Abstract
Background
Animal data suggest the utility beta-hCG followed by erythropoietin to promote brain repair after stroke. The current study directly translated these results by evaluating safety of this sequential growth factor therapy via a three center, single dose, open label, non-controlled, Phase IIa trial.
Methods
Patients with ischemic stroke 24–48 hours old and NIHSS score of 6–24 started a nine day course of beta-hCG (once-daily on days 1, 3 and 5 of study participation) followed by erythropoietin (once-daily on days 7, 8, and 9 of study participation). This study also evaluated performance of serially measured domain-specific endpoints.
Results
A total of 15 patients were enrolled. Two deaths occurred, neither related to study medications. No safety concerns were noted among clinical or laboratory measures, including screening for DVT and serial measures of serum hemoglobin. In several instances, domain-specific endpoints provided greater insight into impairments as compared to global outcome measures.
Conclusions
Results support the safety of this sequential, two growth factor therapy initiated 24–48 hours after stroke onset.
Keywords: Stroke, Treatment, Recovery, Growth Factor
Stroke remains a major source of disability. Most patients do not receive approved acute therapies, often because the time window for intervention is 4.5–8 hours after stroke onset. Further, among those thrombolysed, many nonetheless have significant long-term disability. Therapies are needed that both have a wider treatment time window and reduce disability in a larger fraction of affected subjects1.
Growth factor levels spontaneously increase in many brain regions following a stroke, and are considered an important contributor to the process of spontaneous recovery2, 3. Increased growth factor levels due to exogenous administration, beginning 1–7 days after stroke, can further improve long-term outcome in preclinical experiments4, 5. Kolb et al6 used a sequential two growth factor approach, employing epidermal growth factor and erythropoietin (EPO), to boost levels of new neural stem cells. As an extension of this approach, in a prior study in animals, we used beta-human chorionic gonadotropin (hCG) in place of epidermal growth factor, given the more extensive human experience with hCG. That study7 evaluated the effects of sequentially administered hCG, which promotes proliferation of endogenous neural stem cells7, followed by EPO, which promotes differentiation of these cells into neural stem cells8. Treatment initiated 24 hours after experimental stroke onset in rats was found to be safe and to improve long-term behavioral outcome7. The hormone hCG is in the same growth factor family as nerve growth factor9, crosses the blood brain barrier10 and enters the cerebrospinal fluid11, and its receptor is normally present in adult rat brains12. EPO is a growth hormone13 that also crosses the blood brain barrier14, and its receptor mRNA is readily detectable in CNS neurons and glia15.
The primary aim of the current study was to examine the safety of this sequential hCG plus EPO regimen translated to human subjects with stroke 24–48 hours old. The goal of the intervention was to act as a restorative rather than neuroprotective agent, promoting repair without reducing injury1. This was a three-center, single dose, open label, non-controlled, Phase IIa safety trial.
A secondary aim evaluated domain-specific endpoints. Different neurological domains recover with different rates and extents after stroke, and furthermore restorative therapies might differentially influence recovery of these individual domains. This suggests the potential value of adding measures of separate behavioral domains to recovery assessments16. As a secondary goal, the current study therefore also aimed to examine performance of domain-specific endpoints for measuring safety and detecting behavioral gains.
Methods
Subjects and Overall Study Design
Subjects were consented and enrolled from September, 2006 – February, 2008, in accordance with local Institutional Review Boards, at three North American sites: University of California Irvine Medical Center (n=11), Hoag Memorial Hospital Presbyterian (n=1), and the Foothills Hospital at University of Calgary (n=3). Entry and exclusion criteria are listed in Table 1. This study was registered at clinicaltrials.gov as # NCT00362414.
Table 1.
Entry and exclusion criteria
Entry criteria
|
Exclusion Criteria
|
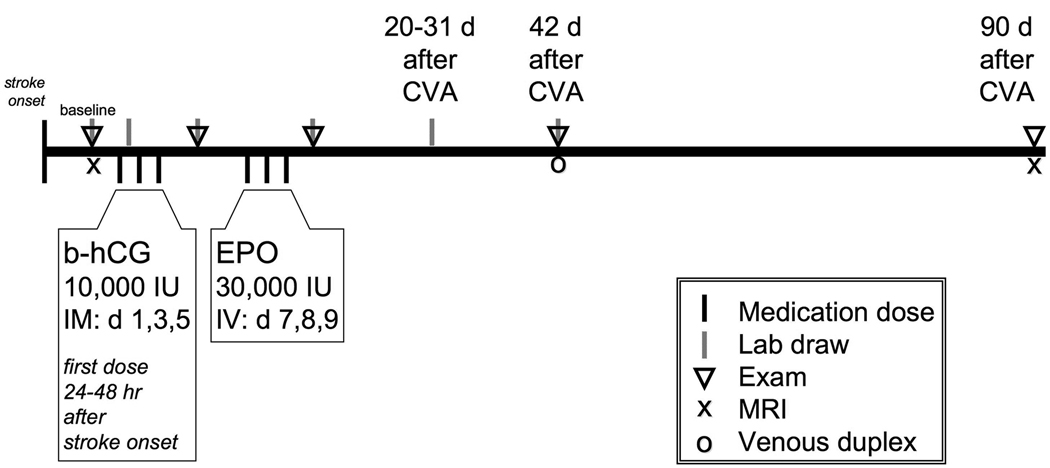
Administration of beta-hCG followed by EPO (“B-E therapy”) is a 9-day course, initiated 24–48 hours after stroke onset, consisting of three once-daily IM doses of hCG at 10,000 IU/dose, one day apart, on Days 1, 3 and 5 of study participation; followed by a one day washout period; followed by three once-daily iv doses of EPO at 30,000 IU/dose on days 7, 8, and 9 of study participation (Figure 1). Patients with weight ≤ 45 kg (n=1) received half dose EPO.
Figure 1.
Study design
The primary outcome measure was safety through day 90, assessed via adverse event reporting, serial exams, blood testing, and a leg vein Doppler at day 42. Secondary outcomes included the NIH Stroke Scale (NIHSS) score, Barthel index, and a battery of domain-specific endpoints (see below) at 90 days.
Study Procedures
Study tests are outlined in Figure 1. Prior to therapy, subjects were screened, consented, and underwent baseline exam, scoring on NIHSS, blood testing, and (when possible) scoring on a battery of domain-specific endpoints. This battery of domain-specific endpoints consisted of the Action Research Arm Test17, Fugl-Meyer Arm and Leg motor scores18, Boston Naming Test19, Line Cancellation Test20, and the Trails A & B Tests21. B-E therapy then commenced, with the first dose starting 24–48 hours after stroke onset. Exam, urinalysis, blood chemistry testing, reticulocyte count, and the battery of domain-specific endpoints were each performed four more times through day 90; measurement of serum blood counts and hemoglobin, five more times; assessment of adverse events, six more times. On day 42, a venous Doppler study of the legs was administered to screen for the presence of any DVT and the Barthel Index was scored. The NIHSS was repeated on day 90, and the Geriatric Depression Scale22 was scored. When possible, MRI data were collected, consisting of diffusion weighted images (DWI) at baseline and FLAIR images at day 90.
Data Analysis
All analyses were two-tailed, with alpha=0.05.
Results
Baseline patient characteristics
A total of 15 patients with moderate stroke were enrolled, with demographic and baseline features as in Tables 2 and 3. An exception allowed enrollment of an 87 year-old. Of the 15 enrollees, acute therapy included iv tPA (n=1), intra-arterial tPA (n=1), and MERCI thrombus retrieval (n=1). The baseline (i.e. pre-therapy) serum hCG level was normal in all cases. Baseline serum iron levels were low in 8 and normal in 7 subjects. Baseline serum ferritin was normal in 11 and elevated in 4. The median time from stroke onset to the start of B-E therapy was 40 hours (range, 19–48 hours). Most of the B-E therapy doses were given as an inpatient, however in a few instances patients received final B-E doses in an outpatient setting.
Table 2.
Baseline clinical features
| Measure | Value |
|---|---|
| n | 15 |
| Age | 72 (23–87) years |
| Gender | 9M/6F |
| Ethnicity | 9 Caucasian, 4 Hispanic, 2 Asian |
| History of | |
| Hypertension | 12 |
| Hyperlipidemia | 9 |
| Diabetes mellitus | 6 |
| Prior stroke | 5 |
| Atrial fibrillation | 4 |
The value for age is the median (with range in parentheses)
Table 3.
Clinical data from baseline to day 90
| Scale | Baseline score | Day 90 score |
|---|---|---|
| NIHSS--all patients (n=15 at baseline, n=12 at d90) |
10 (6–19) | 2 (0–6) |
| Barthel Index (n=11 at baseline, n=12 at d90) |
25 (0–100) | 95 (70–100) |
| Arm Fugl-Meyer Motor Scale (n=12 at baseline, n=12 at d90; normal score= 66) |
32 (2–65) | 62 (4–66) |
| Leg Fugl-Meyer Motor Scale (n=10 at baseline, n=12 at d90; normal score= 34) |
20 (3–34) | 32 (20–34) |
| Boston Naming Test (n=13 at baseline, n=12 at d90; normal=10) |
2 (0–10) | 8 (2–10) |
| Line Cancellation Test (n=8 at baseline, n=12 at d90; normal = 100%) |
96 (20–100) % | 100 (87–100) % |
| Action Research Arm Test (n=9 at baseline, n=11 at d90; normal=57) |
23 (0–57) | 57 (0–57) |
| Trailmaking A, # connected (n=7 at baseline, n=12 at d90; normal=25) |
8 (0–25) | 25 (12–25) |
| Trailmaking B, # connected (n=5 at baseline, n=11 at d90; normal=25) |
3 (0–11) | 22 (6–25) |
The total number of testable subjects was 15 at baseline and 12 at day 90. Data are presented when scoring could be completed. Values are median (range).
Baseline scores were tested < 48 hours after stroke onset, and prior to B-E therapy, except for the Barthel Index, for which baseline testing was done 6 days after study entry.
Note that 8 of 12 subjects had day 90 Barthel Index score ≥95.
Review of the line cancellation test indicated left hemineglect in 4 of 8 subjects at baseline, and in 1 of 12 subjects at day 90.
For the Geriatric Depression Scale at day 90 (n=12), the median score was 5 (range 0–12), with five patients having a score of 6 or greater, which is suggestive of depression.
Safety of B-E therapy
Clinical and laboratory data indicated no safety concerns and no serious adverse event related to study drug. Of the 12 patients assessed through day 90, the median day 90 score for NIHSS was 2; for the Barthel Index, 95, with 8 of 12 subjects having a score ≥ 95 (Table 3).
There were two deaths. One patient suffered a retroperitoneal hemorrhage associated with anticoagulant therapy given for an MI that was concomitant with the index stroke, which ultimately led to multi-organ failure and death after a decision was made to change to palliative care. This patient received a total of three doses of hCG and one dose of EPO. A second patient suffered cardiac arrest three weeks after enrollment.
Two instances of DVT were identified. One patient with preexisting pulmonary disease developed an upper extremity DVT. This patient received 3 doses hCG but because of the DVT he received no EPO. A DVT (asymptomatic, in calf) was diagnosed at the planned (Figure 1) day 42 leg venous Doppler in 1 of the 13 patients for whom this result was available.
One enrollee with atrial fibrillation had 2 additional cerebral emboli prior to EPO, received the full B-E therapy course, and did well (day 90 NIHSS score of 3). One enrollee lacked day 90 follow-up evaluation due to recurrent mediastinal sepsis arising from cardiac surgery.
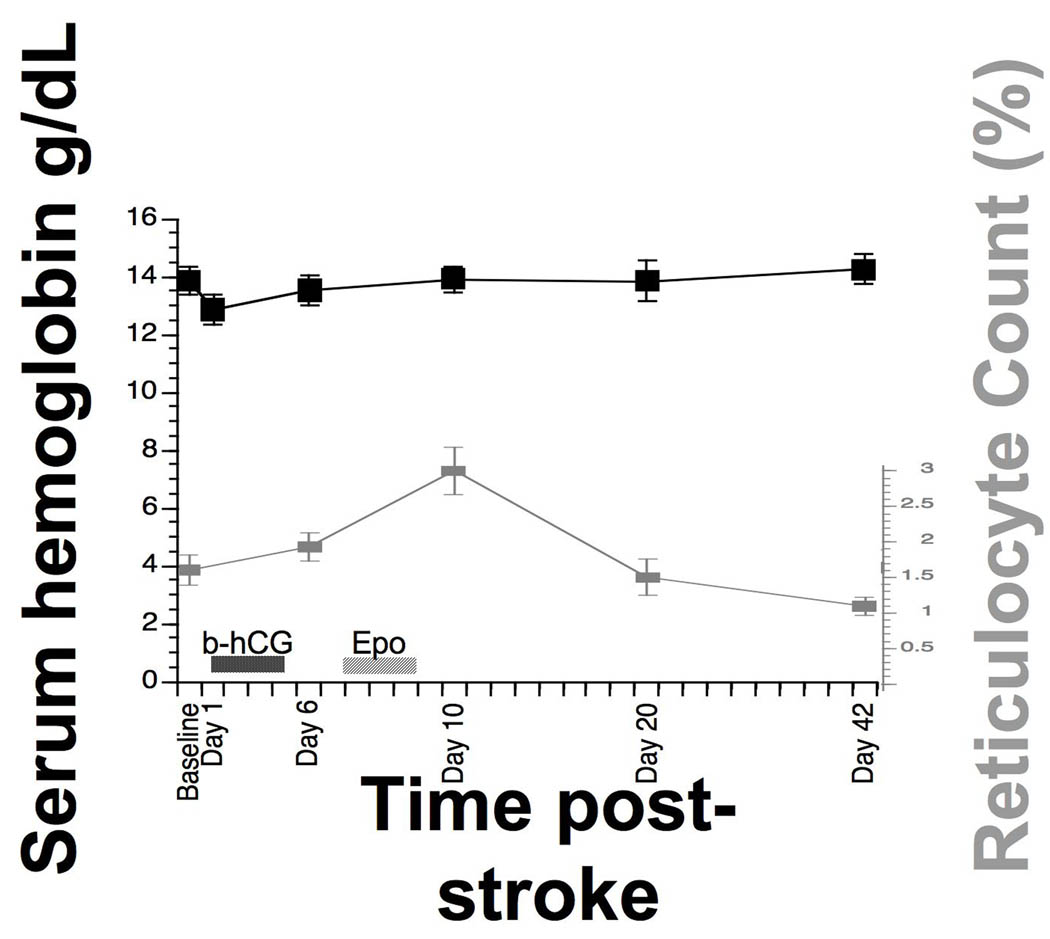
The serum hemoglobin and the reticulocyte count were each stable over time (repeated measures ANOVA, p > 0.1 in each case for main effect of time, see Figure 2).
Figure 2.
Hematological changes in association with B-E therapy. In parallel with B-E therapy, serum hemoglobin and reticulocyte count (mean ± SEM) showed no change over time, as the main effect of time was not significant in repeated measures ANOVA. Note that the initial increase in reticulocyte count from baseline to day 6 preceded EPO administration, and that the normal upper limit for reticulocyte count is 2.5%.
Infarct volume (baseline=DWI, d90=FLAIR, data available at both time points in seven patients) declined over time in 5/7 subjects. The mean baseline infarct volume was 55 ± 38 cc. The mean within-subject change in infarct volume was a drop of 22 ± 34 %.
Serial measurement of domain-specific endpoints
This study also provided an opportunity to examine the performance of domain-specific endpoints, values for which are presented in Table 3. Note that measurement could not be obtained at baseline for some of these scales, typically due to reduced patient sensorium or patient declining to complete the test, with the Boston Naming Test performing best in this regard (data available in 13 of 15 patients) and Trailmaking B the worst (data available in 5 of 15 patients).
In some cases, the domain-specific endpoints provided insight into focal areas of dysfunction among patients with low global disability. For example, of two patients with day 90 Barthel Index score of 95 (little or no disability), one had arm motor Fugl-Meyer score of 37 (moderate-severe arm weakness) and one could name only 4 of 10 items on the Boston Naming Test (moderate aphasia).
The domain-specific endpoints also provided finer resolution of outcomes in some cases. For example, among the 9 subjects with an NIHSS arm motor subscore of 0 at day 90, the range of arm motor Fugl-Meyer scores was 53–66 (out of 66), indicating substantial arm motor deficits among some subjects with a perfect NIHSS arm motor subscore. Domain-specific measures also provided finer resolution of change over time. For example, one patient had modest improvement in NIHSS score, going from baseline score of 10, to day 90 score of 5. In parallel, tremendous gains were measured via the arm motor Fugl-Meyer score, improving by 40 out of 66 possible points.
Discussion
The BETAS study was a three center, single dose, open label, non-controlled, Phase IIa trial that directly translated findings from prior preclinical investigations. The use of sequential growth factor administration was intended to increase endogenous neural stem cell proliferation. The current study enrolled a total of 15 patients with acute ischemic stroke that was overall moderate in severity. B-E therapy lasted 9 days, and was initiated 24–48 hours after stroke onset.
The primary focus of this study was safety, and B-E therapy was found to be safe. Although no placebo group was included in this safety study, clinical outcomes, such as 8 of 12 subjects having Barthel Index score ≥ 95 at day 90, compared favorably with placebo groups from prior stroke clinical trials23. Neither of the two deaths was related to B-E therapy. Infarct volumes overall showed a trend towards reduction over time that were similar to those described elsewhere24.
Hematological effects of three EPO doses were negligible (Figure 2). Clinical applications of erythropoietic stimulating agents such as EPO have recently come under review25. Most of this attention has been focused on applications in oncology and renal failure, where dozens or hundreds of doses are administered, which is much larger than the EPO exposure with B-E therapy. Also, the increased mortality rate described in association with EPO in a recent clinical stroke trial by Ehrenreich et al26 was not seen in the current study. There are several possible explanations for these differences: Ehrenreich et al26 used higher EPO doses as compared to B-E therapy (40,000 vs. 30,000 IU), introduced the EPO at the time when the ischemic insult was actively evolving (EPO started at 6 hours post-stroke vs. EPO started 8–9 days post-stroke in the current study), and gave EPO in parallel with tPA (vs. tPA effects completely resolved by time of EPO dosing in the current study).
Domain-specific endpoints have been useful in acute stroke research27, but have only rarely been used in prior acute stroke trials. These measures have been suggested to have value for detecting differential effects that therapies might have on various functional aspects of stroke recovery.16 Domain-specific endpoints showed strengths in the current study, for example, in several instances provided greater insight than global outcome measures did into final level of impairment. Also, some of the domain-specific endpoints provided finer resolution of specific deficits and their change over time. Domain-specific endpoints also showed weaknesses, as a number of patients could not be satisfactorily evaluated at baseline, a topic that requires further study. In addition, the current battery of domain-specific endpoints did not directly examine quality of life, a priority to address in future studies.
The time window of B-E therapy suggests that it acts as a restorative rather than neuroprotective treatment1. The time window of such therapies suggests the potential to reach a high fraction of patients with stroke. The current study provides data supporting the safety of a sequential two growth factor therapy initiated 24–48 hours after stroke onset. A placebo-controlled, double-blind, phase IIb trial of B-E therapy has been initiated (clinicaltrials.gov ID # NCT00938314).
Acknowledgments
Acknowledgments and Funding
This study was supported by a grant from Stem Cell Therapeutics, and by funds provided by the National Center of Research Resources, 5M011 RR-00827-29, US Public Health Service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CLINICALTRIALS.GOV IDENTIFIER: NCT00362414
Conflicts of Interest Disclosures
Dr. Cramer has received consulting fees from Stem Cell Therapeutics.
References
- 1.Cramer SC. Repairing the human brain after stroke. II. Restorative therapies. Ann Neurol. 2008;63:549–560. doi: 10.1002/ana.21412. [DOI] [PubMed] [Google Scholar]
- 2.Finklestein SP, Caday CG, Kano M, Berlove DJ, Hsu CY, Moskowitz M, Klagsbrun M. Growth factor expression after stroke. Stroke. 1990;21:III122–III124. [PubMed] [Google Scholar]
- 3.Carmichael ST. Gene expression changes after focal stroke, traumatic brain and spinal cord injuries. Curr Opin Neurol. 2003;16:699–704. doi: 10.1097/01.wco.0000102621.38669.77. [DOI] [PubMed] [Google Scholar]
- 4.Kawamata T, Dietrich W, Schallert T, Gotts J, Cocke R, Benowitz L, Finklestein S. Intracisternal basic fibroblast growth factor (bfgf) enhances functional recovery and upregulates the expression of a molecular marker of neuronal sprouting following focal cerebral infarction. Proc Natl Acad Sci. 1997;94:8179–8184. doi: 10.1073/pnas.94.15.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawamata T, Ren J, Chan T, Charette M, Finklestein S. Intracisternal osteogenic protein-1 enhances functional recovery following focal stroke. Neuroreport. 1998;9:1441–1445. doi: 10.1097/00001756-199805110-00035. [DOI] [PubMed] [Google Scholar]
- 6.Kolb B, Morshead C, Gonzalez C, Kim M, Gregg C, Shingo T, Weiss S. Growth factor-stimulated generation of new cortical tissue and functional recovery after stroke damage to the motor cortex of rats. J Cereb Blood Flow Metab. 2007;27:983–997. doi: 10.1038/sj.jcbfm.9600402. [DOI] [PubMed] [Google Scholar]
- 7.Belayev L, Khoutorova L, Zhao KL, Davidoff AW, Moore AF, Cramer SC. A novel neurotrophic therapeutic strategy for experimental stroke. Brain Res. 2009;1280:117–123. doi: 10.1016/j.brainres.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 8.Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21:9733–9743. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei ZM, Rao CV. Neural actions of luteinizing hormone and human chorionic gonadotropin. Semin Reprod Med. 2001;19:103–109. doi: 10.1055/s-2001-13917. [DOI] [PubMed] [Google Scholar]
- 10.Lukacs H, Hiatt ES, Lei ZM, Rao CV. Peripheral and intracerebroventricular administration of human chorionic gonadotropin alters several hippocampus-associated behaviors in cycling female rats. Horm Behav. 1995;29:42–58. doi: 10.1006/hbeh.1995.1004. [DOI] [PubMed] [Google Scholar]
- 11.Davidoff AW, Hill MD, Cramer SC, Yang Y, Moore A. Open labeled, uncontrolled pharmacokinetic study of a single intramuscular hcg dose in healthy male volunteers. Int J Clin Pharmacol Ther. 2009;47:516–524. [PubMed] [Google Scholar]
- 12.al-Hader AA, Tao YX, Lei ZM, Rao CV. Fetal rat brains contain luteinizing hormone/human chorionic gonadotropin receptors. Early Pregnancy. 1997;3:323–329. [PubMed] [Google Scholar]
- 13.Chen J, Chopp M. Neurorestorative treatment of stroke: Cell and pharmacological approaches. NeuroRx. 2006;3:466–473. doi: 10.1016/j.nurx.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354:2034–2045. doi: 10.1056/NEJMra052706. [DOI] [PubMed] [Google Scholar]
- 15.Digicaylioglu M, Bichet S, Marti HH, Wenger RH, Rivas LA, Bauer C, Gassmann M. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci U S A. 1995;92:3717–3720. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cramer SC, Koroshetz WJ, Finklestein SP. The case for modality-specific outcome measures in clinical trials of stroke recovery-promoting agents. Stroke. 2007;38:1393–1395. doi: 10.1161/01.STR.0000260087.67462.80. [DOI] [PubMed] [Google Scholar]
- 17.Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair. 2008;22:78–90. doi: 10.1177/1545968307305353. [DOI] [PubMed] [Google Scholar]
- 18.Fugl-Meyer A, Jaasko L, Leyman I, Olsson S, S S. The post-stroke hemiplegic patient: A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 19.Kent PS, Luszcz MA. A review of the boston naming test and multiple-occasion normative data for older adults on 15-item versions. Clin Neuropsychol. 2002;16:555–574. doi: 10.1076/clin.16.4.555.13916. [DOI] [PubMed] [Google Scholar]
- 20.Albert ML. A simple test of visual neglect. Neurology. 1973;23:658–664. doi: 10.1212/wnl.23.6.658. [DOI] [PubMed] [Google Scholar]
- 21.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19:393–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Beck RW. Screening depressed patients in family practice. A rapid technic. Postgrad Med. 1972;52:81–85. doi: 10.1080/00325481.1972.11713319. [DOI] [PubMed] [Google Scholar]
- 23.Uchino K, Billheimer D, Cramer S. Entry criteria and baseline characteristics predict outcome in acute stroke trials. Stroke. 2001;32:909–916. doi: 10.1161/01.str.32.4.909. [DOI] [PubMed] [Google Scholar]
- 24.Warach S, Kaufman D, Chiu D, Devlin T, Luby M, Rashid A, Clayton L, Kaste M, Lees KR, Sacco R, Fisher M. Effect of the glycine antagonist gavestinel on cerebral infarcts in acute stroke patients, a randomized placebo-controlled trial: The gain mri substudy. Cerebrovasc Dis. 2006;21:106–111. doi: 10.1159/000090208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fishbane S, Nissenson AR. The new fda label for erythropoietin treatment: How does it affect hemoglobin target? Kidney Int. 2007;72:806–813. doi: 10.1038/sj.ki.5002401. [DOI] [PubMed] [Google Scholar]
- 26.Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M, Jahnig P, Herrmann M, Knauth M, Bahr M, Heide W, Wagner A, Schwab S, Reichmann H, Schwendemann G, Dengler R, Kastrup A, Bartels C. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009 doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 27.Hillis A, Wityk R, Barker P, Beauchamp N, Gailloud P, Murphy K, Cooper O, Metter E. Subcortical aphasia and neglect in acute stroke: The role of cortical hypoperfusion. Brain. 2002;125:1094–1104. doi: 10.1093/brain/awf113. [DOI] [PubMed] [Google Scholar]