Abstract
Statins are 3-hydroxyl-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitors used for the treatment of hypercholesterolemia. We report that a particular statin, simvastatin (SIM), exhibits strong in vitro anti-HBV activity. Moreover, a combination of SIM with each of the individual nucleos(t)ide analogues lamivudine (LMV), adefovir (ADV), tenofovir (TEN) and entecavir (ETV), showed synergistic antiviral activity. Combination drug treatments were performed in the HepG2.2.15 cell line. Compound combinations were centered on a mixture designed to deliver approximately equipotent (not necessarily equimolar) concentrations of each agent, based on the ninety percent viral inhibition monotherapy values. SIM interacted favorably with all four licensed anti-HBV nucleos(t)ide analogues, especially at molar ratios that approximate combinations likely to be used clinically. As the relative concentration of SIM was raised to an excess, the overall favorability of the interactions progressively increased.
SIM displayed about equal degrees of synergy with ADV and TDF. The highest degree of synergy was observed at the 300:1 combination of SIM with ETV. Interactions with LMV were the least favorable. The in vitro potential shown here may greatly augment anti-HBV therapy clinically.
Keywords: hepatitis B virus, simvastatin, mevalonate, entecavir, tenofovir
1. Introduction
Hepatitis B virus (HBV) infects an estimated 400 million people, making it the most common chronic infectious disease worldwide. HBV is the predominant cause of hepatocellular cancer (HCC) globally. HCC is officially listed as the third most common cause of cancer death; however, since 80% of HCC cancer registries are located in developing countries where reporting is spotty, it is thought that HCC is the number one cause of cancer death worldwide (Johnson, 2008). Along with complications from cirrhosis, HBV causes more than a million deaths per year.
Therapies available for treatment of HBV are sub-optimal. Pegylated alfa-interferons (IFN) and an assortment of nucleoside and nucleotide analogues (NA) are available. The definition of “sustained response” to any of these treatments varies widely in the literature, but is often defined in hepatitis B “e” antigen (HBeAg) negative patients as achievement of a normal alanine aminotransferase (ALT) and a serum level of Hepatitis B Virus Deoxyribonucleic Acid (HBV DNA) <10,000 IU/ml six months after cessation of treatment. In HBeAg positive patients, an additional response goal is to achieve seroconversion of HBeAg (Pawlotsky et al., 2008).
IFN produces a 20-40% sustained response rate after one year of use. The advantages of IFN are that it is given only as a single 6 – 12 month course; there is no documented resistance of HBV to IFN; and, response leads to a reduced risk of HCC. However, even in responders, infective HBV in the form of covalently closed circular DNA persists and forms a hepatic reservoir for possible relapse of disease and a continued vulnerability for HCC, albeit much lower than in non-responders. The disadvantages of IFN are its side-effects and expense.
Because of the disadvantages associated with IFN treatment, many clinicians since 1997 have turned to NAs. The current FDA-approved ones are lamivudine, adefovir, tenofovir, entecavir, and telbuvidine. The advantages of NAs are tolerability and price. In contrast to IFN, a one-year course of any given NA provides an 11-17% response rate; thus, these agents are often given for many years, a feature which obscures price comparison with the one-time course of IFN. Moreover, the durability of a sustained response is much poorer than the result induced by IFN (Jacobson, 2008; Lok and McMahon, 2007).
Combination of any two anti-HBV treatments together does not improve the response over an individual component alone. IFN plus any NA yet tested does not improve the response rate over the use of IFN monotherapy. Trial combinations of NAs continue; but, so far, while two agents together can increase viral suppression modestly, no increase in HBeAg seroconversion rate has been reported (Jacobson, 2008). One duo treatment has even shown a trend for diminished HBeAg seroconversion rate of 15% versus 22-31% in the combination versus monotherapy arms (lamivudine + telbuvidine), respectively (Lai et al., 2007). The reason for failure of significant additive effect may be that the NAs all target the same viral component, HBV polymerase.
Certain 3-hydroxy-3-methylglutaryl-coenzyme A (HMG CoA) reductase inhibitors, called statins, have been shown to have in vitro antiviral activity against RNA viruses (Ikeda et al., 2006). We have demonstrated the antiviral activity of fluvastatin as monotherapy in chronic hepatitis C patients (Bader et al., 2008). The only DNA virus reported to be susceptible in vitro to a statin, fluvastatin, has been cytomegalovirus in a human endothelial cell assay (Potena et al., 2004). When the S gene coding region for HBV surface antigen was integrated into the genome of a Hep3B cell line, in vitro production of surface antigen was inhibited 60% by lovastatin; however, in cell culture the same authors could not demonstrate reduction of HBV virions (Lin et al., 2003).
The purpose of this study was to investigate the suppression of HBV in a whole virus assay by simvastatin (SIM). We also explored whether SIM would work synergistically with lamivudine (LMV), adefovir (ADV), tenofovir (TDF) or entecavir (ETV) to further inhibit HBV replication.
2. Materials and Methods
2.1 Agents
Two lots of certified reference standard SIM (99.4% purity) were obtained from USP Pharmaceuticals (Rockville, Maryland). Mevalonate was obtained from Sigma-Aldrich, Inc. (St. Louis, MO). ETV, LMV, ADV, LMV are available from Toronto Research Chemicals (North York, Ontario, Canada).
2.2 Cell cultures
Well validated HBV antiviral assays were conducted in a laboratory (B.K.) that routinely performs screening assays for new compounds against HBV. The cell line, HepG2.2.15, chosen for this study has been used to discover all of the currently FDA-approved nucleoside analogues for HBV. Briefly, and as previously described, confluent cultures of 2.2.15 cells were maintained on 96-well flat-bottomed tissue culture plates and were treated with nine consecutive daily doses of SIM. HBV DNA levels were assessed by quantitative dot blot hybridization analysis of HBV DNA after 24 hours (Korba and Milman, 1991). Cytotoxicity was assessed by uptake of neutral red dye 24 hours following the last treatment (Korba and Gerin, 1992).
2.3 Calculations
Fifty and 90 percent inhibition (EC50, EC90) and 50% cell cytotoxicity (CC50) values were calculated by linear regression analysis (MS EXCEL®, QuattroPro®). Standard deviations for EC50 and EC90 values were calculated from the standard errors generated by the regression analyses and represent drug concentrations at which a 2-fold or a 10-fold depression of intracellular HBV DNA occurs. CC50 is usually the drug concentration at which a two-fold lower level of neutral red dye uptake (relative to the average levels in untreated cultures) is observed, but this degree of cytotoxicity was never observed for SIM despite testing a 100X greater concentration than that seen causing EC90. The Selectivity index (SI) was calculated as CC50/EC90. EC90 values were used for calculation of the SI in HBV assays since at least a three-fold depression of HBV DNA levels is typically required to achieve statistical significance in this assay system.
Compound combinations were centered on a mixture designed to deliver approximately equipotent (not necessarily equimolar) concentrations of each agent, based on the EC90 monotherapy values (Korba, 1996). The ratios indicate the relative molar amount of the compounds used for each combination. Molar ratios were held constant throughout serial dilution. Four replicate cultures were assessed at each dilution. The overall type of interaction, as determined by analysis with CalcuSynTM (Biosoft, Inc.), for each combination is indicated next to the corresponding EC50 and EC90 values. EC50 and EC90 values for combinations are expressed in units of SIM.
For the mevalonate experiments, the concentration of mevalonate was held constant at 10uM throughout the dilution series with SIM and LMV. For the mevalonate combinations, EC50 and EC90 values are expressed in units of SIM or LMV, respectively.
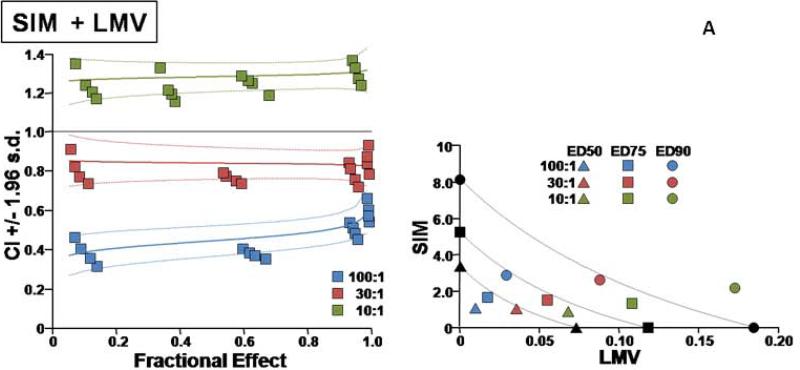
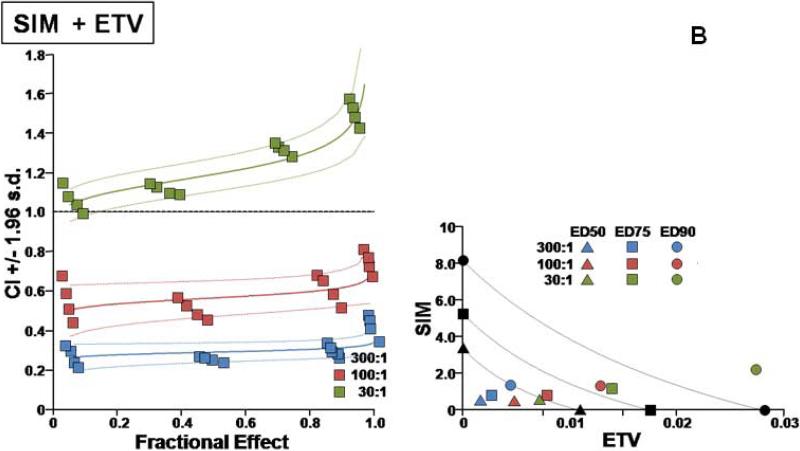
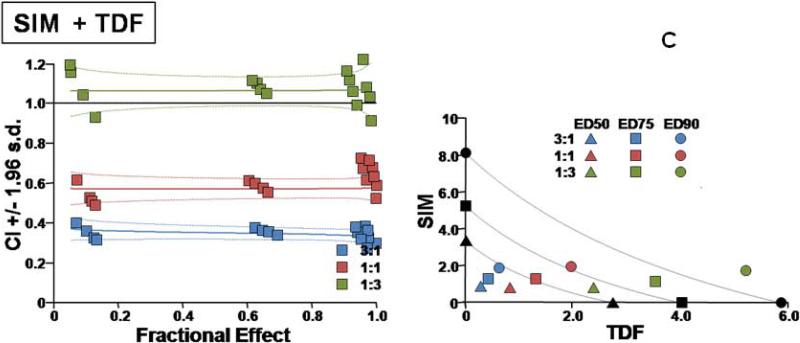
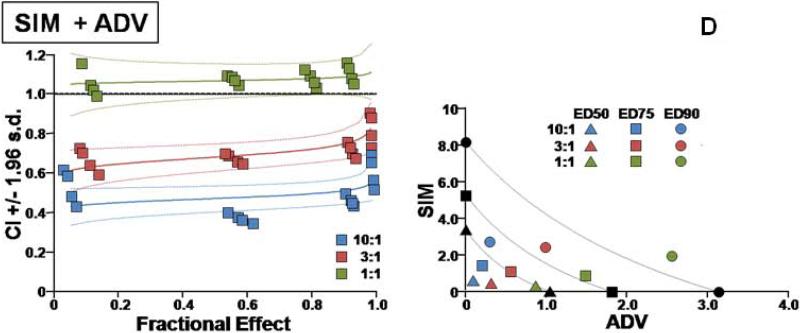
Additional details on the types of interactions for the different combinations are presented in Figures 1 and 2.
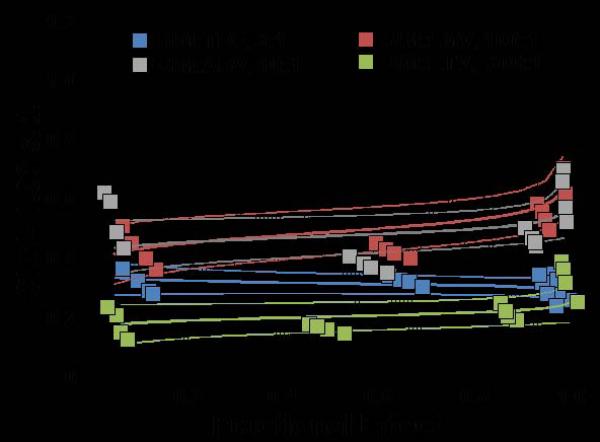
Fig. 1.
Two types of evaluations are presented for combination effect of SIM with each of the NA drugs. (A) LMV, (B) ETV, (C) TDF, and (D) ADV. The left hand panels present CI-Fa (Combination Index-Fraction (of virus) affected) plots). For these plots, a combination index [CI] greater than 1.0 indicates antagonism and a CI less than 1.0 indicates synergism. Evaluations of synergy, additivity (summation), or antagonism at different levels of virus inhibition (e.g. 5% (Fa=0.5) to 99% (Fa=0.99)) are provided by the plotted lines and points. The outer lines denote 1.96 standard deviations for significance evaluations.
The right hand panels present conventional isobolograms. For these plots, ED50, ED75, and ED90 (50%, 75%, and 90% effective antiviral dose) values for the combination treatments are displayed as single points. Three convex curves connecting the axes denote the expected (e.g. additive) EDC50, EDC75, and EDC90 values for drug combinations as calculated from the monotherapies. ED50, ED75, and ED90 values for the combinations that plot to the left (e.g. less than) of the corresponding lines indicate synergy, and values plotting to the right (e.g. greater than) of the corresponding lines indicate antagonism.
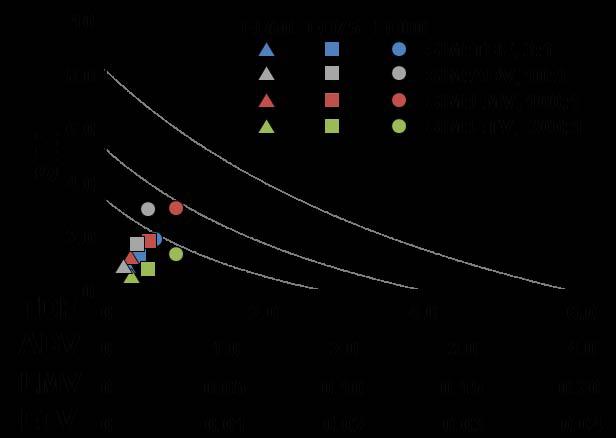
Fig. 2.
Collation of best molar combinations for drugs with simvastatin shown in Fig. 1 for comparison. See explanation under Fig. 1 as to description of left-sided and right-sided panels. SIM = simvastatin LMV = lamivudine, ENT = entecavir, ADV = adefovir, TDF = tenofovir.
3. Results
3.1 Anti-HBV activity
In cell culture, SIM was effective in inhibiting HBV replication (Table 1). SIM alone showed an ED90 of 8.6 μM. SIM interacted favorably (Table 1) with all four licensed anti-HBV nucleosides/tides, especially at molar ratios that approximated relatively equipotent dosing that most closely models combinations likely to be used clinically (first two ratios for each set of combinations). As the relative concentration of SIM in the combinations was raised, the overall favorability of the interactions progressively increased (Fig. 1A, B, C, D). SIM displayed approximately equal degrees of synergy with ADV and TDF. The highest degree of synergy was observed at the 300:1 combination of SIM with ETV. Interactions with LMV were the least favorable overall (Figure 2). The addition of these nucleosides/tides did not increase the cytotoxicity of SIM (>300uM in all cases).
Table 1.
HBV Antiviral Activity of Four Nucleoside Analogues Alone and Added to Simvastatin
| TREATMENT | EC50(uM) | EC90(uM) | INTERACTION |
|---|---|---|---|
| LMV | 0.06 ± 0.007 | 0.14 ± 0.011 | |
| ADV | 1.0 ± 0.2 | 2.9 ± 0.2 | |
| TDF | 2.4 ± 0.2 | 7.7 ± 0.6 | |
| ETV | 0.008 ± 0.001 | 0.03 ± 0.002 | |
| SIM | 2.5 ± 0.3 | 8.6 ± 0.8 | |
| SIM+ ADV ( 10:1) | 0.94 ± 0.05 | 3.1 ± 0.2 | synergistic |
| SIM+ ADV ( 3:1) | 0.95 ± 0.08 | 3.0 ± 0.2 | synergistic |
| SIM+ ADV ( 1:1) | 0.96 ± 0.07 | 3.0 ± 0.3 | additive/antagonistic |
| SIM+ TDF ( 3:1) | 0.78 ± 0.06 | 2.4 ± 0.2 | synergistic |
| SIM+ TDF ( 1:1) | 0.33 ± 0.04 | 1.5 ± 0.2 | synergistic |
| SIM+ TDF ( 1:3) | 0.80 ± 0.07 | 2.3 ± 0.2 | additive/antagonistic |
| SIM+ ETV (300:1) | 0.39 ± 0.03 | 1.6 ± 0.2 | synergistic |
| SIM+ ETV (100:1) | 0.40 ± 0.03 | 1.7 ± 0.3 | synergistic |
| SIM+ ETV ( 30:1) | 0.58 ± 0.06 | 2.8 ± 0.2 | antagonistic |
| SIM+ LMV (100:1) | 0.86 ± 0.07 | 2.7 ± 0.2 | synergistic |
| SIM+ LMV ( 30:1) | 0.89 ± 0.08 | 2.8 ± 0.2 | synergistic |
| SIM+ LMV ( 10:1) | 1.0 ± 0.1 | 3.0 ± 0.2 | antagonistic |
EC50 - μM causing 50% reduction, EC90 = μM causing 90%. The ratios indicate the relative molar amount of the compounds used for each combination. EC50 and EC90 values for combinations are expressed in units of SIM. Additional details on the types of interactions for the different combinations are presented in Figures 1 and 2.
3.2 Mevalonate addition
In a separate study (Table 2), the addition of 10uM mevalonate abolished the anti-HBV effect of SIM throughout the range of SIM molar concentration. Mevalonate monotherapy did not display an anti-HBV effect and did not affect the anti-HBV effectiveness of LMV.
Table 2.
Observations of effect of Mevalonate upon Simvastatin and Lamivudine
| TREATMENT | CC50(uM) | EC50(uM) | EC90(uM) | SI (CC50/EC90) |
|---|---|---|---|---|
| LMV | 2321 ± 102 | 0.048 ± 0.005 | 0.146 ± 0.017 | 15897 |
| SIM | >300 | 2.8 ± 0.3 | 8.1 ± 0.7 | >37 |
| Mevalonate | >10 | >10 | >10 | |
| SIM+10uM mevalonate | >300 | >100 | >100 | |
| LMV+10uM mevalonate | 2203 ± 94 | 0.040 ± 0.003 | 0.132 ± 0.014 | 16689 |
4. Discussion
The HBV infected liver makes a trillion virions every day. SIM is an ideal drug to target the liver since 93% of an oral dose is extracted on the first pass through the liver (Mauro, 1993). Liver tissue levels of SIM have not been reported in humans. In a mammalian model, the hepatic concentration of SIM was observed to be 44X that of serum after 60 minutes (Germershausen et al., 1989). SIM given as a single dose of 40 mg to humans produced peak serum levels of 10 ng/ml; if 44-fold hepatic concentration also occurs in humans, the resulting liver tissue level would then be 3.2 μM (Pentikainen et al., 1992). Thus, the ambient molar concentrations needed to reduce in vitro virion production listed in Table 1 appear to be achievable in vivo with doses that are currently approved by the FDA for cholesterol lowering.
In contrast, NAs target the liver poorly. LMV and ADV have liver/serum ratios of 0.007 and 0.38, respectively (Reddy et al., 2008). Liver/serum ratios for TDF and ETV could not be found in the literature. The excellent liver penetration of SIM may confer an additional benefit when combined with NAs in vivo not evaluable in the current assays.
The total composition of lipid by weight in HBV has been accurately determined only for the abundant surface antigen (HBsAg) component and not for whole virions. Regardless of whether the HBsAg originates from mammalian, human hepatoma cell lines or even yeast, the amount of total lipid constituting HBsAg remains 40% by weight (Diminsky et al., 1997). In any case, this large proportion reveals the substantial need HBV has for cholesterol and its derivatives.
Our observations are similar to those of Delang et al, for an entirely different virus, hepatitis C. They showed synergistic effects for all 10 protease and polymerase inhibitors tested individually in conjunction with simvastatin. However, the latter group did not propose any potential mechanism for this synergistic activity (Delang et al., 2009).
A number of potential mechanisms responsible for the general antiviral effect of statins have been investigated, including inhibiting geranylgeranyl pyrophosphate. Derivatives of the mevalonate pathway, such as geranylgeranyl pyrophosphate are important in the activation of a number of cellular proteins, including small guanosine-5'-triphosphate binding proteins and the Rho family (Ikeda et al., 2006; Schönbeck and Libby, 2004). The specific production of the cholesterol precursor mevalonate by HMG CoA reductase represents a potential bottleneck for blocking cholesterol production. Inhibition of HMG CoA reductase is a well understood pharmacological effect for statins. Others have shown that if this latter reaction is the cause for an effect of a statin, that it can be reversed with addition of mevalonate back to the culture (Ikeda et al., 2006; Ye et al., 2003). The addition of 10uM mevalonate in our test system (Table 2) abolished the anti-HBV effectiveness of SIM. Mevalonate monotherapy did not display an anti-HBV effect nor did it reduce the anti-HBV effectiveness of LMV.
However, since we and others have been unable to make other statins work against HBV, such as fluvastatin (Korba, unpublished results), pravastatin (Bader, unpublished results), or lovastatin (Lin et al., 2003), the reduction of mevalonate is unlikely to be the sole source of anti-HBV effect. Lack of cell penetration by statins cannot explain the lack of anti-HBV activity as fluvastatin, lovastatin and pravastatin have all been shown to penetrate the HepG2 cell line and affect intracellular processes (Ha et al., 2009; Hayashi et al., 1993; Lin et al., 2003).
An anti-cholesterol effect provides a ready explanation as to how SIM inhibits extracellular virion production (i.e. structural assembly) of HBV. How SIM reduces DNA intracellular intermediates of HBV remains to be elucidated. It is unlikely that SIM is working as a polymerase inhibitor, since it does not appear to be structurally related to nucleosides/tides. Thus, the mechanism of anti-HBV action by SIM needs further investigation.
Most hepatologists no longer consider statins to have any significant hepatotoxicity (Cohen et al., 2006). Millions of people have taken SIM safely. While there are rare adverse effects, these side–effects are well delineated. Moreover, it is even possible that SIM may become useful in the treatment of portal hypertension. Abraldes et al have extensively investigated SIM as an agent to treat portal hypertension (Zafra et al., 2004). They recently reported a 30-day double-blind randomized controlled trial using 20 – 40 mg/day of SIM. A significant lowering of portal hypertension and fewer side-effects than placebo were seen in 59 patients with advanced cirrhosis (Abraldes et al., 2009).
Statins have been noted in two large epidemiologic studies in veterans to reduce the risk of HCC by 50% (El-Serag et al., 2009; Khurana et al., 2005). Moreover, because of Veteran's Administration pricing policies during the latter study periods, the predominant statin being prescribed (77%) was SIM (El-Serag et al., 2009). The possibility that SIM may possess anti-HCC activity provides additional rationale for combination therapy.
HBV resistance to the NAs is becoming an increasing problem. We have previously shown equal in vitro efficacy of SIM against wild-type and the clinically relevant HBV resistant strains that encode rtL180M, rtM204V, rtM204I, rtN236T (Bader and Korba, 2008).
5. Conclusions
We report, for the first time, significant in vitro suppression of HBV in a whole virus assay by a particular statin, simvastatin (SIM). We have explored possible mechanisms for the anti-HBV effect of SIM. We also demonstrate the ability of SIM to work synergistically with lamivudine (LMV), adefovir (ADV), tenofovir (TDF) and entecavir (ETV) to further inhibit HBV replication. The in vitro anti-HBV synergism of simvastatin with nucleos(t)ide analogs is robust.
Other evidence suggests that the clinical translation of this observation may lead not only to increased antiviral efficacy, but also slowing of viral resistance and reduction of HCC. The in vitro potential reported here warrants further testing in humans as another possible approach for the eventual reduction of cancer and cirrhosis caused by the most common chronic infectious disease in the world.
Acknowledgements
This study was performed in collaboration with Georgetown University under NIAID contract NO1-AI-30046. Dr Bader would like to acknowledge Dr Michael Bronze's support and encouragement for this study as Chair of the Department of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma.
List of abbreviations
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- IFN
pegylated alfa-interferons
- NA
nucleoside analogue
- HBeAg
hepatitis B “e” antigen
- ALT
alanine aminotransferase
- HBV DNA
hepatitis B virus deoxyribonucleic acid
- DNA
deoxyribonucleic acid
- FDA
Food and Drug Administration (USA)
- HMG CoA
3-hydroxy-3-methylglutaryl-coenzyme A
- RNA
ribonucleic acid
- HBsAg
hepatitis B surface antigen
- SIM
simvastatin
- LMV
lamivudine
- ADV
adefovir
- TDF
tenofovir
- ETV
entecavir
- NIAID
National Institute of Allergy and Infectious Diseases (USA)
- HBcAg
hepatitis B core antigen
- EC50
fifty percent viral inhibition
- EC90
ninety percent viral inhibition
- CC50
50% cell cytotoxicity
- SI
selectivity index
- AST
aspartate aminotransferase
- HBx
“x” protein for hepatitis B virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- Abraldes JG, Albillos A, Bañares R, Turnes J, González R, García-Pagán JC, Bosch J. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651–1658. doi: 10.1053/j.gastro.2009.01.043. [DOI] [PubMed] [Google Scholar]
- Bader T, Fazili J, Madhoun M, Aston C, Hughes D, Rizvi S, Seres K, Hasan M. Fluvastatin inhibits hepatitis C replication in humans. Am. J. Gastroenterol. 2008;103:1383–1389. doi: 10.1111/j.1572-0241.2008.01876.x. [DOI] [PubMed] [Google Scholar]
- Bader T, Korba B. Drug-resistant and wild-type strains of HBV are inhibited equally by simvastatin. Hepatology. 2008;48(Suppl 1):A877. [Google Scholar]
- Cohen DE, Anania FA, Chalasani N. An assessment of statin safety by hepatologists. Am. J. Cardiol. 2006;97(Suppl 8A):77C–81C. doi: 10.1016/j.amjcard.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Delang L, Paeshuyse J, Vliegen I, Leyssen P, Obeid S, Durantel D, Zoulim F, Op de Beeck A, Neyts J. Statins potentiate the in vitro anti-hepatitis C virus activity of selective hepatitis C virus inhibitors and delay or prevent resistance development. Hepatology. 2009;50:6–16. doi: 10.1002/hep.22916. [DOI] [PubMed] [Google Scholar]
- Diminsky D, Schirmbeck R, Reimann J, Barenholz Y. Comparison between hepatitis B surface antigen (HBsAg) particles derived from mammalian cells (CHO) and yeast cells (Hansenula polymorpha): composition, structure and immunogenicity. Vaccine. 1997;15:637–647. doi: 10.1016/s0264-410x(96)00239-3. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Johnson ML, Hachem C, Morgana RO. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterol. 2009;136:1601–1608. doi: 10.1053/j.gastro.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germershausen JI, Hunt VM, Bostedor RG, Bailey PJ, Karkas JD, Alberts AW. Tissue selectivity of the cholesterol-lowering agents lovastatin, simvastatin and pravastatin in rats in vivo. Biochem. Biophys. Res. Commun. 1989;158:667–675. doi: 10.1016/0006-291x(89)92773-3. [DOI] [PubMed] [Google Scholar]
- Ha CE, Ha JS, Theriault AG, Bhagavan NV. Effects of statins on the secretion of human serum albumin in cultured HepG2 cells. J. Biomed. Sci. 2009;16:32. doi: 10.1186/1423-0127-16-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Kurokawa J, Nomura S, Kuga Y, Ohkura Y, Kajiyama G. Effect of fluvastatin sodium (XU62-320), a new inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, on the induction of low-density lipoprotein receptor in HepG2 cells. Biochim. Biophys. Acta. 1993;1167:223–225. doi: 10.1016/0005-2760(93)90166-7. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Abe K, Yamada M, Dansako H, Naka K, Kato N. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology. 2006;44:117–125. doi: 10.1002/hep.21232. [DOI] [PubMed] [Google Scholar]
- Jacobson IM. Combination therapy for chronic hepatitis B: ready for prime time? J. Hepatol. 2008;48:687–691. doi: 10.1016/j.jhep.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Johnson P. Epidemiology of hepatocellular cancer presented at the European Study for the Study of Liver monothematic conference on liver cancer. Prague: 2008. [December 30, 2008]. (at http://www.multiwebcast.com/easl/2008/prague.) [Google Scholar]
- Khurana V, Saluja A, Caldito G, Fort C, Schiff E. Statins are protective against hepatocellular cancer in patients with hepatitis C infection: half a million US veterans’ study. Gastroenterology. 2005;128(Suppl 2):A714. [Google Scholar]
- Korba BE. In vitro evaluation of combination therapies against hepatitis B virus replication. Antiviral Res. 1996;29:49–51. doi: 10.1016/0166-3542(95)00915-9. [DOI] [PubMed] [Google Scholar]
- Korba BE, Milman G. A cell culture assay for compounds which inhibit hepatitis B virus replication. Antiviral Res. 1991;15:217–228. doi: 10.1016/0166-3542(91)90068-3. [DOI] [PubMed] [Google Scholar]
- Korba BE, Gerin JL. Use of a standardized cell culture assay to assess activities of nucleoside analogs against hepatitis B virus replication. Antiviral Res. 1992;19:55–70. doi: 10.1016/0166-3542(92)90056-b. [DOI] [PubMed] [Google Scholar]
- Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, Chen Y, Heathcote EJ, Rasenack J, Bzowej N, Naoumov NV, Di Bisceglie AM, Zeuzem S, Moon YM, Goodman Z, Chao G, Constance BF, Brown NA. Telbivudine versus lamivudine in patients with chronic hepatitis B. N. Engl. J. Med. 2007;357:2576–2588. doi: 10.1056/NEJMoa066422. [DOI] [PubMed] [Google Scholar]
- Lin YL, Shiao MS, Mettling C, Chou CK. Cholesterol requirement of hepatitis B surface antigen (HBsAg) secretion. Virology. 2003;314:253–260. doi: 10.1016/s0042-6822(03)00403-3. [DOI] [PubMed] [Google Scholar]
- Lok A, McMahon B. Chronic hepatitis B. Hepatology. 2007;45:503–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- Mauro VF. Clinical pharmacokinetics and practical applications of simvastatin. Clin Pharmacokinet. 1993;24:195–202. doi: 10.2165/00003088-199324030-00002. [DOI] [PubMed] [Google Scholar]
- Pawlotsky JM, Dusheiko G, Hatzakis A, Lau D, Lau G, Liang TJ, Locarini S, Martin P, Richman DD, Zoulim F. Virologic monitoring of hepatitis B virus therapy in clinical trials and practice: recommendations for a standardized approach. Gastroenterology. 2008;134:405–415. doi: 10.1053/j.gastro.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentikainen PJ, Saraheimo M, Schwartz JI, Amin RD, Schwartz MS, Brunner-Ferber F, Rogers JD. Comparative pharmacokinetics of lovastatin, simvastatin and pravastatin in humans. J. Clin. Pharmacol. 1992;32:136–140. doi: 10.1002/j.1552-4604.1992.tb03818.x. [DOI] [PubMed] [Google Scholar]
- Potena L, Frascaroli G, Grigioni F, Lazzarotto T, Magnani G, Tomasi L, Coccolo F, Gabrielli L, Magelli C, Landini MP, Branzi A. Hydroxymethyl-glutaryl coenzyme. A reductase inhibition limits cytomegalovirus infection in human endothelial cells. Circulation. 2004;109:532–536. doi: 10.1161/01.CIR.0000109485.79183.81. [DOI] [PubMed] [Google Scholar]
- Reddy KR, Matelich MC, Ugarkar BG, Gómez-Galeno JE, DaRe J, Ollis K, Sun Z, Craigo W, Colby TJ, Fujitaki JM, Boyer SH, van Poelje PD, Erion MD. Pradefovir: a prodrug that targets adefovir to the liver for the treatment of hepatitis B. J. Med. Chem. 2008;51:666–676. doi: 10.1021/jm7012216. [DOI] [PubMed] [Google Scholar]
- Schönbeck U, Libby P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation. 2004;109:18–26. doi: 10.1161/01.CIR.0000129505.34151.23. [DOI] [PubMed] [Google Scholar]
- Ye J, Wang C, Sumpter R, Jr, Brown MS, Goldstein JL, Gale M., Jr Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc. Natl. Acad. Sci. U S A. 2003;100:15865–15870. doi: 10.1073/pnas.2237238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra C, Abraldes JG, Turnes J, Berzigotti A, Fernández M, Garcia-Pagán JC, Rodés J, Bosch J. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004;126:749–755. doi: 10.1053/j.gastro.2003.12.007. [DOI] [PubMed] [Google Scholar]