Abstract
Variations in maternal behavior among lactating rats associate with differences in estrogen-oxytocin interactions in the medial preoptic area (mPOA) and in dopamine levels in the nucleus accumbens (nAcc). Thus, stable, individual differences in pup licking/grooming (LG) are abolished by oxytocin receptor blockade or treatments that eliminate differences in the nAcc dopamine signal. We provide novel evidence for a direct effect of oxytocin at the level of the ventral tegmental area (VTA) in the regulation of nAcc dopamine levels. Mothers that exhibit consistently increased pup LG (i.e. high LG mothers) by comparison with low LG mothers show increased oxytocin expression in the mPOA and the paraventricular nucleus of the hypothalamus and increased projections of oxytocin-positive cells from both mPOA and paraventricular nucleus of the hypothalamus to the VTA. Direct infusion of oxytocin into the VTA increased the dopamine signal in the nAcc. Finally, high compared with low LG mothers show greater increases in dopamine signal in the nAcc during bouts of pup LG, and this difference is abolished with infusions of an oxytocin receptor antagonist directly into the VTA. These studies reveal a direct effect of oxytocin on dopamine release within the mesocorticolimbic dopamine system and are consistent with previous reports of oxytocin-dopamine interactions in the establishment and maintenance of social bonds.
Oxytocin modulates dopamine release.
Maternal behavior in the rat is dependent on hormonal changes in late pregnancy that include elevations in estradiol levels (1,2,3,4) and accompanying increases in estrogen receptor activation in the brain regions, such as the medial preoptic area (mPOA) (5). The estrogenic effect is critical for hormonal mediators of maternal behavior such as prolactin or oxytocin (3,6). Estradiol implants into the mPOA stimulate maternal behavior, in part, through effects on oxytocin receptor activity (7,8). Treatment with an oxytocin-antisera or an oxytocin receptor antagonist (OTA) directly into the mPOA blocks the effect of estradiol on maternal behavior (8,9). Intracerebroventricular (ICV) administration of oxytocin stimulates maternal behavior in virgin rats (10). This effect is abolished by ovariectomy and reinstated with estradiol treatment (11), reflecting the interdependence of estrogens and oxytocin in the regulation of maternal behavior in the rat.
Individual differences in specific forms of maternal behavior are also related to estrogen-oxytocin interactions. Lactating female rats that exhibit increased pup licking/grooming (LG; i.e. high LG mothers) show enhanced expression of estrogen receptor-α and increased oxytocin receptor binding in the mPOA by comparison to low LG dams (12,13,14,15). An ICV infusion of OTA on d 3 postpartum completely eliminates the difference in pup LG between high and low LG mothers (13).
An obvious question concerns the relevant neural target for the estrogen-oxytocin signaling. Projections from the mPOA to the ventral tegmental area (VTA), which contains dopamine neurons, regulate maternal behavior in the rat (16). Nursing bouts increase both Fos expression (17) and extracellular dopamine levels in the nucleus accumbens (nAcc) (18,19). Chemical lesions of the dopamine projections to the nAcc (18) or direct infusion of dopamine receptor antagonists into the nAcc (20,21) disrupt maternal behavior. High LG mothers show significantly increased dopamine levels in the nAcc shell during periods of pup LG compared with low LG mothers; treatment of dams with a dopamine reuptake blocker eliminates the group differences in both nAcc dopamine levels and pup LG (19).
Pedersen et al. (9) found that OTA infusions into the VTA impair maternal behavior. These findings and those of Numan and Stolzenberg (22) suggest that oxytocin projections from the mPOA might act directly on the VTA to regulate dopamine release during mother-pup interactions. The results of studies reported here support this hypothesis and suggest that variations in mother-pup interactions are associated with differences in oxytocinergic regulation of the mesolimbic dopamine system.
Materials and Methods
Animals and maternal behavior
The animals were outbred Long-Evans, hooded rats born in our colony and housed in 46 × 18 × 30-cm Plexiglas cages. Food and water were provided ad libitum. The colony was maintained on a 12-h light, 12-h dark schedule with lights on at 0900 h. All procedures were performed according to guidelines from the Canadian Council on Animal Care and approved by the McGill University Animal Care Committee. Animals were mated with males obtained from Charles River Canada (St. Constant, Québec, Canada) and singly housed 2 wk after mating. Maternal behavior was observed for five 75-min periods per day for the first 6 d postpartum at three periods during the light (1000, 1300, and 1700 h) and two during the dark (0600 and 2100 h) phases of the light/dark cycle. The behavior of each mother was scored every 3 min (25 observations/period × 5 periods per day = 125 observations/mother/day) for the following behaviors: mother off pups, mother carrying pup, mother LG any pup, and mother nursing pups in an arched-back posture, a blanket posture in which the mother lays over the pups, or a passive posture in which the mother is lying either on her back or side while the pups nurse (see Ref. 23 for a detailed description). Pup LG included both anogenital and body licking. High LG mothers were defined as females for which the mean frequency scores for LG over the first 6 d postpartum were greater than 1 sd above the cohort mean. Low LG mothers were defined as females for which the mean frequency scores for LG over the first 6 d postpartum greater than 1 sd below the cohort mean.
Fluorogold infusion
Primiparous female rats were precharacterized as high or low LG mothers. Importantly, individual differences in pup LG are highly stable across multiple litters (23). High and low LG mothers reared pups to weaning and were then mated 2 wk later and allowed to give birth. On postpartum d 3, dams were anesthetized using ketamine/xylazine/acepromazine (0.1 ml per 100 g, im). Animals were placed in a stereotaxic apparatus with the incisor bar adjusted to maintain the skull horizontal between bregma and λ. Animals were infused with 1 μl of 4% fluorogold (Sigma, St. Louis, MO), a retrograde tracer, into the VTA (24) (coordinates: 4.8 mm posterior to bregma, 0.8 mm lateral to the midline, and 8.0 mm ventral to the surface of the cortex) using an infusion pump fitted with a 10-μl Hamilton syringe attached to the stereotaxic arm. The infusion rate was 0.2 μl/min, and after the completion of the infusion, the needle remained in place for 10 min. The animals were allowed to recover under a heat lamp for 1 h before being returned to their pups. Antibiotic powder (Cicatrin GSK, Montréal, Canada) was applied to the site of incision and a 1-ml solution of 0.9% saline (sc) was given for fluid replacement.
Oxytocin and fluorogold immunocytochemistry
Brains were taken from perfused, postpartum d 6 dams, postfixed overnight, and then transferred to a 25% sucrose/PBS solution for 2–4 d. Brains were sliced at 30 μm and stored in cryoprotectant (pH 7.4) at −25 C. Free-floating sections were transferred from Eppendorf tubes into wells and washed five times in 0.9% PBS (pH 7.6) for 8 min for preparation for the double-labeling immunocytochemistry (ICC). Sections were incubated for 90 min in 1% hydrogen peroxide and 3% normal goat serum in PBS at room temperature (RT) and then incubated in 0.4% Triton X-100/PBS containing a monoclonal rabbit anti-FG (antifluorogold) antibody for 24 h at RT (1:20,000, AB153; Millipore, Billerica, MA). Sections were then rinsed three times in PBS for 5 min and incubated for 2 h in PBS with 1% normal goat serum containing a biotinylated secondary antibody (goat antirabbit IgG, 1:200; Vector Laboratories, Burlingame, CA). Sections were rinsed three times in PBS for 5 min and incubated in the ABC reagent for 2 h at RT (ABC kit; Vector Laboratories). The sections were removed from the ABC solution and rinsed three times in PBS for 5 min and visualized with 3,3′-diaminobenzidine (DAB) solution [DAB is a water-soluble tetrahydrochloride used for permanent immunohistochemical staining]. Sections were then rinsed five times in PBS for 5 min and incubated for 60 min in 1% hydrogen peroxide and 3% normal horse serum in PBS at RT. After blocking, sections were incubated in mouse monoclonal antioxytocin antibody at RT overnight (1:8000, MAB5296; Millipore). On the third day, sections were rinsed three times in PBS for 5 min and incubated for 2 h in PBS with 1% normal horse serum containing a biotinylated secondary antibody (horse antimouse IgG, 1:200; Vector Laboratories), followed by further rinses (three times in PBS for 5 min) and incubated in the ABC reagent for 2 h at RT (ABC kit; Vector Laboratories). Finally, the sections were removed from the ABC solution and rinsed three times in PBS for 5 min and visualized with NOVA RED solution (Vector Laboratories). Sections were mounted on gelatin-coated slides, dehydrated, and coverslipped. Counts of oxytocin and fluorogold-positive cells (four to five sections/animal) were determined using a computer-assisted densitometry program (MCID Systems; Imaging Research, St. Catherine’s, Ontario, Canada).
Probe and guide cannula implantation
Lactating females were prepared for surgery 1–2 d after parturition. The animals were pretreated with atropine sulfate (0.1 mg/kg, ip) to reduce bronchial secretions, anesthetized with sodium pentobarbital (60 mg/kg, ip), and placed in a stereotaxic apparatus with the incisor bar adjusted to maintain the skull horizontal between bregma and lambda. Electrochemical probes were lowered into the nAcc shell (24) (coordinates: 1.2 mm anterior to bregma, 0.8 mm lateral to the midline, and 7.0 mm ventral to the surface of the cortex). Animals were also implanted with an Ag/AgCl reference electrode in the contralateral parietal cortex. Miniature pin connectors soldered to the voltammetric and reference electrodes were inserted into a Carleton connector (Ginder Scientific, Ottawa, Ontario, Canada). Subsequent to the probe placements, guide cannulae (10 mm long, 23 gauge; Plastic One, Roanoke, VA) were lowered into the VTA (24) (coordinates: 5.2 mm posterior to bregma, 0.8 mm lateral to the midline, and 8.0 mm ventral to the surface of the cortex). The assembly was secured with acrylic dental cement to four stainless steel screws threaded into the cranium. Animals were returned to their home cages after a 2.5-h recovery period and observed to be alert and to engage in full maternal behavior toward pups (i.e. retrieve pups and initiate a nursing bout).
In vivo electrochemical recordings
Electrochemical probes
The voltammetric electrodes consisted of a bundle of three 30-μm-diameter carbon fibers (Textron Systems, Wilmington, MA) extending 50–100 μm beyond the sealed tip of a pulled-glass capillary (outer diameter 0.5 mm), and repeatedly coated with a 5% solution of Nafion (Aldrich, Milwaukee, WI), a perfluorinated ionomer that promotes the exchange of cations such as dopamine but impedes the exchange of interfering anionic species such as ascorbic acid (AA) and 3,4 dihydroxyphenylacetic acid. Each electrode was calibrated to determine dopamine sensitivity and selectivity compared with AA in 0.1 m PBS (pH 7.4) that contained 250 μm AA to mimic brain extracellular conditions. We used only electrodes with a highly linear response (r ≥ 0.997) to increasing concentrations of dopamine and a nominal dopamine to AA selectivity ratio of greater than 1000:1. Electrodes used had a mean (±sem) dopamine to AA selectivity ratio of 1619:1 ± 133:1. These Nafion-coated carbon fiber electrodes retain dopamine sensitivity and selectivity for dopamine against both AA and 3,4-dihydroxyphenylacetic acid for several days after implantation (25).
Electrochemical measurements
Electrochemical recordings were performed using a computer-controlled, high-speed chronoamperometric apparatus (Quanteon, Lexington, KY). An oxidation potential of +0.55 mV (with respect to the reference electrode) was applied to the electrode for 100 msec at a rate of 5 Hz. The oxidation current was digitally integrated during the last 80 msec of each pulse. The sums of every 10 digitized oxidative cycles of the chronoamperometric waveform were automatically converted into equivalent values of dopamine concentration using the in vitro calibration factor. The reduction current generated when the potential was returned to resting level (0.0 V for 100 msec) was digitized and summed in the same manner and served as an index to identify the main electroactive species contributing to the electrochemical signals. With Nafion-coated electrodes and a sampling rate of 5 Hz, the magnitude of the increase in reduction current elicited by an elevation in dopamine concentration is typically 60–80% of the corresponding increase in oxidation current [reduction to oxidation ratio (red:ox)], 0.6–0.8 (26,27,28,29,30,31,32). These studies also indicate that the oxidation of AA is virtually irreversible (red:ox 0–0.1), whereas that of DOPAC is almost entirely reversible (red:ox 1.0); the red:ox for norepinephrine and serotonin are 0.4–0.5 and 0.1–0.3, respectively.
Testing procedure
Electrochemical recordings began on postpartum d 4 and 5. The in vitro calibration factor was entered in the data acquisition software before recordings. Dams were placed in a sound-attenuating chamber containing bedding material and connected to the chronoamperometric instrument by a shielded cable and a low-impedance computator (Airflyte, Bayonne, NJ). A preamplifier configured as a current-to-voltage converter (gain, 1 × 108) was connected onto the head assembly to minimize electrical interference. The pups were introduced into the recording chamber with bedding from the home cage before the dams were connected to the chronoamperometric instrument. Electrochemical recordings were allowed to stabilize for 60 min before drug infusions (see below). The monitoring of behavior began after a 5-min recovery period and continued for 1.5 h after the stabilization period. The time of onset of each behavior, including an LG bout, was recorded to associate ongoing maternal behavior with the electrochemical signal. An LG bout was defined as a continuous period of LG of at least 5 sec in duration (14,19).
Electrochemical data
Because of the inherent variations in the sensitivity of Nafion-coated electrodes, the changes in oxidation current recorded with different electrodes (i.e. in different animals) cannot be assumed to be equivalent. Thus, valid comparisons are possible only if the sensitivity of each electrode is calibrated against a standard and the electrochemical data are expressed as standard equivalent values. The in vivo changes in oxidation current are expressed as nanomoles equivalent values of dopamine concentration. Data are presented as changes in electrochemical signal (nanomoles dopamine equivalent) relative to the signal level after stabilization. The electrochemical signal level at this time (baseline) is 0 nm. A value of 0 nm is not the absolute concentration of extracellular dopamine; rather, the electrochemical data reflect relative changes in dopamine signal associated with bouts of pup LG. Group comparisons are based on the peak dopamine signal increases during each LG bout as well as the dopamine signal amplitude taken at the onset of each bout of LG.
The similarity in the red:ox ration of norepinephrine and dopamine is inherent to electrochemical analyses. However, any contribution of norepinephrine should be minor relative to that of dopamine. The Nafion-coated carbon fiber electrodes used in this study are five to 10 times less sensitive to norepinephrine than to dopamine; thus, increases in extracellular norepinephrine in the order of 1–3 μm, typical only of K+-induced efflux would be required to elicit increases in electrochemical signals comparable with those we report for dopamine. Second, whereas the nAcc shell is innervated by norepinephrine, the density of norepinephrine terminals is considerably lower than that of dopamine. Third, LG-elicited increases in nAcc dopamine signals are potentiated by selective DAT blockade (19), suggesting that dopamine contributes significantly to the nAcc signal increases reported here. Finally, given that the norepinephrine input to nAcc shell originates primarily in the nucleus tractus solitarius and that LG-elicited increases in nAcc electrochemical signals are blocked by intra-VTA application of OTA (see below) would suggest that the relevant signal is that of dopamine.
Histology
Animals were deeply anesthetized with sodium pentobarbital (75 mg/kg, ip) and transcardially perfused with 0.9% saline followed by 10% formalin. The brains were stored in 10% formalin and subsequently cryoprotected in a 30% sucrose-formalin solution. Electrode and cannulae placements were confirmed from 40 μm thionin-stained coronal sections using the atlas of Paxinos and Watson (24). Only animals with electrodes and cannulae placed in the nAcc and VTA, respectively, were included in the study.
Drug infusion into the VTA
A 1-μl Hamilton syringe attached to a polyethylene tube carrying an infusion cannula was used for VTA infusions. On postpartum d 4 lactating females were infused unilaterally with a 1-μl solution of 0.25 μg/μl of the oxytocin antagonist [(β-mercapto-β,β cyclopentamethylenepropionyl1, O-Me-Tyr2, Orn8)oxytocin; OTA; Sigma; no. O6887] or with a 0.9% sterile saline solution. The OTA concentration was based on previous studies examining the effects of OTA infusions into the mPOA or VTA on sexual receptivity (33) or maternal behavior (9) in the rat. Previous studies (9) show a decrease in maternal behavior after a bilateral infusion of OTA in the VTA using the same OTA concentration (2.4 nm). We used a unilateral infusion of OTA into the VTA to limit the behavioral effect and to permit recording of the dopamine signal from the ipsilateral nAcc shell during periods of pup LG. This approach was based on previous studies (34) showing that a unilateral lesion of the VTA does not disrupt maternal behavior unless combined with a lesion of the contralateral mPOA. For virgin females, a 1-μl solution containing 0.24 μg of oxytocin (oxytocin acetate salt hydrate or α-hypophamine, no. O6379; Sigma) in sterile saline was infused unilaterally into the VTA. Adult, nonlactating female rats were infused with 1 μl of oxytocin (0.25 μg/μl) or 0.9% saline into the VTA. This oxytocin concentration is similar to that used in studies examining the effects of central oxytocin on maternal behavior in the rat (11). All infusions were performed over a 3-min time period. The interior of the polyethylene tube was coated with rat bovine serum (Sigma) to minimize the adhesion of the peptide to the tube. Oxytocin and OTA infusions were performed after the stabilization of the electrochemical recordings.
Statistical analysis
A two-tailed t test was performed for all ICC experiments. For the infusion studies, a repeated-measures ANOVA with drug as a between-subject factor and time as a within-subject factor was used. Pair-wise comparisons were conducted with a Bonferroni adjustment for multiple comparisons.
Results
Oxytocin projections from the mPOA to VTA
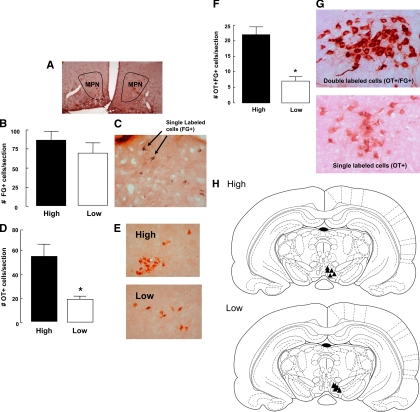
Sections were analyzed for fluorogold staining to examine differences in projections from the mPOA [specifically from the medial preoptic nucleus (MPN) of the mPOA] to the VTA (Fig. 1A). Only animals in which the fluorogold injection site was histologically confirmed to lie within the VTA were included in the study (Fig. 1H; n = 5/group). Statistical analysis revealed no significant differences between the two groups [t (5) = 0.92, P = 0.40] in the number of fluorogold-positive cells in the MPN (Fig. 1, B and C).
Figure 1.
Photomicrograph outlining the boundaries of the MPN of the mPOA used for the quantification of ICC experiments (A). Mean ± sem number of fluorogold-labeled cells in the MPN of lactating high or low LG mothers (n = 5/group) after injection in the VTA on postpartum d 6 (B). Photomicrograph depicting cells that stained positively for fluorogold (FG) (C). Mean ± sem number of MPN cells positive for oxytocin (OT) immunoreactivity in high or low LG mothers (*, P < 0.05) (D). Photomicrograph of oxytocin-positive cells in the MPN of high and low LG mothers on postpartum d 6 (E). Mean ± sem number of oxytocin- and fluorogold-positive cells in the MPN of high or low LG mothers on postpartum d 6 (*, P < 0.001) (F). Photomicrograph of single- (positive for oxytocin but not fluorogold immunoreactivity) or double-labeled (positive for both oxytocin and fluorogold immunoreactivity) cells. NOVA RED was used to visualize oxytocin immunoreactivity and DAB to visualize fluorogold immunoreactivity (G). Schematic illustration of the sites of fluorogold infusions of the five high and five low LG dams. All animals included in the analysis had confirmed infusion placement in the VTA (H).
Immunohistochemistry was performed to examine the number of oxytocin-positive cells (n = 5/group). The results revealed that high LG dams showed a significantly higher number of oxytocin-immunoreactive cells in the MPN of high compared with low LG mothers compared with low LG dams [(t (8) = 2.67, P < 0.05; and Fig. 1, D and E].
Sections from the same d 6 postpartum females were double labeled for both oxytocin and fluorogold immunoreactivity (n = 5/group). Statistical analysis revealed a significantly increased number of fluorogold-positive/oxytocin-positive cells in the MPN from high compared with low LG mothers [t (8) = 6.40, P < 0.001; see Fig. 1, F and G]. These findings reveal an increased number of oxytocin-expressing cells in the MPN with direct projections to the VTA in the high compared with low LG mothers. This difference in the number of oxytocin/fluorogold-labeled cells was primarily due to a higher number of oxytocin neurons in the high LG dams. In the MPN, the percentage of oxytocin neurons that were fluorogold positive was comparable across the groups (high = 37.9%; low = 35.0%). In addition, ODs were used to distinguish between single-labeled (oxytocin positive) and double-labeled (oxytocin/fluorogold positive) staining.
Oxytocin projections from the paraventricular nucleus of the hypothalamus (PVNh) to VTA
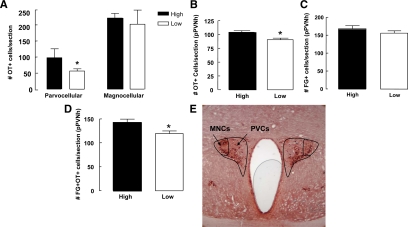
Analysis of the ICC data for singly labeled oxytocin cells revealed a significantly increased number of oxytocin-positive cells in the parvocellular region of the PVNh of high compared with low LG mothers [t (6) = 4.34, P < 0.05; Fig. 2A]. In contrast, we found no differences between high and low LG mothers in the number of oxytocin-positive cells in the magnocellular region of the PVNh on postpartum d 6 [t (6) = 0.42, P = 0.68; Fig. 2A]. The number of oxytocin cells was also analyzed in the supraoptic nucleus of the hypothalamus. High LG mothers showed a significant increase in the number of oxytocin-positive cells compared with low LG mothers at postpartum d 6 [t (8) = 2.48, P < 0.05; Fig. 2B].
Figure 2.
Mean ± sem number of oxytocin-labeled cells (OT+) in the parvocellular and magnocellular regions of the paraventricular nucleus of the hypothalamus PVNh (A) and in the supraoptic nucleus (SON; B) of lactating high and low LG mothers on postpartum day 6 (n = 5/group/*, P < 0.05). Mean ± sem number of fluorogold (FG+)-labeled cells in the parvocellular region of the PVNh (pPVNh) of high or low LG mothers on postpartum d 6 (n = 5/group) (C). Mean ± sem number of oxytocin+/fluorogold+ double-labeled cells in the parvocellular region of the PVNh of high and low LG dams on post partum d 6 (n = 5/group, *, P < 0.05) (D). Photomicrograph outlining the magnocellular (MNCs) and parvocellular (PVCs) regions of the PVNh used for the quantification of immunocytohistochemistry experiments. NOVA RED was used to visualize oxytocin immunoreactivity and DAB to visualize fluorogold immunoreactivity (E).
A single-labeling ICC for fluorogold revealed no significant difference between the number of fluorogold-positive cells in the parvocellular region of the PVNh of high compared with low LG mothers at d 6 postpartum [t (11) = 1.03, P = 0.32; Fig. 2C]. Double-labeling ICC for oxytocin and fluorogold showed a significantly greater number of oxytocin-positive/fluorogold-positive cells in the parvocellular region of the PVNh of high compared with low LG mothers on postpartum d 6 [t (9) = 2.85, P < 0.05; Fig. 2, D and E]. These findings suggest that high LG dams have an increased number of oxytocin projections from the parvocellular region of the PVNh to the VTA compared with low LG dams. The proportion of oxytocin-positive cells in the PVNh that were fluorogold positive did not differ across groups (high = 71.4%; low = 66.7%), suggesting that the difference in the absolute values was due to an increase in the number of oxytocin-positive cells in high LG dams.
The effect of oxytocin on nAcc dopamine transmission
These findings suggest an increased oxytocinergic projection from both the mPOA and the PVNh to the VTA in high LG mothers. We then examined whether oxytocin might act at the level of the VTA to alter the nAcc dopamine signal and whether the activation of nAcc dopamine signal during bouts of LG is mediated by oxytocin receptors in the VTA.
Oxytocin infusion into the VTA increases the dopamine signal in the nAcc
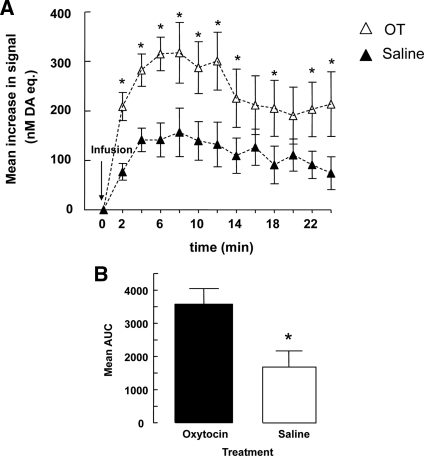
We used in vivo voltametry to examine the acute effects of intra-VTA oxytocin infusions on dopamine signals recorded in the nAcc shell. The results (Fig. 3, A and B) revealed an increased dopamine signal in response to both saline and, to a greater extent, the oxytocin infusion. Thus, a repeated-measures ANOVA revealed a significant main effect of treatment [F (1,6) = 7.99; P = 0.03] and time [F (7,42) = 14.83; P = 0.0001] as well as a significant treatment-by-time interaction effect [F (7,42) = 2.50; = 0.03]. Pair-wise post hoc comparisons confirmed that the increases in the dopamine signal in oxytocin-treated animals were significantly greater than in saline-treated animals at postinjection times 2–14, 18, and 22–24 min (all P values <0.05) but not at later time points, reflecting the relatively short half-life for oxytocin (35). In addition, the difference between the dopamine signal of the oxytocin- and saline-treated animals is partially masked by the acute stress of the infusion, which increases the extracellular dopamine signal. An area under the curve analysis (Fig. 3B) taken from t = 0 (min) to t = 24 (min) confirmed that the increases in the dopamine signal in oxytocin-treated animals were significantly greater than those seen in saline-treated animals [t (6) = 2.81; P < 0.05].
Figure 3.
The mean ± sem increase in dopamine (DA; nanomoles) in the nucleus accumbens shell of virgin females after the infusion of saline or oxytocin (2.4 nmol) into the VTA (n = 5/group). Infusion of oxytocin increased the dopamine (nanomoles) signal at min 2–14, 18, 22, and 24 after infusion by comparison with saline-treated animals (*, P < 0.05) (A). Mean ± sem of total area under the curve (AUC) for dopamine levels measured over the period depicted in A for virgin females infused with oxytocin or saline (*, P < 0.05) (B). Repeated-measures ANOVA was followed by a pair-wise post hoc test.
We then examined whether the differences in the dopamine signal in the nAcc shell between high and low LG mothers during periods of pup LG were associated with oxytocin effects at the level of the VTA. Consistent with previous findings (34), we found no effect of the unilateral OTA infusion into the VTA on the frequency or duration of pup LG (data not shown). Although the characterization of high-low LG mothers relies on multiple observations per day performed over 6 consecutive days, such differences were apparent, even during a single recording period, the equivalent of a single period of observation. As in our previous study (19), the duration of pup LG bouts in high LG mothers was significantly (P < 0.05) longer than that of low LG mothers. This finding is consistent with previous reports (19,23) showing that the differences in pup LG are associated with differences in the duration of individual LG bouts.
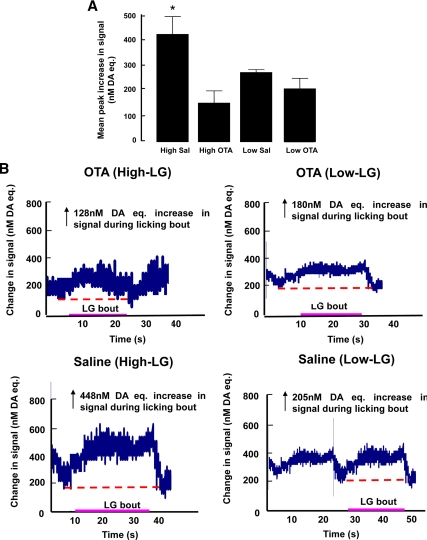
As previously described (19), increases in nAcc dopamine signals were recorded during periods of pup LG and the peak amplitude of such increases differed significantly in high vs. low LG dams (Fig. 4, A–C). We found that the difference in LG-related activation of nAcc dopamine transmission was also associated with a differential sensitivity to intra-VTA blockade of oxytocin receptors with OTA. A two-way ANOVA revealed a main effect of treatment [F (1,11) = 12.66; P = 0.01] such that an intra-OTA infusions significantly attenuated the peak nAcc dopamine responses associated with LG. Most importantly, the analysis revealed a significant treatment by maternal phenotype interaction [F (1,11) = 4.58; P < 0.05]. As expected from our previous studies (19), post hoc analyses revealed that among saline-treated animals, the peak LG-related nAcc increases in the dopamine signal in the nAcc shell during a bout of pup LG were significantly (P < 0.05; Fig. 4A) greater in high LG than low LG mothers. Importantly, intra-VTA OTA infusions significantly attenuated LG-related dopamine signal increases in high LG mothers but had no effect on the dopamine responses seen in low LG mothers. Thus, OTA-treated high and low LG animals showed comparable LG-related increases in nAcc dopamine signals. Interestingly, the mean increase in the amplitude of the dopamine signal in the nAcc of high LG mothers associated with pup LG (∼400 nm) was comparable with that obtained in nonlactating females rats (∼300 nm) after oxytocin infusion into the VTA.
Figure 4.
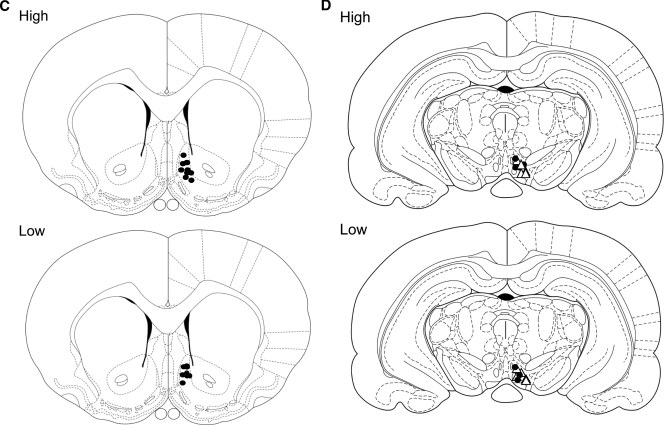
Mean ± sem peak increase in dopamine (DA; nanomoles) in the nAcc shell of high and low LG lactating females on postpartum d 4 or 5 after infusion with either an oxytocin antagonist (OTA) or saline in the VTA (n = 4–5/group). The saline-treated high LG mothers showed a significantly higher dopamine signal (*, P < 0.05) compared with all other groups (A). Representative recordings depicting the change in the dopamine (nanomoles) signal in the nAcc shell of high and low LG mothers before, during, and at the end of a period of pup LG on postpartum d 4/5 after infusion of an oxytocin antagonist (OTA) or saline into the VTA (red dashed line represents baseline) (B). Schematic illustration of the sites of electrochemical probe placement in the high and low LG dams included in the electrochemical and behavioral analysis. All animals included in the analysis had confirmed probe placement in the nAcc shell (n = 4–5/group) (C). Schematic illustration of the sites of cannulae placements in the high and low LG dams included in the electrochemical and behavioral analysis. All animals included in the analysis had confirmed cannulae placement in the VTAea (•, OTA; ▵, saline) (D). A two-way ANOVA was used followed by Bonferroni’s post hoc test. Figure continued on next page.
Discussion
Variations in pup LG among lactating rats is associated with differences in the dopamine signal in the nAcc shell (19). There is an increase in the nAcc dopamine signal that accompanies the onset of pup LG and the termination of the increased dopamine signal corresponds that of pup LG. The magnitude of the increase in the dopamine signal in the nAcc is strongly correlated to the duration of the pup LG bout (19). The differences between high and low LG mothers in the nAcc dopamine signal and in pup LG are abolished by pretreatment with a dopamine reuptake blocker (19). These findings are consistent with previous studies (18,20,21,36,37), revealing the importance dopamine activity in the nAcc for maternal behavior in the rat.
Differences between high and low LG mothers in mesolimbic dopamine activity are associated with the effects of oxytocin at the level of the VTA. Individual differences in pup LG among lactating females derive, in part, from alterations in central oxytocin systems that regulate maternal behavior. Thus, ICV infusions of OTA eliminate the differences in pup LG between high and low LG mothers (13). We found increased oxytocin expression in the parvocellular region of the PVNh of high compared with low LG mothers; the PVNh projects to the mPOA and VTA (38). There were no such differences in the magnocellular neurons that project to the neurohypophysis and regulate milk ejection. Oxytocin commonly acts at oxytocin receptors to increase the firing rate of oxytocin neurons and enhance oxytocin synthesis (i.e. a positive feedback effect) (39,40,41). Lactating high LG mothers show increased oxytocin receptor binding in the mPOA, suggesting greater sensitivity to oxytocin (12,13,15). Increased oxytocin receptor binding in the mPOA of high LG mothers (12,13) combined with a positive feedback effect on oxytocin expression is consistent with the finding of an increased oxytocin expression in the mPOA of high compared with the low LG mothers.
Numan and colleagues (34,42) described the importance of mPOA projections to the VTA for maternal behavior in the rat. Thus, unilateral mPOA lesions combined with lesions of the contralateral VTA impairs maternal behavior (16), as do bilateral OTA infusions into the VTA (9). The tract-tracing study suggests direct projections from oxytocin-positive cells in the mPOA to the VTA as a mechanism for a direct effect of oxytocin on dopamine release from VTA neurons. The results also revealed increased oxytocin projections in high LG mothers (Fig. 1), consistent with the increased oxytocin expression in the mPOA. Whereas these findings focus on the importance of mPOA-VTA projections, it is worth noting an alternative pathway that includes the lateral preoptic area, with subsequent projections to the VTA (43). There was also an increased number of oxytocin-positive cells in the PVNh with projections to the VTA in high compared with low LG mothers.
Infusion of oxytocin directly into the VTA enhanced the dopamine signal in the nAcc shell (also see Ref. 44), suggesting an effect of oxytocin on the dopamine neurons of the VTA. We replicated previous studies showing an increased dopamine signal in the nAcc during periods of pup LG in high compared with low LG mothers (19) and showed that this difference was abolished with an intra-VTA infusion of an oxytocin receptor antagonist (Fig. 4A). These findings suggest that under normal conditions the increased dopamine signal in the nAcc of the high LG mothers is oxytocin dependent. Note, however, that these findings do not necessarily imply a direct effect of oxytocin on dopamine neurons, nor can we exclude the possibility of diffusion from the VTA as a complication.
The oxytocin receptor is expressed in the rat VTA (16) and is a class I G protein-coupled receptor that is primarily coupled via Gq proteins to phospholipase C (45,46) with the capacity to regulate calcium-mediated neuronal events, such as dopamine release. The present findings are also consistent with those showing oxytocin enhanced psychostimulant-induced sensitization (47); such effects are commonly mediated by the activation of the dopamine neurons in the VTA (48). Our findings are also consistent with reports of oxytocin-dopamine interactions in the regulation of social behavior. Thus, an interaction between oxytocin and mesocorticolimbic dopamine systems is implicated in the maintenance of social bonds (e.g. Refs. 49,50,51,52,53). The dopamine signal in the nAcc might mediate an increased expression of appetitive behaviors directed toward pups (22,54). Such maternally initiated (or active) behaviors would include pup LG, and likely pup retrieval, which is also subject to disruption by manipulations that target oxytocin-dopamine pathways (22). The mesocorticolimbic dopamine system modulates behavioral responses to incentive stimuli through projections from the VTA to the nAcc (48,55,56,57,58,59). Pups hold remarkable salience for the lactating female rat and postpartum females bar-press vigorously for access to neonates, a behavior that is abolished by lesions to the mPOA abolish such responses (60). Suckling pups are more reinforcing for lactating rats than is cocaine or food (61,62).
Dopamine levels in the nAcc shell are increased during a nursing bout (18) and 6-hydroxydopamine lesions of either the VTA or nAcc severely disrupt maternal behavior (36,37). Microinjection of mixed dopamine type 1/type 2 receptor antagonists into the nAcc shell impairs maternal behavior, including pup LG (20,21). Activation of the D1 receptor increases FOS expression; maternal contact with pups increases FOS expression in the nAcc (17,63). Our findings suggest that individual differences in maternal behavior associate with variations in the activity of the mesolimbic dopamine system during periods of mother-pup interactions. Thus, Champagne et al. (19) found that infusion of a dopamine reuptake blocker enhanced the dopamine signal in the low LG mothers to that observed in high LG dams and completely eliminated the group differences in pup LG (19). Febo et al. (64) used functional magnetic imaging and showed that suckling from pups increases the BOLD signal in the VTA and nAcc. These effects were reduced with OTA infusion. Likewise functional magnetic imaging studies (65,66, and Popeski N., C. Scherling, A. S. Fleming, J. Lydon, J. C. Pruessner, M. J. Meaney, submitted for publication) with human mothers revealed that activity along dopamine sensitive pathways, including the nAcc, is associated with individual differences in maternal responsivity; increased activity within these systems predicts greater responsivity to infant-related stimuli. Thus, studies across a range of species suggest that individual differences in maternal behaviors derive from variation within mesolimbic oxytocin-dopamine systems.
Footnotes
Disclosure Summary: D.K.S., T.-Y.Z., J.D., A.G., and M.J.M. have nothing to declare.
First Published Online March 12, 2010
For editorial see page 1978
Abbreviations: AA, Ascorbic acid; DAB, 3,3′-diaminobenzidine; ICC, immunocytochemistry; ICV, intracerebroventricular; LG, licking/grooming; mPOA, medial preoptic area; MPN, medial preoptic nucleus; nAcc, nucleus accumbens; OTA, oxytocin receptor antagonist; PVNh, paraventricular nucleus of the hypothalamus; red:ox, reduction to oxidation ratio; RT, room temperature; VTA, ventral tegmental area.
References
- Moltz H, Lubin M, Leon M, Numan M 1970 Hormonal induction of maternal behavior in the ovariectomized nulliparous rat. Physiol Behav 5:1371–1377 [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS 1994 Psychobiology of maternal behavior: contribution to the clinical understanding of maternal behavior among humans. Acta Paediatr Suppl 397:3–8 [DOI] [PubMed] [Google Scholar]
- Bridges RS 1996 Biochemical basis of parental behavior in the rat. Adv Study Behav 25:215–242 [Google Scholar]
- Fleming AS, O'Day DH, Kraemer GW 1999 Neurobiology of mother-infant interactions: experience and central nervous system plasticity across development and generations. Neurosci Biobehav Rev 23:673–685 [DOI] [PubMed] [Google Scholar]
- Giordano AL, Siegel HI, Rosenblatt JS 1989 Nuclear estrogen receptor binding in the preoptic area and hypothalamus of pregnancy-terminated rats: correlation with the onset of maternal behavior. Neuroendocrinology 50:248–258 [DOI] [PubMed] [Google Scholar]
- Pedersen CA 1997 Oxytocin control of maternal behavior. Regulation by sex steroids and offspring stimuli. Ann NY Acad Sci 807:126–145 [DOI] [PubMed] [Google Scholar]
- Numan M, Rosenblatt JS, Komisaruk BR 1977 Medial preoptic area and onset of maternal behavior in the rat. J Comp Physiol Psychol 91:146–164 [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Pfaff DW 1986 Effect of preoptic region implants of dilute estradiol on the maternal behavior of ovariectomized, nulliparous rats. Horm Behav 20:354–363 [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA 1994 Oxytocin activates the postpartum onset of maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci 108:1163–1171 [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange Jr AJ 1979 Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci USA 76:6661–6665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrbach SE, Morrell JI, Pfaff DW 1985 Possible role for endogenous oxytocin in estrogen-facilitated maternal behavior in rats. Neuroendocrinology 40:526–532 [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ 2000 Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol 12:1145–1148 [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ 2001 Variations in maternal care in the rat are associated with differences in estrogen-related changes in oxytocin receptor levels. Proc Nat Acad Sci USA 98:12736–12741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ 2003 Natural variations in maternal care are associated with estrogen receptor α expression and estrogen sensitivity in the MPOA. Endocrinology 144:4720–4724 [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ 2006 Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry 59:1227–1235 [DOI] [PubMed] [Google Scholar]
- Numan M, Smith HG 1984 Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci 98:712–727 [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Swann JM, Stern JM 1998 Forebrain expression of cFos due to active maternal behavior in lactating rats. Neuroscience 82:267–281 [DOI] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S 1993 Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav 45:673–676 [DOI] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ 2004 Individual differences in maternal behavior are mediated by dopamine release in the nucleus accumbens. J Neurosci 24:4113–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keer SE, Stern JM 1999 Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav 67:659–669 [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD 2005 The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci 119:1588–1604 [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS 2009 Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol 30:46–64 [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ 2003 Naturally occurring variations in maternal care in the rat as a mediating influence for the effects of environment on the development of individual differences in stress reactivity. Physiol Behav 79:359–371 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C 1986 The rat brain in stereotaxic coordinates. 2nd ed. New York: Academic Press [Google Scholar]
- Doherty MD, Gratton A 1997 NMDA receptors in nucleus accumbens modulate stress-induced dopamine release in nucleus accumbens and ventral tegmental area. Synapse 26:225–234 [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Friedemann M, Brodie MS, Vickroy TW, Gratton AP, Hoffer BJ, Rose GM 1989 The effects of cholecystokinin (CCK-8) on dopamine-containing nerve terminals in the caudate nucleus and nucleus accumbens of the anesthetized rat: an in vivo electrochemical study. Brain Res 499:157–163 [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Oke AF, Nagy G, Moghaddam B, Adams RN 1984 Nafioncoated electrodes with high selectivity for CNS electrochemistry. Brain Res 290:390–395 [DOI] [PubMed] [Google Scholar]
- Gratton A, Hoffer BJ, Gerhardt GA 1989 In vivo electrochemical studies of monoamine release in the medial prefrontal cortex of the rat. Neuroscience 29:57–64 [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Gratton A 1991 Opioid modulation and sensitization of dopamine release elicited by sexually relevant stimuli: a high speed chronoamperometric study in freely behaving rats. Brain Res 551:20–27 [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Gratton A 1992 Partial dopamine depletion of the prefrontal cortex leads to enhanced mesolimbic dopamine release elicited by repeated exposure to naturally reinforcing stimuli. J Neurosci 12:3609–3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentney RJ, Gratton A 1991 Effects of local Δ and μ opioid receptor activation on basal and stimulated dopamine release in striatum and nucleus accumbens of rat: an in vivo electrochemical study. Neuroscience 45:95–102 [DOI] [PubMed] [Google Scholar]
- Doherty MD, Gratton A 1992 High-speed chronoamperometric measurements of mesolimbic and nigrostriatal dopamine release associated with repeated daily stress. Brain Res 586:295–302 [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Johns JM, Faggin BM, Senger MA, Pedersen CA 1994 Infusion of an oxytocin antagonist into the medial preoptic area prior to progesterone inhibits sexual receptivity and increases rejection in female rats. Horm Behav 28:288–302 [DOI] [PubMed] [Google Scholar]
- Numan M, Sheehan TP 1997 Neuroanatomical circuitry for mammalian maternal behavior. Ann NY Acad Sci 807:101–125 [DOI] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS 1987 Hormonal factors influence the onset of maternal aggression in laboratory rats. Horm Behav 21:253–267 [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K 1991 Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav Neurosci 105:588–598 [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K 1991 The effects of 6-OHDA-induced dopamine depletions in the ventral and dorsal striatum on maternal and sexual behavior in the female rat. Pharmacol Biochem Behav 39:71–77 [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW 1982 Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol 205:260–272 [DOI] [PubMed] [Google Scholar]
- Kombian SB, Hirasawa M, Mouginot D, Pittman QJ 2002 Modulation of synaptic transmission by oxytocin and vasopressin in the supraoptic nucleus. Prog Brain Res 139:235–246 [DOI] [PubMed] [Google Scholar]
- Wang YF, Hatton GI 2007 Dominant role of βγ subunits of G-proteins in oxytocin-evoked burst firing. J Neurosci 27:1902–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Aragona BJ 2004 Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav 83:319–328 [DOI] [PubMed] [Google Scholar]
- Numan MJ, Insel TR 2003 The neurobiology of parental behavior. 3rd ed. New York: Springer [Google Scholar]
- Numan M 1988 Neural basis of maternal behavior in the rat. Psychoneuroendocrinology 13:47–62 [DOI] [PubMed] [Google Scholar]
- Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, Boi A, Ferri GL, Argiolas A 2007 Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur J Neurosci 26:1026–1035 [DOI] [PubMed] [Google Scholar]
- Zingg HH, Laporte SA 2003 The oxytocin receptor. Trends Endocrinol Metab 14:222–227 [DOI] [PubMed] [Google Scholar]
- Gimpel G, Fahrenholz F 2001 The oxytocin receptor system: structure, function and regulation. Physiol Rev 81:629–683 [DOI] [PubMed] [Google Scholar]
- Sarnyai Z 1998 Oxytocin and neuroadaptation to cocaine. Prog Brain Res 119:449–466 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J 1991 Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev 16:223–244 [DOI] [PubMed] [Google Scholar]
- Wang Z, Yu G, Cascio C, Liu Y, Gingrich B, Insel TR 1999 Dopamine D2 receptor-mediated regulation of partner preferences in female prairie voles (Microtus ochrogaster): a mechanism for pair bonding? Behav Neurosci 113:602–611 [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX 2003 Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121:537–544 [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z 2004 The neurobiology of pair bonding. Nat Neurosci 7:1048–1054 [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z 2006 Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci 9:133–139 [DOI] [PubMed] [Google Scholar]
- Insel TR 2003 Is social attachment an addictive disorder? Physiol Behav 79:351–357 [DOI] [PubMed] [Google Scholar]
- Stern JM 1997 Offspring induced nurturance: animal-human parallels. Dev Psychobiol 31:19–37 [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP 1989 Brain dopamine and reward. Annu Rev Psychol 40:191–225 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW 2005 Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8:1481–1489 [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE 1998 What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev 28:309–369 [DOI] [PubMed] [Google Scholar]
- Phillips AG, Ahn S, Howland JG 2003 Amygdalar control of the mesocorticolimbic dopamine system: parallel pathways to motivated behavior. Neurosci Biobehav Rev 27:543–554 [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J 1999 The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev 31:6–41 [DOI] [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS 2000 Mother rats bar-press for pups: effects of lesions of the MPOA and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res 108:215–231 [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI 2001 Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci 115:683–694 [DOI] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan Jr JM, Harder JA, Messenger TL, Febo M 2005 Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci 25:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Fleming AS 2003 The nucleus accumbens shell is critical for the normal expression of pup-retrieval in postpartum female rats. Behav Brain Res 145:99–111 [DOI] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF 2005 Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup boding during suckling. J Neurosci 25:11637–11644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ 2004 Orbital frontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage 21:583–592 [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV 2004 Mothers’ neural activation in response to pictures of their children and other children. Biol Psychiatry 56:225–232 [DOI] [PubMed] [Google Scholar]