Abstract
Thyroid hormones influence brain development through the control of gene expression. The concentration of the active hormone T3 in the brain depends on T3 transport through the blood-brain barrier, mediated in part by the monocarboxylate transporter 8 (Mct8/MCT8) and the activity of type 2 deiodinase (D2) generating T3 from T4. The relative roles of each of these pathways in the regulation of brain gene expression is not known. To shed light on this question, we analyzed thyroid hormone-dependent gene expression in the cerebral cortex of mice with inactivated Mct8 (Slc16a2) and Dio2 genes, alone or in combination. We used 34 target genes identified to be controlled by thyroid hormone in microarray comparisons of cerebral cortex from wild-type control and hypothyroid mice on postnatal d 21. Inactivation of the Mct8 gene (Mct8KO) was without effect on the expression of 31 of these genes. Normal gene expression in the absence of the transporter was mostly due to D2 activity because the combined disruption of Mct8 and Dio2 led to similar effects as hypothyroidism on the expression of 24 genes. Dio2 disruption alone did not affect the expression of positively regulated genes, but, as in hypothyroidism, it increased that of negatively regulated genes. We conclude that gene expression in the Mct8KO cerebral cortex is compensated in part by D2-dependent mechanisms. Intriguingly, positive or negative regulation of genes by thyroid hormone is sensitive to the source of T3 because Dio2 inactivation selectively affects the expression of negatively regulated genes.
Genes positively regulated by thyroid hormones in the postnatal mouse cerebral cortex are sensitive to the T3 entering the brain from the circulation, or being locally generated by D2, whereas genes negatively regulated by the hormone are dependent mostly on locally generated T3.
The effects of thyroid hormones on brain development are the result of their complex and intricate action on the expression of many genes. Thyroid hormone regulation of gene expression in brain has different profiles that are characteristic of specific developmental stages and brain regions (1). In addition, the concentration of the active hormone T3 in brain is controlled by two local mechanisms. One is the rate of entry from blood into brain through specific T4 and T3 transporters located in the blood-brain barrier and the plasma membrane of neural cells (2). The second mechanism is the local control of T3 concentration by the deiodinases type 2 (D2) and type 3, which regulate the balance between its production from T4 and its degradation to 3,3′-diiodothyronine (3). Examination of brain gene expression in mice with disruption of the Dio2 gene led to the conclusion that the T3 generated from D2 may not be equivalent to the T3 reaching the brain from the circulation (4).
The importance of the transporters is best illustrated by the severe phenotype caused by mutations of the specific T4 and T3 transporter MCT8 (monocarboxylate transporter 8, SlC16A2) gene (5). Patients suffer from a severe neurodevelopmental defect and abnormal distribution and metabolism of thyroid hormones (2,6,7,8,9). It is assumed that the neurological syndrome is the consequence of restricted access of T3 to the target neurons (10). Disruption of the Mct8 gene in mice (Mct8KO) also impairs thyroid hormone uptake in brain and results in changes in the serum thyroid hormone profile characteristic of humans with MCT8 mutations. Manifestations of brain hypothyroidism include a decrease in brain T3 content, increased brain D2 activity, increased TRH mRNA in the paraventricular nucleus, and decreased expression of T3 target genes, neurogranin (Nrgn) and Hairless (Hr) (9,11,12,13). However structural and functional defects caused by hypothyroidism in the brain of these animals are minimal. Therefore, efficient compensating mechanisms for the lack of Mct8 must exist in the mouse brain to permit seemingly normal development.
A likely compensating mechanism for the lack of Mct8 involves the elevated D2 activity. In the brain parenchima, D2 is expressed primarily in astrocytes (14,15,16), and its activity is elevated in the settings of hypothyroidism and iodine deficiency, and this helps to maintain the brain T3 content within a safe range provided that there is enough T4 substrate (3). In Mct8KO mice, the transport of T4 through the blood-brain barrier is less compromised than that of T3, due to the presence of transporters such as the organic anion transporter polypeptide 14 (Oatp14, Slco1c1) (17,18) with higher affinity for T4 than for T3. Therefore, it is likely that, due to the elevated level of D2 activity, the T4 that enters the Mct8KO mouse brain is converted with increased efficiency to T3, with the result that the brain T3 content is adequate for development.
Despite the importance of D2 for the local generation of T3, D2-deficient mice (D2KO) also exhibit little evidence of impaired neurofunction, suggesting that enough T3 is reaching the brain through the blood-brain barrier to prevent significant brain damage. It was therefore surprising that D2KO mice and hypothyroid mice have a comparable reduction in their brain T3 content but target gene expression in the D2KO mice was only mildly affected and certainly not to the same degree as that in the hypothyroid mice (4). This indicates that the overall content of tissue T3 does not reflect actual T3 action at the cell level because the latter depends on its presence at specific sites and cells. Furthermore, in hypothyroid mice, much of the T3 in brain is likely to derive locally from T4, whereas in D2KO mice, it must be obtained directly from the circulation because deiodinase type 1 activity in brain is very low or absent. Thus, the finding that, compared with the hypothyroid mouse, gene expression levels are only minimally affected in the brain of the D2KO mouse suggests that T3 action in brain may be dependent in part on its source. Therefore, the different effects on brain gene expression between hypothyroid and D2KO mice suggest differences in the final effect of T3 from the blood and that from the astrocytes.
In this work, we analyzed the roles of Mct8 as a transporter of T4 and T3 into the brain and D2 in providing local T3 by studying gene expression in the cerebral cortex of postnatal mice. First we identified genes that were increased or decreased by hypothyroidism, and therefore negatively or positively regulated by thyroid hormones. Then we studied the effects of thyroid hormone deficiency and the lack of functional Mct8 and/or D2. The results indicate that, in the absence of Mct8, the expression level of most genes is maintained due at least in part to the T3 generated locally by D2. On the other hand, in the absence of D2, the expression levels of the positive genes are maintained solely by T3 obtained from the blood. However, the negative genes are sensitive to D2 deficiency, suggesting that they are dependent on the T3 formed locally from T4 by the D2.
Materials and Methods
Animals
Protocols for animal handling were approved by the local institutional Animal Care Committee, following the rules of the European Union. Animals were housed in temperature (22 ± 2 C)- and light (12-h light, 12-h dark cycle; lights on at 0700 h)-controlled conditions and had free access to food and water. Mct8KO (male genotype, Mct8−/y) mice were generated by Dumitrescu et al. (13) using homologous recombination. Experiments were carried out on wild-type (Wt) and knockout (KO) male litter mates derived from backcrossing of heterozygous females with Wt males of the C57BL/6J strain. Genotypes were confirmed by PCR of tail DNA (38 cycles at 55 C annealing temperature) using the following primers: forward common, 5′-ACAACAAAAAGCCAAGCATT-3′; reverse Wt specific, 5′-GAGAGCAGCGTAAGGACAAA-3′; reverse KO specific, 5′-CTCCCAAGCCTGATTTCTAT-3′. Using this procedure the Wt allele generated a 476-bp PCR product and the null allele a 239-bp product.
After crossing with Wt male mice, Mct8KO heterozygous pregnant dams were given either drinking water or a solution containing 0.02% 1-methyl-2-mercapto-imidazol (Sigma Chemical Co., St. Louis, MO) plus 1% KClO4 ad libitum. These antithyroid drugs were given from gestational d 17, and throughout the lactating period, until the end of the experiment on postnatal day (P) 21. The pups were genotyped at P11 to select for Mct8+/y and Mct8−/y mice from the same litters. For simplicity, throughout this paper, these animals will be referred to as wild-type (Wt) and Mct8KO mice, or the corresponding hypothyroid mice (WtH and Mct8KOH).
Mice deficient in D2 (D2KO; genotype Dio2−/−) and mice deficient in both D2 and Mct8 (Mct8D2KO; genotype D2−/−Mct8−/y) were generated by crossing D1D2KO with Wt and Mct8KO mice (13). Disruption of the Mct8 gene in the context of D2 deficiency decreased the total brain content of T3 from 0.75 ± 0.08 ng in the D2KO to 0.10 ± 0.04 ng in the adult double KO (P < 0.001), i.e. only about 7% the normal content of T3 (4). To produce the male mice used in the experiments, female D2−/−Mct8+/− mice were mated with male D2−/−Mct8+/y mice, producing D2−/−Mct8+/y (D2KO) and D2−/−Mct8−/y (Mct8D2KO) male littermates. The D2 genotype was confirmed by PCR of tail DNA (38 cycles at 57 C annealing temperature) using the following primers: forward common, 5′-ATTTTCTCTTGACCATCCTT-3′; reverse Wt specific, 5′-TATACCAACAGGAAGTCAGC-3′; reverse KO specific, 5′-GAACTTCCTGACTAGGGGAG-3′. This procedure generated a 463-bp fragment from the Wt allele and a 230-bp fragment from the null allele.
Real-time quantitative PCR (qPCR)
The pups were killed by decapitation on P21. The cerebral cortex was rapidly dissected out, frozen on dry ice, and kept at −80 C until RNA isolation. The Trizol procedure (Invitrogen, Carlsbad, CA) was followed, with an additional step of chloroform extraction. The quality of RNA was analyzed using a BioAnalyzer (Agilent, Santa Clara, CA). cDNA was prepared from 250 ng of RNA using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). qPCR assays were performed on TaqMan low-density arrays (Applied Biosystems), format 98a (P/N 4342253). cDNA aliquots corresponding to 10 ng of starting RNA from individual mice were used, with TaqMan universal PCR master mix, No Amp Erase UNG (Applied Biosystems) on a 7900HT fast real-time PCR system (Applied Biosystems). The PCR program consisted in a hot start of 95 C for 10 min, followed by 40 cycles of 15 sec at 95 C and 1 min at 60 C. For analysis we used the 2-Ct method. As internal control we included 18S RNA, and three negative controls, Diablo [diablo homolog (Drosophila)], Ube2b (ubiquitin-conjugating enzyme E2B, RAD6 homology [S. cerevisiae)]6, and Ppia (peptidylprolyl isomerase A). Data were expressed relative to the values obtained for the control Wt, which was given a value of 1.0 after correction for 18S RNA. Differences between means were obtained by one- or two-way ANOVA, depending on the experiment, and the Tukey or Bonferroni’s post hoc tests, respectively. Calculations were done using the GraphPad Prism software (http://www.graphpad.com/prism/).
Results
The goal of this work was to analyze the role of Mct8 as a T3 transporter to the brain and that of D2, which generates T3 from T4 locally, on the regulation of thyroid hormone-dependent gene expression in the cerebral cortex. To isolate candidate genes for these experiments, we first performed microarray analysis using cerebral cortices from control and hypothyroid Wt mice (the procedures and results of this analysis can be found in Supplemental Materials and Methods and Supplemental Table 1 published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). We found that 316 genes were decreased, and 318 genes increased in the hypothyroid mice and therefore represent genes that are putatively up- or down-regulated, respectively, by thyroid hormone. For simplicity we will refer to these two categories as positive and negative genes, respectively.
Nineteen positive and 15 negative (a total of 34) genes were used for further analysis. We first selected known targets of thyroid hormone, such as Hr, Itih3, Nefh, Nefm, and Sema7a. The rest were chosen following the criteria of fold change and relative abundance as explained in Supplemental Materials and Methods. We also crossed our data with the transcriptome database published by Cahoy et al. (14) to identify specific cell type-enriched genes. Seven of the selected genes were enriched at least 5-fold in astrocytes (Aldh1a1, Abcd2, Itga7, Slc1a3, Hapln1, Sult1a1, and Mamdc2), and four genes were enriched more than 5-fold in neurons (Nefm, Nefh, Ppm2c, and Dgkg). The rest was not enriched in any particular cellular subset.
Effect of thyroid hormone deprivation and the role of Mct8
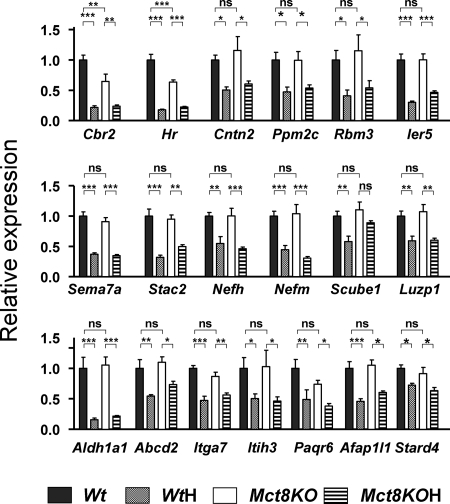
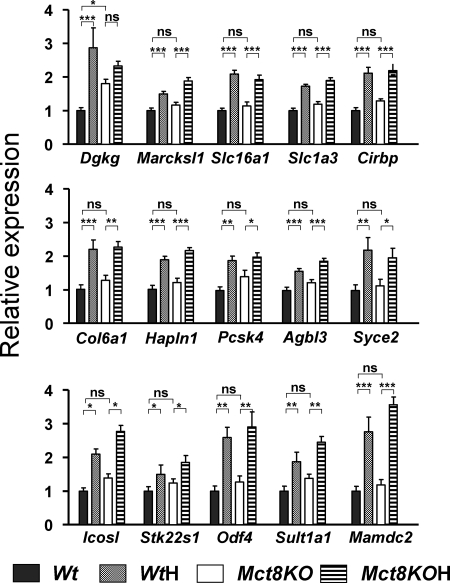
This experiment confirmed that the expression of the selected genes was dependent on the thyroid status and examined the effect of Mct8 deficiency. To this end we analyzed by qPCR four groups of animals: Wt and Mct8KO littermates and WtH and Mct8KOH littermates, obtained from different dams from those used for the arrays. Figure 1 shows the effects of hypothyroidism and Mct8 deficiency on the expression of the 19 positive genes. Of the 19 genes, only Cbr2 and Hr were also sensitive to Mct8 deficiency. The rest had normal expression in the untreated Mct8KO mice. All were also decreased significantly in the Mct8KOH mice compared with the Mct8KO mice, with the exception of Scube1, in which the decrease was not significant. Figure 2 shows the results for the 15 negative genes. Expression of all these genes was increased in the WtH mice but, with the exception of Dgkg, was unchanged in the Mct8KO. Hypothyroidism also increased their expression in the Mct8KO with the exception of Dgkg. The conclusion from these experiments is that the expression of most thyroid hormone-dependent genes in the neocortex of the Mct8-deficient mice is kept normal despite the restriction for T3 entry into the brain.
Figure 1.
Effects of Mct8 deficiency and thyroid hormone deprivation on gene expression in the cerebral cortex: positive genes. Gene expression was measured by PCR on TaqMan arrays, using RNAs from control wild-type mice (Wt), hypothyroid Wt mice (WtH), Mct8−/y mice (Mct8KO), and hypothyroid Mct8−/y mice (Mct8KOH) (n = 6 for all groups). Results are expressed as mean ± sem relative to the control Wt value set as 1.0. Significant differences (*, P < 0.05, **, P < 0.01, ***, P < 0.001) between means were determined by two-way ANOVA, the two factors being genotype and thyroid status. Abcd2, ATP-binding cassette, subfamily D (ALD), member 2; Afap1l1, actin filament-associated protein 1-like 1; Aldh1a1, aldehyde dehydrogenase family 1; Cbr2, carbonyl reductase 2; Cntn2, contactin 2 (axonal); Hr, hairless; Ier5, immediate early response 5; Itga7, integrin-α7; Itih3, inter-α-trypsin inhibitor, heavy chain 3; Luzp1, leucine zipper protein 1; Nefh, neurofilament, heavy polypeptide; Nefm, neurofilament, medium polypeptide; Paqr6, progestin and adipoQ receptor family member VI; Ppm2c, protein phosphatase 2C, magnesium-dependent, catalytic subunit; Rbm3, RNA binding motif protein-3; Scube1, signal peptide, CUB domain, EGF-like 1; Sema7a, sema domain, immunoglobulin domain (Ig), and GPI membrane anchor, (semaphorin) 7A; Stac2, SH3 and cysteine-rich domain 2; Stard4, StAR-related lipid transfer (START) domain containing 4.
Figure 2.
Effects of Mct8 deficiency and thyroid hormone deprivation on gene expression in the cerebral cortex: negative genes. Gene expression was measured by PCR on TaqMan arrays, using RNAs from control Wt mice, hypothyroid Wt mice (WtH), Mct8−/y mice (Mct8KO), and hypothyroid Mct8−/y mice (Mct8KOH) (n = 6 for all groups). Results are expressed as mean ± sem relative to the control Wt value set as 1.0. Significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001) between means were determined by two-way ANOVA, the two factors being genotype and thyroid status. Agbl3, ATP/GTP binding protein-like 3; Cirbp, cold inducible RNA binding protein; Col6a1, collagen, type VI, α1. Dgkg, diacylglycerol kinase, γ; Hapln1, hyaluronan and proteoglycan link protein 1; Icosl, Icos ligand; Mamdc2, MAM domain containing 2; Marcksl1, MARCKS-like 1; Odf4, outer dense fiber of sperm tails 4; Pcsk4, proprotein convertase subtilisin/kexin type 4; Slc1a3, solute carrier family 1 (glial high affinity glutamate transporter), member 3; Slc16a1, solute carrier family 16, member 1 (monocarboxylic acid transporter 1); Stk22s1, serine/threonine kinase 22 substrate 1; Sult1a1, sulfotransferase family, cytosolic, 1A, phenol-preferring, member 1; Syce2, synaptonemal complex central element protein 2.
Role of D2
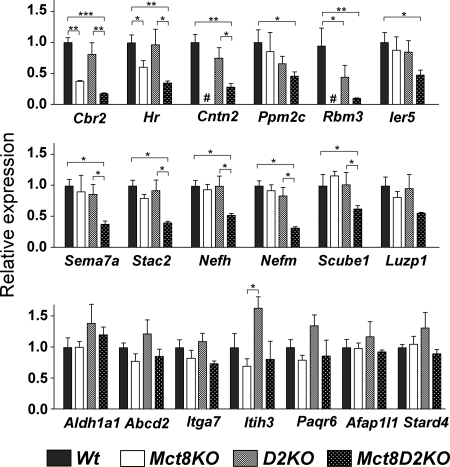
A second experiment was designed to analyze whether the normal expression of thyroid hormone-regulated genes in Mct8KO was due to compensatory generation of T3 by D2 because an increase in D2 activity has been demonstrated in these mice (9,13). Four groups of animals were studied: Wt, Mct8KO, D2KO (genotype Dio2−/−) (4), and Mct8D2KO (genotype Mct8−/yDio2−/−). Comparisons between Wt and Mct8KO gave similar results as shown above for both positive and negative genes. The single exception was Dgkg, which showed no changes in this experiment for unknown reasons. For the positive genes (Fig. 3), isolated D2 deficiency was without effect compared with the Wt animals on most genes studied except for Rbm3. Cntn2 and Ppm2c were also decreased in D2KO mice, but the difference was borderline significant. The combined effect of Mct8 and D2 deficiency resulted in the decreased expression of 11 genes (Cbr2, Hr, Cntn2, Ppm2c, Rbm3, Ier5, Sema7a, Stac2, Nefh, Nefm, and Scube1) and was without effect on eight genes (Luzp1, which was borderline significant, Aldh1a1, Abcd2, Itga7, Itih3, Paqr6, Afap1l1, and Stard4).
Figure 3.
Effects of D2 deficiency on gene expression in the cerebral cortex: positive genes. Gene expression was measured by PCR on TaqMan arrays (microfluidic cards), using RNAs from control Wt mice, Mct8−/y, Mct8−/yDio2−/− mice (Mct8D2KO), and Dio2−/− mice (D2KO) (n = 3 for all groups). Results are expressed as mean ± sem relative to the control Wt value set as 1.0. #, These samples were lost during the procedure. Significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001) between means compared with the control values were determined by one-way ANOVA. All other comparisons for which the significance is not shown were not significant. Gene abbreviations as in Fig. 1.
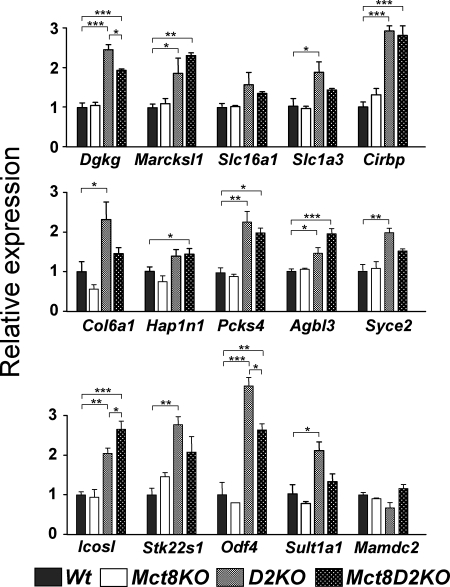
Expression of the negative genes was increased in the D2KO animals with the exception of Slc16a1, Mamdc2, and Hapln1 (Fig. 4). Deficiency of both Mct8 and D2 had the same effect as D2 deficiency alone except that the increase for Slc1a3, Syce, and Stk22sl did not reach statistical significance.
Figure 4.
Effects of D2 deficiency on gene expression in the cerebral cortex: negative genes. Gene expression was measured by PCR on TaqMan arrays (microfluidic cards), using RNAs from control Wt mice, Mct8−/y, Mct8−/yDio2−/− mice (Mct8D2KO), and Dio2−/− mice (D2KO) (n = 3 for all groups). Results are expressed as mean ± sem relative to the control Wt value set as 1.0. Significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001) between means compared with the control values were determined by one-way ANOVA. All other comparisons for which the significance is not shown were not significant. Gene abbreviations as in Fig. 2.
The above data suggest that there is a significant difference in the pattern of regulation between the positive and negative genes. To substantiate this concept, we pooled all raw data from each group and performed one-way ANOVA. For the positive genes there was a significant effect of genotype [F (2,168) = 37.93, P < 0.0001] with a significant difference between the Wt and the Mct8D2KO mice but not between Wt and the Mct8KO and D2KO mice. These data reinforce the observation on individual genes on the lack of effect of isolated Mct8 and D2 deficiency but decreased expression in the deficiency of both Mct8 and D2. In other words, adequate generation of T3 due to the increased level of D2 activity compensated for the defect in T3 transport in Mct8KO mice, and vice versa, indicating that the positive genes can be regulated appropriately by T3 from the blood or by T3 generated from T4 by D2.
For the negative genes, there was also a significant effect of genotype, with F (2,131) = 45.00, P < 0.0001. However, in contrast to the positive genes, there was a significant difference between the Wt and D2KO mice as well as between the Wt and the Mct8D2KO mice. The mean expression of the negative genes in the D2KO mice was not different from the Mct8D2KO mice. This indicates that the negative genes depend on a normal level of D2 activity to generate the T3 required to maintain a normal level of gene expression.
Discussion
The principal goal of this work was to determine to what extent blood T3 reaching the mouse brain trough the Mct8 transporter and that generated locally by T4 deiodination contribute to the hormonal effect on gene expression. To this aim we used Mct8- and D2-deficient mice alone and in combination. mRNAs found to be regulated by thyroid hormone were measured by qPCR in the cerebral cortex of these mice. These mRNAs were identified as being either positively or negatively regulated by thyroid hormone through a preliminary microarray screen of mRNA from cortices of Wt mice that were untreated and mice deprived of thyroid hormone.
Previous work has shown that the mRNA levels of two thyroid hormone target genes, Hr and Nrgn, were decreased in the whole brain and cerebellum (Hr) or the striatum (Nrgn) of Mct8KO mice (9,11,13). In this work, we examined whether other thyroid hormone-dependent genes identified in the P21 mouse cerebral cortex behave as Nrgn and Hr. The most striking finding is that despite the reported reduction of brain T3 content, the expression of thyroid hormone-dependent genes in the Mct8KO mice is generally similar to that in the Wt mice, with exceptions such as Hr and Cbr2.
The lack of significant effect of Mct8 deficiency on the expression of multiple genes regulated by thyroid hormone in mouse cerebrum was somewhat surprising. Yet previous work indicated that mechanisms exist to compensate for the lack of Mct8 in the mouse brain (11,18). One likely mechanism involves D2, which in brain is expressed primarily in astrocytes (14,15). D2 activity is increased severalfold in the brain of Mct8-deficient mice. In the absence of Mct8, transport of T4 is less compromised than transport of T3 (9,11). Therefore, enough T4 reaches the brain in which due to the elevated level of D2 activity, sufficient T3, even though still only 50% of that in Wt mice (9,13), is generated to normalize gene expression.
In fact, further analysis suggests that the T3 generated locally by the D2 is responsible for the normal expression of most of the genes studied in Mct8-deficient mice. As mentioned in the Materials and Methods, in the combined Mct8 and D2 deficiency brain T3 content is reduced to a level that is barely detectable by a highly sensitive RIA. In this situation 11 of 19 positive genes were decreased, suggesting that D2 is involved in the compensation mechanisms for some but not all of these genes. The mechanisms for compensation of these genes are unclear and difficult to explain with the available data, but it is possible that they are very sensitive to low amounts of T3 that might be reaching the target cells, even in the combined absence of Mct8 and D2.
A notable difference was identified between the positive and negative genes. The former were not affected by D2 or Mct8 deficiency alone, whereas the combined defect led to reduced expression. These genes appear to be regulated equally well by T3 obtained directly from the blood or by T3 generated in the cerebral cortex. On the other hand, the negative genes were not affected by Mct8 deficiency but were sensitive to D2 deficiency and the combined D2 and Mct8 deficiency. This result suggests that the negative genes are more dependent on the T3 produced locally than on the T3 obtained directly from the circulation. The hypothesis of dissimilar effects of brain T3 derived from these two sources was also formulated by Galton et al. (4). At present it is difficult to provide a coherent explanation for this phenomenon. It may be related to multiple causes. Most attractive is the notion that the positive and negative genes have different sensitivity to the magnitude of change in thyroid hormone. In other words, positive gene regulation can be sustained by lower concentrations of thyroid hormone derived from the systemic circulation, whereas negative gene regulation requires a higher concentration of thyroid hormone achieved by local conversion of T4 to T3 and uptake from the bloodstream.
Another, although less likely, mechanism is through the nongenomic action of T4 (19). T4 concentration is higher in D2KO mice, which may have a yet-unidentified indirect genomic effect on negative genes. Other factors such as differences in cellular composition cannot be ruled out.
The dissociation between positive and negative regulation in the D2KO mice is reminiscent of that observed in some mutations of the Thrb gene (encoding the thyroid hormone receptor-β subtype) leading to central resistance to thyroid hormone (20,21). When the mutant thyroid hormone receptor is expressed in mice, positive regulation by T3 is preserved, but negative regulation is impaired (20). This phenomenon seems to be due to the altered molecular properties of the mutant receptor and selective impairment of interaction with corepressors. The molecular mechanism for negative regulation of gene expression by T3 is not clear, involving interactions with corepressor proteins, decreased or increased histone acetylation without coactivator recruitment (22), and other histone modifications (21). We can only speculate on how these mechanisms might be influenced by signaling pathways directly or indirectly related to T4 to T3 conversion in the astrocytes. Clearly this is an interesting topic for future exploration.
These studies do not intend to explain the differences in phenotype between patients and mice with transporter defect. A possible explanation is the presence of alternative transporters in mice that are not present in humans, such as Slco1c1 (Oatp14) (18) or Slc7a8 (Lat2) (23). Another possibility in light of this work would be differences in the timing or regional expression of D2 during development between human and mice and therefore on the capacity to compensate for the lack of T3 transport. Compensation may be more efficient in some parts of the brain than in others due to differences in expression between Mct8 and D2 in particular regions of the developing brain. Finally, a species difference in the quantitative requirement of thyroid hormone during development may account for the psychomotor defect characteristic in humans.
Supplementary Material
Acknowledgments
The technical help of Eulalia Moreno and Ana Torrecilla is gratefully acknowledged.
Footnotes
This work was supported by the Center for Biomedical Research on Rare Diseases; Grants SAF2008-01168 and SAF2008-00429E from the Ministry of Science and Innovation, Spain; the European Union Integrated Project CRESCENDO (LSHM-CT-2005-018652) Grants DK15070, DK07011, and DK20595 from the National Institutes of Health; and the Sherman family. A.C. is the holder of a predoctoral fellowship from the Ministry of Science and Innovation of Spain.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 10, 2010
Abbreviations: D2, Type 2 deiodinase; KO, knockout; Mct8, monocarboxylate transporter 8; P, postnatal day; qPCR, quantitative PCR; Wt, wild type.
References
- Bernal J 2005 Thyroid hormones and brain development. Vitam Horm 71:95–122 [DOI] [PubMed] [Google Scholar]
- Heuer H, Visser TJ 2009 Minireview: pathophysiological importance of thyroid hormone transporters. Endocrinology 150:1078–1083 [DOI] [PubMed] [Google Scholar]
- St Germain DL, Galton VA, Hernandez A 2009 Minireview: defining the roles of the iodothyronine deiodinases: current concepts and challenges. Endocrinology 150:1097–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton VA, Wood ET, St Germain EA, Withrow CA, Aldrich G, St Germain GM, Clark AS, St Germain DL 2007 Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient rodent brain during development. Endocrinology 148:3080–3088 [DOI] [PubMed] [Google Scholar]
- Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ 2003 Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 278:40128–40135 [DOI] [PubMed] [Google Scholar]
- Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ 2004 Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364:1435–1437 [DOI] [PubMed] [Google Scholar]
- Grüters A 2007 Thyroid hormone transporter defects. Endocr Dev 10:118–126 [DOI] [PubMed] [Google Scholar]
- Holden KR, Zuñiga OF, May MM, Su H, Molinero MR, Rogers RC, Schwartz CE 2005 X-linked MCT8 gene mutations: characterization of the pediatric neurologic phenotype. J Child Neurol 20:852–857 [DOI] [PubMed] [Google Scholar]
- Trajkovic M, Visser TJ, Mittag J, Horn S, Lukas J, Darras VM, Raivich G, Bauer K, Heuer H 2007 Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest 117:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesema EC, Kuiper GG, Jansen J, Visser TJ, Kester MH 2006 Thyroid hormone transport by the human monocarboxylate transporter 8 and its rate-limiting role in intracellular metabolism. Mol Endocrinol 20:2761–2772 [DOI] [PubMed] [Google Scholar]
- Ceballos A, Belinchon MM, Sanchez-Mendoza E, Grijota-Martinez C, Dumitrescu AM, Refetoff S, Morte B, Bernal J 2009 Importance of monocarboxylate transporter 8 for the blood-brain barrier-dependent availability of 3,5,3′-triiodo-l-thyronine. Endocrinology 150:2491–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cosmo C, Liao XH, Dumitrescu AM, Weiss RE, Refetoff S 2009 A thyroid hormone analogue with reduced dependence on the monocarboxylate transporter 8 (MCT8) for tissue transport. Endocrinology 150:4450–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S 2006 Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology 147:4036–4043 [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA 2008 A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28:264–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadaño-Ferraz A, Obregón MJ, St Germain DL, Bernal J 1997 The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proc Natl Acad Sci USA 94:10391–10396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadaño-Ferraz A, Escámez MJ, Rausell E, Bernal J 1999 Expression of type 2 iodothyronine deiodinase in hypothyroid rat brain indicates an important role of thyroid hormone in the development of specific primary sensory systems. J Neurosci 19:3430–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama D, Kusuhara H, Taniguchi H, Ishikawa S, Nozaki Y, Aburatani H, Sugiyama Y 2003 Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier: high affinity transporter for thyroxine. J Biol Chem 278:43489–43495 [DOI] [PubMed] [Google Scholar]
- Roberts LM, Woodford K, Zhou M, Black DS, Haggerty JE, Tate EH, Grindstaff KK, Mengesha W, Raman C, Zerangue N 2008 Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology 149:6251–6261 [DOI] [PubMed] [Google Scholar]
- Davis PJ, Leonard JL, Davis FB 2008 Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol 29:211–218 [DOI] [PubMed] [Google Scholar]
- Machado DS, Sabet A, Santiago LA, Sidhaye AR, Chiamolera MI, Ortiga-Carvalho TM, Wondisford FE 2009 A thyroid hormone receptor mutation that dissociates thyroid hormone regulation of gene expression in vivo. Proc Natl Acad Sci USA 106:9441–9446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa R, Yamada M, Horiguchi K, Ishii S, Hashimoto K, Okada S, Satoh T, Mori M 2009 Aberrant histone modifications at the thyrotropin-releasing hormone gene in resistance to thyroid hormone: analysis of F455S mutant thyroid hormone receptor. Endocrinology 150:3425–3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Xia X, Liu Y, Oetting A, Walker RL, Zhu Y, Meltzer P, Cole PA, Shi YB, Yen PM 2009 Negative regulation of TSHα target gene by thyroid hormone involves histone acetylation and corepressor complex dissociation. Mol Endocrinol 23:600–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth EK, Roth S, Blechschmidt C, Hölter SM, Becker L, Racz I, Zimmer A, Klopstock T, Gailus-Durner V, Fuchs H, Wurst W, Naumann T, Bräuer A, de Angelis MH, Köhrle J, Grüters A, Schweizer U 2009 Neuronal 3′,3,5-triiodothyronine (T3) uptake and behavioral phenotype of mice deficient in Mct8, the neuronal T3 transporter mutated in Allan-Herndon-Dudley syndrome. J Neurosci 29:9439–9449 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.