Abstract
Abnormal proliferation of vascular smooth muscle cells (VSMC) is a key feature of development of cardiovascular complications, atherosclerosis, and restenosis. Patients with diabetes have higher risk for restenosis after coronary angioplasty than nondiabetic patients due to hyperglycemia-induced release of cytokines such as TNF-α. However, the molecular mechanisms regulating VSMC proliferation remain unclear. Herein, we report that inhibition of the polyol pathway enzyme aldose reductase (AR) prevents high glucose (HG)- and/or TNF-α-induced VSMC proliferation by accumulating cells at the G1 phase of the cell cycle. Treatment of VSMC with AR inhibitor sorbinil prevented HG- as well as TNF-α-induced phosphorylation of retinoblastoma protein and activation of E2F-1. Inhibition of AR also prevented HG- and TNF-α-induced phosphorylation of cyclin-dependent kinase (cdk)-2 and expression of G1/S transition regulatory proteins such as cyclin D1, cyclin E, cdk-4, c-myc, and proliferative cell nuclear antigen. More importantly, inhibition of AR prevented the increased expression of E2F-1 and proliferative cell nuclear antigen in diabetic rat aorta. Treatment of VSMC with the most abundant and toxic lipid aldehyde 4-hydroxy-trans-2-nonenal (HNE) or its glutathione conjugate [glutathionyl (GS)-HNE] or AR-catalyzed product of GS-HNE, GS-1,4-dihydroxynonane, resulted in increased E2F-1 expression. Inhibition of AR prevented HNE- or GS-HNE-induced but not GS-1,4-dihydroxynonane-induced up-regulation of E2F-1. Collectively, these results show that AR could regulate HG- and TNF-α-induced VSMC proliferation by altering the activation of G1/S-phase proteins such as E2F-1, cdks, and cyclins. Thus, inhibition of AR may be a useful therapeutic approach in preventing vascular complications.
Inhibition of aldose reductase prevents proliferation of vascular smooth muscle cells by regulating cell cycle events, indicating that aldose reductase inhibition could prevent vascular complications.
Poor control of diabetes is associated with abnormal growth of vascular smooth muscle cells (VSMC) that contributes to the development of secondary complications such as micro- and macroangiopathy, neuropathy, nephropathy, and retinopathy (1,2,3). Patients with diabetes mellitus have higher risk for cardiovascular diseases and have higher rates of restenosis after coronary angioplasty compared with nondiabetic patients (4). Neointimal hyperplasia and restenosis are the major problems associated with percutaneous transluminal coronary angioplasty (5). A number of growth factors, hyperglycemia, and cytokines such as TNF-α have been shown to trigger a mitogenic response resulting in the activation of cell cycle progression and VSMC proliferation that cause vascular injury (3,6). Therefore, elucidation of the mechanisms of VSMC proliferation under high glucose (HG), known to increase TNF-α levels also, are important for developing drugs against abnormal cell proliferation that could cause secondary diabetic complications.
Diabetes is known to accelerate progression of atherosclerotic lesions in peripheral, coronary, and cerebral arteries and also cause increased restenosis after angioplasty through multiple mechanisms such as hyperglycemia, hyperinsulinemia, and dyslipidemia (3,7,8). Elevated levels of growth factors and cytokines during intimal lesions have been shown to play an important role in the progression of atherosclerosis, neointimal hyperplasia, and restenosis (9,10,11,12). Among the cytokines, TNF-α is the most abundant and main cytokine involved in the VSMC proliferation and apoptosis of vascular endothelial cells (6,9,13,14). During hyperglycemic conditions, increased levels of cytokines such as TNF-α could activate various signaling intermediates such as protein kinase C (PKC), MAPK, Janus kinase, and nuclear factor (NF)-κB that could deregulate VSMC growth and cause atherosclerosis and could also promote restenosis after angioplasty (15,16).
Aldose reductase (AR), a member of the aldo-keto reductase superfamily, is a polyol pathway enzyme that, besides reducing glucose to sorbitol, also reduces lipid peroxidation-derived aldehydes such as 4-hydroxy-trans-2-nonenal (HNE) and their glutathione conjugates (17). Lipid aldehydes and their metabolites generated in larger amounts during hyperglycemia are known to induce various cytokines, chemokines, and growth factors (17,18). These factors in turn cause inflammation-related cytotoxic disorders.
It has been reported that vascular response of arterial injury leads to proliferation and migration of VSMC by activating cell cycle-related proteins including proliferating cell nuclear antigen (PCNA), cyclin D1, cyclin E, cyclin-dependent kinases (cdk)-2 and cdk-4, and transcription factors such as E2F-1 and c-myc (19,20). Members of the E2F family (E2F1–E2F8) are known to play a major role in the cell proliferation, differentiation, and apoptosis (19,20,21). The most important member of the E2F family is E2F-1, which regulates initiation of DNA synthesis and the G1/S transition of cells during cell division (22,23). The crucial G1 gatekeeper, tumor suppressor retinoblastoma protein (Rb) binds to the E2F-1 transcription factor and prevents nuclear translocation and activation of E2F-1 target genes that are involved in G1/S phase transition and DNA replication (24). During G1 to S phase progression of the cell cycle, heterodimer complexes of cyclin D1/cdk-4 and -6 and cyclin E/cdk-2 cause sequential phosphorylation of Rb and release transcriptionally active E2F-1 (23,24,25). However, the role of AR in modulating cell cycle progression in response to hyperglycemia and vascular injury remains unclear. We, therefore, determined the molecular mechanism of involvement of AR in the modulation of cell cycle progression and expression of cell cycle-related proteins such as E2F-1, cyclins, and cdks in response to HG and TNF-α in cultured VSMC and in arteries of diabetic animals.
Materials and Methods
Materials
DMEM, PBS, penicillin/streptomycin solution, trypsin, and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA). Antibodies against E2F-1, E2F-2, cyclin D1, cyclin A, cdk-2, phospho-Rb, phospho-cdk-2, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and consensus oligonucleotide for E2F-1 transcription factor were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Sorbinil and tolrestat were gifts from Pfizer (Groton, CT) and American Home Products (Madison, NJ), respectively. Small interfering RNA (siRNA) transfection reagent RNAiFect was obtained from QIAGEN (Valencia, CA). All other reagents used for EMSA and Western blotting were of analytical grade (Sigma Chemical Co., St. Louis, MO). Esters of glutathionyl (GS)-HNE, and GS-1,4-dihydroxynonene (GS-DHN) were prepared as described earlier (17).
Cell culture
Rat VSMC were isolated from healthy rat aorta and characterized by smooth muscle cell-specific α-actin expression. The VSMC were maintained and grown in endotoxin-free DMEM containing 5.5 mm glucose supplemented with 10% FBS and 1% penicillin/streptomycin at 37 C in a humidified atmosphere of 5% CO2 as described before (6). For HG and TNF-α stimulation experiments, the medium was replaced with fresh medium containing 25 mm glucose (added 19.5 mm glucose to DMEM that already contained 5.5 mm glucose) and 2 nm TNF-α, respectively, in the absence and presence of 10 μm AR inhibitors. We have prepared 10 mm stock AR inhibitors in 25% dimethylsulfoxide and added to the incubation media such that the final concentrations of inhibitors were at 10 μm. The cells at passage numbers 10–14 were used for this study.
Cell viability assays
VSMC grown in DMEM were harvested by trypsinization and plated in a 96-well plate at a density of 2500 cells per well (n = 5 for each treatment). VSMC at 60–70% confluence, transfected with AR siRNA (AAGCAATCGGAGTCTCCAACT) or E2F-1 siRNA (Dharmacon Inc., Lafayette, CO) for control siRNA using RNAiFect reagent (QIAGEN) or preincubated with or without sorbinil (10 μm) in the DMEM containing 0.1% FBS and HG (25 mm) or normal (5.5 mm) glucose for 24 h. We have shown earlier that 10 μm sorbinil inhibits more than 90% of the AR and proliferation of oxidant-induced VSMC (3,6). Low serum levels were maintained during growth arrest of VSMC to prevent slow apoptosis that accompanies complete serum deprivation of these cells. The growth-arrested cells were treated with HG, TNF-α (2 nm), or medium containing both HG and TNF-α for another 24 h. Because in our earlier studies we have shown that incubation of HG or TNF-α caused more than 60% proliferation of VSMC at 24 h, we used the same time point in these studies (3,6). The rate of cell proliferation was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described earlier (6).
EMSA
The EMSA was performed as described before (6). Briefly, consensus oligonucleotide (5′-ATT TAA GT T TCG CGC CCTTTC TCA A-3′) for E2F-1 transcription factor was 5′-end [γ-32P]ATP labeled using T4 polynucleotide kinase. Nuclear extracts prepared from control and treated cells were incubated with labeled consensus oligonucleotide for E2F-1 for 15 min at 37 C, and the DNA-protein complex formed was resolved on 6.5% native polyacrylamide gel. After electrophoresis, the gels were dried by using a vacuum gel dryer and were autoradiographed on x-ray films (Eastman Kodak Co., Rochester, NY).
Western blot analysis
Equal amounts of total cell lysates were separated by 10% SDS-PAGE. After electrophoresis, proteins were electroblotted to nitrocellulose filters and probed with antibodies against E2F-1, E2F-2, cyclin D1, cyclin A, cdk-2, PCNA, phospho-Rb, phospo-cdk-2, and GAPDH. The antigen-antibody complex was detected by enhanced chemiluminescence (Pierce, Piscataway, NJ).
RT-PCR analysis
Total RNA was isolated from VSMC by using RNeasy micro isolation kit (QIAGEN) as described earlier. Total RNA (1.5 μg) sample was reverse transcribed with Omniscript and Sensiscript reverse transcriptase one-step RT-PCR system with HotStarTaq DNA polymerase (QIAGEN) at 55 C for 30 min followed by PCR amplification. The oligonucleotide primer sequences were as follows: 5′-TTCTTGGAGCTGCTGAGCC-3′ (sense) and 5′-TGGTGATGTCATAGATGCG-3′ (antisense) for E2F-1, 5′-GTGCAGAGGGAGATTGTGCC-3′ (sense) and 5′-GCGGCCCAGGTTCCATTTGAG-3′ (antisense) for cyclin D1, 5′-TCAAGAGGCCACAGCAAAC-3′ (sense) and 5′-AAAAGCTACGCTTCAGCTCG-3′ (antisense) for c-myc, 5′-GCCCTCAAAGACCTCATCAA-3′ (sense) and 5′-GCTCCCCACTCGCAGAAAAC-3′ (antisense) for PCNA, and 5′-TGAGACCTTCAACACCCCAG-3′ and 5′-TTCATGAGGTAGTCTGTCAGGTCC-3′ for β-actin. PCR was carried out in a GeneAmp 2700 thermocycler (Applied Biosystems, Foster City, CA) under the following conditions: initial denaturation at 95 C for 15 min and 35 cycles of 94 C for 30 sec, 62 C for 30 sec, and 72 C for 1 min and then 72 C for 5 min for final extension. PCR products were electrophoresed in 2% agarose/1× Tris-Acetate-EDTA gels containing 0.5 μg/ml ethidium bromide. Bands were quantified using Kodak Image Station 2000R loaded with Kodak one-dimensional image analysis software, and the average fold change intensities were calculated.
Cell cycle analysis
VSMC were grown in six-well plates at a density of 1.5 × 105 cells per well. VSMC were preincubated with sorbinil (final concentration 10 μm; made 10 mm stock in 25% dimethylsulfoxide) or carrier in the DMEM containing 0.1% FBS and HG (25 mm) or normal (5.5 mm) glucose for 24 h followed by stimulation with TNF-α (2 nm) for another 24 h. Cell cycle analysis was performed as described earlier (3).
Luciferase reporter assay
The E2F-1 luciferase activity was determined as described elsewhere (25). Briefly, VSMC were plated in 96-well plates at a density of 1 × 104 cells per well (n = 5 for each experiment). After 24 h, cells were transiently cotransfected with 1 μg pGL2-E2F-1-Luc plasmid and 0.1 μg pRL-CMV plasmid using FuGENE 6 transfection reagent for 6 h followed by serum starvation for overnight in presence or absence of sorbinil (10 μm). The E2F-1-luciferase reporter construct containing −728/+77 region of E2F- 1 gene promoter was a generous gift from Dr. Stephen Safe (Texas A&M University, College Station, TX) (26). VSMC were then stimulated with HG (25 mm) and/or TNF-α (2 nm) for 24 h and dual luciferase activity (Promega, Madison, WI) was measured as per the manufacturer instructions using a luminometer. The luciferase activity was normalized against the ratio of firefly luciferase to renilla luciferase units and expressed as relative luciferase activity.
Induction of diabetes in rats and treatment with sorbinil
Diabetes was induced in adult male Sprague Dawley rats by streptozotocin (55 mg/kg ip; n = 6 for each group) as described previously (3). Diabetic and normal rats were injected with sorbinil (25 mg/kg · d ip) or vehicle. After 14 d, rats were killed, and aorta was dissected. All animal protocols were approved by the institutional animal care committee.
Immunohistochemical analysis
The aorta samples were perfusion fixed with 4% paraformaldehyde and stored in 70% ethanol. Five-micrometer sections of paraffin-embedded tissues taken from rat aorta were stained with antibodies against E2F-1, c-myc, or PCNA as described previously using DakoCytomation LSAB+System-HRP kit (Dako, Carpentaria, CA) (6). The images were quantified by the digital image analysis using Scion Image software.
Determinations of reactive oxygen species (ROS)
ROS were quantified as described earlier (27). Briefly, VSMC were grown in 24-well plates at a density of 1.5 × 104 cells per well. At 70–80% confluence, serum-starved VSMC without or with sorbinil (10 μm) were treated with ROS-sensitive fluorophore 2′,7′-dichlorodihydrofluorescein diacetate (DCHF-DA) for 15 min. Subsequently, VSMC were exposed to HG (25 mm), TNF-α(2 nm), or medium containing both HG and TNF-α for 60 min, and fluorescence was measured with a CytoFluorII fluorescence plate reader (PerSeptive Biosystems, Inc., Framingham, MA) at excitation of 485 nm and emission of 528 nm.
Statistical analysis
Data are presented as mean ± sem, and the P values were determined using the unpaired Student’s t test.
Results
Inhibition of AR prevents synergistic HG- and TNF-α-induced VSMC proliferation
As shown in Fig. 1A, treatment of VSMC with HG and/or TNF-α for 24 h significantly stimulated the growth as determined by MTT assay. The increase in growth was attenuated in the presence of AR inhibitor sorbinil (10 μm). In the absence of HG and TNF-α, sorbinil did not affect VSMC growth. To exclude nonspecific effects of pharmacological inhibitor, we ablated the AR message by transient transfection of VSMC with AR siRNA. Transfection of VSMC with AR siRNA but not scrambled AR siRNA caused more than 95% ablation of AR protein (Fig. 1B, inset), and no detectable amount of AR activity was observed in siRNA-transfected cells (data not shown). Consistent with our previous data, transfection of VSMC with AR siRNA significantly (∼75%) inhibited HG- and TNF-α-induced cell growth (Fig. 1B). Enhanced growth of VSMC by synergistic effect of HG and TNF-α was also significantly inhibited by AR inhibition, suggesting that AR is required to mediate HG- and/or TNF-α-induced VSMC growth.
Figure 1.
Inhibition or ablation of AR prevents HG- and/or TNF-α-induced VSMC proliferation. A, Growth-arrested VSMC were preincubated with 10 μm sorbinil or carrier for 24 h in the presence or absence of HG and/or TNF-α. B, VSMC were transfected with AR siRNA or scrambled AR siRNA for 24 h at 37 C in the presence or absence of HG. Subsequently, the cells were stimulated with TNF-α (2 nm) for another 24 h. Cell viability was determined by MTT assay. Inset in B, Western blot analysis of untransfected (c), scrambled (s), and AR siRNA-transfected (a) cell extracts for determination of AR protein levels. Bars, Mean of five individual experiments (n = 5) ± sem; #, P < 0.001 compared with treatment without the inhibitor or scrambled AR siRNA-transfected cells; *, P < 0.001 compared with HG- and/or TNF-α-treated cells. NG, Normal glucose.
Effect of AR inhibition on HG- and TNF-α-induced cell cycle progression in VSMC
Because inhibition of AR prevents HG- and TNF-α-induced VSMC proliferation, we investigated how AR inhibition regulates cell cycle events. Treatment of VSMC with HG- and/or TNF-α-induced entry of cells to the synthesis (S) phase of the cell cycle, suggesting that cells were undergoing proliferation (Fig. 2). Inhibition of AR prevented HG- and/or TNF-α-induced S phase entry, and the cells accumulated at G2-M and G1 phase, indicating that inhibition of AR could prevent entry of cells from G1 to S phase of the cell cycle, which is an important stage required for cell proliferation. Furthermore, our previous studies indicate that approximately 2% of cells were entered in the G2 phase (3); however, in the present study, the number of cells in the G2 phase was approximately 14%. The difference observed could be due to variation in the VSMC passage number (10,11,12,13,14) used in this study.
Figure 2.
AR inhibition prevents HG- and/or TNF-α-induced synthesis phase in VSMC. Growth-arrested VSMC were preincubated with 10 μm sorbinil or carrier for 24 h in the presence or absence of HG followed by stimulation with TNF-α (2 nm) for another 24 h. The cells were trypsinized and washed, and DNA was stained with propidium iodide as described in Materials and Methods. Cell cycle analysis was performed with a minimum of 10,000 events per analysis by using FACScan flow cytometer. The data (inset) represent means ± sem of three independent analyses and shows one of the representative FACScans. #, P < 0.001 compared with treatment without inhibitor; **, P < 0.01 compared with HG- and/or TNF-α-treated cells.
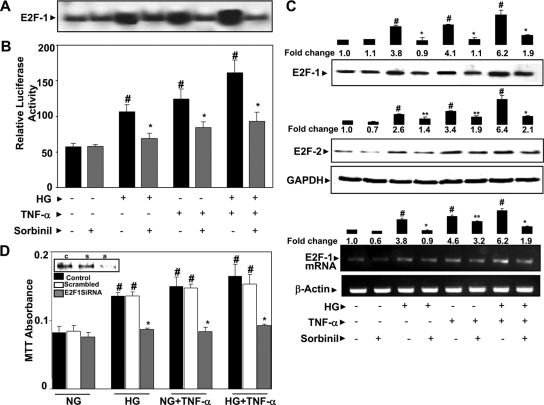
Inhibition of AR prevents HG- and TNF-α-induced E2F-1 DNA-binding activity in VSMC
To further understand how AR inhibition prevents S phase entry of cells, we examined the effect of AR inhibitor on one of the important cell cycle regulatory transcription factors, E2F-1, which binds to the promoter regions of various cell cycle regulatory enzymes such as cyclins and cdks and up-regulates their synthesis (22,23). Treatment of VSMC with HG and/or TNF-α caused a pronounced activation of E2F-1 as determined by EMSA (Fig. 3A). The synergistic effect of HG and TNF-α further enhanced the activation of E2F-1. Preincubation of VSMC with sorbinil significantly prevented the HG- and/or TNF-α-induced activation of E2F-1. We have further confirmed the effect of AR inhibition on transcriptional activity of E2F-1 by luciferase reporter gene assay. As shown in Fig. 3B, inhibition of AR significantly prevented HG and/or TNF-α-induced transcriptional activation of E2F-1. Treatment of VSMC with sorbinil alone did not affect the basal level of E2F-1 activation. These results indicate that inhibition of AR could prevent DNA-binding activity of E2F-1 in VSMC.
Figure 3.
AR inhibition prevents HG- and/or TNF-α-induced E2F-1 DNA-binding activity and expression of E2F-1 and E2F-2 in VSMC. A, Growth-arrested VSMC (n = 5 in each group) were preincubated with 10 μm sorbinil or carrier for 24 h in the presence or absence of HG followed by stimulation with TNF-α (2 nm) for 18 h. The nuclear extracts were prepared, and E2F DNA-binding activity was measured by EMSA. B, Determination of transcriptional activity of E2F-1 by luciferase reporter gene assay. C, The total cell lysate was subjected to Western blot analyses using antibodies against E2F-1 and E2F-2 and GAPDH. Growth-arrested VSMC were preincubated with sorbinil or carrier for 24 h followed by stimulation with HG and/or TNF-α for 4 h to determine E2F-1 mRNA expression by RT-PCR. D, VSMC were transfected with E2F-1 siRNA or scrambled siRNA for 24 h at 37 C. Subsequently, the cells were stimulated with HG or TNF-α or both for another 24 h. Cell viability was determined by MTT assay. Inset in D, Western blot analysis of untransfected (c), scrambled (s), and E2F-1 siRNA-transfected (a) cell extracts for determination of E2F-1 protein levels. Densitometry analysis was performed by using Kodak 1D image analysis software. Bars, Means ± sem of three independent analyses (n = 3); #, P < 0.001 compared with control cells; *, P < 0.001; **, P < 0.05 compared with cells treated with HG and/or TNF-α.
Inhibition of AR prevents HG- and TNF-α-induced expression of E2F-1 and E2F-2
Because E2F proteins stimulate the expression of their own proteins directly by binding to their respective gene promoters (20,21,22,23), we determined whether AR inhibition prevents HG- and/or TNF-α-induced de novo synthesis of important E2F family proteins such as E2F-1. Treatment of VSMC with HG or TNF-α or both HG and TNF-α increased the expression E2F-1, and inhibition of AR prevented it (Fig. 3C). We next measured the expression of another important G1-phase E2F family protein, E2F-2. As shown in Fig. 3C, inhibition of AR by sorbinil prevented the expression of E2F-2 protein. Furthermore, treatment of VSMC with HG and/or TNF-α for 4 h significantly increased the expression of E2F-1 mRNA, and inhibition of AR prevented it (Fig. 3C). These results suggest that AR is involved in the de novo synthesis/expression and activation of E2F-1 and E2F-2. To determine whether E2F-1 transcription factor is necessary for proliferation of VSMC in response to HG and TNF-α, we ablated E2F-1 with E2F-1 siRNA. Transfection of VSMC with E2F-1 siRNA significantly (∼80%) inhibited HG- and TNF-α-induced cell growth (Fig. 3D). Enhanced growth of VSMC by synergistic effect of HG and TNF-α was also significantly inhibited by E2F-1 inhibition, suggesting that E2F-1 promotes VSMC growth.
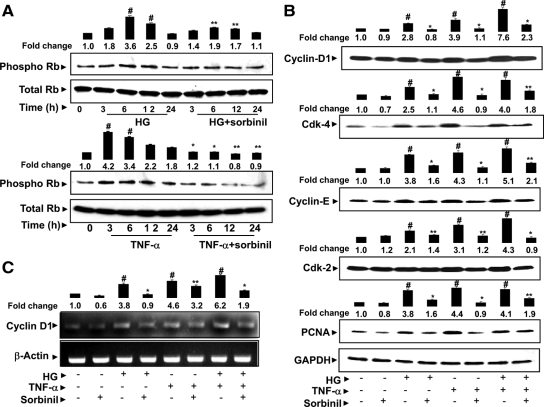
Inhibition of AR prevents HG- and TNF-α-induced phosphorylation of retinoblastoma protein
Because unphosphorylated Rb, a tumor suppressor, binds to E2F-1 and prevents nuclear translocation (24), we next measured the effect of AR inhibition on early Rb (Ser780) phosphorylation. As shown in Fig. 4A, both HG and TNF-α phosphorylated Rb in VSMC, and the maximal phosphorylation was observed at 6 and 3 h, respectively. The increase in phosphorylation of Rb was significantly inhibited by AR inhibition.
Figure 4.
Inhibition of AR prevents HG- and/or TNF-α-induced phosphorylation of Rb and expression of cyclin D1, cdk-4, cyclin E, cdk-2, and PCNA in VSMC. A and B, Quiescent VSMC were preincubated with 10 μm sorbinil for 24 h followed by stimulation with HG or TNF-α for different time intervals (A) and 24 h (B) (n = 5 for each treatment). The pooled total cell lysate was subjected to Western blot analysis using antibodies against phospho-Rb, cyclin D1, cdk-4, cyclin E, cdk-2, PCNA, and GAPDH. C, Growth-arrested VSMC were preincubated with sorbinil or carrier for 24 h followed by stimulation with HG and/or TNF-α for 4 h to determine cyclin D1 mRNA expression by RT-PCR. Densitometry analysis was performed by using Kodak 1D image analysis software. Bars, Means ± sem of three independent analyses; #, P < 0.001 compared with control cells; *, P < 0.001; **, P < 0.05 compared with cells treated with HG and/or TNF-α.
Inhibition of AR prevents HG- and/or TNF-α-induced expression of cyclins and cdks in VSMC
We next determined whether inhibition of AR prevents HG and/or TNF-α-induced expression of cyclins and cdks that are known to regulate entry of cells into the S phase. These include hetero-dimer complexes of mid-G1-phase cyclin D1 and cdk-4 and late-G1-phase cyclin E and cdk-2, which are known to regulate phosphorylation of Rb. As shown in Fig. 4B, the synergistic effect of HG and TNF-α caused a marked increase in the expression of these proteins compared with HG or TNF-α alone. Pretreatment of VSMC with AR inhibitor significantly (60–80%) prevented the expression of cyclin D1, cdk-4, cyclin E, and cdk-2. In addition, inhibition of AR also significantly prevented the induction of the proliferation index marker protein PCNA by HG or TNF-α alone or by both HG and TNF-α. Furthermore, treatment of VSMC with HG and/or TNF-α for 4 h significantly increased the expression of cyclin D1 mRNA, and inhibition of AR prevented it (Fig. 4C). These observations suggest that AR inhibition prevents the expression of the G1 phase of cell cycle regulatory proteins and thereby inhibits the nuclear translocation of E2F-1. Because during proliferation of cells, phosphorylation of cdk-2 at late G1 phase drives G1 to S phase transition by further phosphorylating Rb and stabilizing active E2F-1, we next measured the effect of AR inhibition on the phosphorylation of cdk-2 in VSMC proliferation induced by HG or TNF-α (Fig. 5Α). Both HG and TNF-α caused an increase in phosphorylation of cdk-2 in a time-dependent manner. The maximal level of phosphorylation was observed at the 24-h time point, and inhibition of AR prevented it. The observed phosphorylation could be due to increased levels of total cdk-2; however, when we normalized the values with total cdk-2, the HG- and TNF-α-treated cells showed a slight increase in the phosphorylation of cdk-2, and inhibition of AR prevented it.
Figure 5.
Inhibition of AR prevents HG- and/or TNF-α-induced phosphorylation of cdk-2 and c-myc expression and ROS production in VSMC. A and B, Quiescent VSMC (n = 5 for each treatment) were preincubated with 10 μm sorbinil or carrier for 24 h followed by stimulation with HG or TNF-α for different time intervals (A) and 24 h (B). The pooled total cell lysate was subjected to Western blot analysis using antibodies against phospho-cdk-2, total cdk-2, and c-myc. C, Growth-arrested VSMC were preincubated with sorbinil or carrier for 24 h followed by stimulation with HG and/or TNF-α for 4 h to determine c-myc mRNA expression by RT-PCR. D, For the determination of ROS, growth-arrested VSMC were preincubated with sorbinil or carrier for 24 h followed by stimulation with HG and/or TNF-α for 1 h, and the cells were treated with dichlorodihydrofluorescein for 15 min, washed, and fluorescence determined using excitation and emission wavelengths of 485 and 528 nm, respectively. Densitometric analysis was performed using Kodak 1D image analysis software. Bars, Means ± sem of three independent analyses; #, P < 0.001 compared with control cells; *, P < 0.001; **, P < 0.05 compared with cells treated with HG and/or TNF-α.
Inhibition of AR prevents HG- and TNF-α-induced c-myc expression and ROS production in VSMC
To investigate the role of c-myc, an important transcription factor that binds to consensus sequences of various cyclins, cdks, and E2F family proteins and causes cell cycle transition (28,29), we next measured the effect of AR inhibition on HG- and TNF-α-induced c-myc expression. As shown in Fig. 5B, treatment of VSMC with HG and TNF-α caused a significant increase in the expression of c-myc protein, and inhibition of AR prevented it. We next determined the effect of AR inhibition on transcriptional activation of c-myc. As shown in Fig. 5C, treatment of VSMC with HG and/or TNF-α for 4 h significantly increased the expression of c-myc mRNA, and inhibition of AR prevented it. ROS generated during oxidative stress conditions are known to induce expression of c-myc protein (28). Hence, we next determined whether inhibition of AR affects HG- and/or TNF-α-induced changes in ROS generation. As shown in Fig. 5D, inhibition of AR by sorbinil prevented the ROS generation as measured by dichlorodihydrofluorescein fluorescence, suggesting that inhibition of AR prevents c-myc expression by inhibiting ROS generation in VSMC. These results were correlated with protein levels of cyclin D1 and c-myc.
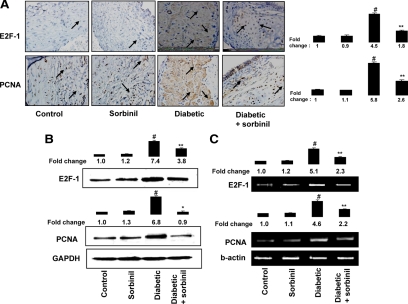
Inhibition of AR prevents E2F-1 and PCNA expression in diabetic rat aorta
We next examined the involvement of AR in the expression of cell cycle proteins E2F-1 and PCNA in an in vivo rat diabetic model. As shown in Fig. 6A, the intensity of E2F-1 and PCNA antibodies staining in the diabetic rat aorta sections was significantly higher compared with nondiabetic rats. Treatment of diabetic rats with sorbinil significantly diminished the expression of E2F-1 and PCNA. Western blot and RT-PCR analysis of aorta samples against E2F-1 and PCNA further confirmed the inhibition of AR prevents the expression of E2F-1 and PCNA at the protein and mRNA levels (Fig. 6, B and C).
Figure 6.
Inhibition of AR prevents E2F-1 and PCNA expression in diabetic rat aorta. Diabetes was induced in adult male Sprague Dawley rats by single streptozotocin injection (55 mg/kg ip), and diabetic and normal rats were treated with sorbinil (25 mg/kg · d ip) or vehicle for 14 d. A, Sections of diabetic and nondiabetic rat aorta stained with anti-E2F-1 and anti-PCNA followed by counterstaining with hematoxylin (magnification, ×400). Photomicrographs of the stained sections were acquired using an EPI-800 microscope (bright field) connected to a Nikon camera. Percent staining was determined by measuring positive immunoreactivity per unit area. Arrows represent the area for positive staining for an antigen. The intensity of antigen staining was quantified by digital image analysis using Scion Image software. B, Equal amounts of aorta tissue homogenates were subjected to Western blot analysis using antibodies against E2F-1, PCNA, and GAPDH. C, RT-PCR analysis of aorta tissues. Bars, Means ± sem (n = 3, representing the number of sections used for analysis, not the number of animals). #, P < 0.001 vs. control; *, P < 0.001; **, P < 0.05 vs. diabetic rats.
Effect of AR inhibition on lipid aldehyde-induced E2F-1 expression in VSMC
We have shown earlier that AR-catalyzed reaction products of GS-HNE could mediate mitogenicity in VSMC (17). Therefore, we examined whether GS conjugates of lipid aldehydes could be involved in the G1/S transition in VSMC. Treatment of VSMC with HNE or cell-permeable esters of GS-HNE or GS-DHN resulted in increased expression of E2F-1 (Fig. 7A). Inhibition of AR by sorbinil significantly prevented the HNE- and GS-HNE-induced E2F-1 expression but had no effect on GS-DHN-induced expression of E2F-1. These results indicate that hyperglycemia- and/or cytokine-induced G1/S phase transition in VSMC could be mediated by the reduced form of lipid aldehyde-glutathione conjugates catalyzed by AR.
Figure 7.
Effect of AR-catalyzed reaction products on E2F-1 expression in VSMC. A, Growth-arrested VSMC were preincubated with sorbinil 10 μm or carrier for 24 h followed by stimulation with HNE, GS-HNE-ester, or GS-DHN-ester for another 24 h (n = 5 for each treatment). The pooled total cell lysate was subjected to SDS-PAGE and Western blots were developed using antibodies against E2F-1 and GAPDH. Densitometry analysis was performed by using Kodak 1D image analysis software. Bars, Means ± sem of three independent analyses; #, P < 0.001 compared with control cells. *, P < 0.001, **, P < 0.05 compared cells treated with HNE or GS-HNE. B, Schematic representation of AR mediation during G1-S phase transition of cell cycle. Oxidative stress-induced lipid peroxidation results in generation of toxic lipid aldehydes such as HNE. AR efficiently reduces HNE and its conjugate with glutathione to DHN and GS-DHN, respectively. The reduced products of aldehydes may be involved in G1/S phase transition of cell cycle, which leads to proliferation of VSMC.
Discussion
Several lines of evidence suggest that inhibition of AR prevents or delays secondary diabetic complications such as cataractogenesis, retinopathy, neuropathy, nephropathy, and microangiopathy (1,2,3). In hyperglycemia, increased flux of glucose via AR causes osmotic and oxidative changes, which in turn trigger a sequence of metabolic changes such as tissue dysfunction, altered intracellular signaling, and extensive cell death or proliferation (2). In addition to reduction of glucose, AR efficiently reduces lipid aldehydes and their glutathione conjugates such as HNE and GS-HNE with a Michaelis-Menten constant (Km) in low 10- to 30-μm range (17,27). Oxidative stress during pathological conditions such as myocardial ischemia, reperfusion, infection, and diabetes is known to up-regulate AR (30,31). Specifically, oxidative stress in diabetes has been shown to be associated with cardiovascular diseases (30,31,32). Diabetic subjects are predisposed to progression of atherosclerotic lesions in peripheral, coronary, and cerebral arteries and also restenosis after angioplasty (30,31,32).
In VSMC, we have shown that inhibition of AR prevents HG-induced progression of cell cycle at S phase (3). Here, we report the molecular mechanism of AR’s involvement in G1/S phase cell cycle transition (Fig. 6D). Increased levels of growth factors and cytokines have been observed during intimal lesions that play important role in the progression of atherosclerosis and restenosis that involve cell proliferation (9,10,11,12,13). Therefore, our demonstration that AR inhibition can arrest cell cycle at S phase and arrest the growth has high clinical significance. Recently, in VSMC, we have reported that AR is required for HG-induced TNF-α synthesis and release (18). These observations suggest that release of TNF-α by HG leading to autocrine stimulation of TNF-α synthesis may be a critical step in the development of the cardiovascular complications of diabetes. Because TNF-α is thought to contribute to the development of insulin resistance as well as to the manifestation and severity of diabetes, and our current and earlier results indicate that inhibition of AR prevents HG-induced TNF-α synthesis and secretion as well as its signals leading to VSMC proliferation (6,18), physiological inhibition of AR could prevents vascular complications of hyperglycemia. Multiple mechanisms involving PKC and NF-κB have been implicated in the context of HG- and ΤΝF-α-induced VSMC proliferation and neointima formation (3,6,14). Our results in various cell lines show that AR mediates the HG- and ΤΝF-α-induced activation of PKC/phospholipase C/diacylglycerol and NF-κB (6,33). In addition, recently using a mouse model of endotoxemia, we have shown that lipopolysaccharide-induced decrease in the functional recovery of myocardial fractional shortening and restoration of contractile function of isolated perfused hearts by inhibiting AR (34). This suggested that AR could play an important role in cardiovascular dysfunction associated with inflammation.
Multiple studies show that E2F-1 is an important transcription factor that regulates the expression of various genes such as PCNA, cyclin D1, cdk-2, cdk-4, c-myc, and c-myb required for G1/S phase cell cycle progression (22,23). Among the E2F family proteins, only three proteins, E2F-1, E2F-2, and E2F-3a, are minimal in quiescent cells and increased in response to growth stimulation (35,36). Furthermore, of these three proteins, E2F-1 has been shown to up-regulate the expression of cell cycle-related genes (20). Numerous studies show that HG or TNF-α induces expression of E2F-1, PCNA, cyclin A, and cyclin D1 (37,38,39). Our data convincingly demonstrate that inhibition of AR could prevent HG- and/or TNF-α-induced expression of E2F-1 and E2F-2 and their endogenous targets such as PCNA, cyclin D1, cyclin E, and cdk-4. As one of the major cytokines, TNF-α is secreted by VSMC after balloon injury as well as by macrophages in atherosclerotic lesions, and TNF-α promotes cell growth and progression of vascular lesions by activating ERK1/2 (3,13,16). Recently, in RAW264.7 murine macrophages, we have shown that inhibition of AR prevents lipopolysaccharide-induced release of cytokines (40), suggesting that AR could play an important role in atherosclerotic lesions and neointima formation.
Our results showing that inhibition of AR prevented the ΗG- or TNF-α-induced phosphorylation of Rb suggest that AR inhibition may have antimitogenic effect in VSMC by inhibiting activation of E2F-1. Kim et al. (41) in mouse embryonic stem cells showed that inhibition of phosphatidylinositol-3-kinase prevents HG-induced expression of the G1-S phase of cell cycle proteins cyclin D1, cyclin E, cdk-2, and cdk-4 and phosphorylation of Rb. Various reports show ROS stimulate VSMC growth by expressing protooncogenes such as c-myc and c-fos (28). Significant inhibition of HG- and/or TNF-α-induced ROS production and c-myc expression by AR inhibition indicates that AR is involved in the cascade of the c-myc pathway in VSMC. Various reports show that HG or TNF-α causes increase in the expression of c-myc protein during cell growth, apoptosis, and differentiation of physiological and pathological conditions (29). In a porcine stent restenosis model, intramural coronary delivery of advance antisense oligonucleotides against c-myc protein showed reduced neointimal formation, suggesting that c-myc is critical in cell division and is involved in the neointima formation (42). In balloon-injured carotid artery of nondiabetic and diabetic rat models (3), we showed that inhibition of AR prevented the cell proliferating index marker protein PCNA expression and neointima formation, indicating that AR is involved in the cell cycle progression. Furthermore, we have shown that diabetic rats are more susceptible to neointima formation than nondiabetic rats (3).
In conclusion, we have demonstrated that inhibition of AR prevents HG- and/or TNF-α-stimulated VSMC proliferation by inhibiting ROS generation and c-myc expression, activation/expression of E2F-1, cyclin D1/cdk-4, and cyclin E/cdk-2 activities. Similarly, AR inhibition also prevents the expression of E2F-1 and PCNA in diabetic rat aorta. These results indicate that inhibition of AR prevents HG-induced proliferation by ameliorating cell cycle events. Understanding this mechanism is potentially important in developing therapeutic strategies to ameliorate or prevent cardiovascular complications, especially in diabetic patients by inhibition or ablation of AR.
Footnotes
This work was supported by National Institutes of Health Grants DK36118 (to S.K.S.) and GM71036 (to K.V.R.) and National Institute of Environmental Health Core Facility Grant (ES-006676).
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 22, 2010
Abbreviations: AR, Aldose reductase; cdk, cyclin-dependent kinase; DHN, 1,4-dihydroxynonene; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GS, glutathionyl; HG, high glucose; HNE, 4-hydroxy-trans-2-nonenal; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NF, nuclear factor; PCNA, proliferative cell nuclear antigen; PKC, protein kinase C; Rb, retinoblastoma protein; ROS, reactive oxygen species; siRNA, small interfering RNA; VSMC, vascular smooth muscle cells.
References
- Krolewski AS, Warram JH, Rand LI, Kahn CR 1987 Epidemiologic approach to the etiology of type I diabetes mellitus and its complications. N Engl J Med 317:1390–1398 [DOI] [PubMed] [Google Scholar]
- Srivastava SK, Ramana KV, Bhatnagar A 2005 Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev 26:380–392 [DOI] [PubMed] [Google Scholar]
- Srivastava S, Ramana KV, Tammali R, Srivastava SK, Bhatnagar A 2006 Contribution of aldose reductase to diabetic hyperproliferation of vascular smooth muscle cells. Diabetes 55:901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsness GW, Peterson ED, Ohman EM, Nelson CL, DeLong ER, Reves JG, Smith PK, Anderson RD, Jones RH, Mark DB, Califf RM 1997 Relationship between diabetes mellitus and long-term survival after coronary bypass and angioplasty. Circulation 96:2551–2556 [DOI] [PubMed] [Google Scholar]
- Van Belle E, Ketelers R, Bauters C, Périé M, Abolmaali K, Richard F, Lablanche JM, McFadden EP, Bertrand ME 2001 Patency of percutaneous transluminal coronary angioplasty sites at 6-month angiographic follow-up: a key determinant of survival in diabetics after coronary balloon angioplasty. Circulation 103:1218–1224 [DOI] [PubMed] [Google Scholar]
- Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Aggarwal BB, Srivastava SK 2002 Aldose reductase mediates mitogenic signaling in vascular smooth muscle cells. J Biol Chem 277:32063–32070 [DOI] [PubMed] [Google Scholar]
- Sheetz MJ, King GL 2002 Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA 288:2579–2588 [DOI] [PubMed] [Google Scholar]
- Selvin E, Coresh J, Golden SH, Brancati FL, Folsom AR, Steffes MW 2005 Glycemic control and coronary heart disease risk in persons with and without diabetes: the atherosclerosis risk in communities study. Arch Intern Med 165:1910–1916 [DOI] [PubMed] [Google Scholar]
- Clausell N, Kalil P, Biolo A, Molossi S, Azevedo M 1999 Increased expression of tumor necrosis factor-α in diabetic macrovasculopathy. Cardiovasc Pathol 8:145–151 [DOI] [PubMed] [Google Scholar]
- Rayment NB, Moss E, Faulkner L, Brickell PM, Davies MJ, Woolf N, Katz DR 1996 Synthesis of TNFα and TGFβ mRNA in the different micro-environments within atheromatous plaques. Cardiovasc Res 32:1123–1130 [DOI] [PubMed] [Google Scholar]
- Braun-Dullaeus RC, Mann MJ, Dzau VJ 1998 Cell cycle progression: new therapeutic target for vascular proliferative disease. Circulation 98:82–89 [DOI] [PubMed] [Google Scholar]
- Walker LN, Bowen-Pope DF, Ross R, Reidy MA 1986 Production of platelet-derived growth factor-like molecules by cultured arterial smooth muscle cells accompanies proliferation after arterial injury. Proc Natl Acad Sci USA 83:7311–7315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner SJ, Libby P 1989 Human vascular smooth muscle cells: target for and source of tumor necrosis factor. J Immunol 142:100–109 [PubMed] [Google Scholar]
- Ramana KV, Bhatnagar A, Srivastava SK 2004 Aldose reductase regulates TNF-α-induced cell signaling and apoptosis in vascular endothelial cells. FEBS Lett 570:189–194 [DOI] [PubMed] [Google Scholar]
- Elkind MS, Cheng J, Boden-Albala B, Rundek T, Thomas J, Chen H, Rabbani LE, Sacco RL 2002 Tumor necrosis factor receptor levels are associated with carotid atherosclerosis. Stroke 33:31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SO, Jeong YJ, Yu MH, Lee JW, Hwangbo MH, Kim CH, Lee IS 2006 Wogonin suppresses TNF-α-induced MMP-9 expression by blocking the NF-κB activation via MAPK signaling pathways in human aortic smooth muscle cells. Biochem Biophys Res Commun 351:118–125 [DOI] [PubMed] [Google Scholar]
- Ramana KV, Bhatnagar A, Srivastava S, Yadav UC, Awasthi S, Awasthi YC, Srivastava SK 2006 Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J Biol Chem 281:17652–17660 [DOI] [PubMed] [Google Scholar]
- Ramana KV, Tammali R, Reddy AB, Bhatnagar A, Srivastava SK 2007 Aldose reductase-regulated tumor necrosis factor-α production is essential for high glucose-induced vascular smooth muscle cell growth. Endocrinology 148:4371–4384 [DOI] [PubMed] [Google Scholar]
- Ahn JD, Morishita R, Kaneda Y, Kim HS, Chang YC, Lee KU, Park JY, Lee HW, Kim YH, Lee IK 2002 Novel E2F decoy oligodeoxynucleotides inhibit in vitro vascular smooth muscle cell proliferation and in vivo neointimal hyperplasia. Gene Ther 9:1682–1692 [DOI] [PubMed] [Google Scholar]
- Kim MJ, Park KG, Lee KM, Kim HS, Kim SY, Kim CS, Lee SL, Chang YC, Park JY, Lee KU, Lee IK 2005 Cilostazol inhibits vascular smooth muscle cell growth by down regulation of the transcription factor E2F. Hypertension 45:552–556 [DOI] [PubMed] [Google Scholar]
- Salam MA, Matin K, Matsumoto N, Tsuha Y, Hanada N, Senpuku H 2004 E2f1 mutation induces early onset of diabetes and Sjögren’s syndrome in nonobese diabetic mice. J Immunol 173:4908–4918 [DOI] [PubMed] [Google Scholar]
- Helin K, Lees JA, Vidal M, Dyson N, Harlow E, Fattaey A 1992 A cDNA encoding a pRB-binding protein with properties of transcription factor E2F. Cell 70:337–350 [DOI] [PubMed] [Google Scholar]
- Shan B, Lee WH 1994 Deregulated expression of E2F-1 induces S-phase entry and leads to apoptosis. Mol Cell Biol 14:8166–8173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins JR 1992 E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science 258:424–429 [DOI] [PubMed] [Google Scholar]
- Hahm ER, Singh SV 2007 Honokiol causes G0–G1 phase cell cycle arrest in human prostate cancer cells in association with suppression of retinoblastoma protein level/phosphorylation and inhibition of E2F1 transcriptional activity. Mol Cancer Ther 6:2686–2695 [DOI] [PubMed] [Google Scholar]
- Wang W, Dong L, Saville B, Safe S 1999 Transcriptional activation of E2F1 gene expression by 17β-estradiol in MCF-7 cells is regulated by NF-Y-Sp1/estrogen receptor interactions. Mol Endocrinol 13:1373–1387 [DOI] [PubMed] [Google Scholar]
- Tammali R, Ramana KV, Singhal SS, Awasthi S, Srivastava SK 2006 Aldose reductase regulates growth factor-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colon cancer cells. Cancer Res 66:9705–9713 [DOI] [PubMed] [Google Scholar]
- Rao GN, Berk BC 1992 Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res 70:593–599 [DOI] [PubMed] [Google Scholar]
- Elouil H, Cardozo AK, Eizirik DL, Henquin JC, Jonas JC 2005 High glucose and hydrogen peroxide increase c-Myc and haeme-oxygenase 1 mRNA levels in rat pancreatic islets without activating NFκB. Diabetologia 48:496–505 [DOI] [PubMed] [Google Scholar]
- Hwang YC, Kaneko M, Bakr S, Liao H, Lu Y, Lewis ER, Yan S, Ii S, Itakura M, Rui L, Skopicki H, Homma S, Schmidt AM, Oates PJ, Szabolcs M, Ramasamy R 2004 Central role for aldose reductase pathway in myocardial ischemic injury. FASEB J 18:1192–1199 [DOI] [PubMed] [Google Scholar]
- Kaiserova K, Srivastava S, Hoetker JD, Awe SO, Tang XL, Cai J, Bhatnagar A 2006 Redox activation of aldose reductase in the ischemic heart. J Biol Chem 281:15110–15120 [DOI] [PubMed] [Google Scholar]
- Ruef J, Liu SQ, Bode C, Tocchi M, Srivastava S, Runge MS, Bhatnagar A 2000 Involvement of aldose reductase in vascular smooth muscle cell growth and lesion formation after arterial injury. Arterioscler Thromb Vasc Biol 20:1745–1752 [DOI] [PubMed] [Google Scholar]
- Tammali R, Ramana KV, Srivastava SK 2007 Aldose reductase regulates TNF-α-induced PGE2 production in human colon cancer cells. Cancer Lett 252:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramana KV, Willis MS, White MD, Horton JW, DiMaio JM, Srivastava D, Bhatnagar A, Srivastava SK 2006 Endotoxin-induced cardiomyopathy and systemic inflammation in mice is prevented by aldose reductase inhibition. Circulation 114:1838–1846 [DOI] [PubMed] [Google Scholar]
- Cobrinik D 2005 Pocket proteins and cell cycle control. Oncogene 24:2796–2809 [DOI] [PubMed] [Google Scholar]
- Maiti B, Li J, de Bruin A, Gordon F, Timmers C, Opavsky R, Patil K, Tuttle J, Cleghorn W, Leone G 2005 Cloning and characterization of mouse E2F8, a novel mammalian E2F family member capable of blocking cellular proliferation. J Biol Chem 280:18211–18220 [DOI] [PubMed] [Google Scholar]
- Féliers D, Frank MA, Riley DJ 2002 Activation of cyclin D1-Cdk4 and Cdk4-directed phosphorylation of RB protein in diabetic mesangial hypertrophy. Diabetes 51:3290–3299 [DOI] [PubMed] [Google Scholar]
- Lee B, Moon SK 2005 Resveratrol inhibits TNF-α-induced proliferation and matrix metalloproteinase expression in human vascular smooth muscle cells. J Nutr 135:2767–2773 [DOI] [PubMed] [Google Scholar]
- Suh SJ, Jin UH, Kim SH, Chang HW, Son JK, Lee SH, Son KH, Kim CH 2006 Ochnaflavone inhibits TNF-α-induced human VSMC proliferation via regulation of cell cycle, ERK1/2, and MMP-9. J Cell Biochem 99:1298–1307 [DOI] [PubMed] [Google Scholar]
- Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK 2006 Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem 281:33019–33029 [DOI] [PubMed] [Google Scholar]
- Kim YH, Heo JS, Han HJ 2006 High glucose increase cell cycle regulatory proteins level of mouse embryonic stem cells via PI3-K/Akt and MAPKs signal pathways. J Cell Physiol 209:94–102 [DOI] [PubMed] [Google Scholar]
- Kipshidze NN, Kim HS, Iversen P, Yazdi HA, Bhargava B, New G, Mehran R, Tio F, Haudenschild C, Dangas G, Stone GW, Iyer S, Roubin GS, Leon MB, Moses JW 2002 Intramural coronary delivery of advanced antisense oligonucleotides reduces neointimal formation in the porcine stent restenosis model. J Am Coll Cardiol 39:1686–1691 [DOI] [PubMed] [Google Scholar]