Abstract
The exact mechanisms through which ghrelin promotes lipogenesis are unknown. Uncoupling protein (UCP)-2 is a mitochondrial protein important in regulating reactive oxygen species; however, recent research shows that it may play an important role fat metabolism. Given that ghrelin increases UCP2 mRNA in white adipose tissue, we examined whether the lipogenic actions of ghrelin are modulated by UCP2 using ucp2+/+ and ucp2−/− mice. Chronic ghrelin treatment either via osmotic minipumps or daily ip injections induced body weight gain in both ucp2+/+ and ucp2−/− mice; however, body weight gain was potentiated in ucp2−/− mice. Increased body weight gain was completely due to increased body fat as a result of decreased fat oxidation in ucp2−/− mice. Ghrelin treatment of ucp2−/− mice resulted in a gene expression profile favoring lipogenesis. In a calorie-restriction model of negative energy balance, ghrelin to ucp2+/+ mice did not increase body weight; however, ghrelin to ucp2−/− mice still induced body weight. These results show that UCP2 plays an important role in fat metabolism by promoting fat oxidation and restricts ghrelin-induced lipogenesis.
The ability of ghrelin to induce weight gain is controlled by a metabolic threshold determined by the energy status of the animal.
Ghrelin is a hormone from the stomach that promotes food intake and adiposity (1). Ghrelin concentrations in the blood are increased during negative energy balance, such as fasting, and decreased during positive energy balance, such as obesity (1,2,3). Thus, ghrelin is a potent hunger signal that maintains appetitive drive during periods of decreased food availability. In the brain, ghrelin promotes food intake by activating orexigenic neuropeptide Y (NPY)/agouti gene-related peptide (AgRP) neurons in the arcuate nucleus of the hypothalamus (4,5). Ghrelin further promotes orexigenic tone via NPY/AgRP secretion of γ-aminobutyric acid inhibitory inputs that synapse onto and suppress anorexigenic proopiomelanocortin neurons (6).
We showed recently that the acute actions of ghrelin on food intake required uncoupling protein (UCP)-2 for a complete food intake response (4). Specifically, ghrelin activated UCP2-dependent mitochondrial function and biogenesis in NPY/AgRP but not POMC neurons. Activation of the AMP-activated protein kinase-carnitine palmitoyltransferase-1 (CPT1)-UCP2 pathway buffered reactive oxygen species levels in NPY/AgRP neurons because UCP2 is important for reducing reactive oxygen species in the brain (7). Whereas this study showed that UCP2 in NPY/AgRP neurons promotes food intake in response to an acute injection of ghrelin, the importance of UCP2 to body weight gain after chronic ghrelin treatment remains unknown.
Exactly how ghrelin promotes adiposity is still currently unclear. Ghrelin potently activates NPY and AgRP gene expression in the hypothalamus (8,9) and intracerebroventricular NPY infusion promotes adiposity (10,11). Thus, the effects of ghrelin on adiposity could be driven through central NPY pathways. In support, there are many genetic models of obesity that show elevated hypothalamic NPY (12,13). However, central ghrelin has direct effects on adipocyte metabolism, which are independent from ghrelin-induced hyperphagia (14). These effects are mediated by fat metabolism genes in white adipose tissue, which are driven through the sympathetic nervous system. Specifically, central ghrelin increased enzymes involved with fat storage such as lipoprotein lipase (LPL), acetyl CoA carboxylase (ACC)-α, stearoyl CoA desaturase-1 (SCD1) and decreased the rate-limiting enzyme in fat oxidation, CPT1α (CPT1a).
Central ghrelin also reduced UCP1 and UCP3 in brown adipose tissue, suggesting decreased thermogenesis and energy expenditure contributes to lipogenesis (14). Although UCP2 was not examined in that study, chronic ghrelin increased UCP2 mRNA expression in white adipose tissue (15), the liver (16), and the pancreas (17). The importance of increased UCP2 mRNA after chronic ghrelin treatment has not been established. In fact, evidence shows that UCP2 knockout mice (ucp2−/−) are more susceptible to weight gain on a high-fat diet (18), and a high-fat diet decreases white adipose tissue UCP2 and UCP1, which can be reversed by the antiobesity effects of juniperus chinensis extract (19).
These observations suggest that UCP2 is an important target to reduce diet-induced obesity. In support of a positive role for UCP2, diet-induced obesity causes severe metabolic dysfunction that decreases life span (20), and UCP2 promotes healthy aging and maintains life span in mice (21). Furthermore, in response to fasting, UCP2 promotes degradation of triacylglycerols, raises plasma free fatty acids (22), and promotes fatty acid oxidation by limiting glycolysis (23). All these lines of evidence collectively imply that UCP2 protects against diet-induced obesity by enhancing peripheral fatty acid metabolism. This creates an interesting conundrum whereby ghrelin increases weight gain and UCP2 expression in peripheral tissues, which then stimulates fatty acid oxidation. This ghrelin-induced UCP2 mRNA expression in white adipose tissue may be a protective mechanism to prevent excessive weight gain and obesity. This study was designed to examine the role of UCP2 in ghrelin-induced weight gain.
Materials and Methods
Mice
The Institutional Animal Care and Use Committee of Yale University approved all experiments. Mice were kept under standard laboratory conditions with free access to standard chow food and water unless otherwise stated. The generation of ucp2−/− (on a C57B6 background) mice has been previously described (24). Mice were divided into two groups for body weight and food intake experiments. In the first set of experiments, ghrelin or saline was delivered to ucp2+/+ or ucp2−/− mice (n = 6–7) via osmotic minipumps (Alzet Osmotic Minipumps, Cupertino, CA) placed sc on the dorsal body surface lateral to the spine. Ghrelin (acylated; NeoMPS, Strasbourg, France) was delivered at 10 nmol/d at 0.5 μl/h for 14 d. Ten nanomoles was chosen as a dose because this dose is known to stimulate food intake (4). Food intake and body weight was recorded every morning at approximately 1000 h for 14 d. In the second set of experiments, ghrelin (10 nmol ip) or saline was injected just before the dark phase at approximately 1800 h every night to ucp2+/+ or ucp2−/− mice (n = 6).
For studies involving calorie restriction, average daily food intake was measured over 1 wk before the beginning of the restriction period. Seventy percent of the average daily food intake was given to the mice in the morning and monitored daily to ensure all food was consumed. Ghrelin was injected ip (10 nmol) at the beginning of the dark phase.
Metabolic studies
Fat and lean body masses were measured by 1H-nuclear magnetic resonance spectroscopy (NMR; Bruker Biospin, Billerica, MA) before and after 14 d of ghrelin injections in 10- to 12-wk-old ucp2+/+ or ucp2−/− mice. Total body fat, muscle, and fluid were calculated and expressed as fold increase at the end of the 14-d treatment relative to the beginning. A comprehensive mouse metabolic monitoring system (CLAMS; Columbus Instruments, Columbus, OH) was used to measure O2 consumption (VO2), CO2 production (VCO2), respiratory exchange ratio (RER), activity, and heat. RER and heat were calculated from VO2 and VCO2 gas exchange data. The RER changes, depending on the primary energy source and is the ratio of VCO2 to VO2. Pure carbohydrate oxidation produces an RER of 1.0, whereas pure fatty acid oxidation produces a RER of 0.7. Heat was calculated as follows: (3.815 + 1.232 × RER) × VO2 (25). Activity was measured on x- and z-axes using infrared beams to count the number of beam breaks during the recording period (25).
Real-time PCR
RNA was isolated from abdominal white adipose tissue using a RNeasy lipid tissue extraction kit (QIAGEN, Valencia, CA) and transcribed to cDNA using the first-strand cDNA kit (Amersham Biosciences, Piscataway, NJ), both following the manufacturers’ instructions. Real-time PCR was performed using SYBR green (Bio-Rad Laboratories, Hercules, CA). Primers used were as follows: UCP2 (forward, 5′-TCT GGA TAC CGC CAA GGT-3′, reverse, 5′-TTG TAG AGG CTG CGT GGA-3′), SCD1 (forward, 5′-TGA AAG CTG AGA AGC TGG TG-3′, reverse, 5′-TGT GGG CAG GAT GAA G-3′), fatty acid synthase (FAS; forward, 5′-TGG GTT CTA GCC AGC AGA GT-3′, reverse, 5′-ACC ACC AGA GAC CGT TAT GC-3′), LPL (forward, 5′-GCC CAG CAA CAT TAT CCA GT-3′, reverse, 5′-GGT CAG ACT TCC TGC TAC GC-3′), ACC (forward, 5′-GCC TCT TCC TGA CAA ACG AG-3′, reverse, 5′-TGA CTG CCG AAA CAT CTC TG-3′), CPT1a (forward, 5′-CCA GGC TAC AGT GGG ACA TT-3′, reverse, 5′-GAA CTT GCC CAT GTC CTT GT-3′), and 18S (forward, 5′-GAA AAT AGC CTT CGC CAT CA-3′, reverse, 5′-CAC CTC ATC CTC CGT GAG TT C-3′). Fold differences in target mRNA expression were measured using the Δ-cycle threshold method by comparison with the housekeeping gene, 18S.
Statistical analysis
All data are presented as mean ± sem. Data were analyzed with two-way ANOVA followed by Bonferroni’s post hoc test or unpaired two-tailed t tests using GraphPad Prism 5 for Mac OS X (GraphPad, San Diego, CA).
Results
The lack of UCP2 potentiates weight gain in response to ghrelin
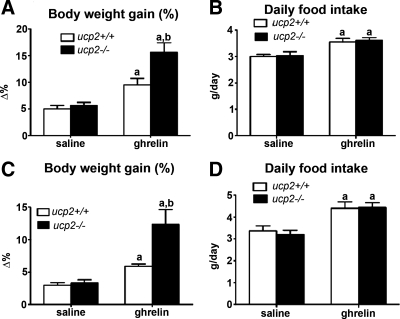
We used two distinct models to test the lipogenic actions of ghrelin on ucp2+/+ and ucp2−/− mice. First, ghrelin was chronically infused using osmotic minipumps at 10 nmol/d over a 14-d period. We clearly observed an increase in body weight in both ucp2+/+ and ucp2−/− mice treated with ghrelin compared with saline, indicating efficacy in each model (Fig. 1A). Interestingly, ghrelin-induced weight gain was significantly increased in ucp2−/− mice relative to ucp2+/+ mice (ucp2+/+, 9.50 ± 1.11% vs. ucp2−/−, 15.6 ± 1.85%, P < 0.05, Fig. 1A). Although ghrelin stimulated food intake in both UCP2 genotypes (ucp2+/+ saline, 2.99 ± 0.07 vs. ucp2+/+ ghrelin, 3.55 ± 0.14; ucp2−/− saline, 3.03 ± 0.16 vs. ucp2−/− ghrelin, 3.61 ± 0.11 g/d, P < 0.05, Fig. 1B), an increased food intake in ucp2−/− mice did not account for the greater weight gain in these mice. We used another cohort of mice, in which ghrelin was injected ip once daily for 14 d at 10 nmol, to verify the results obtained with minipumps. Again, ghrelin effectively promoted weight gain in both ucp2+/+ and ucp2−/− mice; however, the effect was dramatically potentiated in ucp2−/− mice (ucp2+/+, 5.89 ± 0.34% vs. ucp2−/−, 12.34 ± 2.27%, P < 0.05, Fig. 1C). Increased weight gain was not ascribed to differences in food intake in ghrelin-treated ucp2+/+ or ucp2−/− mice (ucp2+/+ saline, 3.37 ± 0.22 vs. ucp2+/+ ghrelin, 4.40 ± 0.28; ucp2−/− saline, 3.20 ± 0.19 vs. ucp2−/− ghrelin, 4.40 ± 0.23, P < 0.05, Fig. 1D). These studies show that the absence of UCP2 exacerbates ghrelin-induced weight gain, which is independent of changes in food intake. This suggests UCP2 regulates weight gain by modifying peripheral energy metabolism.
Figure 1.
The lack of UCP2 increases weight gain in response to ghrelin. A, ucp2−/− mice show increased body weight gain after 14 d of ghrelin delivery via osmotic pumps relative to ucp2+/+ mice (10 nmol/d, n = 6–7, P < 0.05). Data are presented as the percent increase in body weight at the end of the 14-d treatment period relative to the starting body weight. B, Ghrelin via osmotic minipumps increased daily food intake (grams per day) in both ucp2+/+ and ucp2−/− mice; however, no difference between genotypes was observed (n = 6–7, P < 0.05). C, ucp2−/− mice show increased body weight gain, relative to saline injections, after daily ghrelin injections for 14 d (10 nmol, n = 6, P < 0.05). Data are presented as the percent increase in body weight at the end of the 14-d treatment period relative to the starting body weight. D, Ghrelin injections (ip, 10 nmol, n = 6, P < 0.05) increased daily food intake in both ucp2+/+ and ucp2−/− mice, although no difference between genotype was observed. This graph shows that ghrelin promotes greater weight gain in ucp2−/− mice independent of food intake. All data are expressed as mean ± sem. a, Significant with respect to saline controls; b, significant with respect to ucp2+/+ ghrelin.
Ucp2−/− mice show greater fat deposition and altered fat metabolism after ghrelin treatment
Next we used NMR minispectroscopy to determine body composition before and after ghrelin treatment in both ucp2+/+ and ucp2−/− mice. The results showed that ghrelin increases fat deposition in ucp2+/+ and ucp2−/− mice. We observed no differences in body muscle or body fluid. The increased weight gain of ucp2−/− mice relative to ucp2+/+ mice was solely due to increased fat deposition (ucp2+/+ ghrelin, 1.26 ± 0.04- vs. ucp2−/− ghrelin, 1.59 ± 0.14-fold increase, P < 0.05, Fig. 2A), suggesting the lack of UCP2 promotes lipogenesis by altering fat metabolism. To test metabolic parameters and address the issue of altered fat metabolism, we placed ghrelin-treated ucp2+/+ and ucp2−/− mice in metabolic cages. Of all the metabolic parameters examined, we saw a significant increase only in the RER. The ucp2−/− mice exhibited a greater total RER in response to ghrelin compared with ucp2+/+ mice (ucp2+/+ ghrelin, 0.90 ± 0.02 vs. ucp2−/− ghrelin, 0.97 ± 0.02, P < 0.05, Fig. 3A). A high RER represents greater carbohydrate metabolism and our results directly show that UCP2 promotes peripheral fat oxidation because the absence of UCP2 results in elevated carbohydrate metabolism. We observed no differences in total VO2, VCO2, activity, or heat (Fig. 3, B–E), highlighting a specific dysfunction in fat oxidation.
Figure 2.
The lack of UCP2 increases total body fat in response to ghrelin treatment. We measured total body fat, muscle, and fluid using NMR spectroscopy and calculated the fold increase by comparing measurements before and after ghrelin treatment. A, Ghrelin significantly increased total body fat in both ucp2+/+ and ucp2−/− mice (n = 6); however, the increase in ucp2−/− was further significantly elevated when compared with ucp2+/+ mice. B and C, No differences were observed in total body muscle or body fluid after ghrelin treatments. The dotted line represents basal levels measured before beginning the experiment. All data are expressed as mean ± sem. a, Significant with respect to saline controls; b, significant with respect to ucp2+/+ ghrelin.
Figure 3.
Metabolic data from ucp2+/+ and ucp2−/− ghrelin-treated mice. Mice were placed in CLAMS metabolic monitoring systems (Columbus Instruments) for 4 d and treated daily with ghrelin (10 nmol, ip, n = 6/group). A, ucp2−/− mice exhibited a significantly higher RER after ghrelin treatment compared with ucp2+/+ mice, indicating that the lack of UCP2 reduces fat metabolism. RER is calculated as VCO2/VO2. B–E, No significant differences were measured in total VO2, VCO2, activity, or heat. All data are expressed as mean ± sem. a, Significant with respect to saline controls; b, significant with respect to ucp2+/+ ghrelin.
UCP2 regulates genes involved in white adipose tissue fat metabolism
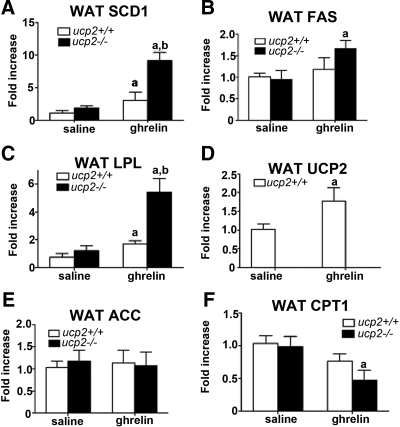
Next we examined important fat metabolism genes to determine how UCP2 regulates fat metabolism in response to ghrelin. Epididymal white adipose tissue was collected from both osmotic minipump and ip-injected treatment models as described in Fig. 1. Recent studies showed that ghrelin increased UCP2 mRNA in white adipose tissue (15) and stimulated fat metabolism by increasing gene expression of lipogenic enzymes including SCD1, FAS, LPL, and ACC and decreased the rate-limiting enzymes in fat oxidation, CPT1a (14). In the miniosmotic pump treatment group, ghrelin-treated ucp2−/− mice exhibited significantly increased white adipose tissue SCD1 (ucp2+/+ ghrelin, 3.05 ± 0.32- vs. ucp2−/− ghrelin, 9.17 ± 1.22-fold increase, P < 0.05, Fig. 4A), FAS (ucp2+/+ ghrelin, 1.19 ± 0.26- vs. ucp2−/− ghrelin, 1.66 ± 0.18-fold increase, P < 0.05, Fig. 4B), and LPL (ucp2+/+ ghrelin, 1.70 ± 0.22- vs. ucp2−/− ghrelin, 5.42 ± 1.02-fold increase, P < 0.05, Fig. 4C) compared with ghrelin-treated ucp2+/+ mice. We saw no effect of ghrelin on ACC, and CPT1a was significantly decreased in ghrelin-treated ucp2−/− vs. saline-treated ucp2−/− mice (ucp2−/− saline, 0.98 ± 0.16- vs. ucp2−/− ghrelin, 0.47 ± 0.15-fold increase, P < 0.05, Fig. 4F).
Figure 4.
UCP2 controls fat metabolism gene expression profile in white adipose tissue after ghrelin delivery (10 nmol/d for 14 d) via osmotic minipumps. A, Ghrelin significantly increases white adipose tissue SCD1 gene expression in both ucp2−/− and ucp2+/+ ghrelin-treated mice relative to saline controls; however, ucp2−/− ghrelin mice show a further significant increase relative ucp2+/+ ghrelin mice (n = 6–7, P < 0.05). B, Ghrelin significantly increases FAS in white adipose tissue of ghrelin-treated ucp2−/− mice compared with saline controls. No effect of ghrelin was observed in ucp2+/+ mice (n = 6). C, Ghrelin significantly increases white adipose tissue LPL mRNA in both ucp2−/− and ucp2+/+ ghrelin-treated mice relative to saline controls; however, ucp2−/− ghrelin-treated mice show a further significant increase relative ucp2+/+ ghrelin mice (n = 6–7, P < 0.05). D, Ghrelin increases UCP2 mRNA in white adipose tissue (n = 6–7, P < 0.05). E, No effect of ghrelin on ACC was observed in either genotype (n = 6–7, P < 0.05). F, CPT1 was decreased in ghrelin-treated ucp2−/− mice relative to saline-treated ucp2−/− mice. Ghrelin had no significant effect on CPT1 mRNA in ucp2+/+ mice (n = 6, P < 0.05). All data are expressed as mean ± sem. a, Significant with respect to saline controls; b, significant with respect to ucp2+/+ ghrelin.
These results, as well as the increase in UCP2 mRNA in response to ghrelin (ucp2+/+ saline, 1.02 ± 0.14- vs. ucp2+/+ ghrelin, 1.78 ± 0.30-fold increase, P < 0.05, Fig. 4D), are consistent with ghrelin’s effects on white adipose tissue (14,15). This shows that the absence of UCP2 promotes a lipogenic gene expression profile that enhances carbohydrate metabolism (Fig. 3), fat deposition (Fig. 2), and weight gain (Fig. 1). We further validated these results (Fig. 5) by examining gene expression in our ghrelin ip treatment model, which also potentiated weight gain in ucp2−/− mice compared with ucp2+/+ mice (Fig. 1). We observed a similar pattern of gene expression to that seen in the minipump model. White adipose tissue SCD1 (ucp2+/+ ghrelin, 1.15 ± 0.34- vs. ucp2−/− ghrelin, 3.33 ± 1.07-fold increase, P < 0.05, Fig. 5A), FAS (ucp2+/+ ghrelin, 1.08 ± 0.16- vs. ucp2−/− ghrelin, 1.73 ± 0.30-fold increase, P < 0.05, Fig. 5B), and LPL (ucp2+/+ ghrelin 1.10 ± 0.24 vs. ucp2−/− ghrelin, 3.06 ± 0.72-fold increase, P < 0.05, Fig. 5C) were all significantly increased in ghrelin-treated ucp2−/− mice relative to ucp2+/+ mice, and CPT1a was significantly reduced (ucp2+/+ ghrelin, 1.02 ± 0.10- vs. ucp2−/− ghrelin, 0.63 ± 0.12-fold increase, P < 0.05, Fig 5E). In Fig. 4F we did not see a similar difference in CPT1a mRNA between ghrelin-treated ucp2+/+ and ucp2−/− as noted in Fig. 5F, although a decreased trend was observed. This could be related to delivery method of ghrelin.
Figure 5.
UCP2 controls fat metabolism gene expression profile in white adipose tissue after ghrelin injection. Ghrelin increases mRNA expression of genes promoting fat storage in white adipose tissue from ucp2−/− relative to ucp2+/+ mice (n = 6, P < 0.05). SCD1 (A), FAS (B), and LPL (C) are all increased after ghrelin injection in ucp2−/− relative to ucp2+/+. D, No difference in white adipose tissue ACC was observed between ghrelin-treated ucp2+/+ and ucp2−/− mice. E, Ghrelin suppressed CPT1 mRNA expression in ucp2−/− relative to ucp2+/+ mice. All data are expressed as mean ± sem. a, Significant with respect to ghrelin-treated ucp2+/+ mice.
Ghrelin increases body gain in ucp2−/− mice independent of food intake
Our initial experiments showed that ghrelin produced greater body weight gain in ucp2−/− mice independent of changes in food intake (Fig. 1). To verify this observation, we used a model of calorie restriction, in which both ucp2+/+ and ucp2−/− mice received 70% of normal food intake. Both calorie-restricted ucp2+/+ and ucp2−/− mice were treated with saline or ghrelin ip (10 nmol) for 14 d, and body weight was recorded. Interestingly, ucp2+/+ mice on a calorie-restricted diet did not gain weight in response to ghrelin (Fig 6A). However, ucp2−/− mice still manifested an increase in body weight, even when food intake was restricted to 70% of controls (Fig. 6B). At the end of the 14-d calorie restriction and ghrelin treatment period, ucp2+/+ saline- or ghrelin-treated mice had lost an equal amount of body weight (ucp2+/+ saline, −11.24 ± 1.96 vs. ucp2+/+ ghrelin, −10.51 ± 0.678 g, n = 6, P = ns, Fig. 6B). Ghrelin significantly increased body weight in calorie restricted ucp2−/− mice compared with ucp2−/− saline-treated mice (ucp2−/− saline, −8.00 ± 2.95 vs. ucp2−/− ghrelin, 0.67 ± 2.44 g, n = 6, P < 0.05, Fig. 6B). This clearly indicates that, at least in ucp2−/− mice, ghrelin can increase body weight independent of food intake.
Figure 6.
Ghrelin induces weight gain in calorie-restricted ucp2−/− but not calorie-restricted ucp2+/+ mice. A, Daily weight gain of saline- and ghrelin-treated ucp2+/+ and ucp2−/− mice. Mice had free access to food until d 4, at which time they were given 70% of ad libitum food intake. Ghrelin (ip, 10 nmol) was given daily for 14 d at 1800 h from the start of the calorie restriction regimen. B, Percent change in body weight of saline and ghrelin-treated calorie-restricted ucp2+/+ and ucp2−/− mice at the end of the experimental period relative to the beginning. All data are expressed as mean ± sem. a, Significant with respect to saline controls; b, significant with respect to ucp2+/+ ghrelin.
Discussion
In this study, we show that the lipogenic actions of ghrelin are enhanced in mice lacking UCP2. This points toward a critical role for UCP2 in promoting fatty acid oxidation in white adipose tissue and lowering body weight. Although we looked at ghrelin-induced adiposity, our results are consistent with other models of adiposity including diet-induced obesity (18), in which ucp2−/− mice gain more weight relative to controls on a high-fat diet. Indeed, we used the lipogenic hormone ghrelin to show that ucp2−/− mice have a reduced capacity to oxidize fatty acids, as shown by RER data, which results in greater fat deposition as shown by NMR spectroscopic analysis. The lack of UCP2 promotes a gene expression profile favoring fat storage because the lipogenic enzymes SCD1, LPL, and FAS are all increased, and CPT1a, the rate-limiting step in fatty acid oxidation, is decreased in ghrelin-treated ucp2−/− relative to ghrelin-treated ucp2+/+ mice. It is not known whether the increase in fat storage gene expression is a direct effect due to loss of UCP2 or a secondary effect due to loss of the ability to oxidize fats through CPT1a. Recent evidence suggests that UCP2 is important in promoting fatty acid oxidation (4,22,23), and overexpression of the closely homologous UCP3 reduced fat-induced insulin resistance (25) by promoting fatty acid oxidation (26,27). We hypothesize that UCP2 is a critical mitochondrial protein that establishes metabolic rate by controlling the capacity to oxidize fatty acids. Furthermore, this study supports the hypothesis that UCP2 in white adipose tissue is a good target to prevent increased fat deposition. Because obesity represents a chronic disease burden and reduced life span (28), restricting weight gain by enhancing UCP2 function may maintain a healthy phenotype and extend life span. Indeed, we have seen that UCP2 increases lifespan as ucp2+/+ mice live longer compared with ucp2−/− mice (21), supporting the idea that the fat-metabolizing capability of UCP2 promotes life span. Furthermore, we observed that UCP2 plays an equally important role in stimulating the AMPK-ACC-CPT1fatty acid oxidation pathway in the hypothalamus (4), suggesting that UCP2 may play a common role in many diverse tissues.
Our results are largely in accord with Theander-Carrillo et al. (14), in which they described a central effect of ghrelin on adipocyte metabolism independent of food intake. In our experiments we observed no difference in ghrelin-induced food intake in either ucp2+/+ or ucp2−/− mice when food was freely accessible. Despite this lack of food intake difference, we observed an increase in weight gain and fat deposition in ucp2−/− mice relative to ucp2+/+. In these experimental conditions, we believe that the lack of UCP2 potentiates the ability of ghrelin to promote weight gain, independent of food intake, by modifying fat metabolism. Moreover, we do not believe this UCP2-dependent action on fatty acid oxidation is specific to the weight-inducing properties of ghrelin. UCP2 would be expected to enhance fat metabolism in response to any lipogenic stimulus whether it be diet-induced obesity, ghrelin, or a genetic ablation (i.e. ob/ob). For example, ucp2−/− mice are more susceptible to weight gain on a high-fat diet (18). Interestingly, when food was not freely available in the calorie-restricted model, we observed that ghrelin had no effect on body weight in ucp2+/+ mice but still initiated weight gain in ucp2−/− mice relative to saline.
Because we observed greater carbohydrate metabolism (increased RER) and a gene expression profile favoring fat deposition in ucp2−/−, we predict that the absence of UCP2 restricted the shift toward fat oxidation in calorie-restricted ucp2−/−, thereby promoting fat deposition. Our data show that in ad libitum-fed mice ghrelin induces weight gain independent of food intake, supporting the work of Theander-Carrillo et al. (14). However, under certain conditions, such as calorie restriction, there is a minimum amount of food intake required for ghrelin to induce weight gain. This metabolic threshold predicts whether ghrelin will induce weight gain and can be modulated by peripheral fat metabolism as ghrelin-treated ucp2−/− mice still gained weight and received the same calorie restriction as ghrelin-treated ucp2+/+ mice.
In light of the work by Theander-Carrillo et al. (14) and Sangiao-Alvarellos et al. (29), in which central ghrelin mediates adipocyte metabolism in a GH independent fashion, the important question arises: does the weight gain effect of ghrelin in the present study stem from centrally or peripherally acting ghrelin? Ghrelin receptors are highly expressed in the central nervous system (30); however, the expression of the primary growth hormone secretagouge receptor (GHSR)-1a was not found in human adipose tissue in one study (31). Subsequent studies demonstrated low-level ghrelin binding to adipose tissue (32), and GHSR1a expression in adipocytes increases with age. Rodriguez et al. (33) showed that ghrelin promoted lipid accumulation in isolated human omental adipocytes by enhancing FAS and LPL, in support of our current studies. This study also found an increase in ACC; however, we did find a similar increase in our study and suggest it may by related to the species (mouse vs. human) or the location of adipose tissue collected (abdominal vs. omental). Recently Davies et al. (34) highlighted that some adipose depots are responsive to ghrelin, whereas others as not. This was not due to differences in GHSR1a receptor expression because similar levels were found in both responsive and unresponsive depots. Although the authors suggested that altering signal transduction pathways may drive the responsiveness, we suggest that central acting ghrelin via the sympathetic nervous system is more important in stimulating adipogenesis and that depot-specific responsiveness to ghrelin arises from sympathetic innervation of neural circuits containing ghrelin receptor neurons in the brain. In line with this notion, GHSR1a levels are 30–40 times higher in the hypothalamus relative to the adipose tissue (34), and different adipose tissue depots receive innervation from separate populations of neurons in the hypothalamus (35). Because ghrelin was delivered iv or via minipumps, the studies by Davies et al. (34) cannot discern between central or peripheral acting ghrelin.
Our data propose an interesting paradox: ghrelin promotes weight gain but at the same time induces UCP2 mRNA, which promotes fatty acid metabolism and weight loss. We postulate that whereas excessive ghrelin treatment will lead to weight gain, the major role of ghrelin is not to induce adiposity or obesity but rather to induce hunger and appetite to shift the organism from a negative energy balance state to a neutral energy balance state. In other words, ghrelin does not promote obesity but rather prevents starvation. The fact that ghrelin is elevated in the circulation during negative energy balance and decreased in the plasma during positive energy balance (1,2,3) supports this hypothesis. Thus, the induction of UCP2 in white adipose tissue by ghrelin appears to be a mechanism developed to prevent excessive weight accumulation. Furthermore, the idea that ghrelin is a potential antiobesity therapeutic target is controversial and fraught with problems. Initial studies showed that both ghrelin receptor and ghrelin knockout mice were resistant to diet-induced obesity (36,37). In more recent studies, congenic (N10) adult ghrelin receptor and ghrelin knockout mice exhibited no resistance to diet-induced obesity (38), suggesting the diet-induced obesity in earlier studies is due to differences in genetic background of the mice used. This is supported by human genetic association studies, whereby common polymorphisms in ghrelin or the receptor genes are not strongly linked to the development of polygenic obesity (39). Moreover, ghrelin promotes learning and memory in the hippocampus (40), defends against depression and stress (41), and enhances reward and motivation through the mesolimbic dopamine system (42). Therefore, targeting ghrelin to control body weight may be ineffective and compromise neuronal and psychological function.
In summary, we show that UCP2 modulates peripheral fat metabolism by preventing excessive lipogenesis. The lipogenic actions of ghrelin are enhanced in ucp2−/− mice; however, we believe UCP2 will limit excessive lipogenesis to any stimulus promoting fat deposition. We observed that UCP2 helps promote fat oxidation and restrict fat accumulation by decreasing a lipogenic expression profile. This observation supports the hypothesis that UCP2 is an important mitochondrial protein establishing metabolic rate (43). Furthermore, these studies support the link between enhanced peripheral fat oxidation and life span because UCP2 positively regulates life span in mice (21). We also show that the ability of ghrelin to induce weight gain is controlled by a metabolic threshold determined by the energy status of the animal.
Footnotes
This work was supported by National Institutes of Health Grant DK060711; a New Zealand Foundation for Research, Science, and Technology fellowship; a Monash Fellowship; and National Health Medical Research Council Grant 546131 (to Z.B.A.).
Disclosure Summary: The authors have nothing to declare.
First Published Online February 26, 2010
Abbreviations: ACC, Acetyl CoA carboxylase; AgRP, agouti gene-related peptide; CPT1, carnitine palmitoyltransferase-1; CPT1a, CPT1α; FAS, fatty acid synthase; GHSR, ghrelin receptor; LPL, lipoprotein lipase; NMR, nuclear magnetic resonance; NPY, neuropeptide Y; RER, respiratory exchange ratio; SCD1, stearoyl CoA desaturase-1; UCP, uncoupling protein; VCO2, CO2 production; VO2, O2 consumption.
References
- Tschöp M, Smiley DL, Heiman ML 2000 Ghrelin induces adiposity in rodents. Nature 407:908–913 [DOI] [PubMed] [Google Scholar]
- Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K 2001 Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86:4753–4758 [DOI] [PubMed] [Google Scholar]
- Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML 2001 Circulating ghrelin levels are decreased in human obesity. Diabetes 50:707–709 [DOI] [PubMed] [Google Scholar]
- Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschöp MH, Shanabrough M, Cline G, Shulman GI, Coppola A, Gao XB, Horvath TL, Diano S 2008 UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 454:846–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Phillips CT, Palmiter RD 2007 NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides 28:214–225 [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL 2003 The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37:649–661 [DOI] [PubMed] [Google Scholar]
- Andrews ZB, Diano S, Horvath TL 2005 Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat Rev Neurosci 6:829–840 [DOI] [PubMed] [Google Scholar]
- Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, Ye Z, Nargund RP, Smith RG, Van der Ploeg LH, Howard AD, MacNeil DJ, Qian S 2004 Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology 145:2607–2612 [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S 2001 A role for ghrelin in the central regulation of feeding. Nature 409:194–198 [DOI] [PubMed] [Google Scholar]
- Baran K, Preston E, Wilks D, Cooney GJ, Kraegen EW, Sainsbury A 2002 Chronic central melanocortin-4 receptor antagonism and central neuropeptide-Y infusion in rats produce increased adiposity by divergent pathways. Diabetes 51:152–158 [DOI] [PubMed] [Google Scholar]
- Chee MJ, Colmers WF 2008 Y eat? Nutrition 24:869–877 [DOI] [PubMed] [Google Scholar]
- Bi S, Ladenheim EE, Schwartz GJ, Moran TH 2001 A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. Am J Physiol Regul Integr Comp Physiol 281:R254–R260 [DOI] [PubMed] [Google Scholar]
- Duan J, Choi YH, Hartzell D, Della-Fera MA, Hamrick M, Baile CA 2007 Effects of subcutaneous leptin injections on hypothalamic gene profiles in lean and ob/ob mice. Obesity (Silver Spring) 15:2624–2633 [DOI] [PubMed] [Google Scholar]
- Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, Castaneda TR, Muzzin P, Schürmann A, Szanto I, Tschöp MH, Rohner-Jeanrenaud F 2006 Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest 116:1983–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubone T, Masaki T, Katsuragi I, Tanaka K, Kakuma T, Yoshimatsu H 2005 Ghrelin regulates adiposity in white adipose tissue and UCP1 mRNA expression in brown adipose tissue in mice. Regul Pept 130:97–103 [DOI] [PubMed] [Google Scholar]
- Barazzoni R, Bosutti A, Stebel M, Cattin MR, Roder E, Visintin L, Cattin L, Biolo G, Zanetti M, Guarnieri G 2005 Ghrelin regulates mitochondrial-lipid metabolism gene expression and tissue fat distribution in liver and skeletal muscle. Am J Physiol Endocrinol Metab 288:E228–E235 [DOI] [PubMed] [Google Scholar]
- Sun Y, Asnicar M, Saha PK, Chan L, Smith RG 2006 Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab 3:379–386 [DOI] [PubMed] [Google Scholar]
- Joseph JW, Koshkin V, Zhang CY, Wang J, Lowell BB, Chan CB, Wheeler MB 2002 Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes 51:3211–3219 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Jung JY, Kim HW, Park T 2008 Anti-obesity effects of Juniperus chinensis extract are associated with increased AMP-activated protein kinase expression and phosphorylation in the visceral adipose tissue of rats. Biol Pharm Bull 31:1415–1421 [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA 2006 Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB, Horvath TL 2009 Uncoupling protein 2 regulates lifespan in mice. Am J Physiol Endocrinol Metab 296:E621–E627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets AR, Fülöp P, Derdák Z, Kassai A, Sabo E, Mark NM, Paragh G, Wands JR, Baffy G 2008 Uncoupling protein-2 modulates the lipid metabolic response to fasting in mice. Am J Physiol Gastrointest Liver Physiol 294:G1017–G1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecqueur C, Bui T, Gelly C, Hauchard J, Barbot C, Bouillaud F, Ricquier D, Miroux B, Thompson CB 2008 Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. FASEB J 22:9–18 [DOI] [PubMed] [Google Scholar]
- Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB 2001 Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell 105:745–755 [DOI] [PubMed] [Google Scholar]
- Choi CS, Fillmore JJ, Kim JK, Liu ZX, Kim S, Collier EF, Kulkarni A, Distefano A, Hwang YJ, Kahn M, Chen Y, Yu C, Moore IK, Reznick RM, Higashimori T, Shulman GI 2007 Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. J Clin Invest 117:1995–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costford SR, Chaudhry SN, Crawford SA, Salkhordeh M, Harper ME 2008 Long-term high-fat feeding induces greater fat storage in mice lacking UCP3. Am J Physiol Endocrinol Metab 295:E1018–E1024 [DOI] [PubMed] [Google Scholar]
- Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, Cooney GJ 2007 Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 56:2085–2092 [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS 2005 A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 352:1138–1145 [DOI] [PubMed] [Google Scholar]
- Sangiao-Alvarellos S, Vázquez MJ, Varela L, Nogueiras R, Saha AK, Cordido F, López M, Diéguez C 2009 Central ghrelin regulates peripheral lipid metabolism in a growth hormone-independent fashion. Endocrinology 150:4562–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK 2006 Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494:528–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M 2002 The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 87:2988 [DOI] [PubMed] [Google Scholar]
- Papotti M, Ghè C, Cassoni P, Catapano F, Deghenghi R, Ghigo E, Muccioli G 2000 Growth hormone secretagogue binding sites in peripheral human tissues. J Clin Endocrinol Metab 85:3803–3807 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Gomez-Ambrosi J, Catalan V, Gil MJ, Becerril S, Sainz N, Silva C, Salvador J, Colina I, Fruhbeck G 2009 Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes (Lond) 33:541–552 [DOI] [PubMed] [Google Scholar]
- Davies JS, Kotokorpi P, Eccles SR, Barnes SK, Tokarczuk PF, Allen SK, Whitworth HS, Guschina IA, Evans BA, Mode A, Zigman JM, Wells T 2009 Ghrelin induces abdominal obesity via GHS-R-dependent lipid retention. Mol Endocrinol 23:914–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Song CK 2007 Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res 48:1655–1672 [DOI] [PubMed] [Google Scholar]
- Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, Sleeman MW 2005 Absence of ghrelin protects against early-onset obesity. J Clin Invest 115:3573–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK 2005 Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 115:3564–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Butte NF, Garcia JM, Smith RG 2008 Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology 149:843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueorguiev M, Lecoeur C, Meyre D, Benzinou M, Mein CA, Hinney A, Vatin V, Weill J, Heude B, Hebebrand J, Grossman AB, Korbonits M, Froguel P 2009 Association studies on ghrelin and ghrelin receptor gene polymorphisms with obesity. Obesity (Silver Spring) 17:745–754 [DOI] [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschöp MH, Horvath TL 2006 Ghrelin controls hippocampal spine synapse density and memory performance. Nature Neurosci 9:381–388 [DOI] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM 2008 The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci 11:752–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschöp MH, Gao XB, Horvath TL 2006 Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 116:3229–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JA, Dickinson K, Brand MD 2001 Mitochondrial uncoupling as a target for drug development for the treatment of obesity. Obes Rev 2:255–265 [DOI] [PubMed] [Google Scholar]