sigmoidoscopy (FS) uses a short flexible scope insert to directly inspect the mucosal surface of a colon segment from the rectum, typically up to the level of the splenic flexure. The procedure typically takes 10 to 30 minutes. Although full oral bowel cleansing can improve the outcome as DCBE does, FS is usually performed following a simple enema preparation. Similar to DCBE, FS does not need sedation. It can have therapeutic capability of resecting found polyps and removing other abnormalities, but fails to detect polyps in the proximal colon, where 40% of all cancers occur, and misses 10–15% sigmoid colon carcinomas [4–6, 37–41]. The risk of bowel perforation is rare, less than 1 in 20,000 cases. As with DCBE, the use of FS has been steadily decreasing due to several reasons, e.g., (i) multiple studies have shown superiority of other methods, such as optical colonoscopy (OC), for detection of polyps and cancers, and (ii) there is lacking of adequately trained examiners. This screening option with therapeutic capability is also recommended by ACS every five years on an asymptomatic patient if no finding. The CPT code for this test is approximately $70 and the actual fee may go up to $600 and higher if resection is performed. To overcome the limitation of FS for the purpose of inspecting the entire colon mucosal surface while retaining its therapeutic capability, OC becomes a choice.
2. Optical Colonoscopy
Since the first reported complete examination of the colon using a flexible fiberoptic endoscope by Wolff and Shinya in 1971 [42], OC has evolved to be the current gold standard for evaluation of the entire colonic mucosal surface with therapeutic capability of resecting detected lesions. Prior to OC, the patient must undergo bowel preparation usually consisting of (i) taking clear liquid diet and (ii) ingesting purgative solutions for colon cleansing the day before examination. Sedation is commonly used to relieve the discomfort during the procedure and, therefore, an escort is needed to accompany the patient to home. The modern colonoscope is equipped with a charge-coupled device or camera and four-way tip controls [43]. The camera can produce images of high-definition television quality with zoom or magnification capability. The four-way tip controls include (1) interrogating a found patch to confirm an abnormal growth if it can not be pushed away; (2) insufflating air to extend the lumen for mucosal inspection and relieving air after inspection, (3) irrigating a concerned region; (4) suckioning to avoid missing lesions under fluid, and (5) inserting biops or polypectomy snare devices.
Currently OC is the definitive test in following up the positive findings of the above screening options, i.e., FOBT, FIT, stool DNA testing, DCBE, FS, and other imaging modalities. In addition, it is also the most common choice of evaluating a variety of signs and symptoms such as (i) unexplained gastrointestional bleeding or iron deficiency anemia, (ii) chronic inflammatory bowel disease, (iii) significant diarrhea of unexplained origin, (iv) abdominal pain, etc. It is also the most common modality of performing interventions such as hemostasis, polypectomy, foreign body removal, balloon dilation, palliative treatment of neoplasms, and others [44, 45]. The current practice of OC is to attempt removal all detected polyps, regardless of histology (adenomatous or hyperplastic).
Since Medicare initiated reimbursement for screening asymptomatic patients in 2001, the volume of OC procedures has greatly increased. It was estimated that 14 million procedures were performed in 2003 [46], which include evaluation of colonic symptoms, follow-up of positive findings of the above less-invasive screening options, and screening of asymptomatic patients. Based on a study by Winawer and colleagues [44], a reduction in the incidence of colon cancer of 76% to 90% would be expected by the use of OC procedures, under the assumption that asymptomatic patients follow the ACS guidelines, i.e., OC every 10 years if no findings and shorter time intervals if abnormalities are found and therapeutic actions are performed. The CPT code for this examination is approximately $250 and the actual fee may go over $5,000 if multiple therapeutic actions are performed.
While it is accurate and can biopsy detected polyps, OC has several drawbacks as a screening option. (1) It is an invasive procedure and sedation may be needed. The use of sedation requires an escort, increases the costs and may induce complications such as cardiac arrhythmias, hypotension, oxygen desaturation, and others [45]. (2) The bowel preparation prior to the procedure is stressful, requiring a full oral laxative colon cleansing, and may cause abdominal discomfort, cramps, nausea, and other symptoms [47–50]. (3) OC is time consuming (especially for elders), ranging from 30 minutes to an hour. (4) It carries a small risk of perforation and death (colonic perforation in one about 1,000 cases and death in one about 5,000 cases [51–55]). (5) It may fail to demonstrate the entire colon in 10–15% of cases and may miss up to 10–20% of polyps < 1 cm [4–6, 56–59]. Overall, the miss rate of OC for large adenomas and cancer has been shown to be about 12% and 5% respectively [56–59].
For the asymptomatic patient population aged over 50, where the prevalence of polyps ranging from 6 to 9 mm would be 8% to 9% and polyps of 10 mm and larger would be 5% to 7% [16–19, 60], screening OC would be expected to be normal in more than 80% of cases. The risk and cost on these normal cases would be unnecessary. Furthermore, it would be expected that of the 16 – 20% of cases in which a polyp is found, only one third would be true adenomas – the other two-thirds would be hyperplastic lesions [16–19, 22, 60, 61].. The resection of the other two thirds hyperplastic polyps by current OC practice may not be necessary.
For patients with non-specific gastrointestinal symptoms, such as anemia and change in bowel habits, OC may find polyps of size 10 mm or greater in about 7% (slightly higher than the findings in the asymptomatic patient population aged over 50) as compared to 17% in patients with a positive FOBT [62]. Therefore, the safe and least expensive screening options of FOBT, FIT and stool DNA testing may have benefit for the patients with non-specific gastrointestinal symptoms prior to the application of OC procedure.
With further technology development, OC can improve its performance as a screening tool, but could not be an optimal option to screen the entire targeted population (i.e., aged over 50 as suggested by the ACS) due to (i) the drawbacks associated with OC (e.g., more than 80% of the screened people would be negative because the prevalence of polyps is less than 20% in the population [16, 18, 60], (ii) the risk of sedation and perforation, (iii) the cost including an escort, (iv) costs associated with unnecessarily removing hyperplastic polyps [51, 53–55]), and (v) the lack of resources (it could take many years and great efforts to train a sufficient number of OC operators to perform the screening task [12, 13, 20, 21, 63]).
Because of the high prevalence of the disease, and the low compliance rate to the currently available screening options, the mortality rate of colorectal cancer remains high. A more effective screening method as compared to the above mentioned options for evaluation of the entire colon and detection of polyps as small as 5 mm is desired. To that end, a great research effort has been seen in the past decades in searching such an alternative. Developing virtual colonoscopy (VC) to supplement the screening role of OC is one example of the research effort.
3. Virtual Colonoscopy
Since 1994, several pilot studies [64–66] evaluating the feasibility of an alternative means using computed tomography (CT) imaging technology for the purpose of screening the entire colon have motivated a great amount of research interests ranging from image formation and segmentation to visualization [67–69], although there was an earlier report [70]. This alternative means, i.e., CT-based virtual colonoscopy or CT colonography (CTC), utilizes computer virtual-reality techniques to navigate inside a three-dimensional (3D) patient-specific colon model reconstructed from abdominal CT images, looking for polyps. It starts by inflating a cleansed colon by room air or CO2 introduced through rectal insert tube [71]. Then abdominal CT slice images are taken in seconds (during a single breath holding) with sub mm resolution in both axial and transverse directions and good image contrast between the colon wall and the lumen (filled by air/CO2). The slice images are stacked together as a volume image, from which the colon model is constructed. Image segmentation is necessary for the construction of an accurate colon model [72]. Computer graphics are heavily involved to navigate or fly through inside the 3D colon model [69]. For the purpose of validating the detection in the 3D colon model, interpretation of the 2D image slices at the three orthogonal (i.e., transverse, sagital and coronal) directions is often included in the VC procedure.
Initial clinical trials on the concept of CTC using laboratory prototypes had shown satisfactory sensitivity and specificity compared to the clinical OC [73–76]. Significant improvement was later demonstrated by large clinical trials using commercially available CTC systems. One example is the DoD clinical trial on 1,233 asymptomatic patients using the commercial V3D-Colon Module system (Viatroxinx Inc., Stony Brook, NY) [77]. A sensitivity 93.9% and specificity 92.2% were achieved vs 91.5% sensitivity of OC for polyps of 8 mm and larger by the same bowel preparation and same day operation of CTC and OC. Other examples are the more recent ACRIN trial [78] and IMPACT trial [79], which included a wider range of subjects, radiologist experience and CT/CTC systems and also generated similar results for polyps of 10 mm and larger. The results of these studies indicate that by a similar full oral bowel cleansing, both CTC and OC have a comparable performance for detecting polyps of 10 mm and larger, indicating that CTC can be a potential screening tool to supplement OC for colorectal cancer prevention.
However, there are several obstacles preventing CTC from becoming a screening modality, as evidenced by the recent refusal of CTC to be included in Medicare coverage: http://www.cms.hhs.gov/mcd/viewpubliccomments.asp?nca_id=220, such as the radiation risk, the challenge in detecting small polyps, the reluctance of going through the full oral bowel cleansing as OC does, the readers’ variation and efficiency, etc. Research to overcome these obstacles is underway and can be summarized as follows.
Current CT scanning for VC procedure is usually operated at a mAs level over 100 and delivers a significant amount of X-ray radiation exposure (two to four rads of dosage) to the patient abdomen [80, 81]. In comparison, a routine X-ray chest radiograph (or X-ray film) delivers approximately 0.5 rads of radiation exposures. For screening purpose, the radiation to the population would be excessively high and could increase the risk of getting cancer and other diseases [82]. Despite the hardware optimization and software improvement for CT advancement in the past decades, the concern on the CT-associated radiation risk remains. Given the current CT technologies, a simple and effective strategy to further reduce the radiation would be to lower the mAs level (i.e., delivering less X-ray photons to the body) during data acquisition [83–85]. (Other approaches to reduce the radiation may include optimization of kVp value, X-ray flux beam collimation, beam modulation or filtering, etc). The low-mAs strategy will lead to higher noise in the acquired data. If there is no adequate treatment of the noise, the reconstructed image would be noisy and frequently contain steak artifacts. Conventional operations utilize low-pass filters to smooth the image noise and have the cost of significant loss of image resolution, because the low-pass filters do not consider the noise properties accurately [86]. Recent effort on modeling the image noise and developing noise-adaptive filters have made some progress, but the gain is limited because of the presence of streak artifacts on the wall-lumen interface [83]. A solution to avoid the artifact is to model the data noise and treat the noise prior or during image reconstruction [84]. A reduction of 50% radiation has been demonstrated [84], i.e., the decrease of the mAs level from 100 to 50. Despite the great effort on this solution in the past decade [83–86], CTC still faces challenges at a mAs level lower than 50. Given the clinical task of detecting polyps as small as 5 mm, the goal of further research effort is to achieve the lowest mAs level on the most recent CT systems.
An alternative solution to minimize the radiation is to use magnetic resonance imaging (MRI) instead of CT for the VC procedure, i.e., MR colonography (MRC). However, this MRC alternative solution has several limitation compared to CTC. It is more costly, more sensitive to motion and other artifacts, and has lower spatial resolution [87]. Compared to other imaging modalities, low-dose CT and high performance MRI are the two most attractive imaging modalities for VC. Great research efforts have been devoted to advance CTC and MRC, especially CTC, toward a viable screening option.
Regarding the issue of detection of small polyps, current modern CT can reach sub-millimeter spatial resolution and acquire a volumetric image of the abdomen in a single breath-holding time period. By theory, the achieved sub-millimeter spatial resolution could resolve polyps protruding to the colon lumen by a size as small as a couple millimeters. In practice, the missing of polyps greater than 5 mm is common in the hands of experts with current CTC system [77, 78]. A major reason for the cause may be due to the imperfect colon cleansing and air/CO2 inflation. The imperfect colon cleansing and air/CO2 inflation will not generate a perfect interface between the colon wall and the air/CO2-filled lumen for detection of polyps at the CT spatial resolution. The complexity of colon anatomical structure may add more difficulties for the detection. For example, a small polyp could be highly likely missing the detection when it is located at the sharp turn of the colon or at the root of a colon folder. Another major reason of missing polyp detection can be the loss of image information by the post-image processing algorithms in current CTC systems (e.g., segmentation for the colon lumen, construction of the colon model, incomplete coverage of the entire colon mucosa surface in endoscopic views, etc). Improvements in these aspects have been under progress and more details are given below.
Because of the similarity in X-ray attenuation among colonic fluid, stool and colon wall, it is almost impossible in the CT images to find a polyp submerged inside the residue fluid (after a routine full oral bowel cleansing). To avoid this problem, the patient is scanned at both supine and prone positions. It is hoped that the residue colonic materials will fall to another side when the patient is turned from one position to the other while the polyp remains at the same location. Unfortunately this is frequently not the case due to many reasons [77, 78, 88]. Clear fluid may move, but some sticky residual stool/fluid may not. The use of two CT scans doubles the radiation.
An alternative solution is to tag the colonic materials for enhanced image contrast between the colonic materials and the colon wall and use computer algorithms to virtually cleanse the colon, called virtual or electronic colon cleansing (ECC) [89, 90]. It includes three major components of (1) image intensity alteration by oral fecal tagging, (2) image segmentation for classifying the tagged image voxels, and (3) post-segmentation operation for cleansing the colon lumen or extracting the colon wall. This alternative solution is a major contribution to the high CTC performance [77, 78], where the scans at supine and prone positions were taken and the routine cathartic bowel preparation of OC was adapted and so the ECC works on the residual colonic materials. Since it works for virtual cleansing of the residues after the routine cathartic bowel preparation, ECC shall also work on virtual cleansing of any fecal materials without the routine cathartic bowel cleansing, leading to cathartic-free CTC -- a substantial relief of the bowel preparation stress of the current CTC practice. In addition, if ECC works on one of the two scans, the other scan may not be needed and, therefore, the radiation can be reduced by a half. If cathartic-free CTC is available, the patient may choose the less-stressful screening procedure first, and only those patients who have clinically significant polyps will undergo the OC intervention after the stressful cathartic bowel preparation. Further development of ECC for single CT scan and cathartic-free CTC would depend on powerful image segmentation and feature extraction. Because of the enhanced image intensities, there are several challenges associated with the alternative ECC solution, e.g., the presence of partial volume (PV) effect at the interface between the colon wall and the fecal materials with non-uniformly enhanced image intensities. The PV effect blurs the interface over a variable range as wide as several image voxels depending on the surrounding the image contrast, causes the loss of details about the interface and, therefore, results in the miss detection of small polyps. The PV effect and the non-uniformly altered image intensity distribution must be handled by the ECC algorithms, e.g., [90].
Differentiation of the colonic materials from the colon wall/polyps could also be made by the use of dual energy scans of a modern CT device (e.g., a dual X-ray source scanner). Two volumetric abdominal images can be acquired simultaneously at two energy levels (e.g., 80 kVp and 140 kVp) respectively at either supine or prone position. It is expected that the colonic materials would have some different image contrasts in the two scans with respect to the colon wall. If the contrasts are not sufficient for segmenting the image voxels of colonic materials, oral contrast agent may be utilized to alter the density of the colonic materials. The ECC role in this dual energy strategy is to segment the colonic materials from multi-spectral CT images, similar to that in MRI where T1-, T2- and proton-weighted images are acquired [91]. Despite the increased X-ray radiation to the patient by dual energy scans, this alternative approach for image contrast may be worthy for investigation.
With accurate handling of the PV effect and the non-uniformly altered image intensity distribution via improved image segmentation, the innovative ECC strategy should further reduce the risk from X-ray radiation, mitigate the challenge in detecting small polyps, and relieve the stress on the bowel preparation toward cathartic-free CTC.
While constructing an accurate colon model from an ECC-cleansed colon lumen of an abdominal volume image for inspection of the entire colon inner surface could be achieved by the commercial V3D Colon Module [77], searching for abnormalities and identifying polyps along the long colon “pipe” would be a challenge task because of the involved intensive user interaction during the fly-through navigation. In addition, the variation among readers with different experience has been widely noticed. Conceptually computer-aided detection (CAD) can reduce the readers’ interaction effort and minimize the variation among readers’ assessments. However, a series of recent studies turned out that developing an effective CAD system is very challenging [92, 93], because of many causes of false positives (FPs), such as imperfect bowel cleansing, complicated colon fold structures, image noise, motion artifacts, etc. Based on our experience in the field, surface-based CAD as reported in most previous CAD papers is not sufficient to reduce the FPs to an accepted level (e.g., < 10 per patient). Morphological and texture features beyond the inner surface inside the mucosal layer and probably even inside the colon wall are needed [94]. High sensitivity CAD with minimal number of FPs remains an active research topic. Recent development of various texture features of the image intensity distribution and virtual biopsy features of the image intensity projection from the colon wall has shown promise for high performance CAD [94, 95].
4. Conclusion
Both OC and VC (i.e., CTC and MRC) have been progressing toward the clinical needs as new technologies are developed and applied to overcome their drawbacks. Each of these two methods has its unique role for the goal of preventing colon cancer. OC will remain the choice for follow-up intervention and therapeutic operation on the patients with symptoms and positive findings from other easy screening options. By its inherent invasive nature, OC will encounter competition from other less invasive modalities for the purpose of mass screening because of the high prevalence but preventive nature of the colon cancer.
For screening purpose, VC has many advantages over other options such as FOBT, FIT, stool DNA testing, and DCBE. Currently it is the most competitive alternative method to OC for colon screening. The competition could lead to a good combination of VC screening with OC follow-up on the positive findings to reduce the incidence rate of the deadly disease [96, 97].
There are a few medical imaging modalities currently available for VC, such as CT and MRI. As time goes on, other medical imaging modalities may become available for VC.
5. Expert Opinion
Because of the high prevalence of colon carcinoma, screening on asymptomatic patients to detect and remove adenomatous polyps is an effective strategy to reduce the mortality. However, the currently available screening options have their limitations and offer a suboptimal solution. While effective for detecting polyps, OC would consume a great deal of resource if ACS guidelines were applied to the target population. It is unlikely that OC will be able to single out asymptomatic patients with adenomatous. Compared to other screening options, VC has the potential to identify patients with adenomatous polyps for colonoscopy. The combination of VC screening with OC follow-up could be a cost-effective means to prevent the deadly disease.
However, there are several challenges in developing VC to accomplish this task. With CT-based VC or CTC, the associated radiation is a concern. Differentiation of the colonic materials from the colon wall remains a large challenge. While MRI-based VC or MRC alleviates the radiation concern and is more capable to differentiate the colonic materials from the colon wall with the potential to obtain more image contrast inside the colon wall, it has lower spatial resolution and prone to motion artifacts. Both CTC and MRC need sophisticated image processing operations to construct the colon model and perform real-time fly-through inside the model, looking for abnormalities. Sophisticated image processing operations would be more essential if differentiation of adenomatous polyps from hyperplastic ones is desired. In other words, the extraction of the colon wall volume via the ECC innovation [90] and the analysis of texture features from image intensity of the wall [95] would be the key steps toward computer-aided detection (CAD) and diagnosis (CADx). If the colon wall volume, rather than only the inner mucosa surface of the wall, can be accurately extracted by a sophisticated ECC pipeline, then almost the entire clinically desired information can be obtained. From the extracted wall volume, we can analyze both geometric and image-density textural information for early sign of abnormality [94, 95]. This will not only improve the current VC detection capability of small polyps protruding into the lumen space, but also the detection of flat polyps which cause the wall thickening, rather than protruding into the lumen, and render an extremely challenging detection task [98].
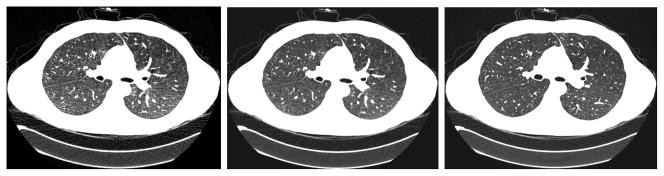
Taking CTC as an example, the strategy of lowering the mAs level during data acquisition and utilizing statistical methods for image reconstruction is an innovation to reduce the risk of radiation [83–85]. Furthermore, use of statistical restoration algorithm to estimate the line integrals or Radon transform of the CT attenuation distribution and inverting the Radon transform for the CT image is another innovation to overcome the challenge of reconstructing the low-mAs data of huge size. Figure 1 shows an example, demonstrating the potential of statistical restoration and/or reconstruction for low-dose CT applications. Figure 2 shows the advantage of the statistical restoration strategy on reconstruction speed.
Fig. 1.
Two sets of projection data were acquired from a patient, one at 20 mAs level and the other at 100 mAs level. These two datasets were first reconstructed by inverting the Radon transform via filtered backprojection method (FBP) which is optimized by varying the involved noise filtration. Then the dataset of 20 mAs level was reconstructed by the same FBP after statistical restoration. (Left) – FBP reconstruction of 20-mAs data without statistical restoration. (Middle) – FBP reconstruction of 20-mAs data with statistical restoration. (Right) – FBP reconstruction of 100-mAs data without statistical restoration. The reconstructed 20-mAs CT image with the statistical restoration is noticeably different from the 20-mAs CT image without the statistical restoration, and approaches to the 100-mAs CT image by a significant similarity.
Fig. 2.
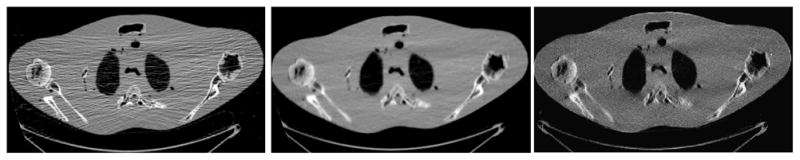
A dataset was acquired from a torso phantom at 10 mAs level. An optimized FBP reconstruction was first performed as a reference. Then the statistical restoration with FBP reconstruction was performed, where the restoration took approximately one minute and the FBP reconstruction consumed less than a minute. Finally, an iterative statistical reconstruction was implemented, which took more than six hours. (Left) – FBP reconstruction with optimized filtration. (Middle) – Statistical restoration and reconstruction. (Right) – Iterative statistical reconstruction. Both the iterative statistical reconstruction and the statistical restoration with FBP reconstruction generated similar image quality, but at a significantly different speed (more than 200 folds).
Utilizing the statistical innovation for low-dose CTC is under progress [85]. Figures 3 and 4 are examples of our preliminary results.
Fig. 3.
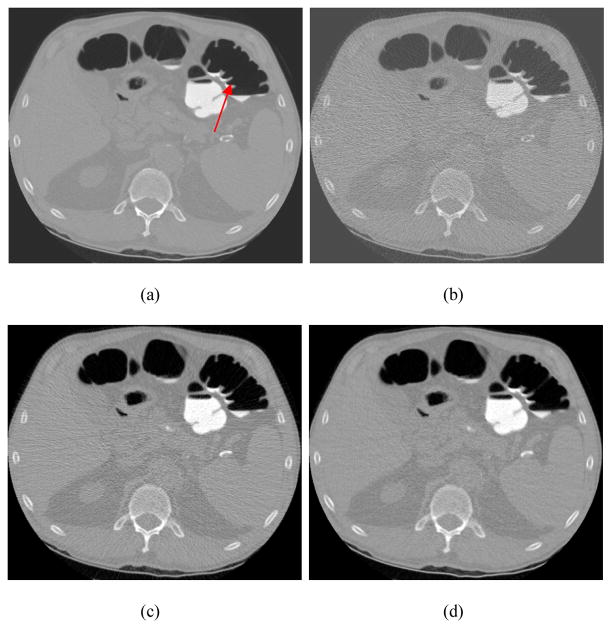
Illustration of one slice of a volumetric image of CTC study. Picture (a) is a standard FBP reconstruction (i.e., using Ramp filter with rectangular window at 100% Nyquist frequency) of routine normal dose projection data at 100 mAs level (the arrow indicates the position of a polyp). Picture (b) is the standard FBP reconstruction of simulated ultra low-dose projection data (by adding noise to the normal dose projection data equivalent to 50 mAs level). Significant image noise is seen. Picture (c) is a conventional FBP reconstruction of the simulated ultra low-dose projection data, where the rectangular window was replaced by the Hanning window with cutoff at 80% Nyquist frequency (the best result by visual judgment on different frequency cutoffs). Picture (d) is standard FBP reconstruction of the simulated ultra low-dose projection data after the statistical sinogram restoration was performed. The displays are in the full range from zero to maximum voxel intensity respectively.
Fig. 4.
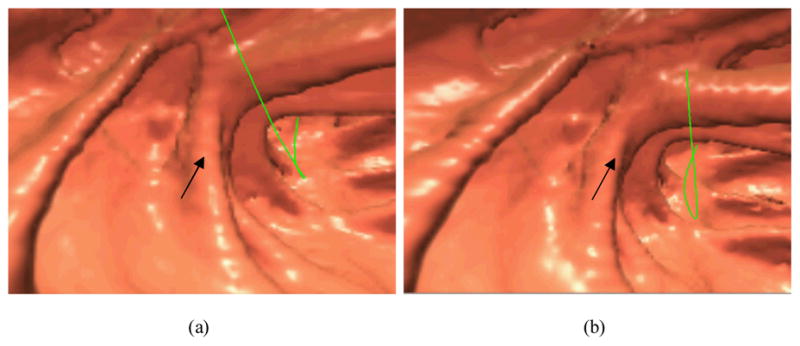
Endoscopic view of a polyp of 5 mm size in a CTC study. Picture (a) shows the result after noise reduction by the Hanning filter in the FBP reconstruction of low-dose CT. Picture (b) shows the result of standard FBP reconstruction after the statistical sinogram restoration. The arrows indicate the position of the polyp. The green line is the central line for guided navigation inside the colon lumen, which is provided by the VC software and facilitates the navigation procedure. Both pictures show the most visually-appealing views.
No matter dual-energy CT, MRI or other imaging modality is used for VC, the ECC strategy will play an essential role to extract the colon wall for polyp detection by either radiologists’ visual inspection (human observer) or CAD (computer observer). The key component in the ECC is the image segmentation. The presence of PV effect and non-uniform fecal tagging render a very challenging task for image segmentation to preserve the details on the mucosa, where clinical information resides. While many segmentation algorithms have been reported in the past, the MAP-EM (maximum a posteriori expectation-maximization) segmentation of tissue mixtures in each image voxel has shown the potential to mitigate the challenge [99, 100]. Figure 5 shows an example, demonstrating that potential. Since the PV layers are accurately identified, see the right of Figure 5, extraction of the colon wall mucosa becomes feasible. The preserved details on the mucosa layer by the MAP-EM mixture segmentation have improved noticeably the detection of small polyps [100].
Fig. 5.
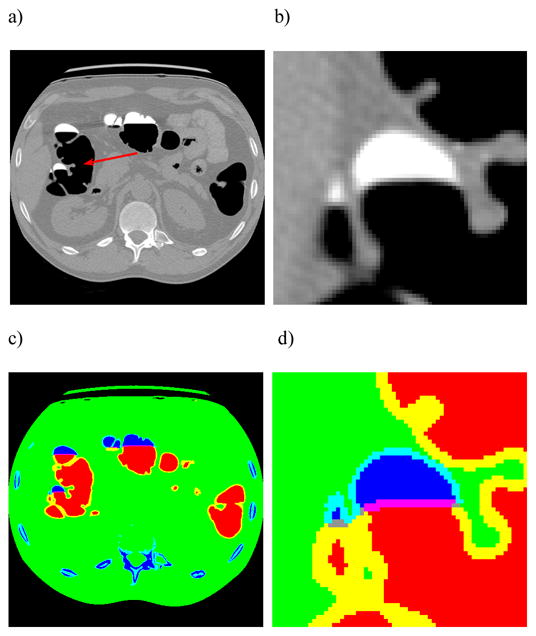
An illustration of the MAP-EM segmentation of tissue mixtures for extraction of the colon wall. Picture (a) shows a slice of an original CTC volume image of the abdomen acquired at the prone position. Picture (b) is a zoom-in view of a small region as indicated by the arrow on the original image of (a). Picture (c) shows the segmented result, where different tissues and their mixtures are represented by colors. Picture (d) is the zoomed-in view of the segmentation of the small region. The red color shows the pure air space and blue indicates the pure tagged colonic materials. The mixture space of air and tagged colonic materials are shown by pink/grey colors. The yellow color indicates the mixture space of air and colon tissues. The light blue color shows the mixture space of tagged colonic materials and colon tissues. The PV layers are accurately identified.
Given the extracted volume of mucosa layer, more useful texture information can be available for development of CAD methods. This benefit for CAD development has been seen in [94, 95]. Further improvement of CAD on small polyps should be expected when more useful texture information is extracted from the volumetric mucosa.
In summary, low-dose CTC and/or high resolution MRC are likely to become a screening modality, supplement to OC to reduce the colon cancer incidence. This is because of the following reasons: (1) Both imaging modalities can generate high quality abdominal volume image, including the entire colon. (2) Given the abdominal image from either imaging modality, extraction of the colon wall is a key operation to achieve high sensitivity and specificity by both the human and computer observers. The under-developing MAP-EM segmentation of tissue mixtures has the potential to accurately identify the colon mucosa, from which an ECC pipeline can be built to cleanse the lumen and extract the wall.
Acknowledgments
The author would like to acknowledge the assistance from Ms. Aimee Minton on the editing of this paper.
Footnotes
Declaration of interest
This work was partially supported by NIH Grant #CA082402 and #CA120917 of the National Cancer Institute.
Contributor Information
Zhengrong Liang, Email: jzliang@mil.sunysb.edu, IEEE Fellow, Professor of Radiology, Computer Science and Biomedical Engineering, School of Medicine, L4-120, Health Sciences Center, Stony Brook University, Stony Brook, NY 11794-8460, USA, (Tel): +1 631-444-7837, (Fax): +1 631-444-6450.
Robert Richards, Email: rrichards@notes.cc.sunysb.edu, Associate Professor, Program Director - GI Fellowship, Department of Medicine/Gastroenterology, Health Science Center, Level 17, Room 060, Stony Brook University, Stony Brook, NY 11794-8173, USA, (Tel): +1 631-444-7623.
References
- 1.American Cancer Society. Cancer Facts & Figures 2008. Atlanta: American Cancer Society; 2008. [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer Statistics – 2008. A Cancer Journal for Clinicians. 2008;58(1):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Hill M, Morrison B, Bussey H. Etiology of Adenoma Carcinoma Sequence in the Large Bowel. Lancet. 1978;1(2):245–247. doi: 10.1016/s0140-6736(78)90487-7. [DOI] [PubMed] [Google Scholar]
- 4.Rickert R, Auerbach O, Garfinkel L, et al. Adenomatous Lesions of the Large Bowel – An autopsy survey. Cancer. 1979;43(11):1847–1857. doi: 10.1002/1097-0142(197905)43:5<1847::aid-cncr2820430538>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Grandqvist S. Distribution of Polyps of the Large Bowel in Relation to Age: A colonoscopic study. Scandinavian Journal of Gastroenterology (SJG) 1981;16(11):1025–1031. doi: 10.3109/00365528109181023. [DOI] [PubMed] [Google Scholar]
- 6.Stryker S, Wolff B, Culp C, et al. Natural History of Untreated Colonic Polyps. Gastroenterology. 1987;93(5):1009–1013. doi: 10.1016/0016-5085(87)90563-4. [DOI] [PubMed] [Google Scholar]
- 7.Winawer S, Zauber A, Diaz B. The National Polyp Study – Temporal sequence of evolving colorectal cancer from normal colon. Gastrointestinal Endoscopy. 1987;33(2):167. [Google Scholar]
- 8.Fearon E, Vogelstein B. A Genetic Model for Colorectal Tumorigensis. Cell. 1990;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 9.Johnson D, Gurney M, Volpe R, et al. A Prospective Study of the Prevalence of Colonic Neoplasms in Asymptomatic Patients with an Age-Related Risk. American Journal of Gastroenterology (AJG) 1990;85(6):969–974. [PubMed] [Google Scholar]
- 10.Simons B, Morrison A, Lev R, Verhoek-Oftendahl W. Relationship of Polyps to Cancer of the Large Intestine. Journal of the National Cancer Institute (JNCI) 1992;84(5):962–966. doi: 10.1093/jnci/84.12.962. [DOI] [PubMed] [Google Scholar]
- 11.Potter J, Slattery M. Colon Cancer: A review of the epidemiology. Epidemiologic Reviews. 1993;15(3):499–545. doi: 10.1093/oxfordjournals.epirev.a036132. [DOI] [PubMed] [Google Scholar]
- 12.Byers T, Levin B, Rotherberger D, et al. ACS Guidelines for Screening and Surveillance for Early Detection of Colorectal Polyps and Cancer: Update 1997, CA. A Cancer Journal for Clinicians. 1997;47(1):154–160. doi: 10.3322/canjclin.47.3.154. [DOI] [PubMed] [Google Scholar]
- 13.Winawer S, Fletcher R, Mayer R. Colorectal Cancer Screening: Clinical guidelines and rational. Gastroenterology. 1997;112(3):594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 14.Robbins D, Itzowitz S. The Molecular and Genetic Basis of Colon Cancer. Medical Clinics of North America (MCNA) 2002;86(6):1467–1495. doi: 10.1016/s0025-7125(02)00084-6. [DOI] [PubMed] [Google Scholar]
- 15.Church J. Clinical Significance of Small Colorectal Polyps. Diseases of the Colon and Rectum. 2004;47(4):481–485. doi: 10.1007/s10350-003-0078-6. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman D, Holub J, Eisen G, et al. Prevalence of Polyps Greater Than 9 mm in a Consortium of Diverse Clinical Practice Settings in the United States. Clinical Gastroenterology and Hepatology. 2005;3(8):798–805. doi: 10.1016/s1542-3565(05)00405-2. [DOI] [PubMed] [Google Scholar]
- 17.Odom S, Duffy S, Barone J, et al. The Rate of Adenocarcinoma in Endoscopically Removed Colorectal Polyps. The American Surgeon. 2005;71(12):1024–1026. [PubMed] [Google Scholar]
- 18.Jass J. Classification of Colorectal Cancer based on Correlation of Clinical, Morphological and Molecular Features. Histopathology. 2007;50(1):113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 19.Yoo T, Park D, Kim Y, et al. Clinical Significance of Small Colorectal Adenoma Less Than 10 mm: The KASID study. Hepatogastroenterology. 2007;54(74):418–21. [PubMed] [Google Scholar]
- 20.Eddy D. Screening for Colorectal Cancer. Annals of Internal Medicine. 1990;113(2):373–384. doi: 10.7326/0003-4819-113-5-373. [DOI] [PubMed] [Google Scholar]
- 21.Ferrucci J. Screening for Colon Cancer: Programs of the American College of Radiology. American Journal of Roentgenology (AJR) 1993;160(6):999–1003. doi: 10.2214/ajr.160.5.8470616. [DOI] [PubMed] [Google Scholar]
- 22.Morimoto L, Newcomb P, Ulrich C, et al. Risk Factors for Hyperplastic and Adenomatous Polyps: Evidence for malignant potential. Cancer Epidemiology, Biomarkers and Prevention. 2002;11(10):1012–1018. [PubMed] [Google Scholar]
- 23.Winawer S, Zauber A. The Advanced Adenomas as the Primary Target of Screening. Gastrointestinal Endoscopy Clinics of North America. 2002;232(3):784–790. doi: 10.1016/s1052-5157(03)00053-9. [DOI] [PubMed] [Google Scholar]
- 24.Gluecker T, Johnson C, Harmsen W, et al. Colorectal Cancer Screening with CTC, Colonoscopy, and Double-Contrast Barium Enema Examination: Prospective assessment of patient perceptions and preferences. Radiology. 2003;227(2):378–384. doi: 10.1148/radiol.2272020293. [DOI] [PubMed] [Google Scholar]
- 25.Levin B, Brooks D, Smith R, Stone A. Emerging Technologies in Screening for Colorectal Cancer: CTC, immunochemical fecal occult blood tests, stool screening using molecular markers. A Cancer Journal for Clinicians. 2003;53(1):44–55. doi: 10.3322/canjclin.53.1.44. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher R. Colorectal Cancer Screening on Stronger Footing. The New England Journal of Medicine (NEJM) 2008;359(12):1285–1287. doi: 10.1056/NEJMe0806029. [DOI] [PubMed] [Google Scholar]
- 27.Ahlquist D, Klee G, McGill D, Ellefson E. Colorectal Cancer Screening Detection in the Practice Setting: Impact of fecal occult blood testing. Archives of Internal Medicine. 1990;150(8):1041–1050. [PubMed] [Google Scholar]
- 28.Hardcastle J, Chamberlain J, Robinson M. Randomized Controlled Trial of Fecal-Occult-Blood Screening for Colorectal Cancer. Lancet. 1996;348(12):1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 29.Kronberg O, Fenger C, Olsen J, et al. Randomized Study of Screening for Colorectal Cancer with Fecal-Occult-Blood Test. Lancet. 1996;348(12):1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 30.Mandel J, Church T, Bond J, et al. The Effect of Fecal Occult Blood Screening on the Incidence of Colorectal Cancer. The New England Journal of Medicine. 2000;343(22):1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 31.Allison J, Sskoda I, Ransom L, et al. Screening for Colorectal Neoplasms with New Fecal Occult Blood Tests: Update on performance characteristics. Journal of the National Cancer Institute. 2007;99(11):1462–1470. doi: 10.1093/jnci/djm150. [DOI] [PubMed] [Google Scholar]
- 32.Imperiale T, Ransohoff D, Itzkowitz S, et al. Fecal DNA verse Fecal Occult Blood for Colorectal Cancer Screening in an Average-Risk Population. The New England Journal of Medicine. 2004;351(26):2704–2714. doi: 10.1056/NEJMoa033403. [DOI] [PubMed] [Google Scholar]
- 33.Ahlquist D, Sargent D, Loprinzi C, et al. Stool DNA and Occult Blood Testing for Screen Detection of Colorectal Neoplasia. Annals of Internal Medicine. 2008;149(7):441–450. doi: 10.7326/0003-4819-149-7-200810070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fork F. Double Contrast Enema and Colonoscopy in Polyp Detection. Gut. 1981;22:971–977. doi: 10.1136/gut.22.11.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morosi C, Ballardini G, Pisani P. Diagnostic Accuracy of the Double-Contrast Enema for Colonic Polyps in Patients with or without Diverticular Disease. Gastrointestinal Radiology. 1991;16(2):345–347. doi: 10.1007/BF01887386. [DOI] [PubMed] [Google Scholar]
- 36.Brady A, Stevenson G, Stevenson I. Colorectal Cancer Overlooked at Barium Enema Examination and Colonoscopy: A continuing perceptual problem. Radiology. 1994;192(2):373–378. doi: 10.1148/radiology.192.2.8029400. [DOI] [PubMed] [Google Scholar]
- 37.Selby J, Friedman G, Quesenberry C, Weiss N. A Case Control Study of Screening Sigmoidoscopy and Mortality from Colorectal Cancer. The New England Journal of Medicine. 1992;326(10):653–657. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 38.Elwood J, Sli G, Schlup M, et al. Flexible Sigmoidoscopy or Colonoscopy for Colorectal Screening: A randomized trial of performance and acceptability. Cancer Detection and Prevention. 1995;19(4):337–347. [PubMed] [Google Scholar]
- 39.Kewenter J, Breringe H, Engaras B, Haglind E. The Value of Flexible Sigmoidoscopy and Double Contrast Barium Enema in the Diagnosis of Neoplasms in the Rectum and Colon in Subjects with Positive Hemoccult: Results of 1,831 rectosigmoidoscopies and double contrast barium enemas. Endoscopy. 1995;27(1):159–163. doi: 10.1055/s-2007-1005655. [DOI] [PubMed] [Google Scholar]
- 40.Newcomb P, Storer B, Morimoto L, et al. Long-Term Efficacy of Sigmoidoscopy in the Reduction of Colorectal Cancer Incidence. Journal of the National Cancer Institute. 2003;95(8):622–625. doi: 10.1093/jnci/95.8.622. [DOI] [PubMed] [Google Scholar]
- 41.Levin T, Farraye F, Schoen R, et al. Quality in the Technical Performance of Screening Flexible Sigmoidoscopy: Recommendations of an international multi-society task group. Gut. 2005;54(6):807–813. doi: 10.1136/gut.2004.052282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolff W, Shinya H. Colonofiberoscopy. Journal of the American Medical Association (JAMA) 1971;217(11):1509–1512. [PubMed] [Google Scholar]
- 43.Hafner M. Conventional Colonoscopy: Technique, indications, limites. European Journal of Radiology (EJR) 2007;61(2):409–414. doi: 10.1016/j.ejrad.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 44.Winawer S, Zauber A, Ho M, et al. Prevention of Colorectal Cancer by Colonoscopic Polypectomy. The New England Journal of Medicine. 1993;329(27):1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 45.American Society for Gastrointestional Endoscopy. Appropriate Use of Gastrointestional Endpscopy. Gastrointestinal Endoscopy. 2000;52(5):831–837. [PubMed] [Google Scholar]
- 46.Seeff L, Richards T, Shapiro J, et al. How Many Endoscopies are Performed for Colorectal Cancer Screening? Results from CDC’s survey of endoscopic capability. Gastroenterology. 2004;127(6):1670–1677. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 47.Bartram C. Bowel Preparation – Principles and practice. Clinical Radiology. 1994;49(2):365–367. doi: 10.1016/s0009-9260(05)81818-5. [DOI] [PubMed] [Google Scholar]
- 48.Ristvedt S, McFarland E, Weinstock L, Thyssen E. Patient Preferences for CTC, Conventional Colonoscopy, and Bowel Preparation. American Journal of Gastroenterology. 2003;98(4):578–585. doi: 10.1111/j.1572-0241.2003.07302.x. [DOI] [PubMed] [Google Scholar]
- 49.“A Consensus Document on Bowel Preparation Prior to Colonoscopy,” prepared by a Task Force from American Society of Colon and Rectal Surgeons (ASCRS), the American Society of Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) on April of 2006.
- 50.Cai S, Zhang S, Zhu H, Zheng S. Barriers to Colorectal Cancer Screening: A case-control study. World Journal of Gastroenterology. 2009;15(12):2531–2536. doi: 10.3748/wjg.15.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith L. Fibreoptic Colonoscopy: Complications of colonoscopy and polypectomy. Diseases of the Colon and Rectum. 1976;11(1):146–150. doi: 10.1007/BF02590825. [DOI] [PubMed] [Google Scholar]
- 52.Stevenson G, Wilson J, Wilkinson J, et al. Pain Following Colonoscopy: Elimination with carbon dioxide. Gastrointestinal Endoscopy. 1992;38(3):564–567. doi: 10.1016/s0016-5107(92)70517-3. [DOI] [PubMed] [Google Scholar]
- 53.Steine S. Which Hurts the Most? A comparison of pain rating during double-contrast barium enema examinations and colonoscopy. Radiology. 1994;191(1):99–101. doi: 10.1148/radiology.191.1.8134605. [DOI] [PubMed] [Google Scholar]
- 54.Orsoni P, Berdah S, Verrier C, et al. Colonic Perforation due to Colonoscopy: A retrospective study of 48 cases. Endoscopy. 1997;29(1):160–164. doi: 10.1055/s-2007-1004156. [DOI] [PubMed] [Google Scholar]
- 55.Weitzman E, Zapka J, Estabrook B, Goins K. Risk and Reluctance: Understanding impediments to colorectal cancer screening. Preventive Medicine. 2001;32(6):502–513. doi: 10.1006/pmed.2001.0838. [DOI] [PubMed] [Google Scholar]
- 56.Rex D, Rahmani E, Haseman J, et al. Relative Sensitivity of Colonoscopy and Barium Enema for Detection of Colorectal Cancer in Clinical Practice. Gastroenterology. 1997;112(1):17–23. doi: 10.1016/s0016-5085(97)70213-0. [DOI] [PubMed] [Google Scholar]
- 57.Bressler B, Paszat L, Vinden C, et al. Colonoscopic Miss Rates for Right-Sided Colon Cancer: A population-based analysis. Gastroenterology. 2004;127(2):452–456. doi: 10.1053/j.gastro.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 58.Pickhardt P, Nugent P, Mysliwiec P, et al. Location of Adenomas Missed by OC. Archives of Internal Medicine. 2004;141(2):352–359. doi: 10.7326/0003-4819-141-5-200409070-00009. [DOI] [PubMed] [Google Scholar]
- 59.Heresbach D, Barrioz T, Lapalus M, et al. Miss Rate for Colorectal Neoplastic Polyps: A prospective multi-center study of back-to-back video colonoscopies. Endoscopy. 2008;40(4):282–290. doi: 10.1055/s-2007-995618. [DOI] [PubMed] [Google Scholar]
- 60.Lieberman D, Moravec M, Holub J, et al. Polyp Size and Advanced Histology in Patients Undergoing Colonoscopy Screening: Implications for CTC. Gastroenterology. 2008;135(4):1100–1105. doi: 10.1053/j.gastro.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim D, Pickhardt P, Taylor A. Characteristics of Advanced Adenomas Detected at CTC Screening: Implications for appropriate polyp size thresholds for polypectomy versus surveillance. American Journal of Roentgenology. 2007;188(6):940–944. doi: 10.2214/AJR.06.0764. [DOI] [PubMed] [Google Scholar]
- 62.Lieberman D, de Garmo P, Fleischer D, et al. Colonic Neoplasia in Patients with Non-Specific GI Symptoms. Gastrointestinal Endoscopy. 2000;51(6):647–651. doi: 10.1067/mge.2000.105082. [DOI] [PubMed] [Google Scholar]
- 63.Gelfand D. Gastrointestinal Radiology: A short history and predictions for future. American Journal of Roentgenology. 1988;150(4):727–730. [Google Scholar]
- 64.Vining D, Gelfand D, Bechtold R, et al. Technical Feasibility of Colon Imaging with Helical CT and Virtual Reality. Annual Meeting of American Roentgen Ray Society; New Orleans. 1994. p. 104. [Google Scholar]
- 65.Hong L, Kaufman A, Wei Y, Viswambharan A, Wax M, Liang Z. IEEE Biomedical Visualization Symposium. IEEE CS Press; CA: 1995. 3D Virtual Colonoscopy; pp. 26–32. [Google Scholar]
- 66.Hara A, Johnson C, Reed J, et al. Detection of Colorectal Polyps by CTC: Feasibility of a novel technique. Gastroenterology. 1996;110(2):284–290. doi: 10.1053/gast.1996.v110.pm8536869. [DOI] [PubMed] [Google Scholar]
- 67.Hong L, Liang Z, Viswambharan A, et al. Reconstruction and Visualization of 3D Models of Colonic Surface. IEEE Transactions on Nuclear Science (TNS) 1997;44(11):1297–1302. [Google Scholar]
- 68.Reed J, Johnson C. Automatic Segmentation, Tissue Characterization, and Rapid Diagnosis Enhancements to the CTC Analysis Workstation. Journal of Digital Imaging. 1997;10(1):70–73. doi: 10.1007/BF03168661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wan M, Tang Q, Kaufman A, Liang Z. Proc IEEE Computer Graphics and Applications. 1999. Volume Rendering Based Interactive Navigation within the Human Colon; pp. 397–400. [Google Scholar]
- 70.Coin C, Wollett F, Coin J, et al. Computerized Radiology of the Colon: A potential screening technique. Computed Radiology. 1983;7(2):215–221. doi: 10.1016/0730-4862(83)90145-2. [DOI] [PubMed] [Google Scholar]
- 71.Shinners T, Pickhardt P, Taylor A, et al. Patient-Controlled Room Air Insufflation versus Automated Carbon Dioxide Delivery for CTC. American Journal of Roentgenology. 2006;186(10):1491–1496. doi: 10.2214/AJR.05.0416. [DOI] [PubMed] [Google Scholar]
- 72.Liang Z, Chen D, Li B, et al. On Segmentation of Colon Lumen for VC. Proc SPIE Medical Imaging. 1999;3660:270–278. [Google Scholar]
- 73.Fenlon H, Nunes D, Schroy P, et al. A Comparison of Virtual and Conventional Colonoscopy for the Detection of Colorectal Polyps. The New England Journal of Medicine. 1999;341(11):1496–1503. doi: 10.1056/NEJM199911113412003. [DOI] [PubMed] [Google Scholar]
- 74.Fletcher J, Johnson C, Welch T, et al. Optimization of CTC Technique: Prospective trial in 180 patients. Radiology. 2000;216(4):704–711. doi: 10.1148/radiology.216.3.r00au41704. [DOI] [PubMed] [Google Scholar]
- 75.Yee J, Akerkar G, Hung R, et al. Colorectal Neoplasia: Performance characteristics of CTC for detection in 300 patients. Radiology. 2001;219(4):685–692. doi: 10.1148/radiology.219.3.r01jn40685. [DOI] [PubMed] [Google Scholar]
- 76.Johnson D, Harmsen W, Wilson L, et al. Prospective Blinded Evaluation of CTC for Screen Detection of Colorectal Polyps. Gastroenterology. 2003;125(2):311–319. doi: 10.1016/s0016-5085(03)00894-1. [DOI] [PubMed] [Google Scholar]
- 77.Pickhardt P, Choi R, Hwang I, et al. Computed Tomographic VC to Screen for Colorectal Neoplasia in Asymptomatic Adults. The New England Journal of Medicine. 2003;349(12):2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 78.Johnson C, Chen MH, Toledano A, et al. Accuracy of CTC for Detection of Large Adenomas and Cancers. The New England Journal of Medicine. 2008;359(12):1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Regge D, Laudi C, Galatola G, et al. Diagnostic Accuracy of Computed Tomographic Colonography for the Detection of Advanced Neoplasia in Individuals at Increased Risk of Colorectal Cancer. The Journal of the American Medical Association. 2009;301(23):2453–2461. doi: 10.1001/jama.2009.832. [DOI] [PubMed] [Google Scholar]
- 80.Brenner D, Georgsson M. Mass Screening with CTC: Should the radiation exposure be of concern? Gastroenterology. 2005;129(2):328–337. doi: 10.1053/j.gastro.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 81.Neri E, Faggioni L, Cerri F, et al. CTC versus Double-Contrast Barium Enema for Screening of Colorectal Cancer: Comparison of radiation burden. 2009 doi: 10.1007/s00261-009-9568-x. Published online by September 24: http://www.springerlink.com/content/100116/?Content+Status=Accepted&sort=p_OnlineDate&sortor der=desc&v=condensed&o=10. [DOI] [PubMed]
- 82.Brenner DJ, Hall EJ. Computed Tomography – An increasing source of radiation exposure. The New England Journal of Medicine. 2007;357(11):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 83.Lu H, Li X, Li L, Chen D, Xing Y, Wax M, Hsieh J, Liang Z. Adaptive Noise Reduction toward Low-Dose CT. Proc SPIE Medical Imaging. 2003;5030:759–766. [Google Scholar]
- 84.Wang J, Li T, Lu H, Liang Z. Penalized Weighted Least-Squares Approach to Sinogram Noise Reduction and Image Reconstruction for Low-Dose X-ray CT. IEEE Transactions on Medical Imaging (TMI) 2006;25:1272–1283. doi: 10.1109/42.896783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang J, Wang S, Li L, Lu H, Liang Z. VC Screening with Ultra Low-Dose CT & Less-Stressful Bowel Preparation: A computer simulation study. IEEE Transactions on Nuclear Science. 2008;55(5):2566–2575. doi: 10.1109/TNS.2008.2004557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rust G, Aurich V, Reiser M. Noise/Dose Reduction and Image Improvements in Screening VC with Tube Currents of 20 mAs with Nonlinear Gaussian Filter Chains. Proc SPIE Medical Imaging. 2002;4683:186–197. [Google Scholar]
- 87.Purkayastha S, Tekkis P, Athanasiou T, et al. Magnetic Resonance Colonography versus Colonoscopy as a Diagnostic Investigation for Colorectal Cancer: A meta-analysis. Clinical Radiology. 2005;60(6):980–989. doi: 10.1016/j.crad.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 88.Laks S, Macari M, Bini E. Positional Change in Colon Polyps at CTC. Radiology. 2004;231(4):761–766. doi: 10.1148/radiol.2313030951. [DOI] [PubMed] [Google Scholar]
- 89.Liang Z, Yang F, Wax M, et al. Inclusion of A Priori Information in Segmentation of Colon Lumen for 3D VC. Conf Record IEEE NSS-MIC, in CD-ROM.1997. [Google Scholar]
- 90.Liang Z. Electronic Colon Cleansing Techniques: Past, present, and future. The 11th International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI) Workshop – “New Era of Virtual Colonoscopy”; New York City, NY. September 6; 2008. pp. 26–32. And “Colonoscopy without Colon Cleansing”, an invitation to SPIE Newsroom: http://spie.org/x31867.xml?highlight=x2416&ArticleID=x31867. [Google Scholar]
- 91.Liang Z, MacFall J, Harrington D. Parameter Estimation and Tissue Segmentation from Multispectral MR Images. IEEE Transactions on Medical Imaging. 1994;13(3):441–449. doi: 10.1109/42.310875. [DOI] [PubMed] [Google Scholar]
- 92.Summers R, Johnson C, Pusanik L, et al. Automated Polyp Detection at CTC: Feasibility assessment in a human population. Radiology. 2001;219(1):51–59. doi: 10.1148/radiology.219.1.r01ap0751. [DOI] [PubMed] [Google Scholar]
- 93.Yoshida H, Nappi J. Three-Dimensional Computer-Aided Diagnosis Scheme for Detection of Colonic Polyps. IEEE Transactions on Medical Imaging. 2001;20(8):1261–1274. doi: 10.1109/42.974921. [DOI] [PubMed] [Google Scholar]
- 94.Wang Z, Liang Z, Li L, et al. Reduction of False Positives by Internal Features for Polyp Detection in CT-Based VC. Medical Physics. 2005;32(12):3602–3616. doi: 10.1118/1.2122447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu H, Liang Z, Barish M, Pickhardt P, et al. Increasing Computer-aided Detection Specificity by Projection Features for CTC. Medical Physics, to appear. 2010 doi: 10.1118/1.3302833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liang Z, Lakare S, Wax M, et al. A Pilot Study on Less-Stressful Bowel Preparation for VC Screening with Follow-up Biopsy by OC. Proc SPIE Medical Imaging. 2005;5746:810–816. [Google Scholar]
- 97.Pickhardt P, Kim D. CTC: Principles and practice of virtual colonoscopy. Saunders, Elsevier; Philadelphia, PA, USA: 2009. [Google Scholar]
- 98.Park S, Lee S, Choi E, et al. Flat Colorectal Neoplasma Definition, Importance, and Visualization on CTC. American Journal of Roentgenology. 2007;188(5):953–959. doi: 10.2214/AJR.06.0436. [DOI] [PubMed] [Google Scholar]
- 99.Liang Z, Wang S. An EM Approach to MAP Solution of Segmenting Tissue Mixtures: A numerical analysis. IEEE Transactions on Medical Imaging. 2009;28(2):297–310. doi: 10.1109/TMI.2008.2004670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang S, Li L, Cohen H, Mankes S, Chen J, Liang Z. An EM Approach to MAP Solution of Segmenting Tissue Mixture Percentages with Application to CT-based VC. Medical Physics. 2008;35(12):5787–5798. doi: 10.1118/1.3013591. [DOI] [PMC free article] [PubMed] [Google Scholar]