Abstract
Background
Disruptions in perception and cognition are characteristic of psychiatric conditions such as schizophrenia. Studies of pharmacological agents that alter perception and cognition in humans might provide a better understanding of the brain substrates of these complex processes. One way to study these states in rodents is with tests that require attention and visual perception for correct performance.
Methods
We examined the effects of two drugs that cause disruptions in perception and cognition in humans—the kappa-opioid receptor (KOR) agonist salvinorin A (salvA; 0.125–4.0 mg/kg) and the non-competitive NMDA receptor antagonist ketamine (0.63–20 mg/kg)—on behavior in rats using the 5-choice serial reaction time task (5CSRTT), a food-motivated test that quantifies attention. We also compared the binding profiles of salvA and ketamine at KORs and NMDA receptors.
Results
SalvA and ketamine produced the same pattern of disruptive effects in the 5CSRTT, characterized by increases in signs often associated with reduced motivation (omission errors) and deficits in processing (elevated latencies to respond correctly). Sessions in which rats were fed before testing suggest that reduced motivation produces a subtly different pattern of behavior. Pretreatment with the KOR antagonist JDTic (10 mg/kg) blocked all salvA effects and some ketamine effects. Binding and function studies revealed that ketamine is a full agonist at KORs, although not as potent or selective as salvA.
Conclusions
SalvA and ketamine have previously underappreciated similarities in their behavioral effects and pharmacological profiles. By implication, KORs might be involved in some of the cognitive abnormalities observed in psychiatric disorders such as schizophrenia.
Keywords: Kappa agonist, NMDA antagonist, Attention, Motivation, Behavior, Model, Rat
Introduction
Disruptions in perception and cognition are characteristic of psychiatric conditions such as schizophrenia and bipolar disorder (Chen and Faraone 2000; Cornblatt and Malhotra 2001; Clark et al. 2002). Pharmacological agents that alter perception and cognition in humans are often used to study the brain substrates of these complex processes. For example, it is often reported that intoxication with the non-competitive NMDA receptor antagonist phencyclidine (PCP) in humans produces virtually all of the symptoms of schizophrenia (Javitt and Zukin 1991; Jentsch and Roth 1999; Morris et al. 2005). Similarly, the non-competitive NMDA receptor antagonist ketamine has been used in humans to study dissociative states and schizophrenia (Lahti et al. 2001; Krystal et al. 2003, 2005). Ketamine also disrupts attention and working memory in humans (Parwani et al. 2005), and related NMDA receptor antagonists (i.e., PCP, MK-801) impair attention and impulse control in rodents (Amitai et al. 2007; Paine et al. 2007). Together these studies suggest that blockade of NMDA receptors is sufficient to produce hallmark signs of schizophrenia. However, recent work suggests that other mechanisms are also sufficient to produce some of these signs, including selective stimulation of kappa-opioid receptors (KORs). Salvinorin A (salvA), the active component of the plant Salvia divinorum, is becoming increasingly recognized for its psychotropic effects in humans (Vortherms and Roth 2006). This drug can induce various symptoms of psychiatric disorders, including dissociation, perceptual distortions, depersonalization, feelings of spatiotemporal dislocation, and anxiety (Valdes 1994; Siebert 1994; González et al. 2006). Considering that receptor screening assays indicate that salvA binds almost exclusively to KORs (Roth et al. 2002; Chavkin et al. 2004), studies of this substance have the potential to provide new insights on the neurobiology of perception and the mechanisms of psychiatric disorders.
Recent developments have piqued interest in ketamine and salvA. Ketamine produces rapid and long-lasting antidepressant effects in humans with treatment-resistant depression (Zarate et al. 2006), raising the possibility that NMDA antagonists might have utility in the treatment of mood disorders. SalvA has become a popular recreational drug that is marketed primarily to adolescents and young adults as a safe and legal hallucinogen (González et al. 2006). Interestingly, there are anecdotal reports that salvA can occasionally produce antidepressant effects in humans (Hanes 2001), although most studies in humans and laboratory animals suggest that salvA and other KOR agonists produce acute states of aversion, dysphoria, and anxiety (Pfeiffer et al. 1986; Wadenberg 2003; Zhang et al. 2005; Carlezon et al. 2006; González et al. 2006). The fact that both ketamine and salvA appear to cause disruptions in perception and cognition provides a rationale for studies in which their effects are directly compared, particularly since it seems conceivable that these effects are somehow related to subsequent effects on mood.
The present studies were designed to compare the effects of salvA and ketamine in the 5-choice serial reaction time task (5CSRTT) in rats. The 5CSRTT is a food-motivated attention test that is analogous to the continuous performance task used to study attention in humans (Rosvold et al. 1956; Robbins 2002). It is well-suited to characterize the effects of psychotropic drugs because it yields metrics that quantify attention, reaction time, motivation, and impulsivity (Robbins 2002; Paine et al. 2007, 2009). We used the KOR antagonist JDTic to evaluate the role of KORs in the effects of salvA and ketamine and, for comparison, we examined the effects of a non-pharmacological manipulation (pre-feeding immediately before testing) designed specifically to affect the food-motivated elements of the task. When we discovered that salvA and ketamine produced many similar effects on behavior in the 5CSRTT, we performed receptor-binding studies (Jensen and Roth 2008) to determine if there are any similarities in their pharmacologic and functional profiles.
Methods
Drugs
Dried S. divinorum leaves were purchased from Salvia Space (Lawrence, KS). SalvA was extracted, isolated, and purified as described previously (Carlezon et al. 2006). Spectroscopic analyses confirmed that the salvA obtained with these methods is chemically identical to that described in other reports (Roth et al. 2002). The samples used for testing were determined by high-pressure liquid chromatography (HPLC) to be >99% pure, and were dissolved in a vehicle of 75% dimethyl sulfoxide (DMSO)–25% distilled water. Ketamine (Sigma, St. Louis MO) was dissolved in physiological saline. JDTic (Research Triangle Park, NC; see Beardsley et al. 2005; Knoll et al. 2007) was dissolved in distilled water. Drugs were administered via intraperitoneal (IP) injection in a volume of 1 ml/kg at doses with behavioral effects in other tests (see below).
Animals
Nineteen male Sprague–Dawley rats (Charles River; 250–300 g at the start of the experiment) were housed in pairs in clear Plexiglas cages on a 12-h/12-h light–dark cycle (lights on at 0700 h). Rats were given 1 week to acclimate to the housing conditions; during this period, food (Purina Rat Chow) and water were freely available. Beginning 24 h prior to training and through the duration of the experiments, rats were food restricted such that they maintained 85% of their free-feeding weight. With the exception of Experiment 3 (below), rats were given a daily ration of chow (~17 g) immediately after training or testing sessions. Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy Press, 1996) and McLean Hospital policies.
Behavioral training
Testing was conducted in six 5CSRTT operant conditioning chambers housed in sound-attenuating ventilated cubicles (Med-Associates, St. Albans VT). Five equally spaced 2.5×2.5×2.2-cm apertures were set into a curved aluminum front wall; each aperture was fitted with a yellow LED stimulus light (6.4 mm in diameter) and an infrared detector (1.0 cm from the front of the aperture). The opposite wall was fitted with a food magazine connected to a 45-mg pellet dispenser; an infrared detector located horizontally across the magazine allowed for the detection of nosepokes into the magazine. The top of the magazine was fitted with a light (1.0 cm in diameter). The house light was located on the ceiling directly above the magazine. The sidewalls and ceiling were made of clear polycarbonate, and the floor was a stainless steel grid.
As described previously (Paine et al. 2007), rats were first trained to retrieve food pellets (45 mg, Bio-Serv #F0021, Frenchtown NJ) from the food magazine. Rats were then trained to detect the presentation of a brief stimulus light at one of five spatial locations. Initially, the duration of the stimulus light (discriminative stimulus; DS) was 30 s, the inter-trial interval (ITI) was 2 s, the limited hold (duration from the onset of stimulus light in which the rat was able to respond) was 30 s and the time-out was 2 s; these were gradually adjusted across training sessions to the final durations described below. Sessions started with the delivery of one food pellet; the first trial commenced when the rat retrieved the food pellet. Nosepoking in the magazine initiated a 5-s ITI during which the house light was turned on. At the end of the ITI, a 1.0-s light stimulus was presented at the rear of one of the five stimulus locations (apertures). Rats had up to 5 s (limited hold) to make a response. A response in this aperture was termed a correct response and resulted in the delivery of one food pellet and illumination of the magazine light; the magazine light remained illuminated for 5 s following food pellet delivery. Nosepokes in the remaining apertures during the limited hold were considered incorrect responses and resulted in a 5-s time-out during which the house light was extinguished. Similarly, failing to respond during the limited hold (i.e., an omission) resulted in a 5-s time-out. Responses occurring prior to stimulus presentation (i.e., during the ITI) were termed premature responses and resulted in a 5-s time-out. Responses occurring during the time-out period had no programmed consequences. Each session consisted of 90 trials or terminated after 30 min, whichever came first. Performance measures of primary interest were: % correct ((correct responses / [correct + incorrect + omitted responses]) × 100), accuracy ((correct responses / [correct + incorrect responses]) × 100), % omissions ([total omissions / number of trials] × 100), premature responses (responses during the ITI), correct response latency (the time from the stimulus onset to a correct response), and reward latency (the time from a correct response to the collection of the food pellet). Subjects were considered to have acquired the task when their behavior stabilized, as reflected by greater than 60% accuracy and fewer than 20% omissions for three consecutive days.
Behavioral testing
A total of four separate experiments were conducted. Experiments 1–3 involved all rats, whereas Experiment 4 involved only a subset from each treatment condition. Those rats not used in Experiment 4 were used in pilot studies not described here.
Experiment 1
Rats received either salvA (n=10) or ketamine (n=9) 10 min prior to testing. Drug doses (salvA, 0.125–4.0 mg/kg; ketamine, 0.625–20.0 mg/kg) were administered in an ascending order, and vehicle was administered last. Drug sessions were separated by at least three drug-free test sessions. Doses of salvA were based on Carlezon et al. (2006) and doses of ketamine were based on Imre et al. (2006).
Experiment 2
After at least three drug-free test sessions, the effects of salvA and ketamine on two variants of the standard 5CSRTT were assessed. Rats received the same drug as in Experiment 1. First, the rats were tested in a version of the 5CSRTT that requires increased attention, where the DS (stimulus light) was shortened from 1.0 to 0.5 s (Short DS). At least 3 days later, rats were tested in a version of the 5CSRTT that requires increased impulse control, where the ITI was increased from 5 to 9 s (Long ITI). Since our working hypothesis was that these versions of the task would be more difficult, we used doses of the drugs that were below those with detectable effects in the standard version of the 5CSRTT: rats were tested once with salvA (1.0 mg/kg) or ketamine (5.0 mg/kg), and once with their respective vehicles.
Experiment 3
After at least seven drug-free test days, we performed a brief environmental manipulation to determine if pre-feeding the rats—presumably reducing motivation for the Bio-Serv pellets used to reward correct performance in the 5CSRTT—would mimic any of the effects of salvA or ketamine. Rats were given their entire daily ration of chow (17 g) 30 min prior to testing in the standard version of the 5CSRTT, as in Experiment 1.
Experiment 4
In a subset of rats (n=8), the ability of a KOR antagonist to block the behavioral effects of salvA and ketamine was assessed. To confirm our initial findings (Experiment 1), rats were first re-tested with salvA (2.0 mg/kg, IP) and ketamine (20 mg/kg, IP) in two test sessions separated by at least 3 days. The order of salvA and ketamine administration was counterbalanced across rats. All rats were then administered JDTic (10 mg/kg, IP), a selective KOR antagonist known to have a slow onset (>24 h) and long duration (>3 weeks) of action (see Knoll and Carlezon 2010). This dose of JDTic has anxiolytic effects, but does not affect locomotor activity in open field tests (Knoll et al. 2007). At 24 and 96 h after JDTic, rats received salvA (2.0 mg/kg, IP) or ketamine (20 mg/kg, IP); the order of drug administration was counterbalanced across rats. The effects of JDTic alone were evaluated in a test conducted 72 h after administration.
Statistical analyses
Since a within-subjects design was used and each rat received multiple treatments, data were analyzed using one-way (Treatment) analyses of variance (ANOVAs) with repeated measures (Experiments 1 and 4) or t tests for correlated samples (Experiments 2 and 3). Significant effects in the ANOVAs were further analyzed using post hoc Fisher’s protected t tests.
In vitro binding studies
Radioligand-binding assays at human-cloned KOR and rat brain σ and NMDA receptors were performed by using the resources of the National Institute of Mental Health Psychoactive Drug Screening Program (NIMH-PDSP). Specifically, KOR radioligand-binding assays were performed using cloned human KOR (hKOR) and [3H] bremazocine as the radioligand. The binding affinities for the σ receptor were determined using rat whole brain homogenates with a protocol adapted from Kovács and Larson (1998) and [3H] pentazocine as the radioligand. Finally, the affinities of the test compounds for the NMDA receptors were obtained using rat whole brain homogenates and [3H] MK-801 as the radioligand. Detailed on-line protocols are available for all assays at the NIMH-PDSP website (http://pdsp.med.unc.edu/UNC-CH%20Protocol%20Book.pdf). Initial screening assays were performed in quadruplicate using a 10-µM test compound, and the percent inhibition of specific binding was determined. Where 10 µM of the test compound inhibited >50% of specific binding, Ki determinations were performed by using six concentrations of unlabeled ligand spanning a 10,000-fold dose range. Ki values were calculated by using GRAPHPAD PRISM and represent the mean ± SEM of quadruplicate determinations. The potencies and efficacies of salvA and ketamine on hKOR were determined by their abilities to regulate [35S] GTPγS binding to membranes of CHO-hKOR cells as previously detailed (Yan et al. 2009).
Results
Behavioral testing
Experiment 1
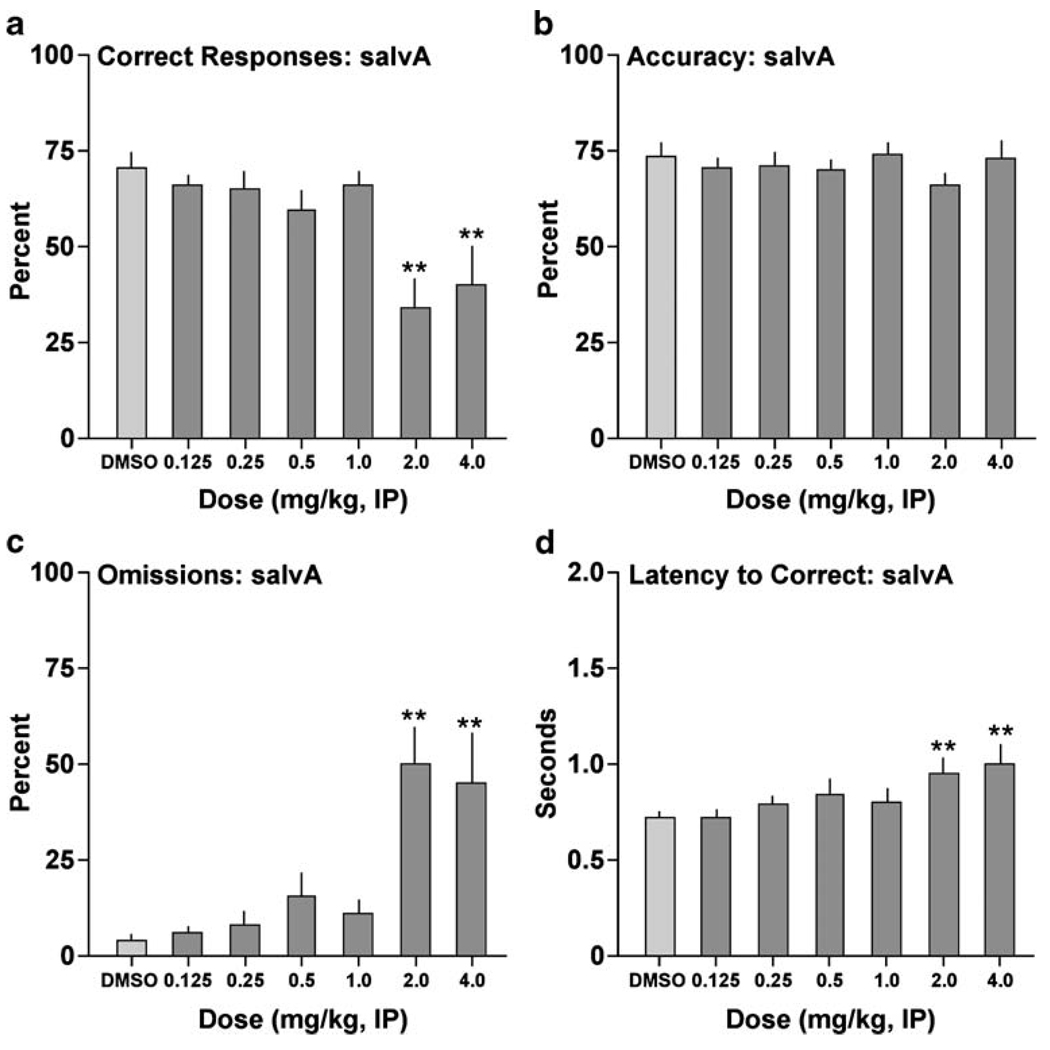
SalvA affected correct responding (F[6,54]=6.99, P<0.01) (Fig. 1a): post hoc analyses revealed that the drug reduced the percentage of correct responses at 2.0 mg/kg (P<0.01) and at 4.0 mg/kg (P<0.01). This effect was not associated with changes in accuracy at any of the doses tested (F[6,54] = 1.22, not significant [n.s.]) (Fig. 1b). Rather, it was associated with effects on omissions (F[6,54]=8.08, P<0.01) (Fig. 1c): salvA produced significant increases in the percentage of trials during which the rats failed to respond at doses of 2.0 mg/kg (P<0.01) and4.0 mg/kg (P<0.01). SalvA also affected correct response latency (F[6,54]=4.48, P<0.01) (Fig. 1d): the drug increased latencies to respond correctly at 2.0 mg/kg (P<0.01) and 4.0 mg/kg (P<0.01). SalvA had no effects on premature responding (F[6,54]=0.83, n.s.; not shown) or the latency to retrieve the reward (F[6,54] = 1.90, n.s.; not shown).
Fig. 1.
Effects of salvA on performance in the 5CSRTT. Rats (N=10) were given IP injections of the drug 10 min before testing. **P<0.01 compared to vehicle (75% DMSO), Fisher’s protected t tests
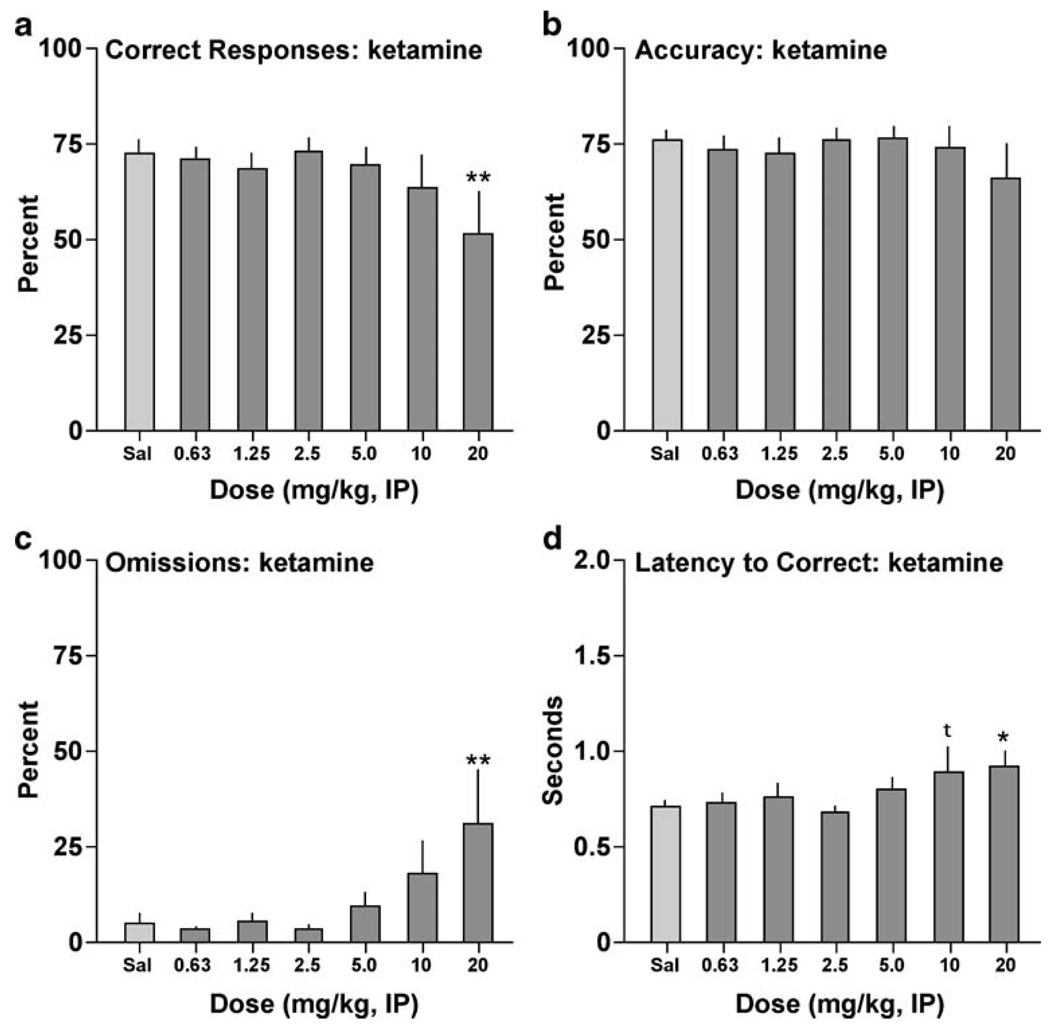
Ketamine produced a similar profile. The drug affected correct responding (F[6,48]=2.43, P<0.05) (Fig. 2a), reducing the percentage of correct responses at 20 mg/kg (P<0.01). Ketamine did not affect accuracy at any of the doses tested (F[6,48]=0.81, n.s.) (Fig. 2b), but it affected omissions (F[6,48]=3.20, P<0.01) (Fig. 2c), producing significant increases in the percentage of trials during which the rats failed to respond at 20 mg/kg (P<0.01). It also affected correct response latency (F[6,48]=2.47, P<0.05) (Fig. 2d), increasing latencies to respond correctly at 20 mg/kg (P<0.05), with a trend at 10 mg/kg (P<0.10). Ketamine had no effects on premature responding (F[6,48] = 0.80, n.s.; not shown) or the latency to retrieve the reward (F[6,48]=0.41, n.s.; not shown).
Fig. 2.
Effects of ketamine on performance in the 5CSRTT. Rats (N=9) were given IP injections of the drug 10 min before testing. *P<0.05, **P<0.01, tP<0.10 compared to vehicle (0.9% saline), Fisher’s protected t tests
Experiment 2
Neither of the manipulations intended to make the 5CSRTT more challenging made performance deficits emerge after treatment with sub-effective doses of the drugs. Administration of salvA (1.0 mg/kg) or ketamine (5.0 mg/kg) did not degrade performance in the short DS (Table 1; all Ps>0.10) or long ITI (Table 2; all Ps>0.10) versions of the task. There was a small but statistically significant effect of ketamine on latency to collect the reward in the short DS task (P<0.05) that is consistent with improved performance on this measure.
Table 1.
Effects of salvA and ketamine on short DS version of the 5CSRTT
| Correct (%) | Accuracy (%) | Omissions (%) | Premature responses | Correct latency (s) | Reward latency (s) | |
|---|---|---|---|---|---|---|
| 75% DMSO | 58.9±3.1 | 63.9±2.5 | 7.7±3.5 | 18.0±3.7 | 0.67±0.04 | 1.43±0.17 |
| SalvA | 64.0±2.4 | 66.6±2.0 | 4.1±1.3 | 14.0±1.9 | 0.73±0.02 | 1.51±0.15 |
| Saline | 61.0±3.1 | 64.4±2.8 | 5.4±2.1 | 27.2±7.7 | 0.62±0.03 | 1.43±0.04 |
| Ketamine | 62.4±4.0 | 66.7±3.2 | 6.9±2.3 | 23.1±5.5 | 0.66±0.04 | 1.34±0.03* |
SalvA (1.0 mg/kg), ketamine (5.0 mg/kg), or vehicle was administered 10 min prior to testing on the short DS version of the 5CSRTT. The discriminative stimulus duration was reduced from the standard 1.0 to 0.5 s for this version of the task.
P<0.05 compared to respective vehicle
Table 2.
Effects of salvA and ketamine on long ITI version of the 5CSRTT
| Correct (%) | Accuracy (%) | Omissions (%) | Premature responses | Correct latency (s) | Reward latency (s) | |
|---|---|---|---|---|---|---|
| 75% DMSO | 68.8±3.3 | 72.0±3.0 | 4.6±1.1 | 33.8±6.0 | 0.65±0.04 | 1.36±0.08 |
| SalvA | 65.0±4.2 | 74.5±3.4 | 11.7±5.7 | 36.0±7.5 | 0.77±0.08 | 1.51±0.11 |
| Saline | 67.2±3.4 | 74.9±2.3 | 10.2±3.9 | 55.6±13.5 | 0.68±0.05 | 1.47±0.06 |
| Ketamine | 69.2±2.5 | 71.8±2.2 | 3.8±1.0 | 54.9±10.3 | 0.68±0.04 | 1.38±0.04 |
SalvA (1.0 mg/kg), ketamine (5.0 mg/kg), or vehicle was administered 10 min prior to testing on the long ITI version of the 5CSRTT. The ITI was increased from the standard 5.0 to 9.0 s for this version of the task.
Experiment 3
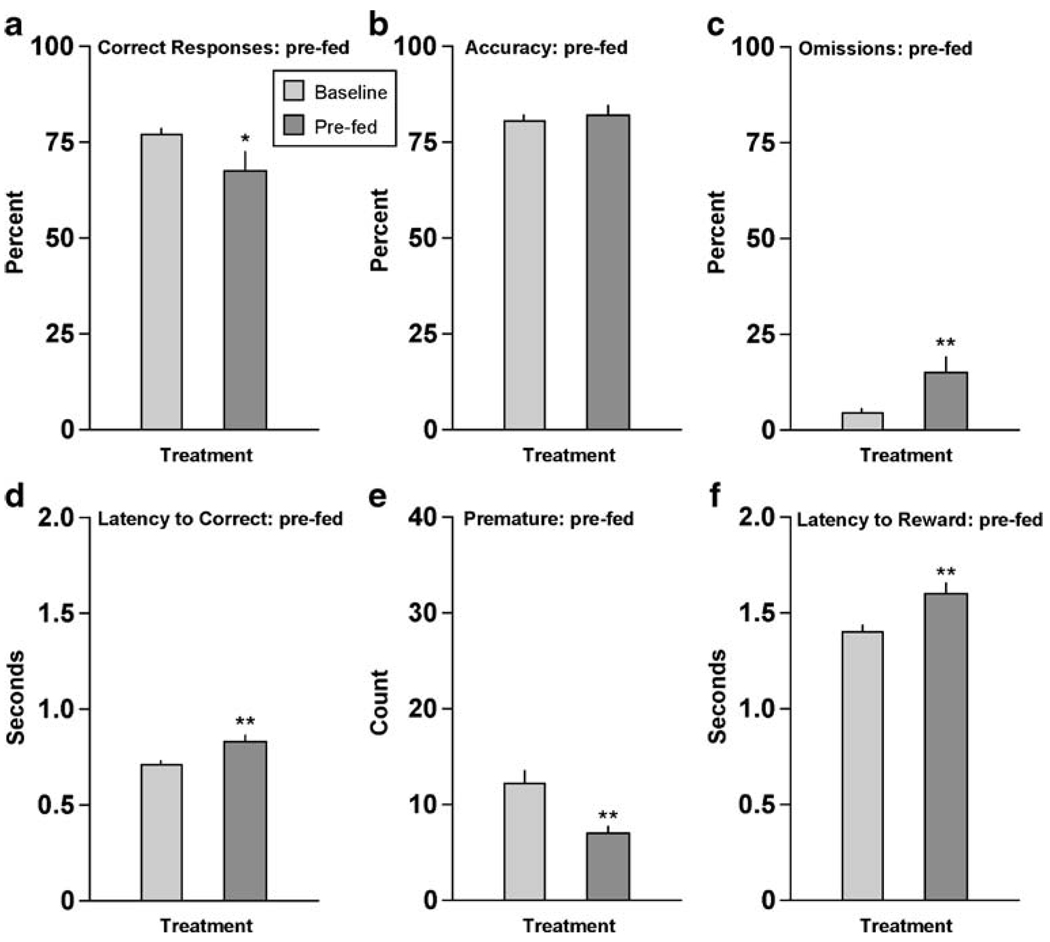
Providing the rats with their normal daily ration of food 30 min before testing produced some of the same effects as active doses of salvA and ketamine in the standard (Experiment 1) version of the 5CSRTT. When compared to baseline (mean performance over the preceding 3 days of testing without any treatments), pre-feeding reduced the percentage of correct responding (t[18] =2.15, P<0.05) (Fig. 3a). As was the case with the drugs, pre-feeding had no effect on accuracy (t[18]=0.62, n.s) (Fig. 3b), but it increased omissions (t[18]=2.97, P<0.01) (Fig. 3c) and latencies to respond correctly (t[18]=4.11, P<0.01) (Fig. 3d). Unlike the drugs, it also reduced premature responding (t[18]=3.21, P<0.01) (Fig. 3e) and increased latencies to collect the food reward (t[18]=7.24, P<0.01) (Fig. 3f).
Fig. 3.
Effects of pre-feeding on performance in the 5CSRTT. Rats (N=19) were given their daily ration of food (~17 g) 30 min before testing. *P<0.05, **P<0.01 compared to baseline (average of the previous 3-day performance), Fisher’s protected t tests
Experiment 4
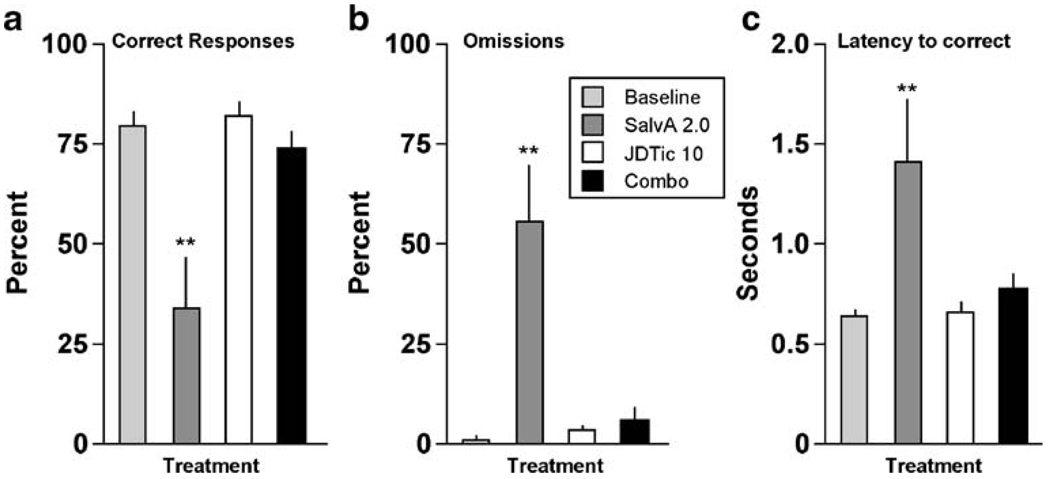
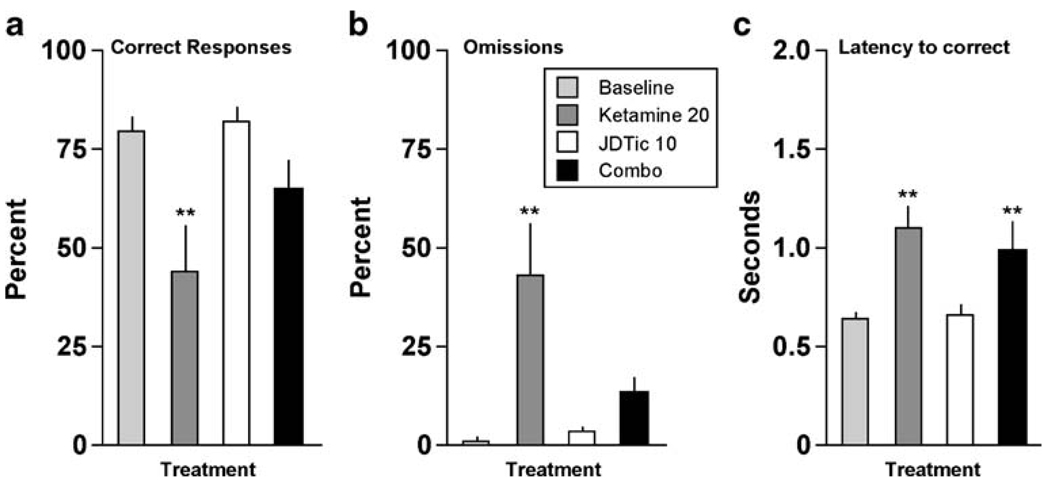
Pretreatment with the selective KOR antagonist JDTic (10 mg/kg, IP, >24 h before testing) blocked all of the effects of salvA (2.0 mg/kg). It blocked the effect on correct responding (F[3,21] = 14.9, P<0.01) (Fig. 4a): the percentage of correct responses was reduced only in the salvA alone group (P<0.01). Similarly, it blocked effects on omissions (F[3,21] = 14.5, P<0.01) (Fig. 4b), with percent omissions elevated only in the salvA alone group (P<0.01), and on latencies to respond correctly (F[3,21]=5.25, P<0.01) (Fig. 4c), with latencies elevated only in the salvA alone group (P<0.01). JDTic alone did not affect any performance measure, nor were there any interactions between JDTic and salvA on any other measures, including accuracy, premature responding, latencies to collect the food reward, or head entries (not shown).
Fig. 4.
Effects of JDTic pre-treatment on the ability of salvA to affect performance in the 5CSRTT (N=8). **P<0.01 compared to baseline (average of the previous 3-day performance), Fisher’s protected t tests
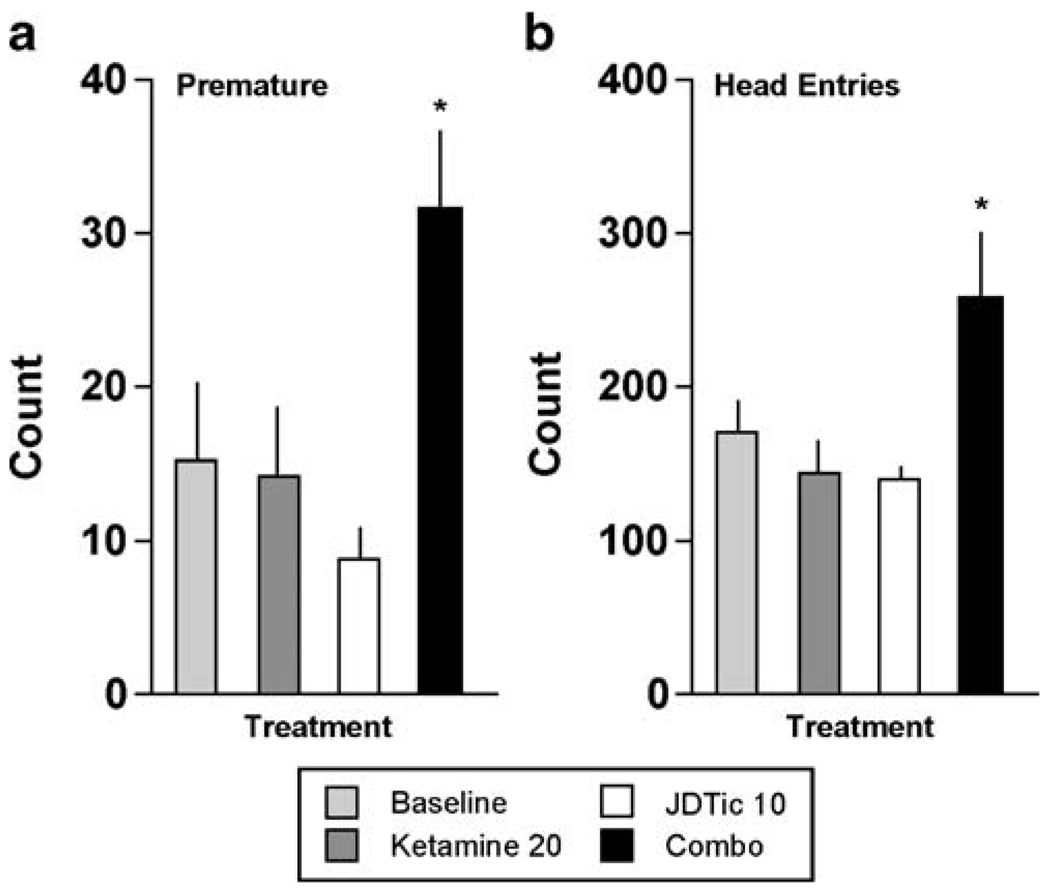
Pretreatment with JDTic also blocked some effects of ketamine (20 mg/kg). It blocked the effect on correct responding (F[3,21]=7.82, P<0.01) (Fig. 5a): the percentage of correct responses was reduced only in the ketamine alone group (P<0.01). Similarly, it blocked effects on omissions (F[3,21]=8.33, P<0.01) (Fig.5b): omissions were elevated only in the ketamine alone group (P<0.01). However, JDTic did not block the effects of ketamine on latencies to respond correctly (F[3,21]=8.46, P<0.01) (Fig. 5c): there were equivalent increases in latencies to respond correctly after ketamine in both the absence (P<0.01) and presence of JDTic (P<0.01). Interestingly, there was evidence of synergistic effects between ketamine and JDTic, as reflected by the emergence of behavioral patterns not caused by either drug alone. An effect on premature responding emerged (F[3,21]=5.38, P<0.01) (Fig. 6a): treatment with ketamine in the presence of JDTic caused a significant increase in premature responding (P<0.01). An effect on the number of head entries into the food magazine—a measure not affected by any drug treatment—also emerged (F[3,21]=4.63, P<0.05) (Fig. 6b): treatment with ketamine in the presence of JDTic caused a significant increase in head entries (P<0.05). There were no interactions between JDTic and ketamine on accuracy or latencies to collect the food reward.
Fig. 5.
Effects of JDTic pre-treatment on the ability of ketamine to affect performance in the 5CSRTT (N=8). **P<0.01 compared to baseline (average of the previous 3-day performance), Fisher’s protected t tests
Fig. 6.
Synergistic effects of JDTic and ketamine in the 5CSRTT (N=8). *P<0.05 compared to baseline (average of the previous 3-day performance), Fisher’s protected t tests
In vitro binding studies
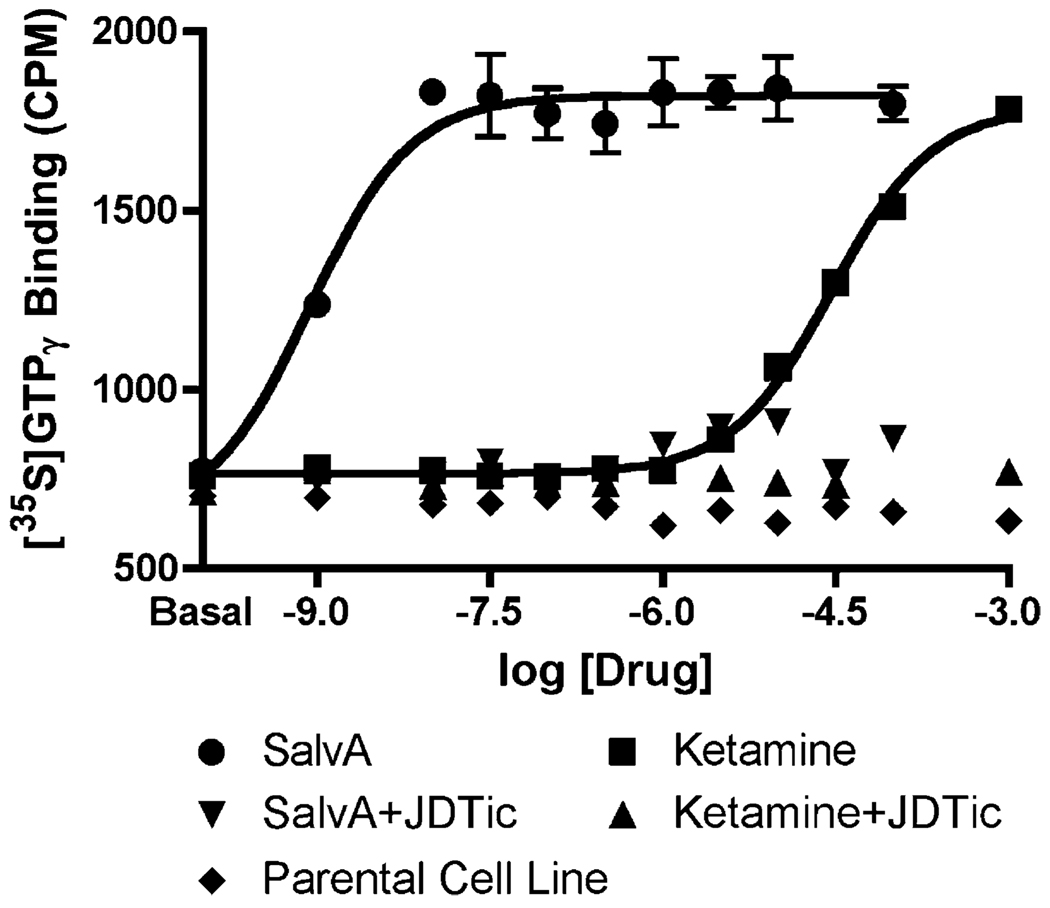
SalvA bound with high affinity to KORs and potently stimulated [35S] GTPγS, while having negligible affinity for σ-opioid and NMDA receptors (Table 3). These findings confirm previous reports describing the potency and selectivity of salvA (Roth et al. 2002; Chavkin et al. 2004). Unexpectedly, ketamine also bound to KORs, though with a substantially lower affinity and potency than at σ-opioid and NMDA receptors. Both salvA and ketamine displayed full agonism at KORs, and the effects of each were completely blocked by 10 nM JDTic (Fig. 7).
Table 3.
Affinities (Ki) and potencies (EC50) of salvA and ketamine
| Human KOR, [3H] bremazocine | Rat σ, [3H] pentazocine | Rat NMDA, [3H] MK-801 | ||
|---|---|---|---|---|
| Ki, nM | EC50, nMa | Ki, nM | Ki, nM | |
| SalvA | 0.44 | 1.5 | -b | -b |
| Ketamine | 25,000 | 29,000 | 5.2 | 890 |
aEC50 values in activating the hKOR to enhance [35 S] GTPγS binding. Ketamine and salvA produced similar maximal response.
SalvA (10 µM) displaced <50% [3 H] radioligand binding (Roth et al. 2002).
Fig. 7.
Functional assay demonstrating that both salvA and ketamine have full agonist effects at KORs, as reflected by regulation of [35S] GTPγS binding to membranes of CHO-hKOR cells. The KOR agonist effects of both drugs were completely blocked by JDTic (10 nM)
Discussion
SalvA and ketamine produced similar effects in the 5CSRTT. Both drugs disrupted performance, as reflected by decreases in the percentage of correct responses. Neither drug affected accuracy, which provides a measure of how the rats perform on trials in which they make a response. Rather, both drugs increased the percentage of trials in which the rats failed to respond (omission errors). This pattern indicates that the decreases in correct responding were caused by “omission errors” (failure to make a response) rather than “commission errors” (responding at an incorrect aperture). Increases in omission errors were accompanied by increases in the latency to make a correct response, an effect that might reflect reduced speed of processing or decision-making (Robbins 2002; Paine et al. 2007). Neither drug affected premature responding or the latency to collect the food reward following correct responses, suggesting the absence of non-specific rate-reducing effects. The pattern of behaviors emerged at a similar rate: the lowest doses of salvA and ketamine that reduced correct responding produced increases in omissions and latencies to make a correct response without significantly affecting the other metrics. Previous work (Paine et al. 2007) demonstrates that the metrics used in this study can vary independently. For example, the NMDA antagonist MK-801 decreases correct responding by increasing omissions. However, it also reduces accuracy and increases premature responding at the same (or even lower) doses, perhaps reflecting the non-specific stimulant effects of the drug. The tricyclic antidepressant desipramine increases omissions and latencies to respond correctly, but it also reduces premature responding and increases latencies to collect the food reward at the same doses, perhaps reflecting non-specific rate-reducing effects of the drug. Of the psychotropic drugs we have tested in the 5CSRTT, only one drug produces an identical pattern of effects as seen here with salvA and ketamine: the selective KOR agonist U69,593, which shares discriminative stimulus properties with salvA (Willmore-Fordham et al. 2007; Baker et al. 2009). Considering the anecdotal similarities between some of the effects of salvA and ketamine in humans (Lahti et al. 2001; Krystal et al. 2003; González et al. 2006), our data raise the possibility that the specific pattern of behaviors seen in the present study—disrupted attentional performance characterized by intact accuracy but increased omissions and decreased processing speed—is a unique behavioral signature of drugs with dissociative effects.
We previously reported that U69,593 disrupts performance in the 5CSRTT (Paine et al. 2007). We speculated that this effect might be related to a reduced motivation for the 45-mg food pellets that reward correct performance in the 5CSRTT. Indeed, KOR agonists decrease the rewarding effects of lateral hypothalamic brain stimulation (Todtenkopf et al. 2004), cocaine (Crawford et al. 1995; Shippenberg et al. 1996; Tomasiewicz et al. 2008), and sexual behavior (Leyton and Stewart 1992). We hypothesized that one way to reduce motivation for the food reward without using a drug treatment would be to pre-feed the rats. In Experiment 3, we provided the rats with their daily ration of food (~17 g) 30 min before testing. Rats maintained at 85% body weight typically eat this amount of food within 5 min. This manipulation produced some of the same effects as salvA and ketamine: it increased omissions and latencies to respond correctly without affecting accuracy. However, pre-feeding also reduced premature responses and increased latency to collect the reward, which were not affected by salvA or ketamine. This pattern of results suggests that the effects of salvA and ketamine can be distinguished from pure reductions in motivation. Our data cannot rule out the possibility that progressively higher doses of salvA and ketamine would eventually cause similar effects on premature responses and latencies to collect the reward. It is important to note that the rate of omissions was three to fourfold greater after treatment with active doses of salvA and ketamine than after pre-feeding (compare Fig. 3c with Figs. 4b and 5b). This suggests that the doses of salvA and ketamine were adequate to cause reductions in premature responding and increases in latencies to collect the reward if these outcomes were inextricably linked to increases in omissions.
The KOR selective antagonist JDTic (10 mg/kg) was administered once, 48 h before testing, because this drug is known to have a slow onset (>24 h) and long duration (>3 weeks) of action (Thomas et al. 2003; Knoll et al. 2007; Knoll and Carlezon 2010). We have shown previously that this dose has anxiolytic effects in the elevated plus maze, but no effect on locomotor activity in an open field (Knoll et al. 2007). Pretreatment with JDTic blocked all of the effects of salvA in the 5CSRTT: it prevented the reductions in correct responding and the increases in omissions and latencies to make a correct response. These data suggest that the ability of salvA to cause these effects is entirely dependent on actions at KORs. Surprisingly, JDTic also blocked some effects of ketamine: it prevented reductions in correct responding and increases in omission errors, although it failed to affect latencies to make a correct response. One explanation for this effect is that a subset of salvA and ketamine effects (reductions in correct responding and increased omissions) is due to stimulation of KORs. Another possibility is that salvA and ketamine cause similar effects through distinct mechanisms, and that JDTic blocks salvA effects directly, but ketamine effects indirectly. In support of this possibility, JDTic and ketamine had synergistic effects on some measures, making behaviors emerge that were not seen with either drug alone. In the presence of JDTic, ketamine increased premature responding and head entries into the food magazine, a measure not affected in our studies by any other drug treatment. Common effects on brain dopamine (DA) may contribute to this effect: as one example, extracellular concentrations of DA in the nucleus accumbens (NAc) are increased by both NMDA antagonists (Imperato et al. 1990; Zhang et al. 1992) and KOR antagonists (Maisonneuve et al. 1994). DA agonists can increase impulsive behavior (reflected by premature responses) (Paine and Olmstead 2004) and stereotyped behavior (reflected by persistent head entries) (Fibiger et al. 1973). No such synergistic effects were seen with JDTic and salvA. The unique pattern of behavior caused by the interaction of JDTic and ketamine again highlights the fact that the behavioral outcomes under study in the 5CSRTT can vary independently.
The ability of JDTic to block at least some effects of ketamine was unexpected, and raised the possibility that ketamine has actions at KORs. High-throughput in vitro screening at the NIMH-PDSP indicates that ketamine binds to human KORs, albeit much less potently than salvA. The salvA data confirm previous reports indicating that this substance is highly selective for KORs (Roth et al. 2002; Chavkin et al. 2004). We report here that salvA has no affinity for σ-opioid and NMDA receptors; additionally, pilot data indicate that it has no affinity for rat DA D2 receptors or the long form of human D2 receptors (B. L. Roth, unpublished observations). Functional assays conducted in parallel indicate that ketamine is a full agonist at KORs, as efficacious as salvA, and that these effects are completely blocked at a concentration of JDTic that also blocks the agonist effects of salvA. The reasons for the smaller difference in potency between salvA and ketamine in vivo are unknown, but may be due to uncharacterized differences in bioavailability and metabolism.
Sub-threshold doses of salvA and ketamine that did not have detectable effects in the standard version of the 5CSRTT also did not degrade performance in versions of the task that were made more difficult by shortening the duration of the light stimulus (short DS) or lengthening the wait between light stimuli (long ITI). For these tests, we administered 1.0 mg/kg salvA because there was a clear distinction between doses with and without effects on the 5CSRTT, whereas we administered 5.0 mg/kg ketamine because there was a detectable (though non-significant) trend for the drug to increase latencies at 10 mg/kg. There was a small but statistically significant effect of 5.0 mg/kg ketamine on latency to collect the reward that is counterintuitive: the drug shortened latencies, reflecting an improvement in performance. One potential explanation for this effect is that ketamine might have motor-activating effects at this dose that are not apparent at higher doses. Indeed, higher doses of ketamine (~80 mg/kg) are often used together with xylazine to produce anesthesia in rats (Todtenkopf et al. 2004; Davis 2008). Each of the modified versions seemed more difficult than the standard versions, considering the differences in baseline performance metrics. For example, in the standard version of the 5CSRTT used in Experiments 1, 3, and 4, baseline correct responding was ~75–80%, whereas it ranged from ~60 to 70% in the short DS and long ITI versions. The fact that this increase in task difficulty did not cause behavioral effects to emerge at sub-threshold doses of salvA or ketamine suggests that certain levels of receptor occupancy are required in order to produce the drug effects seen in the standard version of the 5CSRTT.
The similarities between salvA and ketamine in the 5CSRTT are somewhat surprising when considering some of the other behavioral effects of these drugs in laboratory animals. SalvA and other KOR agonists produce acute depressive-like effects, including increased immobility behavior in the forced swim test, reduced sensitivity to rewarding brain stimulation, and reduced sensitivity to the rewarding effects of drugs of abuse and sexual behavior (Leyton and Stewart 1992; Mague et al. 2003; Todtenkopf et al. 2004; Carlezon et al. 2006, Shippenberg et al. 1996; Tomasiewicz et al. 2008). In the case of salvA, doses of the drug that cause these effects on motivation and cognition also reduce extracellular concentrations of DA in the NAc (Carlezon et al. 2006), an effect often associated with aversion and dysphoria (Carlezon and Thomas 2009). In contrast, ketamine produces acute antidepressant-like effects (Maeng et al. 2007), stimulation of locomotor activity (Hetzler and Wautlet 1985), and increased sensitivity to rewarding brain stimulation (Herberg and Rose 1989) over a range of doses comparable to those used in the present study. It also increases DA efflux in the NAc (Hancock and Stamford 1999), an effect often associated with reward and pleasure (Wise 2008). There is evidence in rats that ketamine and other non-competitive NMDA receptor antagonists (e.g., MK-801, phencyclidine) substitute for the KOR agonist U50,488 in drug discrimination tests (Mori et al. 2006), suggesting similar discriminative stimulus properties in this species. It is conceivable that the drug discrimination test and the 5CSRTT are both most sensitive to the dissociative effects of these drugs in rats. Our data suggest that overlap in the behavioral effects of salvA and ketamine is explained, at least in part, by common actions at KOR receptors.
Ketamine produces rapid and long-lasting antidepressant effects in humans (Zarate et al. 2006). The relationship between the antidepressant effects and the dissociative effects of ketamine (Lahti et al. 2001) is currently unclear. KOR agonists produce acute depressive effects (dysphoria, anxiety) in addition to dissociative effects in humans (Pfeiffer et al. 1986; Wadenberg 2003; González et al. 2006). However, emerging evidence from studies in laboratory animals suggests that prior exposure to KOR agonists can produce long-term effects that are opposite to the acute effects (McLaughlin et al. 2006; Potter et al. 2009), perhaps due to induction of persistent alterations in KOR-linked signaling pathways (see Knoll and Carlezon 2010). Such effects may help to explain anecdotal reports of antidepressant effects in humans (Hanes 2001). Regardless, additional studies of salvA and ketamine on complex behavior may provide deeper insight into the biological basis of mood states and disorders characterized by abnormalities of attention, perception, and cognition.
Acknowledgment
This study is supported by the National Institute of Mental Health (MH063266 to WAC, and RO1DA017204 to BLR).
Contributor Information
Christina L. Nemeth, Behavioral Genetics Laboratory, Department of Psychiatry, Harvard Medical School, McLean Hospital, Belmont, MA 02478, USA
Tracie A. Paine, Behavioral Genetics Laboratory, Department of Psychiatry, Harvard Medical School, McLean Hospital, Belmont, MA 02478, USA
Joseph E. Rittiner, Department of Pharmacology and NIMH Psychoactive Drug Screening Program, University of North Carolina-Chapel Hill School of Medicine, Chapel Hill, NC 27599, USA
Cécile Béguin, Behavioral Genetics Laboratory, Department of Psychiatry, Harvard Medical School, McLean Hospital, Belmont, MA 02478, USA.
F. Ivy Carroll, Research Triangle Institute, Organic and Medicinal Chemistry, Research Triangle Park, NC 27709, USA.
Bryan L. Roth, Department of Pharmacology and NIMH Psychoactive Drug Screening Program, University of North Carolina-Chapel Hill School of Medicine, Chapel Hill, NC 27599, USA
Bruce M. Cohen, Behavioral Genetics Laboratory, Department of Psychiatry, Harvard Medical School, McLean Hospital, Belmont, MA 02478, USA
William A. Carlezon, Jr., Behavioral Genetics Laboratory, Department of Psychiatry, Harvard Medical School, McLean Hospital, Belmont, MA 02478, USA Department of Psychiatry, McLean Hospital, MRC 217, 115 Mill Street, Belmont, MA 02478, USA, bcarlezon@mclean.harvard.edu.
References
- Amitai N, Semenova S, Markou A. Cognitive disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications. Psychopharmacol. 2007;193:521–537. doi: 10.1007/s00213-007-0808-x. [DOI] [PubMed] [Google Scholar]
- Baker LE, Panos JJ, Killinger BA, Peet MM, Bell LM, Haliw LA, Walker SL. Comparison of the discriminative stimulus effects of salvinorin A and its derivatives to U69,593 and U50,488 in rats. Psychopharmacol. 2009;203:203–211. doi: 10.1007/s00213-008-1458-3. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacol. 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas M. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacol. 2009;56 Suppl 1:122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Béguin C, DiNieri JA, Baumann MH, Richards ME, Todtenkopf MS, Rothman RB, Ma Z, Y-W LD, Cohen BM. Depressive-like effects of the κ-opioid receptor agonist Salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ, Roth BL. Salvinorin A, an active component of the hallucinogenic sage Salvia divinorum is a highly efficacious kappa-opioid receptor agonist: structural and functional considerations. J Pharmacol Exp Ther. 2004;308:1197–1203. doi: 10.1124/jpet.103.059394. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Faraone SV. Sustained attention deficits as markers of genetic susceptibility to schizophrenia. Am J Med Genet. 2000;97:52–57. doi: 10.1002/(sici)1096-8628(200021)97:1<52::aid-ajmg7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Clark L, Iversen S, Goodwin G. Sustained attention deficit in bipolar disorder. Br J Psychiatry. 2002;180:133–139. doi: 10.1192/bjp.180.4.313. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Malhotra AK. Impaired attention as an endophenotype for molecular genetic studies of schizophrenia. Am J Med Genet. 2001;105:11–15. [PubMed] [Google Scholar]
- Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP. The effects of the kappa agonist U-50,488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreactivity. Psychopharmacol. 1995;120:392–399. doi: 10.1007/BF02245810. [DOI] [PubMed] [Google Scholar]
- Davis JA. Mouse and rat anesthesia and analgesia. Curr Protoc Neurosci. 2008;42:A.4B.1–A.4B.21. doi: 10.1002/0471142301.nsa04bs42. [DOI] [PubMed] [Google Scholar]
- Fibiger HC, Fibiger HP, Zis AP. Attenuation of amphetamine-induced motor stimulation and stereotypy by 6-hydroxydopamine in the rat. Br J Pharmacol. 1973;47:683–692. doi: 10.1111/j.1476-5381.1973.tb08194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González D, Riba J, Bouso JC, Gómez-Jarabo G, Babanoj MJ. Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug Alcohol Depend. 2006;85:157–162. doi: 10.1016/j.drugalcdep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Hancock PJ, Stamford JA. Stereospecific effects of ketamine on dopamine efflux and uptake in the rat nucleus accumbens. Br J Anaesth. 1999;82:603–608. doi: 10.1093/bja/82.4.603. [DOI] [PubMed] [Google Scholar]
- Hanes K. Antidepressant effects of the herb Salvia divinorum:a case report. J Clin Psychopharmacol. 2001;21:634–635. doi: 10.1097/00004714-200112000-00025. [DOI] [PubMed] [Google Scholar]
- Herberg LJ, Rose IC. The effect of MK-801 and other antagonists of NMDA-type glutamate receptors on brain-stimulation reward. Psychopharmacol. 1989;99:87–90. doi: 10.1007/BF00634458. [DOI] [PubMed] [Google Scholar]
- Hetzler BE, Wautlet BS. Ketamine-induced locomotion in rats in an open-field. Pharmacol Biochem Behav. 1985:653–655. doi: 10.1016/0091-3057(85)90291-6. [DOI] [PubMed] [Google Scholar]
- Imperato A, Scrocco MG, Bacchi S, Angelucci L. NMDA receptors and in vivo dopamine release in the nucleus accumbens and caudatus. Eur J Pharmacol. 1990;187:555–556. doi: 10.1016/0014-2999(90)90387-l. [DOI] [PubMed] [Google Scholar]
- Imre G, Fokkema DS, Den Boer JA, Ter Horst GJ. Dose response characteristics of ketamine effect on locomotion, cognitive function and central neuronal activity. Brain Res Bull. 2006;69:338–345. doi: 10.1016/j.brainresbull.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jensen NH, Roth BL. Massively parallel screening of the receptorome. Comb Chem High Throughput Screen. 2008;11:420–426. doi: 10.2174/138620708784911483. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacol. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA WA., Jr Dynorphin, stress and depression. Brain Res. 2010;1314C:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of κ-opioid receptor antagonists in behavioral models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Kovács KJ, Larson AA. Up-regulation of [3H]DTG but not [3H](+)-pentazocine labeled sigma sites in mouse spinal cord by chronic morphine treatment. Eur J Pharmacol. 1998;350:47–52. doi: 10.1016/s0014-2999(98)00220-9. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacol. 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Abi-Saab W, Perry E, D’Souza DC, Liu N, Gueorguieva R, McDougall L, Hunsberger T, Belger A, Levine L, Breier A. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacol. 2005;179:303–309. doi: 10.1007/s00213-004-1982-8. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacol. 2001;25:455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Leyton M, Stewart J. The stimulation of central kappa opioid receptors decreases male sexual behavior and locomotor activity. Brain Res. 1992;594:56–74. doi: 10.1016/0006-8993(92)91029-e. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2007;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of κ-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Archer S, Glick SD. U50,488, a κ-opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopa-mine in the nucleus accumbens of rats. Neurosci Lett. 1994;181:57–60. doi: 10.1016/0304-3940(94)90559-2. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacol. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Nomura M, Yoshizawa K, Nagase H, Sawaguchi T, Narita M, Suzuki T. Generalization of NMDA-receptor antagonists to the discriminative stimulus effects of κ-opioid receptor agonists U-50,488H, but not TRK-820 in rats. J Pharmacol Sci. 2006;100:157–161. doi: 10.1254/jphs.scj05006x. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Cochran SM, Pratt JA. PCP: from pharmacology to modeling schizophrenia. Curr Opin Pharmacol. 2005;5:101–106. doi: 10.1016/j.coph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Paine T, Olmstead M. Cocaine disrupts both behavioural inhibition and conditional discrimination in rats. Psychopharmacol. 2004;175:443–450. doi: 10.1007/s00213-004-1845-3. [DOI] [PubMed] [Google Scholar]
- Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA., Jr Sensitivity of the 5-choice serial reaction time task to the effects of various psychotropic drugs in Sprague–Dawley rats. Biol Psychiatry. 2007;62:687–693. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Paine TA, Neve RL, Carlezon WA., Jr Attention deficits and hyperactivity following inhibition of cAMP-dependent protein kinase (PKA) within the medial prefrontal cortex of rats. Neuropsychopharmacol. 2009;34:2143–2155. doi: 10.1038/npp.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parwani A, Weiler MA, Blaxton TA, Warfel D, Hardin M, Frey K, Lahti AC. The effects of a subanesthetic dose of ketamine on verbal memory in normal volunteers. Psychopharmacol. 2005;183:265–274. doi: 10.1007/s00213-005-0177-2. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Potter D, Toitman MF, Cohen BM, Carlezon WA, Jr, Chartoff EH. Biphasic effects of the kappa opioid receptor agonist salvinorin A on hedonic state. Soc Neurosci Abs. 2009;39:564.14. [Google Scholar]
- Robbins T. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacol. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Saranson I, Bransome EB, Beck LH. A continuous performance test of brain damage. J Consult Clin Psychol. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Salvinorin A: a potent naturally occurring nonnitrogenous κ opioid selective agonist. Proc Nat Acad Sci USA. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, Heidbreder C. kappa-Opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J Pharmacol Exp Ther. 1996;276:545–554. [PubMed] [Google Scholar]
- Siebert DJ. Salvia divinorum and Salvinorin A: new pharmacologic findings. J Ethnopharmacol. 1994;43:53–56. doi: 10.1016/0378-8741(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Thomas JB, Atkinson RN, Vinson NA, Catanzaro JL, Perretta CL, Fix SE, Mascarella SW, Rothman RB, Xu H, Dersch CM, Cantrell BE, Zimmerman DM, Carroll FI. Identification of (3R)-7-hydroxy-N-((1S)-1-[[(3R, 4R)-4-(3-hydroxyphenyl)-3, 4-dimethyl-1-piperidinyl]methyl]-2-methylpropyl)-1, 2, 3, 4-tetrahydro-3-isoquinolinecarboxamide as a novel potent and selective opioid kappa receptor antagonist. J Med Chem. 2003;46:3127–3137. doi: 10.1021/jm030094y. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacol. 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The kappa-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes LJ., 3rd Salvia divinorum and the unique diterpene hallucinogen, Salvinorin (divinorin) A. J Psychoactive Drugs. 1994;26:277–283. doi: 10.1080/02791072.1994.10472441. [DOI] [PubMed] [Google Scholar]
- Vortherms TA, Roth BL. Salvinorin A: from natural product to human therapeutics. Molec Interv. 2006;6:257–265. doi: 10.1124/mi.6.5.7. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML. A review of the properties of spiradoline: a potent and selective kappa-opioid receptor agonist. CNS Drug Rev. 2003;9:187–198. doi: 10.1111/j.1527-3458.2003.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmore-Fordham CB, Krall DM, McCurdy CR, Kinder DH. The hallucinogen derived from Salvia divinorum, salvinorin A, has kappa-opioid agonist discriminative stimulus effects in rats. Neuropharmacol. 2007;53:481–486. doi: 10.1016/j.neuropharm.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotoxicol Res. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Bikbulatov RV, Mocanu V, Dicheva N, Parker CE, Wetsel WC, Mosier PD, Westkaemper RB, Allen JA, Zjawiony JK, Roth BL. Structure-based design, synthesis, and biochemical and pharmacological characterization of novel salvinorin A analogues as active state probes of the kappa-opioid receptor. Biochem. 2009;48:6898–6908. doi: 10.1021/bi900605n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chiodo LA, Freeman AS. Electrophysiological effects of MK-801 on rat nigrostriatal and mesoaccumbal dopaminergic neurons. Brain Res. 1992;590:153–163. doi: 10.1016/0006-8993(92)91091-r. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacol. 2005;179:551–558. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]