Abstract
The GH receptor (GHR) is expressed on macrophages. However, the precise role of GH in regulation of macrophage function is unclear. We hypothesized that soluble factors including cytokines produced by macrophages in a GH-dependent manner regulate adipogenesis. We confirmed expression and functional integrity of the GHR in the J774A.1 macrophage cells. Conditioned medium (CM) from macrophages inhibited adipogenesis in a 3T3-L1 adipogenesis assay. CM from GH-treated macrophages decreased the inhibitory effect of CM from macrophages on adipogenesis. This effect on preadipocyte differentiation was active only during the first (early) phase of adipocyte differentiation. CM from stromal vascular compartment macrophages of mice with macrophage-specific deletion of the GHR exhibited more inhibitory effect on 3T3-L1 preadipocyte differentiation compared with CM from stromal vascular compartment macrophages of control mice, indicating that intact GH action in primary macrophages also increases preadipocyte differentiation. GH did not increase IGF-1 expression in macrophages. PCR array analysis identified IL-1β as a candidate cytokine whose expression was altered by GH in macrophages. Levels of IL-1β mRNA and protein were significantly decreased in GH-treated J774A.1 macrophages. Nuclear factor-κB stimulates IL-1β gene expression, and GH induced a significant decrease in the levels of phosphorylated nuclear factor-κB in macrophages. IL-1β is a known inhibitor of adipogenesis, and these results support GH-dependent down-regulation of macrophage IL-1β expression as one mechanism for the observed increase in adipogenesis with CM from GH-treated macrophages. We conclude that GH decreases secretion of IL-1β by the macrophage and thus in a paracrine manner increases adipocyte differentiation. These results provide a novel mechanism for GH’s actions in the control of adipogenesis.
Growth hormone action on macrophage enhances adipocyte differentiation.
Pituitary GH is essential for postnatal growth in mammals. In addition to growth, GH affects the metabolism of fat, protein, and carbohydrate (1). At the tissue level, these pleiotropic actions of GH result from the interaction of GH with a specific cell surface receptor, the GH receptor (GHR). The GHR is expressed on monocytes and macrophages, and previous studies have demonstrated GH-dependent effects on macrophage function. For example, GH has been demonstrated to increase the proliferation and alter the morphology of RAW 264.7 macrophages (2). GH primes human phagocytes for enhanced production of reactive oxygen intermediates and hydrogen peroxide (3,4). Mouse peritoneal macrophages and the J774A.1 macrophage cell line respond to GH with a dose-dependent stimulation of cellular uptake and degradation of low-density lipoprotein and enhanced rate of cholesterol esterification (5). GH has also been shown to stimulate the degradation of calcium phosphate biomaterials by human monocyte-macrophages (6). Mononuclear leukocytes and monocytes also synthesize GH, suggesting that in addition to endocrine action of circulating GH, GH synthesized by leukocytes and monocytes can also act in an autocrine/paracrine manner on these cells (7,8).
Obesity is characterized by infiltration, accumulation, and activation of macrophages in adipose tissue (9,10,11). Abnormal production of inflammatory cytokines by macrophages and other cells in adipose tissue is implicated in the pathogenesis of metabolic abnormalities associated with obesity (12). Adipogenesis is impaired in obese individuals, and previous studies have demonstrated that conditioned medium from macrophages inhibits differentiation of preadipocytes, thus compromising the ability of the adipose tissue to function as a fatty acid reservoir (13,14,15,16). This diminished capacity of the adipose tissue to store fatty acids necessitates redistribution of fatty acids to tissues such as liver and muscle and results in insulin resistance and related sequelae of obesity (17,18,19).
GH promotes accretion of lean tissue, reduction in fat mass, and induction of lipolysis (1,20). In both animal models and humans, deficiency of GH or disruption of the GH-GHR axis is characterized by decreased lean body mass and increased adiposity (21). It is also well established that obese individuals have lower levels of circulating GH, which amplifies the deleterious metabolic effects of obesity (22). Conversely, states of GH excess such as acromegaly are associated with decreased fat mass (1). Traditionally these actions of GH are believed to be mediated through activation of GHRs on the adipocyte, which results in increased lipolytic activity via catecholamine-induced lipolysis secondary to up-regulation of β-adrenergic receptors and decreased accumulation of triglyceride in the adipocyte via inhibition of lipoprotein lipase activity (21,23).
Whereas it is known that GHRs are expressed on macrophages, the role of GH action on the macrophage in the pathogenesis of obesity is unknown. We hypothesized that in addition to direct effects on adipocytes, activation of GHRs on macrophage results in alteration of the cytokine profile of macrophages by which paracrine modulation of differentiation and functioning of adipocytes occurs. We report here that GH decreases secretion of IL-1β by the macrophage and thereby increases differentiation of preadipocyte to adipocyte.
Materials and Methods
Materials
DMEM, penicillin/streptomycin/amphotericin B mixture, and Trizol reagent were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS), heat-inactivated FBS, and calf serum was purchased from Atlanta Biologicals (Lawrenceville, GA). Dexamethasone, 1-methyl-3-isobutylxanthine, and insulin were obtained from Sigma-Aldrich (St. Louis, MO). Antibodies for phospho-Janus kinase 2 (JAK2) (used in 1:500 dilution), JAK2 (used in 1:1000 dilution), ERK (used in 1:1000 dilution), MAPK (used in 1:1000 dilution), and phospho-p65 (S536) nuclear factor-κB (NF-κB; used in 1:1000 dilution) were purchased from Cell Signaling Technology (Danvers, MA), and anti-IL-1β (used in 1:1000 dilution) antibody was procured from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-GHR antibody, AL-47 (24,25), was used in 1:1000 dilution for Western blot analyses. Ovine GH was obtained from National Hormone and Pituitary Program (Torrance, CA).
Culture of murine 3T3-L1 preadipocytes and J774A.1 macrophages
3T3-L1 (American Type Culture Collection, Manassas, VA) murine preadipocytes (14–18 passages) were cultured at 37 C under 10% CO2 in DMEM with high glucose (Life Technologies, Inc., Carlsbad, CA) supplemented with 10% calf serum and antibiotics (100 mg/ml penicillin and 0.1 mg/ml streptomycin). J774A.1 murine macrophages (American Type Culture Collection) cells were cultured at 37 C under 5% CO2 in DMEM supplemented with 10% heat-inactivated FBS and antibiotics.
Preparation of conditioned media
J774A.1 macrophages were starved overnight in serum-free DMEM supplemented with 1% BSA and treated with or without GH (500 ng/ml) for 8 h. Subsequently these macrophages were thoroughly washed with DMEM media to remove traces of GH and then cultured in fresh serum-free DMEM medium, which was collected 24 h later (conditioned medium). The conditioned media from GH-treated (GH-CM-Mac) and untreated (CM-Mac) macrophages were centrifuged at 1000 × g for 5 min to remove cell debris and the supernatants stored at −20 C. Conditioned media from stromal vascular compartment (SVC) fraction of adipose tissue was prepared 2 d after cell isolation when fresh culture media were added to the cells and conditioned media harvested 24 h later.
Differentiation of 3T3-L1 preadipocytes
Cells were cultured on six- or 12-well plates and allowed to proceed to confluence. Differentiation was initiated 2 d after the cells reached confluency by treating the cells with induction cocktail containing 1 μm dexamethasone, 0.5 mm 3-isobutyl-1-methylxanthine, and 1 μg/ml insulin in DMEM with 10% FBS in the presence of conditioned medium or normal culture medium. The differentiation cocktail was removed after 2 d, and the cells were cultured in DMEM with 10% FBS and 1 μg/ml insulin. The medium was changed every 2 d and on d 8, the cells were fixed with 10% formalin and stained with 0.3% Oil Red O. In certain instances the fat content was quantified by eluting Oil Red O with isopropanol and measuring the OD at 492 nm.
Western blot analysis
Macrophage cells (J774A.1) were treated with or without GH and harvested at indicated time points. Cells were washed twice with cold PBS, lysed in radioimmunoprecipitation assay buffer (50 mm Tris-HCl, pH 7.5; 150 mm NaCl; 2 mm EGTA; 0.1% Triton X-100; and 0.1 mm NaOV4). Equal amounts of solubilized protein were resolved on SDS-PAGE and transferred onto nitrocellulose membrane. Nonspecific binding sites were blocked with 5% skim milk powder and incubated with indicated primary antibodies. Blots were developed with chemiluminescence (ECL Plus; GE Healthcare, Piscataway, NJ) after incubation with appropriate horseradish peroxidase-conjugated secondary antibodies.
PCR array
Macrophage cells (J774A.1) were treated with or without GH and harvested after 24 h of treatment. Total RNA was isolated using Trizol reagent and then repurified using a column (RNeasy minikit; QIAGEN, Valencia, CA) according to the manufacture’s protocol. In-column deoxyribonuclease digestion was performed for each samples to remove genomic DNA. Equal amount of RNA from each sample was subjected to first-strand cDNA synthesis using ReactionReady kit (Super Array Bioscience Corp., Frederick, MD). PCR array analysis was performed using RT2 profiler PCR array (mouse chemokines and receptors, no. PAMM-022) on the 7000 Prism using RT2 real-time SYBR Green PCR master mix (Applied Biosystems, Foster City, CA). The total volume of the PCR was 25 μl. The thermocycler parameters were 95 C for 10 min, followed by 40 cycles of 95 C for 15 sec and 60 C for 1 min.
Real-time quantitative PCR assay
Total RNA was isolated and purified as described above and quantitative PCR was performed using either QuantiTect SYBR Green RT-PCR kit (QIAGEN; no. 204243) or TaqMan kit (Applied Biosystems) as indicated. The RT-PCRs were repeated in triplicate for each sample using Applied Biosystems 7000 Prism detection system. Primers for the SYBR Green RT-PCR assay, designed using Primer Express 2.0 software, were as follows: PPARγ forward, 5′-TCACAACAGCTGACCCAATGG-3′, reverse, 5′-GCAGGTGCTAC TTTGATCGCACTT-3′; aP2 forward, 5′-GGAAAGTCGACCACAATAAAGAGAA-3′, reverse, 5′-TGTGGAAGTCACGCCTTTCAT-3′; adiponectin forward, 5′-GGCCGTGATGGCAGAGAT-3′, reverse, 5′-GTCTCACCCTTAGGACCAAGAA-3′; and IL-1β forward, 5′-GGACCCATATGAGCTGAAAGC-3′, reverse, 5′-TCGTTGCTTGGTTCTCCTTGT-3′. The TaqMan methodology was used to measure the L2 transcript of the GHR gene as previously described (26). mRNA expression of each gene was normalized using the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a housekeeping gene, and data were compared between GH-treated and untreated macrophages according to the 2−ΔΔCt method (27).
Targeted deletion of GHR in macrophage/monocyte
The generation of the homozygous GHR exon 4 floxed mice (Ghrfl/fl) has been previously described (28). EIIaCre mice were obtained from the Transgenic Core facility of University of Michigan. B6.129P2-Lyz2tm1(cre)Ifo/J, which expresses Cre (Cyclization Recombination) recombinase from the endogenous Lyzs locus, was purchased from Jackson Laboratory (Bar Harbor, ME). In addition to the macrophage, the Cre recombinase from the endogenous Lyzs locus is expressed in cells from myeloid cell lineage, including monocytes, and granulocytes (29). GHR null (GhrΔ/Δ) mice were produced by cross-breeding Ghrfl/fl with EIIaCre. Ghrfl/fl was crossed with the B6.129P2-Lyz2tm1(cre)Ifo/J mice to obtain Ghrfl/fl;LysM-cre(+/−) mice, which were then crossed with GHR null (GhrΔ/Δ) mice to generate GhrΔ/fl;LysM-cre(+/−) (designated as GHRMacD) and GhrΔ/fl;LysM-cre(−/−) (designated as control) mice; genotyping was carried out by PCR analysis (28). Animals were housed in a specific pathogen-free animal facility at the University of Michigan, and all experiments were approved by the University of Michigan Institutional Animal Care and Use Committee.
Isolation of primary macrophages
The SVC is a mixture of macrophage, dendritic, natural killer and endothelial cells (30). Primary macrophages from SVCs of adipose tissue were isolated from GHRMacD and control mice at approximately 12 wk of age using standard techniques and protocols (31). Briefly, abdominal fat pads were collected and minced. The tissues were incubated with 0.01 mg/ml Liberase Blendzyme 3 collagenase (Roche Diagnostics, Indianapolis, IN) for 30–40 min and filtered through a 100-μm nylon cell strainer. The strained cell mixtures were centrifuged at 1000 × g for 10 min, and the pellets that contain SVCs were collected and treated with ACK lysis buffer (Lonza, Walkersville, MD) to remove erythrocytes. The SVCs were cultured in DMEM containing 10% heat-inactivated serum.
Statistical analysis
Data are presented as mean ± se unless otherwise indicated. Mann-Whitney U and Kruskal-Wallis nonparametric tests were performed to analyze statistical significance of the difference between the distributions of two or multiple independent samples, respectively, using SPSS software, version 11.5 for Windows (SPSS, Inc., Chicago, IL). P ≤ 0.05 was considered significant.
Results
GH-dependent activation of signaling pathways in J774A.1 macrophage cells
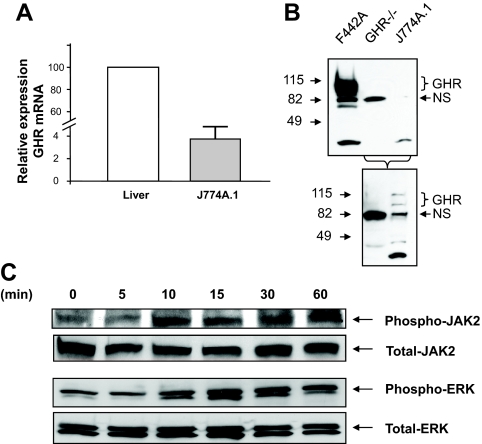
Expression of GHR in J774A.1 was confirmed by TaqMan RT-PCR (Fig. 1A) and Western blot analysis (Fig. 1B). To verify the functional integrity of GHR expressed in macrophages, J774A.1 cells were exposed to ovine GH and cells harvested at different time points (Fig. 1C). The levels of specific proteins in the GH signaling pathway were quantified by Western blot analysis. These results revealed that stimulation of these cells with GH resulted in time-dependent increase in the levels of phospho-JAK2 and phospho-ERK, suggesting that canonical GHR signaling pathways are intact in these cells.
Figure 1.
J77A4.1 cells express functional GHRs. A, Expression of GHR in J774A.1 macrophages. RNA was extracted from liver or J774A.1 cells and steady-state abundance of the major (L2) transcript of the GHR gene measured by TaqMan RT-PCR. Expression of the housekeeping gene GAPDH was used as an internal control to normalize results. The relative amounts of the transcript in J774A.1 cells are depicted relative to the expression in liver. The results (n = 3) are depicted as mean ± sem. B, Equal amounts of protein from F442A cells, GHR−/− liver or J774A.1 cells were size-fractionated by 4–15% SDS-PAGE and immunoblotted with anti-GHR AL47 antibody. Specific signals were visualized using the enhanced chemiluminescence (ECL) system. The position of the specific GHR (∼115 kDa) and nonspecific (NS; ∼82 kDa) bands are indicated. Bottom panel, Overexposure of the immunoblot to demonstrate absence (GHR−/− liver) or presence (J774A.1 cells) of GHR expression. C, GH stimulates canonical GHR signaling pathways in macrophages. J774A.1 macrophages were starved overnight and then exposed to ovine GH (500 ng/ml) for the indicated time periods. Whole-cell protein extracts were prepared and equal amounts of protein size fractionated by SDS-PAGE. Immunoblotting was performed with anti-phospho-JAK2, -JAK2, -phospho-ERK antibody, or -total ERK antibody. Specific signals were visualized using the ECL system. The identity of the protein is indicated.
Factors released from GH-treated macrophages improve 3T3-L1 adipogenesis
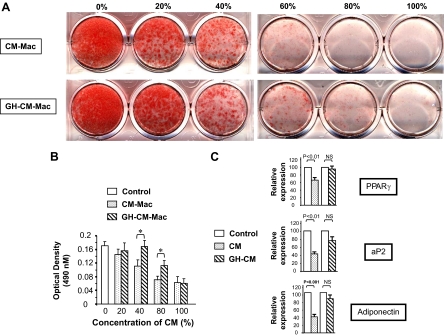
Previous studies demonstrated that conditioned medium from macrophage (cell line or primary cells) inhibits adipogenesis in 3T3-L1 cells and human abdominal preadipocytes (13,14,15,16). The inference from these studies is that secreted factors released by macrophages are responsible for the impairment of adipogenesis. To determine whether GH influences secretion of these factors by macrophage, 3T3-L1 preadipocytes were induced to differentiate in the presence of graded concentration (0–100%) of conditioned medium from either unstimulated macrophages (CM-Mac; Fig. 2A, top row) or GH-treated macrophages (GH-CM-Mac; Fig. 2A, bottom row) and adipocyte differentiation measured by Oil Red O staining (Fig. 2A) and quantification of fat content by eluting Oil Red O with isopropanol and measuring OD at 492 nm (Fig. 2B).
Figure 2.
Stimulation of macrophages with GH decreases the inhibitory activity of conditioned medium (CM) from macrophages on differentiation of 3T3-LI preadipocytes. A and B, 3T3-L1 preadipocytes were induced to differentiate in the presence of 0–100% of CM-Mac or GH-CM-Mac. Eight days after differentiation, the cultures were stained with Oil Red O (A) and the fat content quantified by eluting Oil Red O with isopropanol and measuring the OD at 492 nm (B; mean ± sem, n = 4). *, P < 0.05. C, PPARγ, aP2, and adiponectin mRNA expression. Total RNA was isolated from 3T3-L1 adipocytes (8 d after differentiation) grown in regular CM (control), CM-Mac, or GH-CM-Mac. PPARγ, aP2, and adiponectin mRNA abundance was measured by Syber Green RT-PCR analysis. Expression of the housekeeping gene GAPDH was used as an internal control to normalize results. The results (n = 3–5) are depicted as mean ± sem. The steady-state abundance of the transcript is depicted relative to the abundance in cells grown in regular culture medium. NS, Not significant.
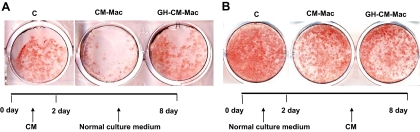
In accordance with published reports (14), conditioned medium from macrophage inhibited adipogenesis. However, 3T3-L1 preadipocytes exposed to conditioned medium from GH-treated macrophage exhibited improved adipogenesis. Peroxisome proliferator-activated receptor (PPAR)-γ is a key adipogenic transcription factor whose expression is increased during differentiation of preadipocytes to adipocytes. Consistent with the results obtained from the adipogenesis assay, exposure of the preadipocytes to conditioned medium from GH-treated macrophage resulted in higher expression levels of PPARγ mRNA, compared with cells exposed to non-GH-treated conditioned medium (Fig. 2C). Additionally the expression profile of two other markers of adipocyte differentiation, adipocyte P2 (aP2) and adiponectin, also exhibited similar changes. Differentiation of 3T3-L1 preadipocytes into adipocytes occurs in two phases, induction and after induction (32). To determine whether the observed effect of conditioned media from GH-treated macrophages was dependent on the phase of adipogenesis, 3T3-L1 preadipocytes were exposed to the conditioned medium during either the induction (first day of initiation of differentiation; Fig. 3A) or postinduction (third day after initiation of differentiation; Fig. 3B) phase. These results revealed that the observed GH-dependent effect on adipogenesis required preadipocytes to be exposed to the conditioned medium during the induction phase of differentiation.
Figure 3.
The effect of conditioned medium (CM) from GH-treated macrophages on the differentiation of 3T3-L1 preadipocytes is restricted to the induction phase of adipogenesis. 3T3-L1 cells were incubated with CM (mixed 1:1 with culture medium) from either macrophages (CM) or GH-CM-Mac for the first 2 d (A) or last 6 d (B) after addition of the differentiation cocktail mixture. Differentiation of adipocytes was monitored by staining with Oil Red O staining 8 d after differentiation. C, Control.
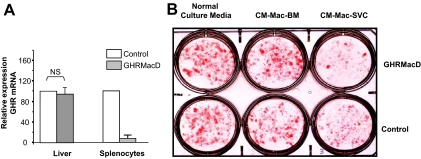
The above-described studies demonstrating the effect of GH on factors secreted by the macrophage into the conditioned medium were conducted with macrophage cell line J774A.1. We sought to confirm our results with primary bone marrow and SVC macrophages. In preliminary experiments we noticed that the inhibitory effect of conditioned medium from macrophages on predipocyte was observed only with SVC macrophages and not with bone-marrow derived macrophages (Fig. 4B), and hence, further studies were performed only with SVC macrophages. Because bovine fetal and calf serum contain significant concentrations of GH and we were investigating GH-dependent effects, we were precluded from using cells grown in serum containing medium. Furthermore, we observed that the inhibitory effect of macrophage-conditioned medium was lost when SVC macrophages were cultured under serum-deprived conditions. The use of charcoal-stripped serum was also unsuccessful for similar reason. Because of these drawbacks and experimental constraints for using primary macrophages to assay for direct effects of GH on factors secreted by the macrophage, we created a mouse model (GHRMacD) with targeted deletion of the GHR in macrophages/monocytes. Quantitative RT-PCR analysis of GHRMacD mice confirmed that macrophage GHR mRNA expression was extinguished, with expression in liver remaining unchanged (Fig. 4A). Using primary macrophages from this model, we observed a more pronounced inhibitory effect on the differentiation of 3T3-L1 preadipocytes with conditioned medium from SVC macrophages (CM-Mac-SVC) from GHRMacD mice compared with macrophage from control mice (Fig. 4B). Hence, these studies with primary macrophages suggest that GH action in macrophage increases preadipocyte differentiation and are consistent with the results obtained using the J774A.1 macrophage cell line.
Figure 4.
The absence of GHR diminishes the salutary effect of conditioned medium from SVC macrophages on the differentiation of 3T3-L1 preadipocytes. A, Expression of GHR in GHRMacD [GhrΔ/fl; LysM-cre (pos)] and control [GhrΔ/fl; LysM-cre (neg)] mice. RNA was extracted from liver or splenocytes isolated from GHRMacD and control mice and steady-state abundance of GHR mRNA measured by TaqMan RT-PCR. Expression of the housekeeping gene GAPDH was used as an internal control to normalize results. The results (n = 3) are depicted as mean ± sem. The relative amount of the transcript is depicted relative to the expression in the control mice. B, The effect of conditioned medium from SVC macrophages on the differentiation of 3T3-L1 preadipocytes. 3T3-L1 cells were incubated with normal culture medium or conditioned medium from either bone marrow-derived macrophages (CM-Mac-BM) or SVC macrophages (CM-Mac-SVC) isolated from either GHRMacD [GhrΔ/fl; LysM-cre (pos)] or control [GhrΔ/fl; LysM-cre (neg)] mice. Differentiation of adipocytes was monitored by Oil Red O staining 8 d after differentiation. The results are representative of results obtained with two separate isolations of SVC macrophages from the respective mouse models. NS, Not significant.
GH does not influence IGF-I expression in J774A.1 macrophages
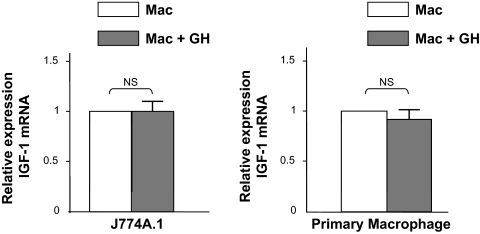
GH is the principal endocrine regulator of IGF-I expression and IGF-I enhances adipogenesis in 3T3-L1 cells. To investigate the possibility that the observed increase in adipogenesis by the GH-treated conditioned medium was due to increase in IGF-I levels in the conditioned medium, we used RT-PCR to measure the expression levels of IGF-I mRNA in both J774A.1 and primary macrophages stimulated with or without GH (Fig. 5). In both these cell types, GH did not significantly stimulate the expression of IGF-I, indicating that the observed improvement on adipogenesis is not mediated by IGF-I.
Figure 5.
GH’s effects on macrophage are IGF-I independent. Total RNA was isolated from either J774A.1 or primary murine macrophages (from adipose tissue SVC) exposed to GH (500 ng/ml for 24 h). IGF-I mRNA abundance was measured by Syber Green RT-PCR analysis. Expression of the housekeeping gene GAPDH was used as an internal control to normalize results. The results (n = 3–5) are depicted as mean ± sem. The amounts of the transcript are depicted relative to the expression in cells not exposed to GH. NS, Not significant.
Expression of IL-1β is down-regulated in cells treated with GH
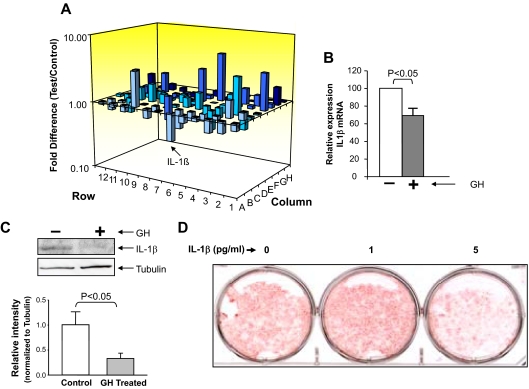
Our results indicate that GH can alter the secretion of factor(s) from macrophages that increases adipocyte differentiation. We used PCR array technology to identify candidate genes that may be responsible for the observed effect of GH-treated conditioned medium on adipogenesis. These results revealed that exposure of J774A.1 macrophages to GH for 24 h resulted in alteration of expression of several factors with decreased expression of IL-1β being one of the changes so identified (Fig. 6A). Because previous studies suggested that IL-1β inhibits adipogenesis (33), we chose to pursue this lead. Syber Green RT-PCR analysis of IL-1β mRNA expression confirmed the PCR array results (Fig. 6B). Furthermore, Western blot analysis indicated that exposure of macrophages to GH resulted in decrease in Pro-IL-1β protein expression in these cells (Fig. 6C).
Figure 6.
GH-dependent decrease in IL-1β expression and secretion in macrophages. A, J774A.1 macrophages were stimulated with either vehicle or GH (500 ng/ml) for 24 h and total RNA extracted and subjected to analysis by SuperArray. A three-dimensional representation of the average changes (positive and negative) for two independent experiments is depicted. The bar representing IL-1β is indicated by an arrow. B, Total RNA was isolated from J774A.1 stimulated with either vehicle or GH (500 ng/ml) for 24 h, and IL-1β mRNA abundance was measured by Syber Green RT-PCR analysis. Expression of the housekeeping gene GAPDH was used as an internal control to normalize results. The results (n = 3) are depicted as mean ± sem. The amounts of the transcript are depicted relative to the expression in cells not exposed to GH. C, GH-dependent decrease in IL-1β expression in J774A.1 cells. J774A.1 cells were stimulated with either vehicle or GH (500 ng/ml) for 24 h, and the whole-cell lysates were size fractionated by SDS-PAGE. Immunoblotting was performed with anti-IL-1β or tubulin antibody. Specific signals were visualized using the enhanced chemiluminescence (ECL) system. The identity of the protein is indicated. D, IL-1β can inhibit adipogenesis at low concentrations. Adipogenesis assay were performed in 3T3-L1 cells treated with IL-1β at 0, 1, and 5 pg/ml concentration. Differentiation of preadipocytes was determined by Oil Red O staining 8 d after differentiation.
IL-1β inhibits adipogenesis at a concentration of 5 pg/ml
ELISA indicated that the maximal concentration of secreted IL-1β in the conditioned medium from macrophages was less than 10 pg/ml (data not shown). To confirm that IL-1β exhibits antiadipogenic effects at these concentrations, 3T3-L1 cells were treated with IL-1β at various concentrations and adipogenesis monitored by Oil Red O staining. As shown in Fig. 6D, IL-1β could significantly inhibit adipogenesis at concentration of 5 pg/ml, thereby supporting GH-dependent down-regulation of macrophage IL-1β expression as a mechanism for the observed increase in adipogenesis with GH-treated conditioned medium.
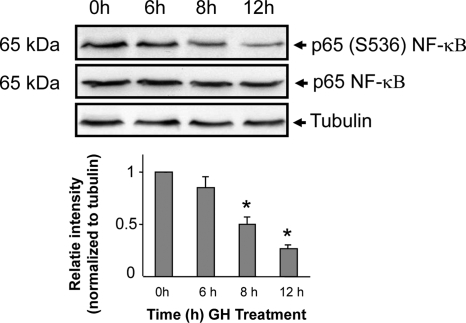
GH attenuates NF-κB phosphorylation
The transcription factor NF-κB regulates the expression of several genes including proinflammatory cytokines, proteins of innate immunity, and acute-phase reactants such as IL-1, IL-2, TNF-α, and IL-12 (34,35). To investigate whether GH’s effect of inhibiting IL-1β secretion by macrophage was mediated by NF-κB, we measured the abundance of phosphorylated NF-κB in macrophages stimulated with GH. These results revealed that GH induced a significant decrease in the levels of phosphorylated NF-κB in macrophages (Fig. 7). This effect was time dependent because the change in levels of phosphorylated NF-κB was observed only after 8 h of exposure to GH; the levels of phosphorylated NF-κB were unchanged at 15 min, 30 min, 1 h, and 6 h after exposure to GH (data not shown and Fig. 7).
Figure 7.
GH attenuates phosphorylation of NF-κB. Top panel, J774A.1 macrophages were starved overnight and then exposed to ovine GH (500 ng/ml) for the indicated time periods. Whole-cell protein extracts were prepared and equal amounts of protein size fractionated by SDS-PAGE. Immunoblotting was performed with anti-phospho-p65 NF-κB (Ser 536) and total NF-κB antibodies. To verify equivalence of sample loading, the abundance of tubulin was also measured by Western blot analysis. Specific signals were visualized using the enhanced chemiluminescence (ECL) system. The identity and molecular weights of the proteins are indicated. Bottom panel, Densitometric measurements (mean ± sem; n = 4) are depicted. *, P < 0.001 compared with 0 h value, which was assigned a value of 1.
Discussion
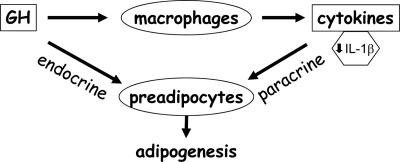
The current investigation reveals a novel mechanism for GH action on adipocyte differentiation. Our results establish that direct GH action on the macrophage results in alteration in the cytokine profile of the macrophage and consequent paracrine modulation of adipocyte differentiation (Fig. 8). We demonstrate improved differentiation of 3T3-L1 preadipocytes in the presence of conditioned medium from GH-treated macrophages compared with 3T3-L1 cells treated with conditioned medium from macrophages exposed to vehicle only. Furthermore, in primary macrophages, absence of the GHR accentuated the inhibitory effect of macrophage conditioned medium on the differentiation of 3T3-L1 preadipocytes. Our results implicate GH-dependent decrease in expression and secretion of IL-1β by the macrophage as a mechanism responsible for this effect of GH on adipocyte differentiation. NF-κB is one of the principal regulators of IL-1β synthesis, and activation of NF-κB stimulates IL-1β synthesis. Our results reveal GH-dependent inhibition of NF-κB activation in the macrophage and thus provide a molecular basis for GH-dependent decrease in expression of IL-1β in the macrophage.
Figure 8.
Model for direct and indirect role of GH in adipogenesis. GH can act directly on the preadipocyte to influence adipogenesis. The studies in the current report also indicate a novel indirect mode of GH action on the preadipocyte wherein GH acts on the adipose tissue macrophage to decrease IL-1β secretion by the macrophage. IL-1β is a known inhibitor of adipogenesis, and thus, GH’s action on the macrophage serves to enhance adipogenesis.
The current studies delineate a novel biological effect of GH action on macrophage by demonstrating that GH alters factors secreted by the macrophage to increase differentiation of 3T3-L1 preadipocytes. It is well established that in invertebrates, nonmammalian vertebrates, and humans, macrophages and cells of the macrophage lineage synthesize GH and express GHRs, thus making these cells targets for autocrine, paracrine, and endocrine actions of GH (7,8). Conditioned medium from macrophage inhibits the differentiation of 3T3-L1 and human abdominal preadipocytes (14,16) by restricting clonal expansion during the induction phase of preadipocyte differentiation (36). Our results indicate that GH’s effect on the ability of the conditioned medium from the macrophage to alter preadipocyte differentiation is present only during the induction phase, thus suggesting that GH’s action on the macrophage derepresses clonal expansion during this induction phase. Our studies demonstrate effect of GH on both macrophage cell lines and SVC primary macrophages. However, SVC also includes a number of other cell types such as multipotent mesenchymal stromal, endothelial, and immune cells. Prior reports demonstrated that cells of the immune system are also targets for GH action (3,4,37,38,39,40), and recent reports highlighted the role of immune cells in modulation of the inflammatory response in adipose tissue (41). Hence, we cannot exclude the possibility that some of the effects of GH on SVC primary macrophages observed in our study could be due to GH action on nonmacrophage cells present in SVC.
The ability of conditioned medium from macrophage to inhibit differentiation of 3T3-L1 cells implicates factor(s) secreted by the macrophage for this effect on adipogenesis (14,16). Whereas many of the biological actions of GH are mediated via generation of IGF-I, we established that in our model system GH does not significantly increase IGF-I expression in the macrophage, thus negating the possibility that IGF-I could be responsible for the observed effect of GH on preadipocyte differentiation. It is well known that GH has direct effects on the preadipocyte (42,43,44). Hence, there was a possibility that the observed effects of the GH-treated conditioned medium could be attributable to trace amount of GH remaining in the conditioned medium despite twice washing of the cells before harvesting the conditioned medium. We excluded this possibility by demonstrating that conditioned medium from GH-treated macrophage preincubated with anti-GH antibody to neutralize residual GH failed to block the improved adipogenesis observed in 3T3-L1 preadipocytes (data not shown).
We used PCR array technology to search for candidate factors secreted by the macrophage that could be responsible for the observed GH-dependent increase in adipocyte differentiation. Our results indicate that GH altered the expression of many proteins in the macrophage. Among those proteins whose expression was decreased in a GH-dependent manner, IL-1β is known to inhibit adipocyte differentiation (45), and hence, our findings implicate decreased IL-1β secretion by the macrophage as a mechanism for the observed GH-dependent increase in adipocyte differentiation. In support of this conclusion, we verified that IL-1β in concentrations as low as 5 pg/ml inhibits differentiation of 3T3-L1 preadipocytes to adipocytes (Fig. 6D). It is noteworthy that the PCR array analysis identified several macrophage proteins whose expression was altered in a GH-dependent manner (Fig. 6A). Hence, the current report does not exclude roles for factors other than IL-1β in mediating GH’s effect on adipogenesis, and further studies will have to investigate the significance of these other candidate factors identified in the PCR array analysis.
IL-1β is a proinflammatory cytokine produced by activated macrophages and monocytes. Unlike classical secretory proteins, IL-1β, which lacks a secretory signal sequence, is secreted via a leaderless pathway (46). IL-1β is secreted via a two-step process. In the first step, microbial products such as pathogen-associated molecular pattern molecules induce gene expression and accumulation of the precursor protein pro-IL-1β. In the second step, stimuli such as extracellular ATP or pathogenic dusts (asbestos or silica) trigger assembly of the multiprotein complex inflammasome with sequential activation of IL-1β-converting enzyme/caspase-1, cleavage of pro-IL-1β, and secretion of mature IL-1β (47). Recently much interest has been focused on the role of redox system in IL-1β secretion (48). It is noteworthy that GH can increase production of reactive oxygen intermediates and hydrogen peroxide in macrophages (3,4). However, at the present time, the precise relationship between GH’s effect on production of reactive oxygen intermediates and the observed effect on IL-1β secretion from macrophages is unclear. Previous studies with bovine thymic stromal cells (49) or peritoneal macrophages (50,51) reported that GH up-regulates IL-1β expression. We also observed that GH can increase IL-1β expression if macrophages are prestimulated by lipopolysaccharide (data not published). Hence, the effect of GH on IL-1β expression may be determined by the cell type and functional state of the cell. One of the key factors regulating expression of the IL-1β gene is NF-κB, and suppression of IL-1β using NF-κB p65 antisense oligonucleotide inhibited 2,4,6-trinitrobenzene sulfonic acid-induced IL-1β expression (52). We investigated the effect of GH on NF-κB activation in J774A.1 macrophages to elucidate the mechanism for GH-dependent decrease in IL-1β. Our results reveal a time-dependent decrease in phosphorylation of NF-κB, thus supporting a model wherein GH decreases IL-1β expression via inhibition of NF-κB.
Obesity is considered to be a chronic inflammatory state (53,54). Recent studies highlighted the central role played by adipose-tissue macrophages in regulating the differentiation and function of the adipocyte (55). The concentration of macrophage in adipose tissue is proportional to the size of the fat mass (31). Inflammatory cytokines secreted from macrophage participate in the pathogenesis of the metabolic syndrome (56). The central role of the macrophage in adipocyte development and function underscores the need to understand the role of various hormones and factors that alter macrophage function. Our studies indicate that GH’s actions on the macrophage increases adipocyte differentiation and thus serves to protect against the deleterious metabolic effects of obesity. This novel action of GH sheds light on the biological significance of the observation that GH secretion is decreased in obesity (22). Our results suggest that decreased GH secretion or action will increase the deleterious effects of obesity by decreasing the number of adipocytes and consequently restricting the capacity of the adipose tissue to store fatty acids, a scenario that is believed to result in the development of insulin resistance (18,19,57). Our studies also provide a mechanistic explanation for the observation that the GH-deficient state in children is associated with a decrease in the number of adipocyte (21).
In summary, this report describes a novel action of GH on adipocyte macrophage. Our results demonstrate that GH action on macrophage increases adipocyte differentiation. This action of GH is mediated via decreased secretion of IL-1β by the macrophage acting in paracrine manner on the adipocyte. Furthermore, our results indicate that GH-dependent inhibition of NF-κB could play a role in the decreased secretion of IL-1β after GH stimulation of macrophage. We propose that GH’s action on the macrophage plays a role in the control of adipogenesis.
Acknowledgments
The authors acknowledge the assistance and the generous provision of reagents by Drs. Carey Lumeng (University of Michigan) and Stuart Frank (University of Alabama at Birmingham).
Footnotes
This work was supported by National Institutes of Health Grant DK49845 (to R.K.M.) and Grant P60DK-20572 from the Michigan Diabetes Research and Training Center.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 25, 2010
Abbreviations: aP2, Adipocyte P2; CM-Mac, conditioned media from untreated macrophages; Cre, Cyclization Recombination; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GH-CM-Mac, conditioned media from GH-treated macrophages; GHR, GH receptor; JAK, Janus kinase; NF-κB, nuclear factor-κB; PPAR, peroxisomal proliferator-activated receptor; SVC, stromal vascular compartment.
References
- Møller N, Jørgensen JO 2009 Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 30:152–177 [DOI] [PubMed] [Google Scholar]
- Smith JR, Benghuzzi H, Tucci M, Puckett A, Hughes JL 2000 The effects of growth hormone and insulin-like growth factor on the proliferation rate and morphology of RAW 264.7 macrophages. Biomed Sci Instrum 36:111–116 [PubMed] [Google Scholar]
- Warwick-Davies J, Lowrie DB, Cole PJ 1995 Growth hormone is a human macrophage activating factor. Priming of human monocytes for enhanced release of H2O2. J Immunol 154:1909–1918 [PubMed] [Google Scholar]
- Warwick-Davies J, Lowrie DB, Cole PJ 1995 Growth hormone activation of human monocytes for superoxide production but not tumor necrosis factor production, cell adherence, or action against Mycobacterium tuberculosis. Infect Immun 63:4312– 4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Z, Hertz P, Maor G, Oiknine J, Aviram M 1992 Growth hormone and insulin-like growth factor-I increase macrophage uptake and degradation of low density lipoprotein. Endocrinology 131:430–435 [DOI] [PubMed] [Google Scholar]
- Guicheux J, Kimakhe S, Heymann D, Pilet P, Daculsi G 1998 Growth hormone stimulates the degradation of calcium phosphate biomaterial by human monocytes macrophages in vitro. J Biomed Mat Res 40:79–85 [DOI] [PubMed] [Google Scholar]
- Weigent DA, Blalock JE 1991 The production of growth hormone by subpopulations of rat mononuclear leukocytes. Cell Immunol 135:55–65 [DOI] [PubMed] [Google Scholar]
- Hattori N 2009 Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Horm IGF Res 19:187–197 [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS 2003 Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 112:1785–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyda M, Stulnig TM 2007 Adipose tissue macrophages. Immunol Lett 112:61–67 [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Campbell LV 2008 Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des 14:1225–1230 [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS 2005 Inflammation, stress, and diabetes. J Clin Invest 115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant VA, Gagnon A, Yarmo M, Sorisky A 2008 The antiadipogenic effect of macrophage-conditioned medium depends on ERK1/2 activation. Metabolism 57:465–472 [DOI] [PubMed] [Google Scholar]
- Constant VA, Gagnon A, Landry A, Sorisky A 2006 Macrophage-conditioned medium inhibits the differentiation of 3T3-L1 and human abdominal preadipocytes. Diabetologia 49:1402–1411 [DOI] [PubMed] [Google Scholar]
- Permana PA, Menge C, Reaven PD 2006 Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem Biophys Res Commun 341:507–514 [DOI] [PubMed] [Google Scholar]
- Lacasa D, Taleb S, Keophiphath M, Miranville A, Clement K 2007 Macrophage-secreted factors impair human adipogenesis: involvement of proinflammatory state in preadipocytes. Endocrinology 148:868–877 [DOI] [PubMed] [Google Scholar]
- Park SH, Kim BI, Kim SH, Kim HJ, Park DI, Cho YK, Sung IK, Sohn CI, Kim H, Keum DK, Kim HD, Park JH, Kang JH, Jeon WK 2007 Body fat distribution and insulin resistance: beyond obesity in nonalcoholic fatty liver disease among overweight men. J Am Coll Nutr 26:321–326 [DOI] [PubMed] [Google Scholar]
- Goossens GH 2008 The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav 94:206–218 [DOI] [PubMed] [Google Scholar]
- Jensen MD 2008 Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 93:S57–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes VY, Urban RJ, Jiang J, Marcell TJ, Helgeson K, Mauras N 2001 Recombinant human growth hormone and recombinant human insulin-like growth factor I diminish the catabolic effects of hypogonadism in man: metabolic and molecular effects. J Clin Endocrinol Metab 86:2211–2219 [DOI] [PubMed] [Google Scholar]
- Nam SY, Lobie PE 2000 The mechanism of effect of growth hormone on preadipocyte and adipocyte function. Obes Rev 1:73–86 [DOI] [PubMed] [Google Scholar]
- Williams T, Berelowitz M, Joffe SN, Thorner MO, Rivier J, Vale W, Frohman LA 1984 Impaired growth hormone responses to growth hormone-releasing factor in obesity. A pituitary defect reversed with weight reduction. N Engl J Med 311:1403–1407 [DOI] [PubMed] [Google Scholar]
- Richelsen B 1997 Action of growth hormone in adipose tissue. Horm Res 48(Suppl 5):105–110 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guan R, Jiang J, Kopchick JJ, Black RA, Baumann G, Frank SJ 2001 Growth hormone (GH)-induced dimerization inhibits phorbol ester-stimulated GH receptor proteolysis. J Biol Chem 276:24565–24573 [DOI] [PubMed] [Google Scholar]
- Jiang J, Wang X, He K, Li X, Chen C, Sayeski PP, Waters MJ, Frank SJ 2004 A conformationally sensitive GHR [growth hormone (GH) receptor] antibody: impact on GH signaling and GHR proteolysis. Mol Endocrinol 18:2981–2996 [DOI] [PubMed] [Google Scholar]
- Menon RK, Shaufl A, Yu JH, Stephan DA, Friday RP 2001 Identification and characterization of a novel transcript of the murine growth hormone receptor gene exhibiting development- and tissue-specific expression. Mol Cell Endocrinol 172:135–146 [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ 2008 Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108 [DOI] [PubMed] [Google Scholar]
- Fan Y, Menon RK, Cohen P, Hwang D, Clemens T, DiGirolamo DJ, Kopchick JJ, Le Roith D, Trucco M, Sperling MA 2009 Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem 284:19937–19944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I 1999 Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8:265–277 [DOI] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R 2009 CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15:914–920 [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante Jr AW 2003 Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire FM, Smas CM, Sul HS 1998 Understanding adipocyte differentiation. Physiol Rev 78:783–809 [DOI] [PubMed] [Google Scholar]
- Lagathu C, Yvan-Charvet L, Bastard JP, Maachi M, Quignard- Boulangé A, Capeau J, Caron M 2006 Long-term treatment with interleukin-1β induces insulin resistance in murine and human adipocytes. Diabetologia 49:2162–2173 [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D 1996 NF-κB: ten years after. Cell 87:13–20 [DOI] [PubMed] [Google Scholar]
- Caamaño J, Hunter CA 2002 NF-κB family of transcription factors: central regulators of innate and adaptive immune functions. Clin Microbiol Rev 15:414–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmo MN, Landry A, Molgat AS, Gagnon A, Sorisky A 2009 Macrophage-conditioned medium inhibits differentiation-induced Rb phosphorylation in 3T3-L1 preadipocytes. Exp Cell Res 315:411–418 [DOI] [PubMed] [Google Scholar]
- Gala RR 1991 Prolactin and growth hormone in the regulation of the immune system. Proc Soc Exp Biol Med 198:513–527 [DOI] [PubMed] [Google Scholar]
- Chappel S 1999 Growth hormone in immune reconstitution. J Acquir Immune Defic Syndr Hum Retrovirol 20:423–431 [DOI] [PubMed] [Google Scholar]
- Kooijman R, Hooghe-Peters EL, Hooghe R 1996 Prolactin, growth hormone, and insulin-like growth factor-I in the immune system. Adv Immunol 63:377–454 [DOI] [PubMed] [Google Scholar]
- Weigent DA 1996 Immunoregulatory properties of growth hormone and prolactin. Pharmacol Ther 69:237–257 [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Maillard I, Saltiel AR 2009 T-ing up inflammation in fat. Nat Med 15:846–847 [DOI] [PubMed] [Google Scholar]
- Harant I, Beauville M, Crampes F, Riviere D, Tauber MT, Tauber JP, Garrigues M 1994 Response of fat cells to growth hormone (GH): effect of long term treatment with recombinant human GH in GH-deficient adults. J Clin Endocrinol Metab 78:1392–1395 [DOI] [PubMed] [Google Scholar]
- Wabitsch M, Braun S, Hauner H, Heinze E, Ilondo MM, Shymko R, De Meyts P, Teller WM 1996 Mitogenic and antiadipogenic properties of human growth hormone in differentiating human adipocyte precursor cells in primary culture. Pediatr Res 40:450–456 [DOI] [PubMed] [Google Scholar]
- Morikawa M, Nixon T, Green H 1982 Growth hormone and the adipose conversion of 3T3 cells. Cell 29:783–789 [DOI] [PubMed] [Google Scholar]
- Simons PJ, van den Pangaart PS, van Roomen CP, Aerts JM, Boon L 2005 Cytokine-mediated modulation of leptin and adiponectin secretion during in vitro adipogenesis: evidence that tumor necrosis factor-α- and interleukin-1β-treated human preadipocytes are potent leptin producers. Cytokine 32:94–103 [DOI] [PubMed] [Google Scholar]
- Rubartelli A, Cozzolino F, Talio M, Sitia R 1990 A novel secretory pathway for interleukin-1β, a protein lacking a signal sequence. EMBO J 9:1503–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pétrilli V, Dostert C, Muruve DA, Tschopp J 2007 The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol 19:615–622 [DOI] [PubMed] [Google Scholar]
- Tassi S, Carta S, Vené R, Delfino L, Ciriolo MR, Rubartelli A 2009 Pathogen-induced interleukin-1β processing and secretion is regulated by a biphasic redox response. J Immunol 183:1456–1462 [DOI] [PubMed] [Google Scholar]
- Tseng YH, Kessler MA, Schuler LA 1997 Regulation of interleukin (IL)-1α, IL-1β, and IL-6 expression by growth hormone and prolactin in bovine thymic stromal cells. Mol Cell Endocrinol 128:117–127 [DOI] [PubMed] [Google Scholar]
- Tripathi A, Sodhi A 2008 Prolactin-induced production of cytokines in macrophages in vitro involves JAK/STAT and JNK MAPK pathways. Int Immunol 20:327–336 [DOI] [PubMed] [Google Scholar]
- Sodhi A, Tripathi A 2008 Prolactin and growth hormone induce differential cytokine and chemokine profile in murine peritoneal macrophages in vitro: involvement of p-38 MAP kinase, STAT3 and NF-κB. Cytokine 41:162–173 [DOI] [PubMed] [Google Scholar]
- Wu F, Chakravarti S 2007 Differential expression of inflammatory and fibrogenic genes and their regulation by NF-κB inhibition in a mouse model of chronic colitis. J Immunol 179:6988–7000 [DOI] [PubMed] [Google Scholar]
- Caspar-Bauguil S, Cousin B, Galinier A, Segafredo C, Nibbelink M, André M, Casteilla L, Pénicaud L 2005 Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett 579:3487–3492 [DOI] [PubMed] [Google Scholar]
- Blüher M 2008 The inflammatory process of adipose tissue. Pediatr Endocrinol Rev 6:24–31 [PubMed] [Google Scholar]
- Bourlier V, Bouloumie A 2009 Role of macrophage tissue infiltration in obesity and insulin resistance. Diabetes Metab 35:251–260 [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR 2007 Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56:16–23 [DOI] [PubMed] [Google Scholar]
- Guilherme A, Virbasius JV, Puri V, Czech MP 2008 Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9:367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]