Abstract
In an effort of achieving suitable biomaterials for peripheral nerve regeneration, we present a material design strategy of combining a crystallite-based physical network and a crosslink-based chemical network. Biodegradable polymer disks and conduits have been fabricated by photo-crosslinking three poly(ε-caprolactone fumarate)s (PCLF530, PCLF1250, and PCLF2000), which were synthesized from the precursor poly(ε-caprolactone) (PCL) diols with nominal molecular weights of 530, 1250, and 2000 g.mol−1, respectively. Thermal properties such as glass transition temperature (Tg), melting temperature (Tm), and crystallinity of photo-crosslinked PCLFs were examined and correlated with their rheological and mechanical properties. Furthermore, in vitro degradation of uncrosslinked and crosslinked PCLFs in PBS crosslinked PCLFs in 1 N NaOH aqueous solution at 37 °C was studied. In vitro cytocompatibility, attachment, and proliferation of Schwann cell precursor line SPL201 cells on three PCLF networks were investigated. Crosslinked PCLF2000 with the highest crystallinity and mechanical properties was found to best support cell attachment and proliferation. Using a new photo-crosslinking method, single-lumen crosslinked PCLF nerve conduits without defects were fabricated in a glass mold. Crosslinked PCLF2000 nerve conduits were selected for evaluation in a 1-cm gap rat sciatic nerve model. Histological evaluation demonstrated that the material was biocompatible with sufficient strength to hold sutures in place after 6 and 17 weeks of implantation. Nerve cable with myelinated axons was found in the crosslinked PCLF2000 nerve conduit.
Keywords: Poly(ε-caprolactone fumarate), Photo-crosslinking, Peripheral nerve regeneration, Cell responses
1. Introduction
Peripheral nerve injuries have been a challenging clinical problem for surgeons.[1–5] Autologous nerve graft is still the gold standard in repairing injured peripheral nerve. However, it has several disadvantages such as limited sources of donor nerve, the need of a second surgery to obtain the donor nerve, loss of nerve function in transplantation, and mismatch between the injured nerve and donor nerve.[1–7] Therefore, synthetic nerve conduits have attracted great attention and numerous biocompatible polymers have been used to fabricate nerve conduits to bridge the gap between injured peripheral nerve stumps.[1–7] Suitable nerve conduit materials should satisfy several general properties in regards of feasibility of fabrication, sterilizability, capability of resisting tear, and suturability.[1]
Crosslinkable polymeric biomaterials have many advantages because they can be injected and hardened in situ to fill tissue defects or fabricated into pre-formed scaffolds.[8,9] Recently we have developed a series of crosslinkable, degradable polymers and polymer composites for hard and soft tissue engineering applications.[10–17] Among these crosslinkable polymers, poly(ε-caprolactone fumarate) (PCLF) (Scheme 1) is a copolymer synthesized through the polycondensation of PCL diol and fumaryl chloride in the presence of a proton scavenger triethylamine or potassium carbonate (K2CO3).[10,12] In this study, PCLFs were synthesized from three starting PCL diols having nominal molecular weights of 530, 1250, and 2000 g.mol−1 with K2CO3. We have reported the structural characterizations and physical properties of these PCLFs and found they could be crosslinked by either thermal crosslinking or photo-crosslinking without the need of additional crosslinkers.[12]
Scheme 1.
Chemical structure of PCLF. (n, m, and p are integers to indicate the number of repeating units).
This report will focus on the physical properties of these three photo-crosslinked PCLFs as well as the evaluations of them as candidate materials for peripheral nerve regeneration. For investigating in vitro cell-material interactions, PCLFs have been fabricated into two-dimensional (2D) flat disks and the tested cell type was SPL201 cell, a conditionally immortalized Schwann cell precursor line for myelinating axons.[18] In in vivo animal testing, three-dimensional (3D) crosslinked PCLF nerve conduits have been fabricated using a novel molding method to guide axon growth between the distal and proximal nerve ends in a rat sciatic nerve defect model.
Because of distinct crystallinity and melting point, these three photo-crosslinked PCLFs have different mechanical properties at room temperature or body temperature. Such crystallite-enhanced surface stiffness will be emphasized in this report as a critical role in both regulating cell responses and determining the suitability for peripheral nerve regeneration. Therefore, this study not only supplies novel photo-crosslinkable biomaterials with great potentials for peripheral nerve regeneration but also testifies the material design strategy of using double polymer networks formed by both crosslinks and crystallites to modulate biomaterial properties and regulate cell responses.
2. Materials and methods
2.1. Materials
Unless noted otherwise, all chemicals used in this study were purchased from Sigma-Aldrich Co (Milwaukee, WI). PCLF samples were synthesized using α,ε-telechelic PCL diols with nominal number-average molecular weight (Mn) of 530, 1250, and 2000 g.mol−1.[12] The obtained PCLFs are named as PCLF530, PCLF1250, and PCLF2000, having weight-average molecular weight (Mw) of 6050, 15800, and 12900 g.mol−1, and Mn of 3520, 9000, and 7300 g.mol−1, respectively.
2.2. Photo-crosslinking of PCLF and fabrication of nerve conduits
Photo-crosslinking was initiated with ultraviolet (UV) light (γ=315–380 nm) in the presence of a photo-initiator, phenyl bis(2,4,6-trimethyl benzoyl) phosphine oxide (BAPO, IRGACURE 819™, Ciba Specialty Chemicals, Tarrytown, NY). In this study, 75 μL of BAPO/CH2Cl2 (300 mg/1.5 mL) solution was used for pre-dissolved PCLF/CH2Cl2 solution (1.5 g/500 μL). BAPO/PCLF weight ratios were calculated to be 10 mg/g. Homogeneous PCLF/BAPO/CH2Cl2 mixture was transferred into a mold consisting of two glass plates (2.1 mm, thickness) and a Teflon spacer (0.37 mm, thickness). The filled mold was placed under UV light with a distance of ~7 cm from the lamp head for 30 min to allow crosslinking. Crosslinked PCLF sheets were removed from the mold after cooled down to ambient temperature. Strips and disks with different dimensions were cut from the sheets for different experimental purposes.
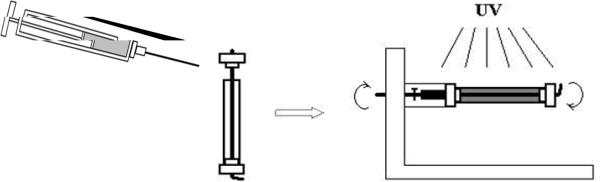
For fabricating nerve conduits for implantation, homogenous PCLF/BAPO/CH2Cl2 mixture mentioned earlier was injected from a syringe to a custom mold formed using a glass tube, a stainless steel wire, and two Teflon end-caps, as depicted in Scheme 2. The mold loaded with viscous polymer solution was rotated under UV light for 30 min to facilitate crosslinking. The crosslinked polymer nerve conduits were soaked in acetone for two days to wash away the solvent and sol fraction. Then the polymer conduits were dried in a vacuum oven.
Scheme 2.
Fabrication of a photo-crosslinked PCLF conduit.
2.3. Gel fraction and swelling ratio measurements
The procedure of determining gel fraction and swelling ratios of crosslinked PCLFs can be found in our previous reports.[12,14] Briefly, two crosslinked PCLF disks (5 mm × 0.34 mm, diameter × thickness) per solvent were immersed in excess CH2Cl2, ethanol, and water for two days. The swelling ratios and gel fractions of these crosslinked PCLF disks were calculated using the equations of (Ws-Wd)/Wd×100% and Wd/W0×100%, respectively, where W0 is the original weight of disk, Wd is the dry weight of disk after being soaked in CH2Cl2, and Ws is the weight of fully swollen disk. CH2Cl2 was the solvent for determining gel fractions.
2.4. Microscopic observation of nerve conduits
Cross-sectional and longitudinal surface morphologies of crosslinked PCLF nerve conduits were examined by cold-field emission scanning electron microscopy (SEM) (S-4700, Hitachi Instruments Inc., Tokyo, Japan). All samples were imaged at 3 kV accelerating voltage and 9500 nA emission current.
2.5. Thermal characterizations
Thermal properties of crosslinked PCLFs (~2 mg) were measured using a differential scanning calorimeter (DSC) (Q1000, TA Instruments) in a nitrogen atmosphere. The sample was first heated from room temperature to 100 °C and cooled to −90 °C at 5 °C/min. Then a subsequent heating run was performed from −90 to 100 °C at 10 °C/min.
2.6. Rheological measurements
Frequency sweep mode was applied to obtain the linear viscoelastic properties of crosslinked PCLFs, including storage and loss moduli G' and G” as well as viscosity η as functions of frequency, on a rheometer (AR2000, TA Instruments) in the frequency (ε) range of 0.5–100 rad/s at 37 °C. In the measurements, a small strain (γ=0.01) was applied and an 8 mm diameter parallel plate flow cell was used. The gap was around 0.5 mm, depending on the sample thickness.
2.7. Mechanical measurements
Tensile, compression, and 3-point bending properties of photo-crosslinked PCLF specimens were implemented by a dynamic mechanical analyzer (DMA2980, TA instruments) at room temperature. In tensile experiments, crosslinked PCLF strips (~30 mm × ~2 mm × ~0.4 mm, length × width × thickness) were elongated at 0.5 N.min−1 up to a maximum static force of 18 N. Five specimens were measured and averaged for each sample. In order to test how much the crosslinked PCLFs can hold the suture, pull-out tests were performed on a laboratory-developed mechanical analyzer for the polymer disks (5 mm × 0.35 mm, diameter × thickness) with a micro-surgical Nylon suture loop (10-0 Ethilon, Ethicon Co., Somerville, NJ) through the center. The detailed procedure for the pull-out tests was given in our earlier report.[14] Three specimens for each sample were measured at a pull-out rate of 0.1 mm.s−1. In compression measurements, modulus was measured on small polymer disks (4.1 mm × 0.36 mm, diameter × thickness) at a compression rate of 2 N.min−1 up to a maximum static force of 18 N. Flexural modulus of crosslinked PCLF conduits was measured using three-point bending mode in DMA. Polymer conduits have an average outer diameter ranging from 2.04 mm for PCLF530 conduits to 1.92 mm for PCLF2000 conduits, and a fixed length of 10.23 mm. In indentation tests, polymer disks were indented with a 1-mm flat-ended cylindrical tip on an ELF3100 micro-indenter (Bose Inc., Minnetonka, MN), assuming they were in unconfined compression. The indentation was performed at a rate of 0.2 mm.s−1 with a maximum force of 20 N. Three indents were performed on each specimen and the indentation depth ranged from 0.1 to 0.3 mm. The concern about disk thickness was included in the calculation according to our earlier report.[14]
2.8. Contact angle measurement and protein adsorption
A static contact angle system (Kruss, Model DSA10) with water as the liquid phase was used to test the hydrophilicity of the crosslinked PCLF disks. Approximately 1 μL of water (pH=7.0) was injected onto the disk surface. Contact angle measurement was made after a static time of 30 s. A tangent method was used to calculate the contact angle in degrees. For each composition, three disks were used and six data points were taken for average and standard deviation calculation.
As described in an earlier paper for protein adsorption measurement,[14] pre-wetted crosslinked PCLF disks (11 mm × 0.37 mm, diameter × thickness) were immersed in cell culture medium for 4 hr at 37 °C. Then the disks were transferred into 48-well plates (one disk per well) and 600 μL of phosphate buffer saline (PBS) was used to wash the disks three times. Five minutes of gentle agitation was applied and PBS was discarded after each wash. Two hundred forty microliters (240 μL) of 1% sodium dodecyl sulfate (SDS) solution was put into these wells for 1 hr at room temperature. The SDS solution was collected in a plastic vial and new SDS solution was put into the wells for another 1 hr. This procedure was repeated twice and all the SDS solution was collected in a plastic vial. The concentrations of protein in the collected SDS solutions were determined on a microplate reader (SpectraMax Plus 384, Molecular Devices, Sunnyvale, CA) using a MicroBCA protein assay kit (Pierce, Rockford, IL). Albumin in the kit was used to prepare solutions in SDS with eight known concentrations in order to construct a standard curve.
2.9. In vitro degradation
In vitro degradation of both uncrosslinked and crosslinked PCLFs was performed as follows. Uncrosslinked PCLFs (0.2 g) or crosslinked PCLF disks (~20 mg) were immersed in 10 mL PBS in glass vials and incubated at 37 °C for 71 weeks. After the degraded samples were fully washed and dried, the molecular weights were measured using GPC every 3–5 weeks for uncrosslinked PCLFs while the weights were measured and compared with their initial weights for crosslinked PCLFs. Degradation of crosslinked PCLF disks in 1 N sodium hydroxide (NaOH) aqueous solution at 37 °C was also studied and the weight change was recorded every day till the disappearance of the sample.
2.10. In vitro cytocompatibility, cell attachment, and proliferation
Rat SPL201 cells were used to evaluate the cytocompatibility of the PCLF material for nerve regeneration. Cryo-preserved SPL201 cells were thawed and plated on polystyrene flasks in culture medium that was composed of Dulbecco's modified eagle medium (Gibco, Grand Island, NY), 10% fetal bovine serum (FCS) (Sera-Tech, Germany), 50 μg gentamycin (Sigma, St. Louis, MO), and 10 ng/ml human recombinant EGF (Pepro Tech Inc., Rocky Hill, NJ). After plating, the cell suspension was incubated for 12 hr in a 5% CO2, 95% relative humidity incubator at 37 °C. Cell culture medium was changed after 24 hours of plating the cells from waking them up. Cells were split when they were 80% confluent. Trypsin with a concentration of 0.025% was used to bring the cells off from the flasks. The passage number was usually between 8 and 20.
The cytotoxicity evaluation proceeded by harvesting the SPL201 cells from the flasks and seeding them in 24-well plates at a density of 2×104 cells/cm2 with 300 μL/cm2 of primary medium. Crosslinked PCLF disks were sterilized in excess 80% ethanol solution overnight with gentle shaking. Then the disks were dried under vacuum and washed with PBS at least three times prior to use. The cells were exposed through culture medium to the sterile polymer disks (5 mm × 0.34 mm, diameter × thickness) inside the trans-wells for 1, 4, and 7 days. SPL201 cells plated with the same density without exposure to the polymer disks were positive controls and empty wells were negative controls. UV absorbance at 490 nm was determined on the incubated MTS assay solution (CellTiter 96 Aqueous One Solution, Promega, Madison, WI) using a microplate reader and cell viability was indicated by comparing the absorbance with the positive control's value.
SPL201 cell attachment and proliferation were performed to demonstrate the effect of material properties on cell responses. Sterile crosslinked PCLF disks were placed on the bottom of 48-well tissue culture plates using sterilized inert silicon-based high temperature vacuum grease (Dow Corning, Midland, MI) to avoid floating in the culture medium. Cells were seeded onto the polymer disk surface at a plating number of 15,000 cells/cm2. After 1, 4, and 7 days, culture medium was removed from the wells and the polymer disks were washed with PBS twice. The attached cells were fixed in paraformaldehyde (PFA) solution for 10 min. After PFA solution was removed, the cells were washed twice with PBS and permeabilised with 0.2% Triton X-100. The cells were stained using rhodamine-phaloidin for 1 hr at 37 °C and DAPI at room temperature for photographing with an Axiovert 25 Zeiss light microscope (Carl Zeiss, Germany).
2.11. In vivo implantation and histological evaluation
All the animal procedures were conducted under anesthesia and governed by an IACCUC protocol with special emphasis placed on post-operative care to alleviate potential pain and reduce autonomy. Single-lumen polymer nerve conduits made from photo-crosslinked PCLF2000 (as described in Section 2.2) were used for in vivo implantation because they were sufficiently flexible and tough to resist tear. The crosslinked PCLF2000 conduits were sterilized in 80% ethanol solution for 30 min and dried under vacuum overnight before they were implanted in a 1-cm gap in the rat sciatic nerve as described previously.[19] Briefly, the sciatic nerve was cut 5 mm distally from the tendon of the internal obturator muscle. A further 5-mm section of the sciatic nerve was replaced with a crosslinked PCLF2000 conduit. The nerve ends were inserted approximately 1 mm into the nerve conduit and held by a pair of sutures at both ends to leave a 1-cm gap in the nerve. After 6 and 17 weeks, the sciatic nerve and polymer nerve conduit were re-exposed and fixed in situ for 30 min in trumps fixative (2% PFA and 1% glutaraldehyde in PBS, pH=7.2) using the methods adapted from the literature.[20] The polymer nerve conduits with approximately 2 mm of both proximal and distal sciatic nerve ends were cut and removed prior to an immediate 24-hr fixation in trumps fixative. The polymer nerve conduits were embedded in SPUR using standard histological techniques. In brief, the nerve conduits were washed twice in PBS, treated with 1% osmium tetroxide (OsO4) for 1 hr, washed three times in water, and immersed in 2% uranyl acetate for 30 min. The nerve conduits were then washed in H2O and subsequently dehydrated. Once dehydrated, the samples were incubated in increasing concentrations of SPUR in ethanol. Finally after the immersion in 100% SPUR for 1 hr, the samples were mounted on a block mold and polymerized for 2–3 weeks at 40 °C. Thick sections were taken from the mid-part of the conduits, stained with toluidine blue and p-phenylene diamine, and evaluated for the presence of myelinated axons, macrophages, and fibroblasts.
3. Results and Discussion
3.1. Gel fraction, swelling ratios, and thermal properties
Before crosslinking, PCLFs are paste- or wax-like and lack sufficient mechanical properties for biomedical applications at room temperature or body temperature because their molecular weights are not high enough. After crosslinking, PCLFs form networks with higher mechanical properties. Because the composition of crosslinkable fumarate segment is lower for the PCLF prepared with a higher molecular weight of PCL precursor,[12] the gel fraction of crosslinked PCLF (Table 1) decreases from 77% for PCLF530 to 69% for PCLF1250 and then 53% for PCLF2000. The chemical structure of PCLF (Scheme 1) indicates that the distance between two neighboring crosslinks in PCLF networks is determined by both the PCL precursor's molecular weight and also the BAPO/polymer ratio.[22] Five amounts of photo-initiator BAPO have been employed in the photo-crosslinking to examine the effect of BAPO/polymer ratio on the physical properties of the PCLF networks.[22] In this study, BAPO/polymer ratio is 10 mg/g, the swelling ratio of crosslinked PCLF in CH2Cl2 shown in Table 1 increases progressively from 4.0±0.2 for crosslinked PCLF530 to 16.8±0.84 for crosslinked PCLF2000. Crosslinked PCLFs do not swell in water but swell slightly in ethanol with swelling ratios ranging from 11% to 25%.
Table 1.
Physical properties of photo-crosslinked PCLF networks
| PCLF530 | PCLF1250 | PCLF2000 | |
|---|---|---|---|
| Tg (°C) | −54.7 | −55.1 | −59.0 |
| Tm or Tc (°C)a | / | 31.6 (−2.6) | 42.0 (16.8) |
| ΔHm (J.g−1)a | 0 | 38.9 (39.7) | 48.4 (50.7) |
| χcb | 0 | 0.30 | 0.37 |
| Gel fraction | 0.77±0.01 | 0.69±0.004 | 0.53±0.07 |
| Swelling ratio in ethanol | 0.25±0.01 | 0.11±0.005 | 0.20±0.01 |
| Swelling ratio in CH2Cl2 | 4.0±0.2 | 9.1±0.46 | 16.8±0.84 |
| Tensile modulus (MPa) | 0.87±0.07 | 30.7±10.7 | 138±17 |
| Stress at break (MPa) | 0.48±0.40 | 3.7±0.5 | 7.1±0.3 |
| Elongation at break (%) | 50±11 | 155±39 | 17±2.3 |
| Pull-out force (N) | 0.05±0.002 | 0.24±0.03 | 0.42±0.03 |
Tm and ΔHm out of parenthesis, and Tc and ΔHm in parenthesis were determined in the heating and cooling runs of DSC, respectively.
Uncrosslinked PCLFs are all semi-crystalline with molecular-weight-dependent melting point (Tm) and crystallinity (χc) increase from 27.5 °C and 37.4% for PCLF530 to 43.9 °C and 47.2% for PCLF1250, then to 45.7 °C and 50.6% for PCLF2000.[12] Their glass transition temperatures (Tg) are around −60 °C.[12] In PCLF networks, both crosslinks and crystallites affect their thermal and mechanical properties.[22] Meanwhile, crystallites can be prohibited by crosslinks as demonstrated in the crystallization of ε-caprolactone blocks within a crosslinked microdomain structure of poly(ε-caprolactone)-block-polybutadiene.[23,24] Crosslinked PCLF530 is amorphous because crystallization is completely suppressed by the crosslinks. In contrast, crosslinked PCLF1250 and 2000 are still semi-crystalline with lower crystallinities (30% and 37%, respectively) and Tm (31.6 and 42.0 °C, respectively) compared with their uncrosslinked counterparts.
3.2. Rheological and mechanical properties
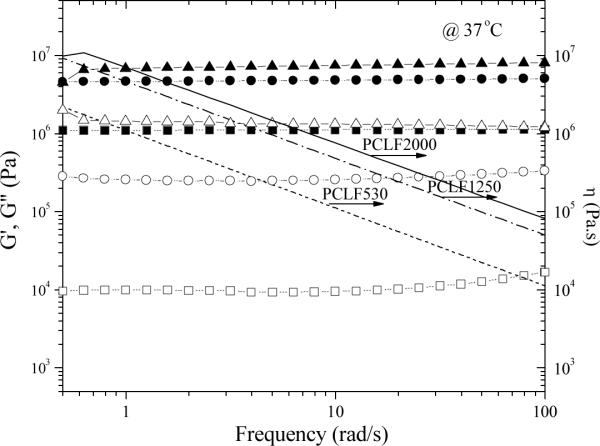
Fig. 1 shows dynamic frequency sweep results of three crosslinked PCLFs at 37 °C, revealing their rheological properties especially shear modulus at body temperature. All these three crosslinked PCLFs demonstrate rubbery behavior with storage modulus G' always larger than loss modulus G“ and no frequency dependence for G' in the frequency range of 0.5–100 rad/s. Shear thinning behavior can be observed for viscosity η. When the measurement temperature is 60 °C, all the PCLF networks are amorphous and the shear modulus is only determined by the average molecular weight between the two neighboring crosslinks or crosslinking density in the polymer network.[25] Consequently, crosslinked PCLF530 possesses the highest shear modulus at 60 °C. Similar to their roles in thermal properties, both crosslinks and crystallites also determine crosslinked PCLFs' rheological and mechanical properties.[22] Because crosslinked PCLF2000's Tm is 42.0 °°C (Table 1), its crystalline structure largely remains at 37 °C and consequently G' and η are the highest (7.25 MPa) among these three samples in Fig. 1. Crosslinked PCLF1250 shows a continuous decrease in G', G”, and η with increasing measurement temperature from 20 to 37 and 60 °C because crosslinked PCLF1250's Tm is 31.6 °C and crystalline domains start to melt partially at 37 °C. In contrast, crosslinked PCLF530 is amorphous, its G', G”, and η are lower than those of semi-crystalline crosslinked PCLF1250 and PCLF2000. The magnitude of G' (4.75 MPa) for crosslinked PCLF1250 is over four times higher than that (1.11 MPa) for crosslinked PCLF530 at 5.0 rad/s because the crystallites enhance the PCLF1250 network significantly as physical fillers or associations.
Fig. 1.
Storage modulus G' (solid symbols), loss modulus G” (open symbols), and viscosity η (lines) vs. frequency for crosslinked PCLFs at 37 °C. Crosslinked PCLF530: squares and dotted lines; crosslinked PCLF1250: circles and dashed lines; crosslinked PCLF2000: triangles and solid lines.
Mechanical properties of crosslinked PCLFs from tensile and pull-out measurements are listed in Table 1. The other mechanical properties determined from indentation, compression, and 3-point bending measurements are shown in Fig. 2. Because crosslinked PCLF530 is amorphous without the enhancement of crystallites, its tensile modulus and stress at break are as low as 0.87±0.07 and 0.48±0.40 MPa. For crosslinked PCLF2000 having the highest crystallinity and Tm among these three crosslinked PCLFs, it has the highest tensile modulus and stress at break as well as pull-out force with crystallites behaving like fillers and forming a physical network. Crosslinked PCLF2000 has a low gel fraction of 53% at the BAPO/polymer ratio of 10 mg/g, so it is brittle with and a low elongation at break of 17±2.3%. Nevertheless, it has a high tensile modulus of 138±17 MPa and a high tensile strength at break of 7.1±0.3 MPa, which exceed the tensile properties of fresh transected and coapted adult rat sciatic nerve (4 MPa for tensile modulus, 0.78 MPa for strength at break, and 30% for elongation at break).[26]
Fig. 2.
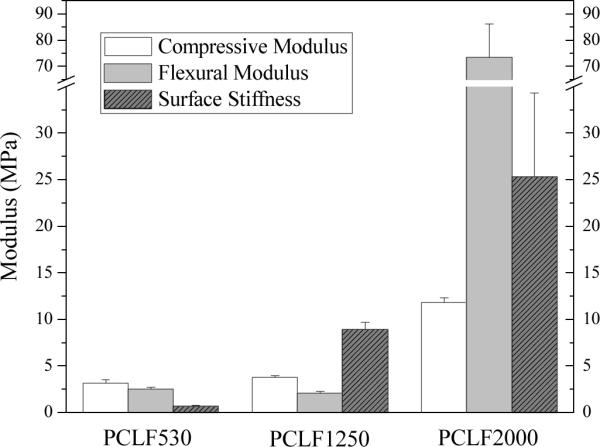
Compression, flexural, and surface moduli of crosslinked PCLF specimens at room temperature.
For peripheral nerve regeneration, a suitable material should have sufficient pull-out force to hold the suture and ensure the connection between the nerve conduits and the proximal and distal nerve ends during the implantation.[1,14] The pull-out force increases from 0.05±0.002 N for crosslinked PCLF530 to 0.24±0.03 N for crosslinked PCLF1250. Due to a higher crystallinity, PCLF2000 network has a much higher pull-out force of around 0.42 N, suggesting that crosslinked PCLF2000 is more suitable for being used as nerve conduit material than the other two crosslinked PCLFs. As shown in Fig. 2, the flexural modulus of crosslinked PCLF conduit increases significantly from 2.54±0.18 MPa for crosslinked PCLF530 and 2.10±0.17 MPa for crosslinked PCLF1250 to 73.4±12.7 MPa for crosslinked PCLF2000. Compression modulus and surface stiffness increase from 3.17±0.31 and 0.69±0.03 MPa for crosslinked PCLF530 to 3.76±0.18 and 8.94±0.73 MPa for crosslinked PCLF1250, and then to 11.8±0.53 and 25.3±9.0 MPa for crosslinked PCLF2000, respectively.
3.3. Protein adsorption, hydrophobicity, and in vitro degradation
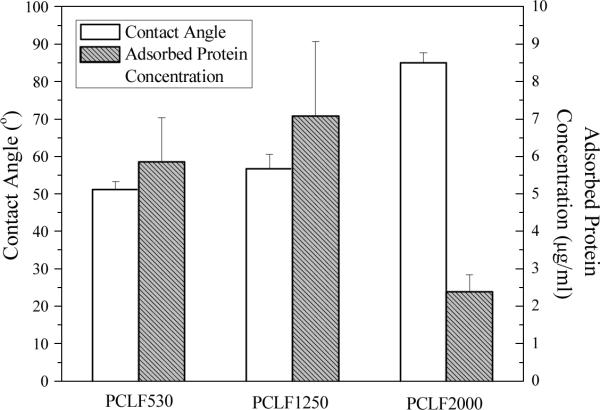
Surface energies of biomaterials are frequently correlated with the onset of biological parameters such as cell adhesion and wettability when implanted.[27] Contact angle of water on the biomaterial surface is used to evaluate surface energy and hydrophilicity. Fig. 3 shows both contact angle of water on the surface of crosslinked PCLFs and the surface's capability of adsorbing protein from cell culture medium. The contact angle for crosslinked PCLF530 is 51±2°, in agreement with the value of 54° reported earlier for crosslinked PCLF530 synthesized using a different proton scavenger triethylamine.[14] The contact angle increases to 57±4° and 85±3° for crosslinked PCLF1250 and PCLF2000, respectively, indicating the hydrophobicity is higher when the crystallinity is higher. Both hydrophilicity and surface roughness influence the capability of the surface to adsorb proteins from culture medium.[27] Though a higher roughness can be observed for crosslinked PCLF2000 disks (Section 3.5 and Fig. 7), its high hydrophobic characteristic may prohibit culture medium from spreading over and consequently less protein will attach, which was reported for the photo-crosslinked blends consisting of polypropylene fumarate (PPF) and PCLF. At different BAPO/polymer ratios, the capacity of adsorbing protein from the culture medium varies without a clear trend among these three crosslinked PCLFs.
Fig. 3.
Contact angle of water and protein adsorption on crosslinked PCLF disks.
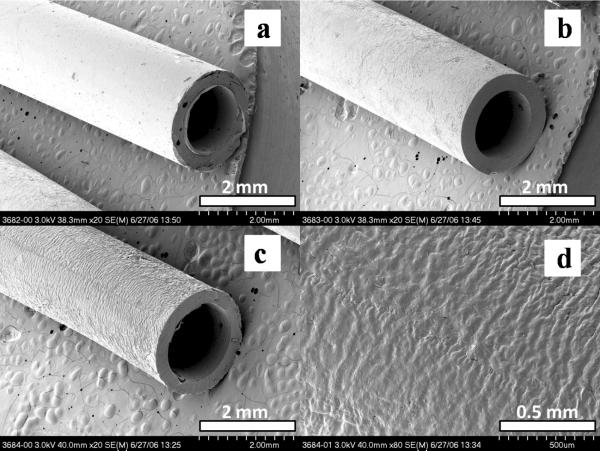
Fig. 7.
SEM pictures of crosslinked (a) PCLF530, (b) PCLF1250, and (c) PCLF2000 conduits, and (d) outer surface of crosslinked PCLF2000. The scale bars in (a–c) stand for 2 mm and the scale bar in (d) is 0.5 mm.
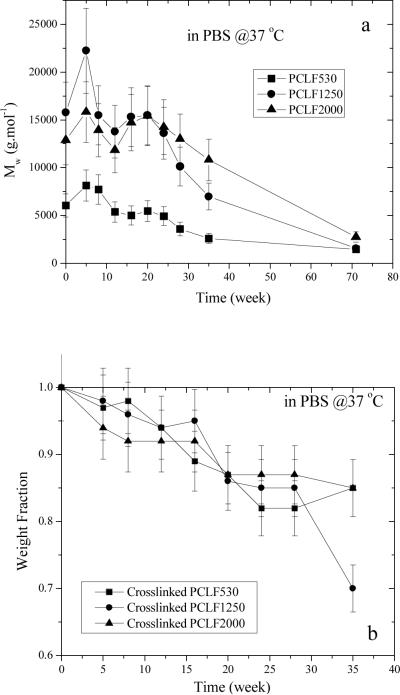
Hydrolytical degradation of both uncrosslinked and crosslinked PCLFs was studied in PBS at 37 °C. The decrease in Mw for uncrosslinked PCLFs over 71 weeks is shown in Fig. 4a. Mw did not decrease in the first 20 weeks for all the PCLFs as both degradation and partial crosslinking might occur at the beginning. After 20 weeks, degradation is dominant as indicated by the continuous decrease in Mw. For crosslinked PCLFs, weight change was examined in both PBS and 1 N NaOH aqueous solution at 37 °C. In PBS, three crosslinked PCLFs degraded at similar rates and only a decrease of 15–30% in weight fraction can be observed after 35 weeks. After 71 weeks, 70–80% of the original samples had been degraded. The in vitro degradation of crosslinked PCLFs in PBS at 37 °C is in agreement with the in vivo degradation of PCL sutures in literature.[28] As shown in the inset of Fig. 4b, the degradation of crosslinked PCLFs in NaOH solution is much faster than in PBS. Moreover, crosslinked PCLF530 demonstrates the highest degradation rate with no residue after 3 days. Crosslinked PCLF1250 is slower and crosslinked PCLF2000 is the slowest in degradation due to higher crystallinities; nevertheless, both samples degrade completely in the NaOH solution after 2 weeks.
Fig. 4.
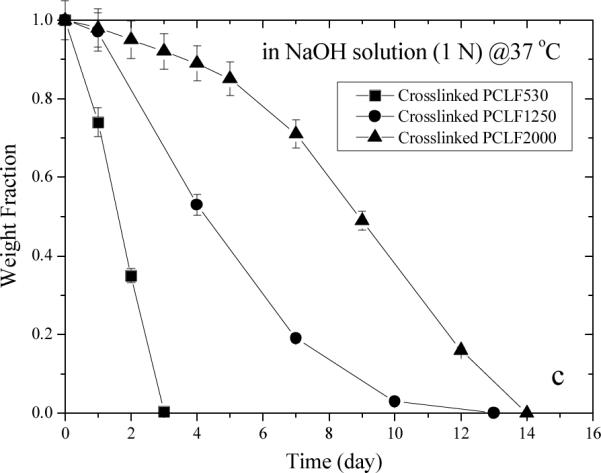
Degradation of (a) uncrosslinked PCLF and crosslinked PCLF samples in (b) PBS and (c) 1 N NaOH aqueous solution at 37 °C.
3.4. In vitro cell studies
For the PCLFs synthesized using a different proton scavenger triethylamine, we have reported excellent cell viability using human fetal osteoblast cells, rat bone marrow stromal cells, and SPL201 cells.[10,14] In the present study, only SPL201 cells are used to evaluate cell compatibility of three crosslinked PCLFs for peripheral nerve regeneration. As discussed in the degradation of crosslinked PCLF disks in PBS at 37 °C, there is no detectable weight change in one week. Therefore, the mechanical properties and topology largely maintain in the present in vitro cell studies. Fig. 5 shows the SPL201 cell viability in the presence of crosslinked PCLF disks for 1, 4, and 7 days. The fractions of viable cells normalized to the positive control, i.e., cells cultured on tissue culture polystyrene (TCPS) without exposure to polymer disks, are close to 100% within experimental errors for all three crosslinked PCLFs. This result indicates that all three crosslinked PCLFs have excellent compatibility to SPL201 cells and do not release toxic small molecules in the duration of one week.
Fig. 5.
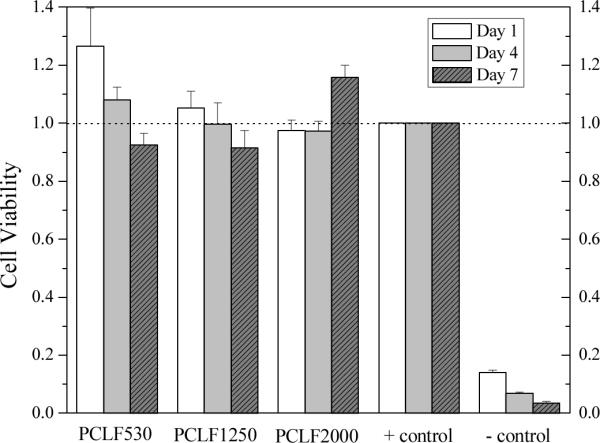
SPL201 cell viability in the presence of crosslinked PCLF disks as compared to cell-seeded tissue culture polystyrene (TCPS) without polymer specimen as positive (+) control and empty TCPS without cells as negative (−) control.
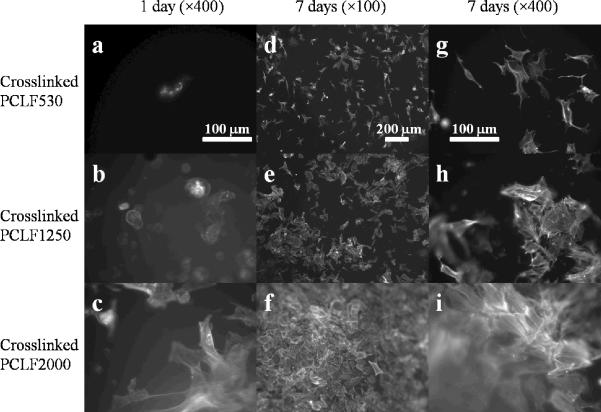
SPL201 cell morphology at 1 day and 7 days post-seeding on the crosslinked PCLF disks is shown in Fig. 6. Consistent with our earlier report,[14] amorphous crosslinked PCLF530 disks cannot support cell adhesion and proliferation well (Figs. 6a, d, g). In contrast, much more attached cells with spread-out phenotype and distinct actin stress fibers can be observed on semi-crystalline crosslinked PCLF2000 disks at both 1 and 7 days (Figs. 6b, e, h). At 37 °C, crosslinked PCLF1250 having a very close Tm (31.6 °C) is still semi-crystalline and its shear modulus and surface stiffness are between those of crosslinked PCLF530 and 2000, as indicated in Figs. 1 and 2. The number of attached cells and their phenotype on the crosslinked PCLF1250 disks (Figs. 6b, e, and h) suggest that crosslinked PCLF1250's capability of supporting cells to attach and proliferate is also between the other two crosslinked PCLFs.
Fig. 6.
SPL201 cell morphology after 1 day (×400) and 7 days (×100 and ×400) of culture on crosslinked PCLF disks. The scale bars in (a), (d), and (g) stand for 100, 200, and 100 μm, respectively, and they are applicable for the images in the same column.
It is generally believed that three major factors, i.e. chemical, topological, and mechanical factors, influence cell responses to biomaterials.[27] As recently elaborated in numerous reports, material's surface stiffness can play an important role through strong mechanical feedback from cell-substrate adhesion sites.[29–32] Crosslinked PCLFs are majorly (>90%) composed of PCL with different molecular weights; however, they have different surface energies or hydrophilicity, capabilities for adsorbing protein from culture medium, and mechanical properties because of the difference in the crystallinity. The contact angle of ~50° for crosslinked PCLF530 and 1250 is believed to favor cell attachment and proliferation better than a larger value of ~90° for crosslinked PCLF2000.[27] However, because crosslinked PCLF2000 has a lower gel fraction compared with crosslinked PCLF530 and 1250, its surface is rougher than the other two samples after extraction of the sol fraction using acetone, which can be directly seen in SEM images of the outer surface of the fabricated nerve conduit in Fig. 7d. Therefore, crosslinked PCLF2000 disks have two advantages: 1) higher surface stiffness; 2) increased surface roughness that encourage SPL201 cells to attach and proliferate on its surface in the time frame of over 1 week, although its hydrophilicity and capability for adsorbing proteins are the poorest among these three crosslinked PCLFs. Note that it is not unusual to observe no certain correlation between the contact angle or protein adsorption and cell attachment.[14,33]
Differences in cell behavior were reported on less crystalline versus more crystalline poly-L-lactide substrates previously.[34] The influence of nanometer-scale roughness on proliferation was also revealed on the osteoblast response to polymer crystallinity using poly(L-lactic acid).[35,36] Although crosslinked PCLF1250 and 2000 are semi-crystalline, the existence of nanometer-scale roughness in them might not be possible because the fabrication method of using glass molds did not allow crystallizable PCL segments to form surface features at nanometer scale. It can be speculated that crystallinity plays a more important role in regulating cell responses through enhancing the PCLF network's mechanical properties. It should be also noted that crosslinking process influences the final physical properties of crosslinked PCLF significantly.[22] Particularly, incomplete thermal crosslinking will result in a tacky surface, a higher crystallinity, a lower gel fraction, and a higher surface roughness even for PCLF530 that has the highest density of crosslinkable segments among the three PCLFs in this study. Cells are expected to respond to the thermally crosslinked PCLF disks differently.
3.5. Scaffold fabrication and in vivo nerve regeneration
Previously we used thermal crosslinking of PCLF530 in Teflon molds to fabricate polymer nerve conduits.[37] After 1 month of implantation of thermally crosslinked PCLF disks, a high rate of biocompatibility was suggested by a very thin layer of fibrous capsule around the sample, and the absence of some adverse reactions such as chronic infection in the blood, abnormalities in the renal capsules or kidney glomerulus, and interstitial thickening around the blood vessels.[10] However, the fabrication method of using thermal crosslinking is time-consuming (12 hr at 60 °C) and inefficient. In addition, the polymer conduits fabricated using thermal crosslinking often contain defects and bubbles because of inhomogeneous distribution and solvent evaporation. The new method presented in this report uses photo-crosslinking of polymers in a glass mold shown in Scheme 2. As elaborated in Section 2.2, defect-free conduits with single or multiple channels can be easily fabricated from homogeneous PCLF/BAPO/CH2Cl2 solutions after 30-min photo-crosslinking. Single-lumen nerve conduits made from three crosslinked PCLFs using this method are shown in Figs. 7a–c. Because crosslinked PCLF2000 has a gel fraction of 53%, its outer surface is with wrinkles and roughness is at the scale of 50 μm after being soaked in acetone, as demonstrated in Fig. 7d. Little swelling of these conduits was found in PBS solution; however, they swell slightly in 80% ethanol solution during sterilization, which is indeed helpful for leachable and potentially toxic molecules to be removed in this step if there are some.
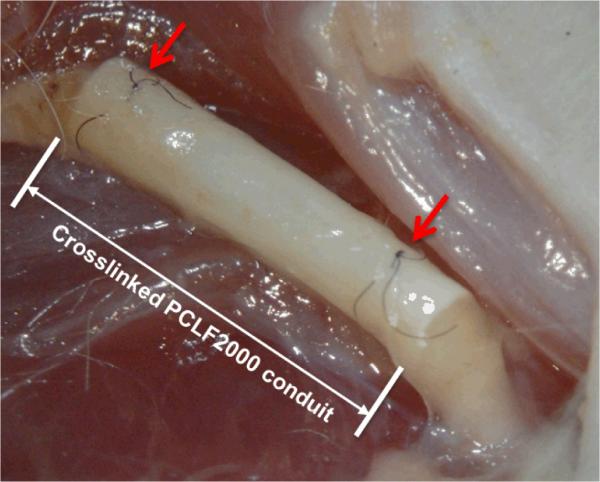
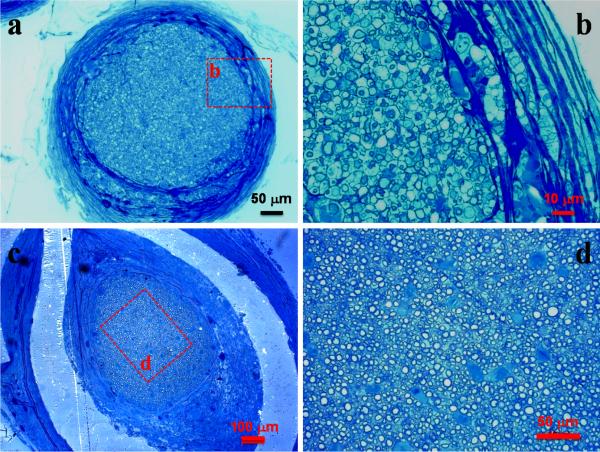
We deliberately selected the toughest crosslinked PCLF2000 nerve conduit to evaluate in vivo biocompatibility of crosslinked PCLFs. By controlling its crosslinking density and crystallinity, the PCLF2000 network is sufficiently strong to hold the suture while still suturable and flexible that is preferred in the repair of large gaps. Furthermore, the mechanical properties of crosslinked PCLF also support cells to attach and proliferate best among these three PCLFs. After 6 and 17 weeks of implantation, the structure of nerve conduit was still intact and only a think capsule of fibrous tissue grew and covered the crosslinked PCLF2000 nerve conduit (Fig. 8). Histological analysis also suggests that the nerve conduits are biocompatible with the surrounding tissue by showing only a small layer of fibrous tissue around the conduits. As demonstrated in Figs. 9a and 9c, the cross-sections of the crosslinked PCLF2000 nerve conduits at the middle point (5 mm) from the proximal anastomosis confirm the presence of a regenerating axon cable 6 and 17 weeks post-implantation. With a larger magnification in Figs. 9b and 9d, myelinated axons can be observed in the mid-part of the channels of the nerve conduit after implantation. Fasciculation of myelinated axons increased number of myelinated axons can be found after a longer period of implantation, 17 weeks, in Fig. 9d when compared with 6 weeks in Fig. 9b. Numerical analysis of the axons in the regenerated nerves section in Figs. 9c and 9d was conducted. The area of regenerative tissue is 0.2438 mm2, the total axon count is 3405, and the average myelin thickness is 1.3 μm.
Fig. 8.
Gross view of a crosslinked PCLF2000 nerve conduit sutured with the proximal and distal sciatic nerve strumps (arrow headed) at 17 weeks post-implantation.
Fig. 9.
Micrographs of toluidine blue-stained cross-sections of regenerated nerve cable at the mid-portion of a crosslinked PCLF2000 single-lumen conduit 6 weeks (a, b) and 17 weeks (c, d) after implantation (a: ×20, b: ×100, c: ×10, d: ×40). The scale bars in (a, b, c, d) stand for 50, 10, 100, and 50 μm.
This pilot study demonstrates that crosslinked PCLF2000 is an excellent candidate material for substituting autologous nerve conduit because it satisfies the major requirements in fabrication, degradation rate, flexibility, toughness, and biocompatibility. Furthermore, it is rather convenient to incorporate crosslinked PCLF2000 nerve conduits with such features as multiple guidance channels, porous permeable wall structure, supporting cells, and nerve growth factors. Beyond the scope of the present report that supplies preliminary data on biocompatibility, more extensive implantations with functional tests are to be performed in our laboratory using these photo-crosslinked PCLF2000 nerve conduits and a detailed investigation of nerve regeneration with statistical analysis will be reported later.
The strategy of combining networks from both crosslinks and crystallites in this study suggests a good method to achieve biomaterials with appropriate properties for both in vitro cell studies and in vivo peripheral nerve regeneration. Our laboratory recently focuses on the injectable polymeric systems with controllable mechanical properties for investigating the role of mechanical factor in regulating cell responses and tissue ingrowth. Besides three PCLFs with different crystallinities and melting points reported herein, the systems include a series of copolymers consisting of PPF and PCL, PPF/PCLF blends,[14] and PPF [17] or PCLF composites with hydroxyapatite nano-particles. The results are consistent with each other in terms of regulating cell behavior and all suggest the role of surface stiffness can be crucial in designing and selecting polymeric materials for biomedical applications.
4. Conclusions
Three polycaprolactone fumarates (PCLFs) with different nominal molecular weights (530, 1250, and 2000 g.mol−1) of their precursor polycaprolactone (PCL) diols have been photo-crosslinked to explore their applications in guided peripheral nerve regeneration. As different crosslinked PCLFs have different crystallinity and melting point, their mechanical properties vary significantly. Among these three photo-crosslinked PCLFs, crosslinked PCLF2000 synthesized from a PCL diol with 2000 g.mol−1 possesses the highest Tm and crystallinity, consequently the best rheological and mechanical properties at body temperature. All three crosslinked PCLFs show no cytotoxicity to rat SPL201 cells. Crosslinked PCLF2000 disks support SPL201 cell attachment and proliferation most efficiently although they are hydrophobic with the highest contact angle of 85±3° and have the lowest capability of adsorbing protein from culture medium among the three crosslinked PCLFs. The role of crystallinity in enhancing cell proliferation can be interpreted as the result of crystalline-domain-induced greater mechanical properties, particularly, surface stiffness. Therefore, crosslinked PCLF2000 nerve conduits have been made using a novel photo-crosslinking fabrication method for evaluating in vivo biocompatibility and functionality of guiding axon growth in a rat sciatic nerve defect model. The materials are biocompatible with the rat tissue without showing inflammatory reactions or existence of macrophages. Nerve cable with myelinated axons has been found in the crosslinked PCLF2000 nerve conduit after 6 and 17 weeks of implantation. Besides physical and degradation properties of PCLF networks, the present study supplies a strategy of combining both chemical and physical networks to achieve biomaterials with suitable mechanical properties to support cell responses and tissue ingrowth.
Acknowledgments
This work was performed at Mayo Clinic with the supports from the Mayo Foundation and NIH (R01 AR45871 and R01 EB003060). The authors thank Jarred J. Nesbitt and Dr. Huan Wang at Mayo Clinic for technical assistance in SPL201 cell culture and animal surgery, respectively. We also thank Drs. Jack L. Lewis and Narendra K. Simha at the University of Minnesota for allowing the use of micro-indenter.
References
- [1].Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- [2].Heath CA, Rutkowski GE. The development of bioartificial nerve grafts for peripheral-nerve regeneration. Tibtech. 1998;16(4):163–8. doi: 10.1016/s0167-7799(97)01165-7. [DOI] [PubMed] [Google Scholar]
- [3].Kannan RY, Salacinski HJ, Butler PEM, Seifalian AM. Artificial nerve conduits in peripheral-nerve repair. Biotechnol Appl Biochem. 2005;41:193–200. doi: 10.1042/BA20040137. [DOI] [PubMed] [Google Scholar]
- [4].Meek MF, Coert JH. US Food and Drug Administration/Conformit Europe-approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves. Ann of Plast Surg. 2008;60(4):466–72. [PubMed] [Google Scholar]
- [5].Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004;26:151–60. doi: 10.1179/016164104225013798. [DOI] [PubMed] [Google Scholar]
- [6].Hadlock T, Elisseeff J, Langer R, Vacanti J, Cheney M. A tissue-engineered conduit for peripheral nerve repair. Arch Otolaryngol Head Neck Surg. 1998;124:1081–6. doi: 10.1001/archotol.124.10.1081. [DOI] [PubMed] [Google Scholar]
- [7].Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000;6(2):119–27. doi: 10.1089/107632700320748. [DOI] [PubMed] [Google Scholar]
- [8].Anseth KS, Shastri VR, Langer R. Photopolymerizable degradable polyanhydrides with osteocompatibility. Nature Biotech. 1999;17(2):156–9. doi: 10.1038/6152. [DOI] [PubMed] [Google Scholar]
- [9].Ifkovits JL, Burdick JA. Photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13(10):2369–95. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- [10].Jabbari E, Wang SF, Lu LC, Gruetzmacher JA, Ameenuddin S, Hefferan TE, Currier BL, Windebank AJ, Yaszemski MJ. Synthesis, material properties and biocompatibility of a novel self-crosslinkable poly(caprolactone fumarate) as an injectable tissue engineering scaffold. Biomacromolecules. 2005;6(5):2503–11. doi: 10.1021/bm050206y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang S, Lu L, Gruetzmacher JA, Currier BL, Yaszemski MJ. A biodegradable and crosslinkable multiblock copolymer consisting of poly(propylene fumarate) and poly(ε-caprolactone): Synthesis, characterization, and physical properties. Macromolecules. 2005;38(17):7358–70. [Google Scholar]
- [12].Wang S, Lu L, Gruetzmacher JA, Currier BL, Yaszemski MJ. Synthesis and characterizations of biodegradable and crosslinkable poly(ε-caprolactone fumarate), poly(ethylene glycol fumarate), and their amphiphilic copolymer. Biomaterials. 2006;27(6):832–41. doi: 10.1016/j.biomaterials.2005.07.013. [DOI] [PubMed] [Google Scholar]
- [13].Wang S, Lu L, Yaszemski MJ. Bone-tissue-engineering material poly(propylene fumarate): Correlation between molecular weight, chain dimensions, and physical properties. Biomacromolecules. 2006;7(6):1976–82. doi: 10.1021/bm060096a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang S, Kempen DH, Simha NK, Lewis JL, Windebank AJ, Yaszemski MJ, Lu L. Photo-cross-linked hybrid polymer networks consisting of poly(propylene fumarate) and poly(caprolactone fumarate): controlled physical properties and regulated bone and nerve cell responses. Biomacromolecules. 2008;9(4):1229–41. doi: 10.1021/bm7012313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee KW, Wang S, Lu L, Jabbari E, Currier BL, Yaszemski MJ. Fabrication and characterization of poly(propylene fumarate) scaffolds with controlled pore structures using three-dimensional printing and injection molding. Tissue Eng. 2006;12(10):2801–11. doi: 10.1089/ten.2006.12.2801. [DOI] [PubMed] [Google Scholar]
- [16].Lee KW, Wang S, Fox B, Ritman EL, Yaszemski MJ, Lu L. Poly(propylene fumarate) bone tissue engineering scaffold fabrication using stereolithography: Effects of resin formulations and laser parameters. Biomacromolecules. 2007;8(4):1077–84. doi: 10.1021/bm060834v. [DOI] [PubMed] [Google Scholar]
- [17].Lee KW, Wang S, Yaszemski MJ, Lu L. Physical properties and cellular responses to crosslinkable poly(propylene fumarate)/hydroxyapatite nanocomposites. Biomaterials. 2008;29(19):2839–48. doi: 10.1016/j.biomaterials.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Smith PM, Buchstaller J, Schweitzer B, Franklin RJM, Suter U, Taylor V. SpL201: A conditionally immortalized Schwann cell precursor line that generates myelin. Glia. 2001;36(1):31–47. doi: 10.1002/glia.1093. [DOI] [PubMed] [Google Scholar]
- [19].de Ruiter GC, Spinner RJ, Alaid AO, Koch AJ, Wang H, Malessy MJ, Currier BL, Yaszemski MJ, Kaufman KR, Windebank AJ. Two-dimensional digital video ankle motion analysis for assessment of function in the rat sciatic nerve model. J Peripher Nerv Syst. 2007;12(3):216–22. doi: 10.1111/j.1529-8027.2007.00142.x. [DOI] [PubMed] [Google Scholar]
- [20].Onishi A, Offord K, Dyck PJ. Studies to improve fixation of human nerves. 1. Effect of duration of glutaraldehyde fixation on peripheral nerve morphometry. J Neurol Sci. 1974;23(2):223–6. doi: 10.1016/0022-510x(74)90225-1. [DOI] [PubMed] [Google Scholar]
- [21].Brandrup J, Immergut EH, editors. Polymer handbook. 3rd ed. Wiley; New York: 1989. [Google Scholar]
- [22].Wang S, Yaszemski MJ, Gruetzmacher JA, Lu L. Photo-Crosslinked Poly(ε-caprolactone fumarate) Networks: Roles of Crystallinity and Crosslinking Density in Determining Mechanical Properties. Polymer. 2008 doi: 10.1016/j.polymer.2008.10.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nojima S, Tanaka H, Rohadi A, Sasaki S. The effect of glass transition temperature on the crystallization of ε-caprolactonestyrene diblock copolymers. Polymer. 1997;39(8–9):1727–34. [Google Scholar]
- [24].Nojima S, Hashizume K, Rohadi A, Sasaki S. Crystallization of ε-caprolactone blocks within a crosslinked microdomain structure of poly(ε-caprolactone)-block-polybutadiene. Polymer. 1997;38(11):2711–8. [Google Scholar]
- [25].Sperling LH. Introduction to Physical Polymer Science. 3rd ed. Wiley; New York: 2001. [Google Scholar]
- [26].Borschel GH, Kia KF, Kuzon WM, Dennis RG. Mechanical properties of acellular peripheral nerve. J Surgical Res. 2003;114(2):133–9. doi: 10.1016/s0022-4804(03)00255-5. [DOI] [PubMed] [Google Scholar]
- [27].Harbers GM, Grainger DW. Cell-material interactions: fundamental design issues for tissue engineering and clinical considerations. In: Guelcher SA, Hollinger JO, editors. Introduction to Biomaterials. CRC Press; Boca Raton: 2005. pp. 15–45. [Google Scholar]
- [28].Perrin DE, English JP. Polycaprolactone. In: Domb AJ, Kost J, Wiseman D, editors. Handbook of Biodegradable Polymers. Harwood Academic Publishers; Amsterdam: 1997. pp. 63–77. [Google Scholar]
- [29].Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- [30].Pelham RJ, Wang Y-L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad. Sci. USA. 1997;94:13661. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Flanagan LA, Ju Y-E, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. NeuroReport. 2002;13:2411–5. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pelham RJ, Wang Y-L. Cell locomotion and focal adhesions are regulated by the mechanical properties of the substrate. Biol Bull. 1998;194:348–9. doi: 10.2307/1543109. [DOI] [PubMed] [Google Scholar]
- [33].Saad B, Keiser OM, Welti M, Uhlschmid GK, Neuenschwander P, Suter UW. Multiblock copolyesters as biomaterials: in vitro biocompatibility testing. J Mater Sci Mater Med. 1997;8:497–505. doi: 10.1023/a:1018582311361. [DOI] [PubMed] [Google Scholar]
- [34].Park A, Griffith-Cima L. In vitro cell response to differences in poly-L-lactide crystallinity. J Biomed Mater Res. 1996;31:117–30. [PubMed] [Google Scholar]
- [35].Simon CG, Jr, Eidelman N, Kennedy SB, Sehgal A, Khatri CA, Washburn NR. Combinatorial screening of cell proliferation on poly(L-lactic acid)/poly(D,L-lactic acid) blends. Biomaterials. 2005;26:6906–15. doi: 10.1016/j.biomaterials.2005.04.050. [DOI] [PubMed] [Google Scholar]
- [36].Washburn NR, Yamada KM, Simon CG, Jr, Kennedy SB, Amis EJ. High-throughput investigation of osteoblast response to polymer crystallinity: influence of nanometer-scale roughness on proliferation. Biomaterials. 2004;25:1215–24. doi: 10.1016/j.biomaterials.2003.08.043. [DOI] [PubMed] [Google Scholar]
- [37].de Ruiter GC, Onyeneho IA, Liang ET, Moore MJ, Knight AM, Malessy MJA, Spinner RJ, Lu L, Currier BL, Yaszemski MJ, Windebank AJ. Methods for in vitro characterization of multichannel nerve tubes. J Biomed Mater Res. 2008;84A:643–51. doi: 10.1002/jbm.a.31298. [DOI] [PubMed] [Google Scholar]