Abstract
Maintenance of glucose homeostasis depends on adequate amount and precise pattern of insulin secretion, which is determined by both β-cell secretory processes and well-developed microvascular network within endocrine pancreas. The development of highly organized microvasculature and high degrees of capillary fenestrations in endocrine pancreas is greatly dependent on vascular endothelial growth factor-A (VEGF-A) from islet cells. However, it is unclear how VEGF-A production is regulated in endocrine pancreas. To understand whether signal transducer and activator of transcription (STAT)-3 is involved in VEGF-A regulation and subsequent islet and microvascular network development, we generated a mouse line carrying pancreas-specific deletion of STAT3 (p-KO) and performed physiological analyses both in vivo and using isolated islets, including glucose and insulin tolerance tests, and insulin secretion measurements. We also studied microvascular network and islet development by using immunohistochemical methods. The p-KO mice exhibited glucose intolerance and impaired insulin secretion in vivo but normal insulin secretion in isolated islets. Microvascular density in the pancreas was reduced in p-KO mice, along with decreased expression of VEGF-A, but not other vasotropic factors in islets in the absence of pancreatic STAT3 signaling. Together, our study suggests that pancreatic STAT3 signaling is required for the normal development and maintenance of endocrine pancreas and islet microvascular network, possibly through its regulation of VEGF-A.
STAT3 signaling is required for the normal development and maintenance of endocrine pancreas and islet microvascular network.
Insulin is the major anabolic hormone that regulates glucose homeostasis. Insulin is stored and secreted from β-cells via a complex and highly regulated process. Under physiological conditions, an elevation of blood glucose triggers rapid uptake of glucose into pancreatic β-cells through glucose transporter (Glut)-2. Glucose metabolism inside β-cells results in increased ATP to ADP ratio, which leads to ATP-sensitive potassium channel closure, membrane depolarization, and subsequent opening of voltage-gated calcium channels and the rise in cytoplasmic calcium concentration (1). Glucose-stimulated insulin secretion consists of two phases: a 10- to 15-min rapid first phase and a less prominent but sustained second phase (2). The first phase requires a rapid and marked elevation of intracellular calcium, whereas the second phase requires amplifying signals from glucose metabolism in addition to oscillatory intracellular calcium (3). Upon exocytosis of insulin-containing secretory granules, insulin is released into blood stream and carried to target organs, such as liver and muscle. Maintenance of glucose homeostasis depends on adequate amount and precise pattern of insulin secretion, which is determined by both β-cell secretory processes and the extensive and well-developed microvascular network within the endocrine glands. Although islets constitute only about 1% of the total pancreatic volume, endocrine pancreas receives up to 5–10% of total pancreatic blood flow (4,5). Pancreatic islets are supplied with arterial blood from arterioles, which form a dense glomerulus-like capillary network after penetrating the islet capsule, leaving all endocrine cells within one-cell distance from arterial blood (6). Furthermore, the islet capillaries have 10 times more fenestrations than those in the exocrine parenchyma (7). The unique structural organization of the endocrine pancreas and microvascular network ensures efficient release of insulin into circulating system and timely delivery to target tissues.
The development of highly organized microvascular network and high degrees of capillary fenestrations in the endocrine pancreas is largely dependent on vascular endothelial growth factor (VEGF)-A from islet cells (8,9,10,11). In β-cell-specific VEGF-A knockout (KO) mice, development of the islet microvasculature and islet capillary fenestrations is impaired, leading to reduced insulin response in vivo and glucose intolerance (12). On the other hand, overexpression of VEGF-A results in pancreas hypervascularization, and hyperplasia of pancreatic islets (13).
Levels of VEGF-A is up-regulated in human pancreatic cancer specimens, and the up-regulation correlates with elevated STAT3 activation (14), which is critical for angiogenesis of pancreatic tumors (15). These studies suggest that signal transducer and activator of transcription (STAT)-3 signaling may promote pancreatic tumor angiogenesis, growth, and metastasis by regulating VEGF expression. Furthermore, a putative binding site for STAT3 has been identified on the VEGF promoter, providing further evidence for a direct role of STAT3 in the regulation of VEGF expression (16).
Given the potential regulatory role of STAT3 in VEGF-A production, we reason that STAT3 signaling in the pancreas may be necessary during the normal development of islet microvascular network and pancreatic islets through its regulation of VEGF-A production. We tested this hypothesis by using a pancreas-specific STAT3 KO mouse model, in which STAT3 signaling in the pancreas is eliminated from the very early stage of pancreas development. We found that pancreas-specific deletion of STAT3 resulted in glucose intolerance and impaired insulin secretion in vivo, whereas insulin secretion from perifused isolated islets was normal. The KO mice exhibited reduced vascular density along with decreased VEGF-A expression level in islets in the absence of STAT3-signaling in the pancreas. Together our results establish a direct role of pancreatic STAT3 signaling to the normal development and maintenance of endocrine pancreas and islet microvascular network through its regulation of VEGF-A expression.
Materials and Methods
Animal welfare
All animal experiments were performed in accordance with the protocol approved by the Institutional Animal Care and Use Committee of Agency for Science, Technology, and Research Biomedical Sciences Institutes. The mice were bred and housed in our animal facilities with standard rodent diet and water provided ad libitum.
Generation of pancreas-specific STAT3 KO mouse
Pancreas-specific STAT3 KO mice (p-KO) were generated by crossing STAT3fl/fl mice containing Cre transgene under pancreas-duodenum homeobox gene 1 (Pdx1) promoter (Pdx1-Cre) with STAT3fl/fl mice. STAT3fl/+ was a generous gift from Drs. Kiyoshi Takeda and Shizuo Akira (Osaka University, Osaka, Japan) and Pdx1-Cre from Dr. Doug Melton (Harvard University, Boston, MA). Littermates with genotypes of STAT3fl/fl/Pdx1-Cre (P-KO) and STAT3fl/fl (control) were used in this study. Genotyping was performed on tail DNA by PCR using the following primers: 5′-TGC TTC TGT CCG TTT GCC GGT-3′ and 5′-CTA AGT GCC TTC TCT ACA CCT-3′ for Pdx1-Cre (500 bp); 5′-CCT GAA GAC CAA GTT CAT CTG TGT GAC-3′ and 5′-CAC ACA AGC CAT CAA ACT CTG GTC TCC-3′ for STAT3 alleles (PCR fragment of 280 and 350 bp for wild type and mutant allele, respectively).
Glucose and insulin tolerance tests
Intraperitoneal glucose tolerance tests (IPGTTs) were performed on 8- to 12-wk-old mice. Animals were studied after 18 h fasting with free access to water. Blood glucose levels were tested before and at 15, 30, 60, 90, and 120 min after ip injection of 20% d-glucose solution at 2 g/kg body weight. For insulin tolerance tests, fed animals were ip injected with human biosynthetic insulin Astrapid HM Penfill (1 U/kg body weight; Novo Nordisk, Copenhagen, Denmark). Glucose concentration was measured before and at 15, 30, 60, and 90 min after insulin injection. For measurement of glucose-stimulated insulin secretion in vivo, mice were ip injected with 20% d-glucose solution at 2 g/kg body weight after 18 h fasting. Blood glucose was measured before and at 8, 15, and 30 min after glucose injection. Blood glucose levels were monitored by measuring tail-vein blood samples using Accu-Chek Advantage blood glucose meter (Roche Diagnostics GmbH, Mannheim, Germany). Insulin levels were measured in plasma samples obtained from tail-vein blood after mixing with 2 μl of 0.5 m EDTA on ice and centrifugation at 10,000 × g for 10 min by using the Ultrasensitive mouse insulin ELISA kit (Mercodia, Uppsala, Sweden).
Immunohistochemistry
Pancreatic tissue was harvested, fixed in 4% paraformaldehyde for 3–4 h at 4 C, washed in PBS, and incubated in 30% sucrose-PBS solution at 4 C overnight. The tissue was then embedded in Tissue-Tek optimum cutting temperature compound medium (Ted Pella, Redding, CA), frozen, and stored at −80 C. Five-micrometer cryosections mounted on Polysine glass slides (Menzel GmbH, Freiburg, Germany) were washed in PBS with 0.1% Triton X-100, blocked with 5% goat serum for 30 min, and incubated with primary antibodies overnight at 4 C. A monoclonal rat antimouse CD31 (platelet endothelial cell adhesion molecule-1) antibody (Invitrogen, Carlsbad, CA) at 1:200 dilution, guinea pig antihuman insulin at 1:200 dilution (Dako, Carpinteria, CA), polyclonal rabbit anti-VEGF-A (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:100 dilution, and polyclonal rabbit anti-STAT3 (Cell Signaling Technology, Beverly, MA) at 1:100 dilution were used for CD31, insulin, VEGF-A, and STAT3 detection, respectively. After washing, appropriate combinations of secondary antibodies, Alexa Fluor 594-conjugated goat antirabbit Ig, Alexa Fluor 488-conjugated goat anti-guinea pig, and Alexa Fluor 488-conjugated goat antirat at 1:400 dilution were applied and incubated for 40 min. Primary and secondary antibodies were diluted in PBS containing 5% goat serum. After immunolabeling, the sections were washed and mounted using Vectashield medium with 4′,6′-diamino-2-phenylindole (Vector Laboratories, Burlingame, CA). Digital images of the sections were acquired on an LSM510 confocal laser scanning microscope (Zeiss, Jena, Germany).
Evaluation of vascular density and expression of VEGF-A by immunostaining
Five-micrometer cryosections from different parts of the pancreas were immunostained with antibody against endothelial marker CD31 (platelet endothelial cell adhesion molecule-1) or VEGF-A. After immunostaining, confocal images of 10 serial sections were acquired using a Zeiss LSM510 META confocal microscope (objectives ×40, ×63; Carl Zeiss). All images were acquired with identical settings on the laser-scanning system. Vascular density was assessed as area of positive CD31 signal above a preset threshold value relative to the whole pancreatic area using Image Pro-Plus software (Media Cybernetics, Inc., Bethesda, MD).
Pancreatic islet isolation and insulin secretion
Pancreatic islets were isolated from mice by liberase digestion as previously described (17). Isolated islets were cultured overnight in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal calf serum, 2 mm l-glutamine, 15 mm HEPES, 1% streptomycin and penicillin, and 11.1 mm glucose. Subsequent handling was performed using Krebs-Ringer HEPES (KRH) buffer containing (in millimoles): 130 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.56 CaCl2, 20 HEPES, and supplemented with 1 mg/ml BSA and 3 (basal) or 20 mm (stimulatory) glucose. After 1 h incubation in KRH buffer containing 3 mm glucose, 20 islets were loaded in a chamber and continuously perifused for another 30 min with the same buffer at flow rate of 1 ml/min at 37 C and then stimulated with KRH buffer containing 20 mm glucose. Perifusion fractions were collected every 3 min for 40 min, starting at 6 min before switching to 20 mm glucose. Insulin concentration in fractions was measured using mouse insulin ELISA (Mercodia).
Combined fluorescence measurements of reduced nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide phosphate [NAD(P)H]
This was performed essentially as previously described (17). Briefly, islets were incubated for 60 min at 3 mm glucose and then placed in a perifusion chamber on an inverted microscope, and continuously superfused with the same buffer at 37 C. After 10 min of perifusion with 3 mm glucose, islets were stimulated with 20 mm glucose. NAD(P)H fluorescence was captured at 5-sec intervals on a CoolSNAP HQ2 charge-coupled device camera (Photometrics, Tucson, AZ) using the following optical filters from Omega: 380DF30, 415DCLP, and 440AF21. Fluorescence increase was measured as the difference between the level immediately before the start of increase and the early plateau phase. Lag time was determined as the interval between the beginning of stimulation and the appearance of fluorescence rise.
Ca2+ measurements
Fura-2-loaded isolated islets were placed in a perifusion chamber on the stage of a Eclipse TE2000-U (Nikon, Tokyo, Japan) and continuously superfused with KRH buffer at 37 C. The islets were positioned to be close to the inflow tube. Fura-2 was excited at 340 and 380 nm using a λ-DG-4 (Sutter Instrument Co., Navato, CA) and emitted signal projected onto a charge-coupled device camera (CoolSNAP HQ2; Photometrics) behind a band pass filter (535DF35; Omega Optical, Tarzana, CA). Islets were perifused with KRH containing 3 mm glucose for 15 min before the buffer was switched to KRH containing 20 mm glucose for an additional 30 min. Images were collected every 2 sec, and fluorescence signals from individual cells were measured as a function of time using Metafluor software (Molecular Devices, Sunnyvale, CA). Ca2+ concentration was calculated from 340:380 nm ratio (18). Lag time for intracellular free calcium concentration ([Ca2+]i) rise was defined as the time from the buffer switch to the first value above baseline average, which was calculated during the 2 min before stimulation. Calcium rise was calculated as the difference between the average basal value and highest peak value.
Western blot analysis
Brain and pancreas samples were removed from mice, frozen in liquid nitrogen, and kept at −80 C until use. Frozen tissue samples were homogenized in a Tris-based lysis buffer at 4 C. Protein was quantified by using DC protein assay (Bio-Rad, Hercules, CA). Samples containing 80 μg of protein were loaded in each well, resolved on a 12% sodium dodecyl sulfate-polyacrylamide gel, and transferred to a nitrocellulose membrane. The membranes were incubated with antibody against STAT3 (1:2000; Cell Signaling) or tubulin (1:1000; Sigma, St. Louis, MO) overnight at 4 C. After incubation with secondary horseradish peroxidase-conjugated antibody, the signals from protein bands were visualized by using enhanced chemiluminescence chemiluminescent system (Amersham Biosciences, Piscataway, NJ).
RNA extraction and quantitative RT-PCR
Quantitative real-time PCR was performed using SYBR Green chemistry and gene-specific primers on an Applied Biosystems Prism 7500 sequence detection system or StepOnePlus real-time PCR system (Applied Biosystems) (19). Total RNA was extracted from about 150 islets of control and p-KO mice with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. RNA samples were reverse transcribed into cDNAs using the TaqMan reverse transcription reagents kit (Roche Molecular Systems, Pleasanton, CA). β-Actin was used as the internal standard to determine relative mRNA levels. Sequences of the primers that were used in quantitative PCR analyses are available on request.
Quantification of VEGF-A content
For estimation of VEGF-A content, batches of 12 isolated islets were incubated for 24 h in a medium with 11.1 mm glucose and then sonicated in 200 μl Tris-based lysis buffer on ice. After quantification with Bio-Rad DC protein assay, 20 μl of lysate containing equal total amount of protein were used for the assay. VEGF-A content in the islet lysate was measured using mouse VEGF ELISA kit (IBL-Hamburg, Hamburg, Germany).
Statistical analysis
The data are presented as means ± sem. Comparisons of data were made by using two-tailed Student’s t test for independent data. The level of statistical significance was set at P < 0.05.
Results
Generation of pancreas-specific STAT3 KO mice
To investigate whether STAT3 signaling in the pancreas was involved in the regulation of pancreatic islet development and maintenance, including development of microvascular network in the islets, we generated p-KO mice by crossing STAT3fl/fl mice with and without Pdx1-Cre transgene (Fig. 1A) (8,20). p-KO mice and littermate STAT3fl/fl (control) were used in the experiments. Pdx1 promoter drives specific expression of Cre recombinase in both endocrine and exocrine pancreas, with no detectable expression in the hypothalamus, in contrast to the rat insulin promoter (RIP)-Cre mouse line, in which Cre is expressed in both pancreas and hypothalamus (8,21,22). Under the Pdx1 promoter, Cre expression in the pancreatic epithelium starts at early embryonic stages, which ensures STAT3 deletion before islet formation (8).
Figure 1.
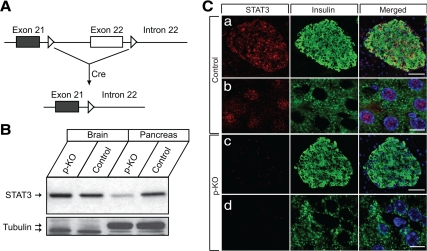
Generation of p-KO mice and assessment of STAT3 deletion in pancreatic tissue. A, Exon 21 and part of exon 22 of STAT3 gene were flanked by loxP sites (open triangle) in STAT3fl/fl mice. In p-KO mice, Cre-mediated recombination removes sequences between the loxP sites (KO) and results in specific inactivation of STAT3 gene in the pancreas without affecting STAT3 expression in other tissues. B, STAT3 protein levels were assessed by Western blot analysis using polyclonal STAT3 antibody. Equal amounts of brain and pancreatic tissue extracts from KO and control mice were resolved by SDS-PAGE. STAT3 was barely detectable in the pancreas of STAT3 KO mice, whereas it was expressed at a similar level in the brain as control. Tubulin was used as loading control. C, Immunohistochemical analysis of STAT3 and insulin expression was performed on 5-μm pancreatic cryosections of 12-wk-old p-KO and control mice. STAT3 (red) was localized throughout the islet area in control but was undetectable in p-KO mice. Scale bar, 50 (a and c) and 5 μm (b and d).
We evaluated the efficiency of STAT3 deletion in the pancreas of p-KO mice. STAT3 was expressed at abundant level in extracts from pancreatic tissue of control mice but reduced to barely detectable level in the pancreas of the p-KO mice (Fig. 1B). The detected residual STAT3 protein in the p-KO mice was most likely from contaminating endothelial and blood cells because protein extracts were prepared from snap-frozen samples of whole pancreata. STAT3 was shown to be expressed in these cells, and STAT3 expression in blood vessels was not affected in p-KO mice (Refs. 23 and 24 and data not shown). Consistent with the restricted expression of Pdx1-cre in the pancreas, we did not observe STAT3 reduction in the brain of p-KO mice, as evidenced by the comparable levels of STAT3 mRNA (data not shown) and protein (Fig. 1B) in samples obtained from brains of p-KO and control mice. To further assess the extent of STAT3 deletion in pancreas, particularly in pancreatic islets, we performed immunohistochemistry on pancreatic cryosections of control and p-KO mice. STAT3 immunoreactivity was readily detectable throughout the islet area of control mice, with intense signal concentrated in the nucleus of endocrine cells, such as β-cells (Fig. 1C), whereas only scattered background staining was observed in p-KO pancreas, which could be due to signals from endothelium or residual blood cells (Fig. 1C) (23,24). STAT3 levels decreased to almost background in both islets and exocrine tissues of p-KO mice (Fig. 1C and data not shown). These results demonstrated that STAT3 was efficiently deleted only in pancreas but not in brain or blood vessels.
p-KO mice exhibit impaired glucose tolerance but normal insulin sensitivity
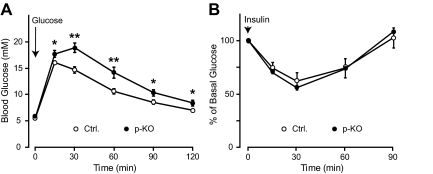
To assess the effect of STAT3 deletion in the pancreas on systemic glucose homeostasis, we measured resting and fasting glucose levels, and performed IPGTTs on overnight-fasted p-KO mice and their control littermates between 8 and 12 wk old. There was no difference in fasting (5.1 ± 0.2 mmol/liter, n = 15, p-KO; 5.2 ± 0.1 mmol/liter, n = 23, control; P = NS) or resting (9.8 ± 0.4 mmol/liter, n = 12, p-KO; 9.6 ± 0.3 mmol/liter, n = 25, control; P = NS) glucose levels between p-KO and control mice. We then tested glucose response in the p-KO and control mice by using IPGTTs. Female p-KO mice showed significantly higher glucose levels at all time points after glucose injection (Fig. 2A), indicating that they were glucose intolerant. However, we did not observe similar glucose intolerance in male p-KO mice because the responses from male mice were highly variable among the four groups tested (data not shown); therefore, female mice were used in subsequent experiments. To ensure that the glucose intolerance phenotype was not due to Pdx1-directed Cre recombinase expression, we compared Pdx1-cre transgenic mice with wild-type control and found no difference in glucose levels and glucose tolerance between the two groups (data not shown). We also tested whether p-KO mice had lower sensitivity to insulin compared with control by ip insulin tolerance test. Insulin induced a similar drop in glucose levels in p-KO mice and their control throughout the tests, indicating that insulin sensitivity was normal in p-KO mice (Fig. 2B). To further evaluate insulin signaling in p-KO and control mice, we examined Akt phosphorylation in liver and soleus muscle after insulin injection and found that insulin induced a similar degree of Akt phosphorylation in the liver and muscle preparations (data not shown). Together, these data demonstrated that the delayed glucose clearance in p-KO mice was not due to impaired liver or muscle responses to insulin but most likely the result of impaired glucose-stimulated insulin secretion.
Figure 2.
Impaired glucose tolerance and normal insulin sensitivity in STAT3 KO mice. A, Glucose levels of p-KO mice and control littermates were measured before and at 15, 30, 60, 90, and 120 min after ip glucose injection. p-KO mice (filled circle, n = 14) showed higher glucose levels than control (open circle, n = 12) after glucose challenge. *, P < 0.05; **, P < 0.01. B, Insulin tolerance tests were performed on fed p-KO (filled circle, n = 11) and control (open circle, n = 11) mice. Blood glucose levels were similarly affected by insulin at all time points in p-KO and control mice. Data are presented as means ± sem.
p-KO mice show impaired glucose-stimulated insulin secretion in vivo
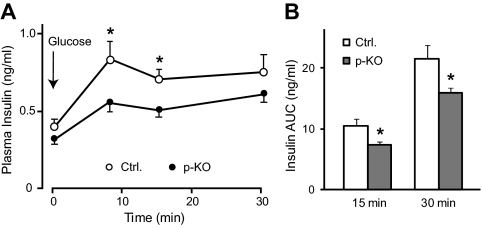
To determine whether glucose-induced insulin secretion was impaired in p-KO mice, we measured insulin secretion before and at 8, 15, and 30 min after a bolus injection of glucose at 2 g/kg body weight in p-KO and control mice. After overnight fasting, basal insulin levels were not different between p-KO and control mice. However, p-KO mice showed consistently lower insulin levels after glucose challenge (Fig. 3A), indicating impaired glucose-induced insulin secretion. Net insulin secretion by glucose stimulation was calculated as area under the curve after baseline subtraction. Both the first phase (15 min) and total (30 min) insulin secretion was reduced in p-KO mice (Fig. 3B), which could account for the delayed glucose clearance during IPGTT.
Figure 3.
STAT3 KO mice exhibited impaired glucose-induced insulin release in vivo. A, Plasma insulin levels were measured before and at 8, 15, and 30 min after ip glucose injection in p-KO (filled circle, n = 12) and control mice (open circle, n = 11) after overnight fasting. Insulin levels were lower in p-KO mice after glucose challenge. B, Total glucose-stimulated insulin secretion, calculated by integrating area under curve in A, was reduced in p-KO mice (gray bars, n = 12) during the first 15 min and the entire 30 min of stimulation compared with control mice (white bars, n = 11). Data are presented as means ± sem. *, P < 0.05.
p-KO mice exhibit normal glucose-stimulated insulin secretion in isolated islets in vitro
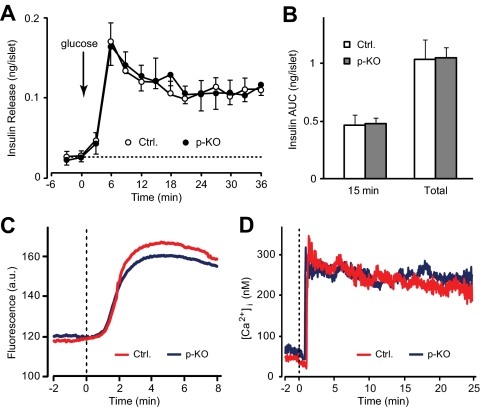
We then tested whether impaired glucose-stimulated insulin secretion persisted in isolated islets, which would indicate β-cell secretory defects. Insulin secretion was measured in isolated and overnight-cultured islets from p-KO and control mice. No difference in insulin release was observed between p-KO and control islets during the initial 6 min before stimulation. Increase of glucose concentration in perifusion buffer from 3 to 20 mm resulted in a sharp rise in insulin release, which reached peak at 6 min after stimulation, followed by a sustained but less-prominent elevation in insulin levels. Insulin secretion patterns were indistinguishable between p-KO and control islets (Fig. 4A). Glucose-induced insulin secretion in vitro was calculated as the sum of insulin amount per islet in all fractions during the first 15 min, corresponding to the first phase of insulin secretion, or during the entire period of stimulation (total) after subtraction of basal secretion. There was no difference in the first phase or total insulin secretion in isolated islets from p-KO and control mice (Fig. 4B).
Figure 4.
Normal insulin secretion from perifused isolated islets of STAT3 KO mice. A, Glucose-stimulated insulin secretion was measured in isolated islets from p-KO and control mice. Groups of 20 islets were incubated for 1 h in KRH buffer containing 3 mm glucose (basal) before switching (arrow) to 20 mm glucose (stimulatory). There was no difference in basal and glucose-stimulated insulin secretion in islets from p-KO (filled circle, n = 4) and control (open circle, n = 4) mice. B, Net glucose-stimulated insulin secretion, calculated as the sum of insulin amount per islet in all fractions during the first 15 min (first phase) or the entire stimulation period (total) after baseline subtraction. No difference was observed in the first phase or total insulin secretion between p-KO (gray bars, n = 4) and control (white bars, n = 4) mice. Data are presented as means ± sem. C, Representative traces of NAD(P)H fluorescence from p-KO (blue) and control (red) mouse islets (n = 12 for each group). The islets were perifused in 3 mm glucose before the perfusion buffer was switched to 20 mm glucose (dotted line). Both p-KO and control groups displayed similar time course. D, Representative traces of calcium recordings from p-KO (blue) and control (red) mouse islets (n = 18 and 13 for p-KO and control, respectively). The islets were initially perifused in 3 mm glucose before the perfusion buffer was switched to 20 mm glucose (dotted line). Cytosolic calcium levels were measured using fura-2AM. Both p-KO and control groups exhibited similar calcium responses.
To further investigate possible defects in β-cell secretory processes in p-KO mice, we examined proteins and cellular signals involved in the insulin secretion process. Quantitative PCR analysis showed that expression levels of Glut2, glucokinase, and insulin receptor substrate-1 and -2 were not affected by STAT3 deletion in p-KO mice (fold change vs. control: 1.15 ± 0.10, 1.12 ± 0.05, 1.06 ± 0.07, and 1.10 ± 0.09 for Glut2, glucokinase, and insulin receptor substrate-1 and -2, respectively; n = 4 independent experiments in triplicates, P = NS). Combined redox signal from NAD(P)H in glucose-stimulated islets, a measure of mitochondrial and glycolytic function (25), was used to examine the metabolic response in p-KO mice. NAD(P)H fluorescence response to 20 mm glucose followed a similar pattern (Fig. 4C). There was no difference in the timing of response (58.8 ± 4.9 vs. 63.5 ± 4.4 sec, p-KO vs. control, n = 12 and 11 for p-KO and control, respectively, P = NS) and the relative fluorescence increase from basal levels (50.5 ± 5.9 vs. 49.6 ± 6.5, p-KO vs. control, n = 12 and 11 for p-KO and control, respectively, P = NS) between p-KO and control islets. These results indicate that mitochondrial and glycolytic function, a key determinant of downstream secondary signals such as [Ca2+]i rise, was normal in p-KO β-cells.
We then recorded calcium changes to high glucose in fura-2-loaded p-KO and control mouse islets. A typical cytoplasmic calcium response in pancreatic islets consists of a short silent period, initial lowering in [Ca2+]i followed by a sharp rise, and subsequent decrease. After that, the dynamics of [Ca2+]i changes normally follows an oscillatory pattern (Fig. 4D) (26). When stimulated with 20 mm glucose, calcium responses in p-KO and control islets were nearly identical (Fig. 4D). There was no difference in lag time after stimulation (56 ± 3 vs. 51 ± 4 sec, p-KO vs. control, n = 17 and 12 for p-KO and control, respectively, P = NS), nadir of initial lowering (16 ± 3 vs. 19 ± 3 nm, p-KO vs. control, n = 17 and 12 for p-KO and control, respectively, P = NS), calcium increase from baseline level (134 ± 23 vs. 126 ± 21 nm, p-KO vs. control, n = 17 and 12 for p-KO and control, respectively, P = NS), or the rate of calcium oscillations (0.3 ± 0.02 vs. 0.3 ± 0.03 min−1, p-KO vs. control, n = 17 and 12 for p-KO and control, respectively, P = NS). These data demonstrate that p-KO mice produced normal cytoplasmic calcium responses on glucose stimulation. Taken together, p-KO mice had no detectable defects in glucose uptake, glucose metabolism, and calcium responses in pancreatic islets; thus, the impaired insulin secretion in vivo was not due to defective β-cell secretory processes but most likely was the result of impaired collection of released insulin into the circulatory system.
Decreased vascular density in the pancreas and reduced VEGF-A expression level in pancreatic islets of p-KO mice
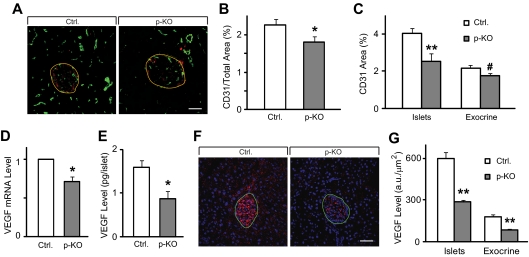
To investigate the involvement of STAT3 in the formation of pancreatic microvascular network, we labeled blood vessels with endothelial cell-specific marker CD31 and assessed vascular density in the pancreas of p-KO and control mice (Fig. 5A). Total area of CD31 signal relative to the whole pancreas was significantly reduced in STAT3 KO mice (Fig. 5B), suggesting that microvascular network in the pancreas of p-KO mice was less developed than that of control mice. Vascular density, as indicated by CD31-labeled area, in endocrine pancreas appeared to be more severely affected by STAT3 deletion, whereas it was marginally reduced in exocrine tissue (Fig. 5C). To ensure that the observed reduction in vascular density was not the result of reduced CD31 level caused by STAT3 deletion, we tested the vascular density with two additional endothelial markers, isolectin-B4 and VE-cadherin in the pancreas of p-KO and control mice (27). Quantification of vascular density by isolectin-B4 and VE-cadherin labeling yielded similar results as by CD31 staining (Supplemental Figs. 1 and 2 published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org).
Figure 5.
Reduced islet microvascular density and lower VEGF-A expression level in STAT3 KO mice. A, Five-micrometer cryosections of p-KO and control mouse pancreas were immunolabeled with STAT3 (red) and endothelial marker CD31 (green). B, Vascular density, measured as CD31-stained area relative to the whole pancreas was reduced in p-KO (gray bar, n = 3 mice) compared with control mice (white bar, n = 3 mice). Images taken from 10 pancreatic sections of each animal were used for analysis. C, Vascular density in the islets (e.g. inside yellow boundary in A) and exocrine pancreas (e.g. outside yellow boundary in A) was similarly assessed as in B, Vascular density was significantly reduced in the islets (**, P < 0.01) and marginally reduced in the exocrine tissue (#, P = 0.06). Data are presented as means ± sem. Images taken from 10 pancreatic sections of each mouse, three mice per group, were used for analysis. D, VEGF-A mRNA levels were analyzed by quantitative real-time PCR from total RNA extracted from isolated islets. P-KO mice (gray bar, n = 4) have lower VEGF-A expression than control mice (white bar, n = 4). *, P < 0.05. E, VEGF-A content in isolated islets from p-KO mice (gray bar, n = 3) was significantly lower than that from control mice (white bar, n = 3). VEGF-A level was determined by ELISA. Data are presented as means ± sem. *, P < 0.05. F, Five-micrometer cryosections of p-KO and control mouse pancreas were immunolabeled with VEGF (red) and 4′,6′-diamino-2-phenylindole (blue). Green boundary denotes islets. G, VEGF-A levels in the islets and exocrine pancreas were measured by integrating the gray levels inside and outside the islets. VEGF-A levels in the islets and exocrine pancreas decreased significantly in p-KO mice. **, P < 0.01. Multiple images were taken from pancreatic sections of each mouse, and three mice per group were used for analysis.
Because VEGF-A is essential in the regulation of capillary network formation, we tested whether reduced vascular density in p-KO pancreas was associated with reduced VEGF-A expression level. Quantitative real-time PCR showed that VEGF-A mRNA level in pancreatic islets was significantly lower in p-KO than in control mice (Fig. 5D). Consistent with the quantitative PCR result, VEGF-A protein level in pancreatic islets was also lower in p-KO mice (Fig. 5E). To examine whether reduced VEGF-A expression was limited to endocrine pancreas, we stained pancreatic sections with an antibody against VEGF-A and quantified fluorescence intensity of VEGF-A signal in the islets and exocrine pancreas (Fig. 5F). In agreement with VEGF-A protein measurement in pancreatic islets (Fig. 5E), we found that VEGF-A levels in the islets decreased to the similar extent (Fig. 5G). Similar reduction of VEGF-A levels was also observed in exocrine tissue (Fig. 5G). We also tested whether other vasotropic factors were affected by STAT3 deletion by measuring expression levels of FGF1 (fibroblast growth factor 1), EGF (epidermal growth factor), and IGF-I (insulin-like growth factor-I) by quantitative real-time PCR using total RNA extracted from pancreas. There was no difference in expression levels of FGF1, EGF, or IGF-I between control and p-KO pancreas (fold change vs. control: 0.92 ± 0.13, 1.18 ± 0.10, and 1.15 ± 0.26 for FGF1, EGF, and IGF-I, respectively; n = 4 independent experiments in triplicates, P = NS). These data suggest that vascular density may be negatively affected by STAT3 deletion via reduced VEGF-A expression in p-KO mice.
Discussion
STAT3 is activated by cytokines and growth factors and is important for a wide range of biological responses (28). Because STAT3 deletion results in early embryonic lethality (29), previous genetic studies relied on cre-loxP system to create tissue-specific knockout mice for investigation of STAT3 functions in various tissues, including pancreas (20,21,24,30,31,32,33,34). So far, two conditional STAT3 KO (STAT3fl/fl) mouse lines have been generated and used in various reports. One KO line targeted exons 18–20 encoding SH2 domain (21,33), whereas the other one floxed exons 21 and 22 encoding a tyrosine residue and MAPK recognition site (Refs. 20, 21, 32, and 35 and the current study). The targeted exons in both mouse lines encode protein fragments (SH2 domain, tyrosine, and MAPK recognition site) that are essential for STAT3 activation; therefore, conditional deletion generated on both STAT3fl/fl mouse line results in inactivation of STAT3 signaling (20,35). Mice with RIP-Cre-mediated STAT3 inactivation (STAT3/RIP-KO) are mildly hyperglycemic, hyperinsulinemic, hyperphagic, and glucose intolerant (32,34). In addition to β-cells, RIP-Cre transgene is expressed in the hypothalamus; therefore, STAT3 was also deleted in the hypothalamus in these studies (32,34). Because both β-cells and hypothalamus are important in regulating glucose metabolism, it is not clear the relative contribution of STAT3 in β-cells and hypothalamus to the observed phenotypes. To clearly identify the function of STAT3 in β-cells without hypothalamic interference, we generated pancreas-specific STAT3 KO using the well-established Pdx1-cre, cre expression being limited only in the pancreas (8). Efficient STAT3 deletion in the pancreas of p-KO mice was confirmed by immunostaining and Western blotting, whereas STAT3 levels in other tissues, such as brain and blood vessels, were not affected. In Western blotting, we did not observe a truncated STAT3 protein in p-KO mice as predicted by Cre recombination of the target gene vector (20), consistent with a previous report (32).
Glucose homeostasis is regulated by the major anabolic hormone, insulin. Maintenance of glucose homeostasis depends on adequate supply and efficient delivery of insulin to target organs and tissues, which is determined by both β-cell secretory processes and the extensive and well-developed microvascular network within the endocrine glands. Microvasculature in endocrine pancreas depends on proper VEGF-A level for its development and maintenance (10,11,36). STAT3 activation is essential for VEGF expression and subsequent angiogenesis in pancreatic cancer (15). However, it was not clear whether STAT3 is involved in normal islet vascularization. Therefore, we tested whether pancreas-specific STAT3 deletion affected pancreas microvascular network and whether the action was mediated through VEGF-A. In this study, we found that in vivo insulin response during glucose tolerance test was impaired and delayed in p-KO mice, although insulin secretion from isolated p-KO pancreatic islets was normal. We further found impaired vascular network development and reduced VEGF-A levels in the pancreas of p-KO mice, thus linking STAT3 to the regulation of VEGF-A, a key regulator of vascular development. Our findings that islet vascularization is impaired in the absence of STAT3 signaling and that VEGF-A expression, but not other vasotropic factors, is reduced in p-KO mouse pancreas provide evidence for a direct role of STAT3 in the formation and maintenance of islet microvascular network and extend previous studies on VEGF-A conditional KO mice by Brissova et al. (12) and Iwashita et al. (37) to link STAT3 as an upstream signaling molecule to VEGF-A expression.
Consistent with the previous VEGF-A KO study by Lammert et al. (8), we observed increased proportion of small islets in the p-KO mice, although the total endocrine islet area relative to the whole pancreas remained similar to that in the control mice (Kostromina, E., and W. Han, unpublished observations). Furthermore, mice with RIP-Cre mediated STAT3 deletion also exhibited abnormal α-cell distribution in the islets (34). These observations suggest that STAT3 might be directly involved in islet development, in addition to its role in regulating the formation and maintenance of islet microvascular network. Further studies are needed to delineate the precise role of STAT3 in regulating islet development and function.
In this study, we observed a dichotomy of in vivo and in vitro insulin secretion in p-KO mice. Similar findings were also reported previously in VEGF-A conditional KO mice: Brissova et al. (12) found that vascular alterations in islets could lead to reduced insulin output in the absence of β-cell dysfunction, and Iwashita et al. (37) reported that abnormal quantity and quality of blood vessels could be a cause of impaired insulin secretion without impairment of β-cell function. The fact that isolated islets function normally but p-KO mice have impaired insulin secretion suggests that the decreased in vivo insulin response could be caused by blunted delivery of glucose to the islets and/or reduced efficiency in insulin collection and output from islet microvascular network to system circulation, a notion that is supported by the observation that microvascular density inside the islets is reduced in p-KO mice.
Although we clearly identified glucose intolerance and impaired insulin secretion and vascular defects in the pancreas of the p-KO mice, a previous study using Pdx1-Cre-mediated STAT3 KO mice found that STAT3 was not required for glucose homeostasis or body weight regulation (21). The lack of phenotype in pancreas-specific STAT3 KO mice was unexpected, considering that STAT3 can be activated in β-cells by leptin and other cytokines (21,38,39,40,41). Furthermore, some of the phenotypes reported in STAT3/RIP-KO mice were unlikely the result of hypothalamic STAT3 deletion, i.e. reduced VEGF-A expression in β-cells (34). The discrepancy between our present study and the previous report could be due to the fact that we used different STAT3fl/fl mice. Although both floxed mice should give rise to STAT3 inactivation (20,35), we cannot exclude the possibility that subtle differences between the two floxed lines may have caused the observed differences.
In the present report, we focused our study on female mice because the responses from male mice were highly variable during the initial rounds of glucose tolerance tests. It is not uncommon to observe the sexual dichotomy in metabolic studies at the whole animal level (42,43), including one of our earlier studies that investigated calcium-dependent insulin secretion in synaptotagmin-7 KO mice (17). To test whether STAT3 signaling performs similar functions in male and female mice, we stained pancreatic sections from male p-KO and control mice and quantified vascular density as we did for the female mice. STAT3 deletion led to similar reduction in vascular density in the pancreas of both male and female p-KO mice (Supplemental Fig. 3), confirming that the same STAT3 signaling mechanism applies to both sexes.
In summary, we studied pancreatic STAT3 function by using a Pdx1-Cre mediated STAT3 KO mice, with STAT3 deletion specifically in the pancreas before islet formation during embryonic development. We demonstrated that p-KO mice exhibit delayed glucose clearance and impaired insulin release. Our major finding in this study is that vascular density in the pancreas is reduced in the absence of STAT3 signaling along with decreased pancreatic VEGF-A transcription and translation, suggesting a functional role of pancreatic STAT3 signaling in the normal development and maintenance of islet microvascular network through its regulation of VEGF-A production.
Supplementary Material
Acknowledgments
We thank Dr. Britton Chance for many insightful discussions, Drs. Christer Betsholtz and Konstantin Gaengel (Karolinska Institute) for advice on endothelial markers; Jian'er Lin for excellent technical support; Dr. Clement Khaw (Singapore Bioimaging Consortium-Nikon Imaging Center) for assistance with fluorescent microscope and image processing; and Dr. Xiao Yong and Candy Zhuang for help with the confocal microscope and image analysis.
Footnotes
This work was supported by the Biomedical Research Council, Agency for Science, Technology, and Research, Singapore (to W.H.), and National Institutes of Health Grant R01-DK60137 (to C.L.).
Present address for C.L.: Merck Research Laboratories, Rahway, NJ.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 9, 2010
Abbreviations: [Ca2+]i, Intracellular free calcium concentration; Glut, glucose transporter; IPGTT, ip glucose tolerance test; KO, knockout; KRH, Krebs-Ringer HEPES; NAD(P)H, reduced nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide phosphate; Pdx1, pancreas-duodenum homeobox gene 1; Pdx1-Cre, Cre transgene under Pdx1 promoter; p-KO, pancreas-specific deletion of STAT3; RIP, rat insulin promoter; STAT, signal transducer and activator of transcription; VEGF, vascular endothelial growth factor.
References
- Ashcroft FM, Proks P, Smith PA, Ammala C, Bokvist K, Rorsman P 1994 Stimulus-secretion coupling in pancreatic β cells. J Cell Biochem 55(Suppl):54–65 [DOI] [PubMed] [Google Scholar]
- Rorsman P, Renström E 2003 Insulin granule dynamics in pancreatic β cells. Diabetologia 46:1029–1045 [DOI] [PubMed] [Google Scholar]
- Henquin JC, Ishiyama N, Nenquin M, Ravier MA, Jonas JC 2002 Signals and pools underlying biphasic insulin secretion. Diabetes 51(Suppl 1):S60–S67 [DOI] [PubMed] [Google Scholar]
- Carlsson PO, Andersson A, Jansson L 1996 Pancreatic islet blood flow in normal and obese-hyperglycemic (ob/ob) mice. Am J Physiol 271:E990–E995 [DOI] [PubMed] [Google Scholar]
- Svensson AM, Sandler S, Jansson L 2003 Role of superoxide anion in pancreatic islet blood flow regulation in anesthetized rats. Eur J Pharmacol 459:59–64 [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S 1988 Morphological evidence for pancreatic polarity of β-cell within islets of Langerhans. Diabetes 37:616–621 [DOI] [PubMed] [Google Scholar]
- Henderson JR, Moss MC 1985 A morphometric study of the endocrine and exocrine capillaries of the pancreas. Q J Exp Physiol 70:347–356 [DOI] [PubMed] [Google Scholar]
- Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA 2003 Role of VEGF-A in vascularization of pancreatic islets. Curr Biol 13:1070–1074 [DOI] [PubMed] [Google Scholar]
- Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM 2006 VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 290:H560–H576 [DOI] [PubMed] [Google Scholar]
- Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W 1998 Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol 140:947–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J 2003 The biology of VEGF and its receptors. Nat Med 9:669–676 [DOI] [PubMed] [Google Scholar]
- Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, Baldwin HS, Nicholson W, Bader DM, Jetton T, Gannon M, Powers AC 2006 Pancreatic islet production of vascular endothelial growth factor-A is essential for islet vascularization, revascularization, and function. Diabetes 55:2974–2985 [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D 2001 Induction of pancreatic differentiation by signals from blood vessels. Science 294:564–567 [DOI] [PubMed] [Google Scholar]
- Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H 2002 Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21:2000–2008 [DOI] [PubMed] [Google Scholar]
- Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL, Xie K 2003 Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene 22:319–329 [DOI] [PubMed] [Google Scholar]
- Cheranov SY, Karpurapu M, Wang D, Zhang B, Venema RC, Rao GN 2008 An essential role for SRC-activated STAT-3 in 14,15-EET-induced VEGF expression and angiogenesis. Blood 111:5581–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson N, Lao Y, Maximov A, Chuang JC, Kostromina E, Repa JJ, Li C, Radda GK, Südhof TC, Han W 2008 Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice. Proc Natl Acad Sci USA 105:3992–3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY 1985 A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450 [PubMed] [Google Scholar]
- Kurrasch DM, Huang J, Wilkie TM, Repa JJ 2004 Quantitative real-time polymerase chain reaction measurement of regulators of G-protein signaling mRNA levels in mouse tissues. Methods Enzymol 389:3–15 [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S 1998 Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol 161:4652–4660 [PubMed] [Google Scholar]
- Lee JY, Hennighausen L 2005 The transcription factor Stat3 is dispensable for pancreatic β-cell development and function. Biochem Biophys Res Commun 334:764–768 [DOI] [PubMed] [Google Scholar]
- Gannon M, Shiota C, Postic C, Wright CV, Magnuson M 2000 Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis 26:139–142 [DOI] [PubMed] [Google Scholar]
- Levy DE, Lee CK 2002 What does Stat3 do? J Clin Invest 109:1143–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano A, Wolfgang MJ, Gao Q, Jacoby J, Chai GX, Hansen W, Iwamoto Y, Pober JS, Flavell RA, Fu XY 2003 Endothelial cells require STAT3 for protection against endotoxin-induced inflammation. J Exp Med 198:1517–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Jetton TL, Ying G, Magnuson MA, Piston DW 1996 Quantitative subcellular imaging of glucose metabolism within intact pancreatic islets. J Biol Chem 271:3647–3651 [DOI] [PubMed] [Google Scholar]
- Bergsten P 1998 Glucose-induced pulsatile insulin release from single islets at stable and oscillatory cytoplasmic Ca2+. Am J Physiol 274:E796–E800 [DOI] [PubMed] [Google Scholar]
- Wallgard E, Larsson E, He L, Hellström M, Armulik A, Nisancioglu MH, Genove G, Lindahl P, Betsholtz C 2008 Identification of a core set of 58 gene transcripts with broad and specific expression in the microvasculature. Arterioscler Thromb Vasc Biol 28:1469–1476 [DOI] [PubMed] [Google Scholar]
- Akira S 2000 Roles of STAT3 defined by tissue-specific gene targeting. Oncogene 19:2607–2611 [DOI] [PubMed] [Google Scholar]
- Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S 1997 Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA 94:3801–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moh A, Iwamoto Y, Chai GX, Zhang SS, Kano A, Yang DD, Zhang W, Wang J, Jacoby JJ, Gao B, Flavell RA, Fu XY 2007 Role of STAT3 in liver regeneration: survival, DNA synthesis, inflammatory reaction and liver mass recovery. Lab Invest 87:1018–1028 [DOI] [PubMed] [Google Scholar]
- Inoue H, Ogawa W, Ozaki M, Haga S, Matsumoto M, Furukawa K, Hashimoto N, Kido Y, Mori T, Sakaue H, Teshigawara K, Jin S, Iguchi H, Hiramatsu R, LeRoith D, Takeda K, Akira S, Kasuga M 2004 Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat Med 10:168–174 [DOI] [PubMed] [Google Scholar]
- Cui Y, Huang L, Elefteriou F, Yang G, Shelton JM, Giles JE, Oz OK, Pourbahrami T, Lu CY, Richardson JA, Karsenty G, Li C 2004 Essential role of STAT3 in body weight and glucose homeostasis. Mol Cell Biol 24:258–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY 2004 Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA 101:4661–4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorogawa S, Fujitani Y, Kaneto H, Hazama Y, Watada H, Miyamoto Y, Takeda K, Akira S, Magnuson MA, Yamasaki Y, Kajimoto Y, Hori M 2004 Insulin secretory defects and impaired islet architecture in pancreatic β-cell-specific STAT3 knockout mice. Biochem Biophys Res Commun 319:1159–1170 [DOI] [PubMed] [Google Scholar]
- Raz R, Lee CK, Cannizzaro LA, d'Eustachio P, Levy DE 1999 Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci USA 96:2846–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issbrücker K, Marti HH, Hippenstiel S, Springmann G, Voswinckel R, Gaumann A, Breier G, Drexler HC, Suttorp N, Clauss M 2003 p38 MAP kinase—a molecular switch between VEGF-induced angiogenesis and vascular hyperpermeability. FASEB J 17:262–264 [DOI] [PubMed] [Google Scholar]
- Iwashita N, Uchida T, Choi JB, Azuma K, Ogihara T, Ferrara N, Gerber H, Kawamori R, Inoue M, Watada H 2007 Impaired insulin secretion in vivo but enhanced insulin secretion from isolated islets in pancreatic β cell-specific vascular endothelial growth factor-A knock-out mice. Diabetologia 50:380–389 [DOI] [PubMed] [Google Scholar]
- Rønn SG, Hansen JA, Lindberg K, Karlsen AE, Billestrup N 2002 The effect of suppressor of cytokine signaling 3 on GH signaling in β-cells. Mol Endocrinol 16:2124–2134 [DOI] [PubMed] [Google Scholar]
- Mattson MP 2001 Lose weight STAT: CNTF tops leptin. Trends Neurosci 24:313–314 [DOI] [PubMed] [Google Scholar]
- Morton NM, Emilsson V, de Groot P, Pallett AL, Cawthorne MA 1999 Leptin signalling in pancreatic islets and clonal insulin-secreting cells. J Mol Endocrinol 22:173–184 [DOI] [PubMed] [Google Scholar]
- Hekerman P, Zeidler J, Bamberg-Lemper S, Knobelspies H, Lavens D, Tavernier J, Joost HG, Becker W 2005 Pleiotropy of leptin receptor signalling is defined by distinct roles of the intracellular tyrosines. FEBS J 272:109–119 [DOI] [PubMed] [Google Scholar]
- Kido Y, Burks DJ, Withers D, Bruning JC, Kahn CR, White MF, Accili D 2000 Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Invest 105:199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaekura K, Julyan R, Wicksteed BL, Hays LB, Alarcon C, Sommers S, Poitout V, Baskin DG, Wang Y, Philipson LH, Rhodes CJ 2003 Insulin secretory deficiency and glucose intolerance in Rab3A null mice. J Biol Chem 278:9715–9721 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.