Abstract
Numerous clinical and experimental studies have linked stress to changes in risk factors associated with the development of physiological syndromes, including metabolic disorders. How different mediators of the stress response, such as corticosterone (CORT), influence these changes in risk remains unclear. Although CORT has beneficial short-term effects, long-term CORT exposure can result in damage to the physiological systems it protects acutely. Disruption of this important physiologic signal is observed in numerous disparate disorders, ranging from depression to Cushing’s syndrome. Thus, understanding the effects of chronic high CORT on metabolism and physiology is of key importance. We explored the effects of 4-wk exposure to CORT dissolved in the drinking water on the physiology and behavior of male mice. We used this approach as a noninvasive way of altering plasma CORT levels while retaining some integrity in the diurnal rhythm present in normal animals. This approach has advantages over methods involving constant CORT pellets, CORT injections, or adrenalectomy. We found that high doses of CORT (100 μg/ml) result in rapid and dramatic increases in weight gain, increased adiposity, elevated plasma leptin, insulin and triglyceride levels, hyperphagia, and decreased home-cage locomotion. A lower dose of CORT (25 μg/ml) resulted in an intermediate phenotype in some of these measures but had no effect on others. We propose that the physiological changes observed in the high-CORT animals approximate changes observed in individuals suffering from the metabolic syndrome, and that they potentially serve as a model for hypercortisolemia and stress-related obesity.
Disruption of normal cyclicity of stress hormone corticosterone results in rapid weight gain and changes in metabolism and physiology, providing a potentially useful animal model to investigate the mechanisms by which stress and stress hormones result in obesity and metabolic dysfunction.
The increase in obesity observed in modern Western society is becoming an important public health issue. Causes for this relatively recent spike in obesity, such as changes to a higher fat diet and an increasingly sedentary lifestyle, have been suggested (1). The stress of living in a modern industrialized society is also a potential contributing factor, which could interact with other environmental conditions to compound their effects. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis has been documented in many metabolic disorders (2). Although stress has been linked to obesity (1), these links are not always clear, as numerous studies show that chronic stress results in a blunted weight gain, or even weight loss (3,4,5). It is becoming increasingly evident that disruption of the adrenal hormone corticosterone (CORT) can result in numerous metabolic changes, perhaps the best known of which is the marked obesity that is present in patients suffering from hypercortisolemia due to Cushing’s syndrome (6,7,8,9). There are also numerous connections between malfunctions in the HPA axis and incidence of the metabolic syndrome (10,11,12), which is generally described as a series of physiological markers that put an individual at greater risk of negative cardiovascular outcomes, obesity, and diabetes (13,14). How disrupted HPA functioning contributes to the development of these risk factors remains unknown.
To clarify how chronic treatment with CORT alters the physiology of an organism, we treated adrenally intact adult male mice with CORT dissolved in their drinking water for 4 wk. This allowed for noninvasive alterations of plasma CORT, whereas retaining some integrity in the diurnal rhythm. Thus, our methodology has the added benefit of resulting in a late-night increase in plasma CORT, mimicking one of the most predictive factors in Cushing’s disease (15). Other methods for manipulating HPA function (e.g. constant release pellets, daily injections, or chronic stress) have an array of possible confounds which makes it difficult to distinguish the effects of CORT from the effects of stress. Although there have been informative studies on metabolism using chronic CORT pellets in rats (16,17,18), most of these were undertaken in adrenalectomized animals, providing experimental confounds due to elimination of other adrenal factors (such as aldosterone and norepinephrine). Additionally, the metabolic phenotype could be influenced by a disruption in the maintenance of proper sodium balance that accompanies adrenalectomy.
In the present study, animals treated with high levels of CORT became markedly obese and showed several other physiological hallmarks of the metabolic syndrome, such as increased plasma leptin and insulin, increased plasma triglycerides, and impaired glucose tolerance. We propose that the physiological phenotype that developed after this treatment approximates many of the changes observed in the metabolic syndrome and that it could become a useful model for understanding how disruption of the HPA and plasma CORT levels may contribute to the development of a syndrome which is increasing in prevalence.
Materials and Methods
Animals, housing, and CORT treatment
Adult male mice (C57/BL6; 19–21 g, 35 d old) were ordered from Charles River Laboratories (Kingston, NY). Animals for all experiments (except for feeding and locomotor experiments) were group-housed (n = 5/cage) for 7 d in standard cages (28.5 × 17 × 13 cm), on a 12-h light, 12-h dark cycle (lights off at 1800 h). A 2lux red light allowed for animal maintenance in the dark phase. Temperature in the room was maintained at about 21 ± 2 C. During the acclimation period, standard rodent chow and tap water were available ad libitum. After the 7-d acclimation phase, ad libitum chow remained available, although drinking water was replaced with a solution containing 25 μg/ml (low) or 100 μg/ml (high) free-CORT (Sigma, St. Louis, MO) dissolved in 100% ethanol (because CORT is hydrophobic), and then diluted in regular tap water to a final ethanol concentration of 1% or a 1% ethanol solution alone (vehicle). Animals were weighed once a week during cage change, at which time solutions were replaced, and otherwise left undisturbed.
After the 4-wk CORT treatment, animals were killed by rapid decapitation, and organs and tissues were rapidly removed (n = 5–10/group). Trunk blood was collected in BD Vacutainer K3 EDTA coated glass tubes (VWR, West Chester, PA), placed on ice, and centrifuged at 1500 rpm for 15 min at 4 C. Plasma was removed and stored at −70 C until used for analyses. For time of day analyses, animals were killed at one of four time points (n = 5/group · time) during the light and dark phases (time of lights on, midlight, time of lights off, and middark; each 6 h apart) and collapsed over a 12-h period. All experimental procedures involving animals were approved by the Rockefeller University Institutional Animal Care and Use Committee.
Plasma measures
Plasma measures were conducted using the specifications of the assay’s respective manufacturer instructions. For plasma CORT, RIAs were run using an antibody kit (MP Biomedicals, Inc., Solon, OH). Samples were run in duplicate, and results are reported as nanograms per milliliter. The assay provided an intraassay coefficient of variation of 11%, with a lower limit of detectability of 16.7 ng/ml. For plasma leptin and insulin, ELISAs were used (Millipore, Inc., Billerica, MA). Samples were run in duplicate, and the results are reported as pg/ml. The lower levels of detectability were 0.219 ng/ml for the leptin assay and 0.405 ng/ml for the insulin assay.
For plasma triglyceride levels, a standard enzymatic hydrolysis kit was used (Cayman Chemicals, Ann Arbor, MI). Samples were run in duplicate, and the results reported as mg/dl. The lower level of detectability was 2.58 mg/dl.
Food consumption and home-cage activity
After 3 wk of CORT treatment, during which food consumption was measured weekly, individually housed mice (n = 5/group) were transferred to a home-cage behavior monitoring system (Accuscan Instruments, Trabue, OH), in a 12-h light, 12-h dark cycle (off at 1800 h). Each animal’s cage was surrounded with a set of infrared photobeams. Disruption of a beam was recorded as an activity count. Data were collected with a PC using Versamax software (Accuscan Instruments). Mice were allowed to acclimate to this environment for 5 d, after which the last 4 d of behavioral recordings were used for analysis. Data were collected in 60-min bins around the clock and then reported as pooled daytime (0600–1800 h) or nighttime (1800–0600 h) activity.
Glucose tolerance test
Group-housed 100 μg/ml CORT (n = 10)- or vehicle (n = 10)-treated mice were used. Just before lights off on testing day, food was removed from the cages. Eight hours into the fast, mice were weighed, and a tail blood sample was obtained and analyzed using the OneTouch Ultra Blood Glucose Monitoring System (LifeScan, Inc., Milpitas, CA). Filtered sterilized d-glucose was injected ip (200 mg/ml), at a dose of 2 g/kg in normal saline (kept at 37 C before injection). Blood was collected at baseline, 15, 30, 60, and 120 min by gentle massage of the tail, and spotting the blood onto the glucometer strip. Mice were returned to the housing colony after the test.
Statistics
One-way or two-way ANOVAs [some with repeated measures (RM)] were used, as indicated, to analyze data (GraphPad Prism, San Diego, CA). Post hoc analyses were undertaken using Tukey-honestly significant difference or Bonferroni corrected t tests where appropriate. Pearson’s coefficients were used to determine correlations, with r2 values reported. Comparisons not reaching statistical significance, although consistent with dose-response-like effects, are noted in the text with their respective P values. Results were considered statistically significant at the P < 0.05 level.
Results
Chronic CORT treatment results in rapid increases in body weight
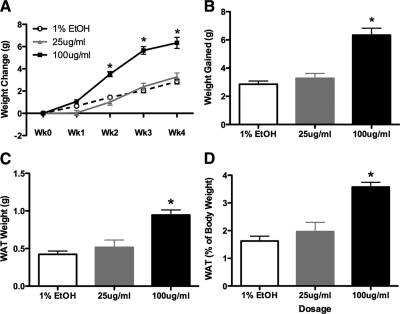
Animals treated with CORT in the drinking water for 4 wk show a dose-dependent weight gain (two-way RM ANOVA; Fig. 1A). Main effects were detected for both time (F4,220 = 304.59, P < 0.0001) and for dosage (F2,220 = 4.49, P = 0.0157). There was also a statistically significant time by dosage interaction (F8,220 = 26.28, P < 0.0001) on body weight. After the first week of treatment, the high-CORT group shows no change in body weight, whereas the low CORT group shows a slight weight loss (although not statistically significant, P = 0.11) when compared with vehicle-treated controls. However, in the following weeks, the weight gain was greatly accelerated in the 100 μg/ml group, with treated animals showing far greater gains over their baseline start weight than do the 25 μg/ml group or the vehicle control. Interestingly, the 25 μg/ml group showed a blunted weight gain over the course of the experiment, showing no differences compared with vehicle-treated controls by the end of the 4-wk treatment.
Figure 1.
Effects of CORT on body weight and WAT. A, CORT treatment results in rapid weight gain over the 4 wk of treatment in high-CORT (100 μg/ml) animals, but there is no significant effect on low-CORT (25 μg/ml) animals. B, Cumulative weight gain in CORT-treated animals, showing the total weight gain is higher in high-CORT animals when compared with low-CORT or vehicle animals. C, Weight of gonadal WAT is significantly increased after 4 wk of CORT treatment. D, Relative contribution of WAT to total body weight is also significantly increased in high-CORT animals. Asterisks indicate statistical significance at the P < 0.05 level.
At the end of 4 wk of treatment, CORT results in a significant cumulative weight gain over baseline (one-way ANOVA, F2,42 = 38.33, P < 0.0001; Fig. 1B), although this effect is dependent on dose, with high-CORT animals gaining significantly more weight over baseline than either low-CORT- or vehicle-treated animals, and low-CORT- and vehicle-treated animals not statistically different from each other. Specifically, the 100 μg/ml group gained almost 50% more weight than controls (6.33 ± 0.4988 vs. 2.858 ± 0.2242 g; Tukey, P < 0.05), whereas the 25 μg/ml group had gained an intermediate amount (3.274 ± 0.3507 g), although this was not statistically different from the vehicle group by the end of treatment. We did not detect any effect of the vehicle treatment on body weight, even after 4 wk, compared with animals housed similarly but on normal tap water (data not shown).
Visceral white adipose tissue (WAT) is increased in high-CORT animals
To determine how CORT alters the relative amounts of WAT, we excised and weighed visceral (gonadal) fat after 4 wk of treatment (Fig. 1C). CORT treatment increased gonadal WAT (one-way ANOVA, F2,12 = 14.59, P = 0.0006). Post hoc analyses indicated that high-CORT-treated animals had greater amounts of WAT compared with both vehicle and low-CORT animals (Tukey, P < 0.05; Fig. 2D). Furthermore, this WAT also made up a larger proportion of their total body weight (one-way ANOVA, F2,12 = 19.30, P = 0.0002) when compared with low-CORT- and vehicle-treated animals (Fig. 1D).
Figure 2.
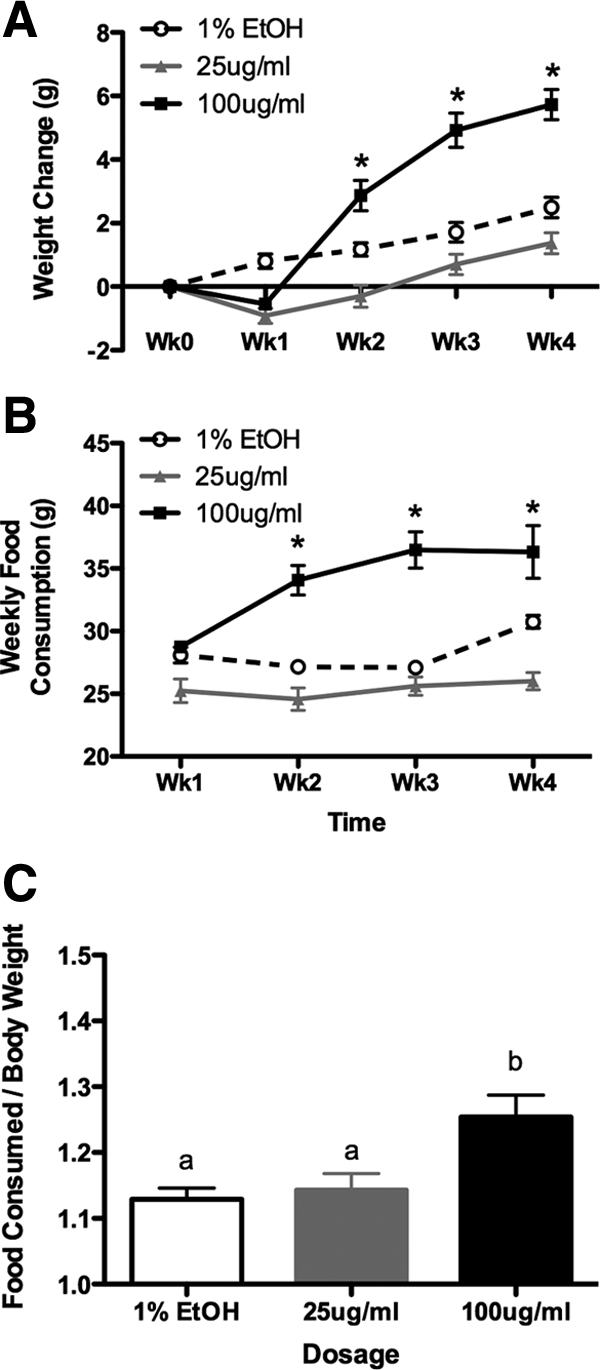
Single housing does not affect weight gain due to CORT treatment, and CORT treatment results in hyperphagia. A, Weight change in response to CORT is largely unaffected in single-housed animals, with high-CORT animals still showing a significant weight gain compared with both other treatments. Although low-CORT animals seem to have a slight weight decrease, these differences are not statistically significant. B, Food consumption is altered by CORT treatment, with high-CORT animals showing a significant increase of the amount of food consumed over the 4-wk treatment period. C, High-CORT animals consume more food per gram of body weight per week than either vehicle or low-CORT animals. Asterisks indicate statistical significance at the P < 0.05 level. Bars sharing the same letter are not statistically different from each other.
CORT treatment results in the atrophy of thymus and adrenal glands
Thymus and adrenal glands were weighed at the end of the 4-wk treatment as a bioassay of the physiological effects of the CORT (Table 1). Both dosages resulted in a clear decrease in the weight of all of these organs compared with the vehicle-treated animals (one-way ANOVA; Thymus, F2,19 = 22.28, P < 0.0001; Adrenals: F2,12 = 40.83, P < 0.0001). This difference is exaggerated when considered in the context of the greatly different body weights of the animals at the end of the treatment.
Table 1.
Effect of CORT treatment on peripheral organ weight
| 1% EtOH vehicle | 25 μg/ml CORT (low) | 100 μg/ml CORT (high) | |
|---|---|---|---|
| Adrenal | 7.72 ± 0.3121 mg (n = 5) | 3.28 ± 0.4954 mga (n = 5) | 2.84 ± 0.4389 mga (n = 5) |
| Thymus | 64.21 ± 6.399 mg (n = 8) | 25.25 ± 1.156 mga (n = 8) | 20.22 ± 3.995 mga (n = 6) |
Effect of CORT treatment on the weight of peripheral organs after 4 wk of treatment. Both low- and high-CORT doses result in the atrophy of adrenal glands (bilateral) and thymus.
Statistically different from vehicle animals at P < 0.0001 levels.
Housing conditions alter the pattern of weight changes, although the relationship between treatment groups remains the same
To determine the effects of housing conditions on the CORT effect on body weight, and to facilitate the analysis of food consumption over the experimental period, animals were individually housed (in the same sized cage as group-housed animals) for the duration of the experiment. Main effects of time (two-way RM ANOVA, F3,36 = 99.46, P < 0.0001) and dosage (F2,36 = 18.79, P = 0.0002), as well as a time by dosage interaction (F6,36 = 32.12, P < 0.0001) on body weight were found. In contrast to group housing, individually housed animals in both high- and low-CORT groups lost some weight after the first week of treatment, although this did not reach statistical significance (Bonferroni, P > 0.05; Fig. 2A). However, in the subsequent weeks, high-CORT animals started to gain weight, eventually exceeding the vehicle animals by the end of the 4 wk (P < 0.05). Low-CORT animals continued to lose weight in the second week of treatment (P < 0.05) and then rebounded, reaching only a fraction of the total weight gain experienced by the high-CORT animals (P < 0.05), and not statistically different from controls by wk 4 (P > 0.05).
CORT treatment results in hyperphagia
High-CORT animals showed increased food consumption when compared with vehicle or low-CORT animals, for the duration of the experiment (Fig. 2B). Main effects of time (two-way RM ANOVA; F3,36 = 15.42, P < 0.0001) and dosage (F2,36 = 30.62, P < 0.0001), as well as a time by dosage interaction (F6,36 = 9.40, P < 0.0001) on food consumption were found. As a proportion of food consumed by body weight, high-CORT animals ate significantly more food per gram of body weight (one-way ANOVA, F2,12 = 6.41, P = 0.0104; Fig. 2C) over the course of the experiment.
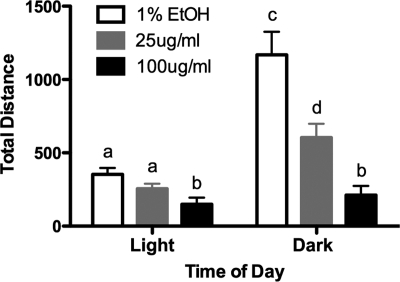
CORT treatment reduces overall home-cage locomotor activity
To probe general behavioral output in CORT-treated animals, we measured home-cage activity in individually housed animals, binned hourly (Fig. 3). Main effects were observed for time of day (two-way RM ANOVA, F1,24 = 35.36, P < 0.0001) and dosage (F2,24 = 23.86, P < 0.0001), as well as a time by dosage interaction effect (F2,24 = 10.16, P = 0.0006) on locomotor activity. As expected, during the light (inactive) period, all treatment groups showed low spontaneous locomotor activity, with no differences between the groups (P > 0.05). However, in the dark (active) period, both low- and high-CORT animals showed depressed activity patterns, with low-CORT animals showing a smaller (although not statistically significant) day-night increase than vehicle animals (P < 0.05), and high-CORT animals showing the lowest level of home-cage activity, with no overall difference between amount of daytime and nighttime activity (P > 0.05).
Figure 3.
CORT treatment reduces general home-cage activity. Graph depicts daily home-cage activity as measured by infrared beam breaks in the x, y, and z planes, averaged over four consecutive days. Daytime activity is depressed by high CORT, although not in vehicle or low-CORT animals. At night, activity is significantly attenuated in both the low- and high-CORT animals, with high-CORT animals showing the lowest amount of home-cage activity. Although there were statistically significant differences between daytime and nighttime activity in vehicle and low-CORT animals, this normal diurnal change in the pattern of activity was abolished in high-CORT animals. Bars sharing the same letter are not statistically different from each other.
Plasma CORT levels are increased in CORT-treated animals
To determine the circulating levels of CORT after our treatment, we measured plasma CORT at two times of day. Animals were evaluated at the light-dark transition (the “morning” to a nocturnal species) and at the dark-light transition (the “evening”; Fig. 4). There were main effects of time of day (two-way ANOVA, F1,51 = 6.21, P = 0.016) and of dosage (F2,51 = 51.05, P < 0.0001) on plasma CORT levels. Although low-CORT-treated animals had equivalent levels of plasma CORT in their morning (light-dark), they had elevated levels of plasma CORT in the evening (dark-light), although the latter did not reach statistical significance (P = 0.14). However, circulating CORT levels in the high-CORT animals were significantly elevated to supraphysiological levels (approaching stress induced levels) at both times of day, although in this case, the levels were lower in the morning than in the evening (P < 0.05).
Figure 4.
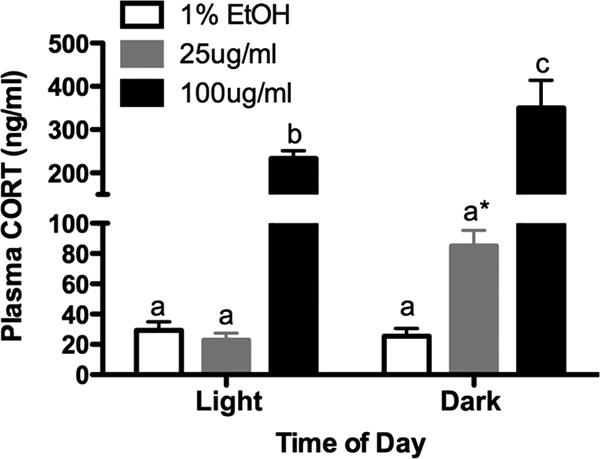
CORT treatment results in changes in the diurnal pattern of plasma CORT levels. Graph depicts plasma CORT levels taken at the end of the 4-wk treatment, during the light (inactive) or dark (active) phases. Although low-CORT animals had slightly elevated plasma CORT during the dark period (although not statistically significant), high-CORT animals had elevated plasma CORT at both time points, in addition to the light-dark difference. Bars sharing the same letter are not statistically different from each other. Asterisk indicates P = 0.14.
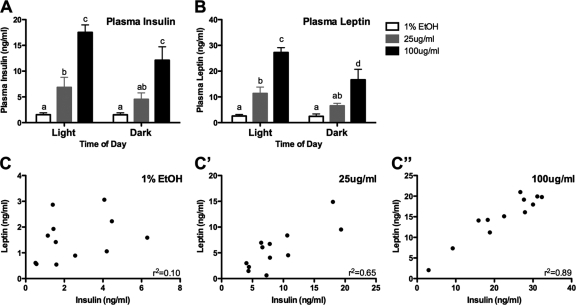
Chronic CORT treatment results in elevated and correlated plasma leptin and insulin levels
Because CORT is a key regulator of glucose stores and because of the metabolic phenotype we describe, we decided to investigate the effects of CORT treatment on both plasma insulin and leptin levels in free-fed animals. Both hormones were assayed during the light and dark periods. There was a dose-dependent effect of CORT on insulin, with high-CORT animals showing very high levels (F2,30 = 38.05, P < 0.0001; Fig. 5A), as well as a main effect of time of day (two-way ANOVA, F1,30 = 4.41, P = 0.0508). An identical pattern was observed in leptin levels (Fig. 5B), with high-CORT animals showing the highest leptin levels (F2,30 = 42, P < 0.0001). We also found a main effect of time of day (two-way ANOVA, F1,30 = 8.58, P < 0.0064), with no statistically significant interaction. The high leptin levels in these animals, along with hyperphagia and high levels of WAT, suggested leptin resistance. We preformed a study to examine leptin resistance by administering leptin ip to vehicle and high-CORT animals (see Supplemental Methods published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). We found that the levels of induced pSTAT3 are blunted in the arcuate nucleus of high-CORT animals (Supplemental Fig. 1), suggesting the development of leptin resistance.
Figure 5.
Levels of plasma insulin and plasma leptin are altered by CORT treatment. A and B, Graphs depict changes in plasma insulin (A) and leptin (B) in response to 4 wk of CORT treatment. Both treatments result in elevated plasma levels in both hormones, although levels in high-CORT animals are substantially higher. Bars sharing the same letter are not statistically significant from each other. C and C″, Plots show correlation between an animal’s insulin and leptin levels, as a function of CORT dosage (collapsed across time of day). Although there is no statistically significant correlation of insulin and leptin in vehicle-treated animals, both low- and high-CORT treatment result in statistically significant correlations (P < 0.01), with r2 values of 0.65 and 0.89, respectively.
Correlations of an individual animal’s insulin level to their leptin level (collapsed across time of day), as a function of their CORT dosage, revealed an interesting relationship (Fig. 5C). Although there was no correlation between insulin and leptin levels in vehicle-treated animals (r2 = 0.11, P = 0.2936; Fig. 5C), low-CORT animals showed a strong insulin-leptin correlation (r2 = 0.65, P = 0.0015; Fig. 5C′), and high-CORT animals showed an even greater correlation (r2 = 0.89, P < 0.0001; Fig. 5C″).
High-CORT results in elevated plasma triglycerides and impaired glucose clearance after challenge
In addition to obesity, high plasma triglyceride levels are a hallmark of the metabolic syndrome. Plasma triglycerides were measured at the end of the 4-wk CORT treatment in each of the groups. We found that CORT treatment resulted in elevated levels of plasma triglycerides (one-way ANOVA, F2,15 = 10.66; P = 0.0013), with the high-CORT-treated animals showing much higher levels than either vehicle- or low-CORT-treated animals (Tukey, P < 0.05), but no differences were detected between vehicle- and low-CORT-treated animals.
An important aspect of metabolic syndrome is impairment of glucose clearance after a glucose challenge, which denotes a prediabetic or diabetic state. The pattern of the body weight, endocrine, and plasma triglyceride measures indicated the most robust differences were between vehicle and high-CORT animals. We tested glucose tolerance in these groups (Fig. 6) to determine the effects of high-CORT exposure on the ability of animals to clear glucose from their bloodstream. Animals received a single bolus of glucose delivered ip, with samples taken at 15, 30, 60, and 90 min after challenge. After 2 wk of exposure to high CORT or vehicle, we found a robust glucose clearance profile over time in both groups (two-way RM ANOVA, F4,60 = 63.60, P < 0.0001) with no effect of treatment (F1,60 = 0.06, P > 0.05) and no interaction (F4,60 = 1.37, P > 0.05). However, after 4 wk of CORT treatment, high-CORT animals showed a sluggish clearance of plasma glucose. There was a significant main effect of time (F4,68 = 32.95, P < 0.0001) and dosage (F1,68 = 8.37, P = 0.0101), with a significant interaction (F4,68 = 5.59, P = 0.006). Although vehicle animals started to return to baseline by 30 min, and fully returned to baseline by 60 min (P > 0.05), plasma glucose in high-CORT animals remained elevated until the 60-min time point (P < 0.01). At the 120-min time point, high-CORT animals still had significantly elevated plasma glucose compared with baseline (P < 0.05).
Figure 6.
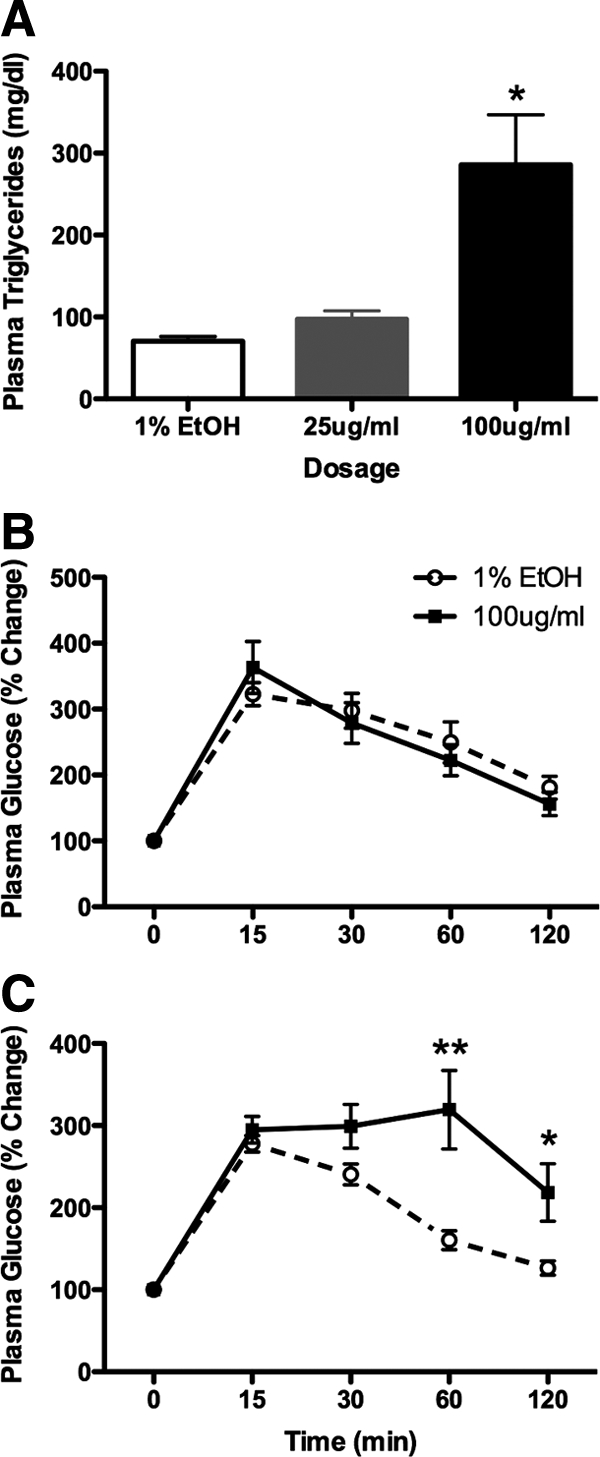
High-CORT treatment results in elevated plasma triglycerides and impaired glucose tolerance. A, Graph depicts plasma triglyceride levels in CORT-treated animals after 4 wk of treatment. High-CORT animals show significantly elevated plasma triglycerides, with over 3-fold higher levels than vehicle-treated animals. Asterisk indicates P < 0.05. B and C, Plasma glucose levels after acute glucose challenge in fasted vehicle or high-CORT mice after either 2 wk (B) or 4 wk (C) of treatment. Although there is no effect of CORT treatment at the 2-wk time point, at the 4-wk time point, high-CORT animals show severely compromised glucose tolerance, with plasma glucose remaining high even 120 min after challenge. Asterisks indicate statistical significance at *, P < 0.05 and **, P < 0.01 levels.
Discussion
The present study analyzed the effects of elevated CORT on the physiological functions of the mouse, using a method to noninvasively maintain a daily (albeit shifted) rhythm in plasma CORT. This also recapitulates an important aspect of hypercortisolemia due to Cushing’s syndrome, specifically, high late-night plasma glucocorticoids (15). Mice exposed to high levels of CORT in their drinking water modeled many of the physiological and behavioral effects observed in hypercortisolemia in humans, namely, increased body weight and adiposity and decreased behavioral output (9). We found increases in food consumption which, when coupled with decreased locomotor activity, may synergize to augment the specific effects of CORT on other factors that affect metabolism, such as thermogenesis, liver function, glucose mobilization in body tissues, and direct effects on adipocytes (20,21,22,23). Physiologically, we demonstrated that high-CORT treatment for 4 wk results in marked hypercortisolemia, hyperinsulinemia, and hyperleptinemia, along with high plasma triglyceride levels. Remarkably, high-CORT treatment also resulted in highly correlated plasma insulin and leptin, which is not observed in vehicle-treated animals. We also showed that high-CORT animals have a functional metabolic deficit, as measured by an impaired glucose clearance after a glucose challenge. This phenotype is remarkably similar to the phenotype observed in the metabolic syndrome (10).
Strengths of drinking water CORT delivery
We delivered CORT in the drinking water to augment endogenous CORT in mice with intact adrenal glands while at the same time maintaining a diurnal periodicity of plasma CORT. Such an approach is not possible with clamped levels of CORT via sc pellet implants. Moreover, in future studies, we are interested in probing how the organism can recover after removal of exogenous CORT, a task that would be rendered impossible if animals were adrenalectomized. We also wanted to evaluate the effects of CORT without confounding it with the other nonglucocorticoid responses that necessarily accompany daily injection and handling stress that accompany daily CORT administration. It is important to note that our CORT treatment is not intended to model chronic stress per se but, instead, to evaluate the effects of chronic hypercortisolemia on physiology and behavior. The present model has several benefits over chronic stress or other chronic CORT treatments. By delivering CORT in the drinking water, a daily variation in plasma CORT is maintained (although at supraphysiological levels). Although timed daily injections of CORT may also result in similar peak levels after the injection, such treatments require daily experimental interventions that serve as a repeated stress. Moreover, the effects of a bolus of CORT are very different from the gradual rise and fall that drinking water exposure provides. Our model also used animals with intact adrenals. Although the adrenal glands atrophy during the course of the treatment (see Table 1), after a 4-wk CORT washout, they return to near normal weight (our unpublished observation). This could provide a useful way to probe the long-term effects of short-term chronic CORT exposure on physiology, and a way to determine how HPA-axis reactivity has been altered by such a treatment after CORT has been removed. We acknowledge that this methodology is not without drawbacks (e.g. potentially different metabolism of oral vs. adrenal CORT, lack of specific control of total dosing in each individual mouse), but the experimental benefits outweigh the potential costs, with these technical considerations taken into account.
An additional strength in our current model is the recapitulation of some of the key temporal aspects of Cushing’s syndrome. Although chronically high levels of cortisol in the plasma is a key aspect of Cushing’s syndrome, the most reliable measure for diagnosis is very high late night (i.e. 2200–2400 h) plasma cortisol (15). Our high-CORT animals parallel these aspects of the syndrome, with both high baseline levels of CORT (∼200 ng/ml), as well as a peak in CORT at the end of the night (rather than at the beginning). Such a pattern could not be reproduced with pellets alone. Moreover, how this “shift” in the diurnal pattern of CORT could contribute to the physiological and metabolic problems observed in this model is an important area for future research.
Chronic CORT treatment and metabolic dysregulation
We show a profound metabolic phenotype after chronic (4 wk) treatment with CORT, causing a clear dysregulation of multiple metabolic systems. Although high CORT resulted in obesity and dramatically increased plasma leptin and insulin, the effect of CORT dosage on the correlation between leptin and insulin levels is particularly interesting. Leptin is secreted by adipocytes and plays an important role in regulation of food intake, in part by serving as a signal of the amount of adipose tissue in the organism. High levels of leptin suggest that metabolic needs are being met, or exceeded, and hence feeding is suppressed. Animals lacking leptin (e.g. the ob/ob mouse), or animals lacking the receptor for leptin (e.g. the db/db mouse), become remarkably obese, even on normal chow (24,25,26). The very high leptin levels observed in our high-CORT mice are equivalent to those observed in diet-induced obese mice, and coupled with their hyperphagia, high body weight, and impaired pSTAT3 induction in response to leptin challenge (see Supplemental Fig. 1), suggest that high-CORT treatment results in leptin resistance.
In comparison, insulin serves as a key signal of plasma glucose levels, with high levels of insulin signaling organs and tissues to take up glucose from the bloodstream. In type 2 diabetes, individuals become resistant to insulin signals, and insulin resistance is posited to play an important role in the development of the metabolic syndrome (27,28). Overall, the levels of insulin and leptin were very high in high-CORT animals, suggesting the development of a resistance to the actions of both of these hormones. This result is in agreement with recent findings in an adrenalectomized and CORT pellet-treated rat model, where elevated WAT, plasma insulin, and plasma leptin were found, but with no concomitant body weight increase reported (18).
Although we found that levels of plasma insulin and leptin were not strongly correlated in vehicle-treated animals, as CORT dosage increased, a correlation between these hormones became clearer. This pattern of results leads us to suggest that high CORT can drive the production of insulin and leptin, which may eventually result in the organism becoming resistant to one or both. Mechanistically, it remains unknown whether CORT directly drives production and release of insulin and/or leptin from the pancreas and adipocytes, respectively, or whether it is secondary effects of CORT treatment that results in these hormones being increased. However, as we describe below, there is evidence for direct glucocorticoid effects on leptin production, both in vitro and in vivo (29,30,31).
Interactions between CORT and insulin and leptin
The interaction between CORT and insulin is an important issue, particularly when one considers that in some cases, these two hormones may act to resist each others’ effects, whereas in other cases, they may act in an additive or synergistic fashion (16). The work of Dallman et al. (32,33,34) has been central to the investigation of interactions between the stress axis and metabolism. Importantly, this group has shown that stress and stress hormones can alter food preferences, with a dose-dependent effect of glucocorticoids on sucrose, saccharin, and lard in adrenalectomized animals (17,35,36). Insulin is usually anorectic, with inhibitory actions on orexigenic neuropeptide-Y neurons. This is coupled with excitatory effects on anorexigenic proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus. However, when streptozotocin (i.e. diabetic) adrenalectomized CORT-treated rats are given a choice between regular chow and lard, insulin actually increases the animals’ consumption of lard, in a dose-dependent manner (17). This highlights the important interactive role between metabolic and stress hormones. It further underscores that disruptions in one hormone system, coupled with disruptions in another hormone system, can lead to unexpected and integrative outcomes.
Our leptin findings also raise important questions. It is clear that the high-CORT animals become obese and show very high levels of leptin. However, in the low-CORT group, although animals do not to gain significant weight or elevated WAT levels, they still show a modest elevation in plasma leptin. The origins of this elevated leptin in the low-CORT group are unknown. However, significant work has been undertaken looking at both the in vivo and in vitro stimulation of leptin production by CORT. In vivo dexamethasone can stimulate plasma leptin expression (29,30) and elevate adipose tissue expression of leptin (37). In parallel, in vitro studies have demonstrated that dexamethasone can increase leptin mRNA in adipose cultures within 24–48 h (31), although these effects seem to be modulated by the origin of the adipose depot. Thus, elevated leptin levels observed in our model could be due to a driving aspect of CORT on leptin itself.
Chronic CORT in the drinking water as a model of the metabolic syndrome
Our results suggest that high-CORT-treated animals are suffering from several aspects of the metabolic syndrome, which is defined as a series of risk factors and physiological markers that place an individual in greater risk of negative cardiovascular outcomes associated with obesity (14). Important factors contributing to the development of the metabolic syndrome include decreased behavioral output, leptin and/or insulin resistance, and increased triglyceride levels, as well as obesity. All of these measures are elevated in high-CORT animals. Importantly, high-CORT animals also show impaired glucose tolerance, suggesting a significant alteration in glucose utilization, likely related to the development of the hyperinsulinemic and potentially insulin resistant state. That this reduced glucose tolerance is not present after 2 wk of treatment suggests that it is not merely high circulating plasma CORT that impairs glucose clearance, but that long-term exposure to high-CORT levels gradually alters the regulation of glucose metabolism.
Thus, we propose that the high-CORT treatment used in the present study could serve as a method to induce metabolic syndrome in the mouse. This method takes effect in a relatively short period of time (4 wk) compared with most other methods, including high-fat feeding (4–5 months), which may be confounded with age effects. The mechanisms by which chronic CORT results in these metabolic changes are undoubtedly complex and interrelated. Moreover, they likely involve both the central and peripheral actions of glucocorticoids. Centrally, the feeding phenotype could be related to changes in the POMC system. It has been shown that low dose CORT (equivalent to our 25 μg/ml dose) can exacerbate the obesity and metabolic phenotype observed in POMC deficient mice, but not in wild-type mice (38,39), similar to what we report. In this context, we propose that low-CORT treatment could “set the stage” for further metabolic or physiologic insults to precipitate a shift from a mild phenotype, to one showing obesity and other signs of metabolic syndrome.
Contrasting chronic CORT and chronic stress
In the present work, high CORT (100 μg/ml) resulted in animals gaining a significant amount of weight over a relatively short time, accompanied by atrophy of the thymus and adrenal glands. When compared with the chronic stress literature, these results may seem somewhat puzzling. Multiple types of chronic stressors result in marked body weight loss and hypertrophy of the adrenal glands in rat (5,40,41,42,43). Although having effects similar to chronic stress (e.g. thymus involution), chronic CORT exposure in the drinking water resulted in increased body weight and adrenal atrophy. Because stress results in the mobilization of many other hormones and factors, including epinephrine and norepinephrine, ACTH, CRH, vasopressin, and β-endorphins, their interactive (or counteractive) effects may change the metabolic outcome. Thus, the effects in the present study may represent more an effect of chronic CORT than an effect of chronic stress.
Considerations of long-term effects after short-term chronic CORT
Interesting work by Gourley et al. (44) has used a similar methodology to probe the behavioral effects of short-term CORT in the drinking water on motivated behaviors after a “washout” period (i.e. the longer term ramifications after CORT levels return to baseline). They found that after a 20-d CORT treatment and a 3-wk washout, animals treated with CORT showed decreased responding in an operant task of motivation, which was acquired before the CORT treatment, and that these effects are reversed when animals are treated with the antidepressant amitriptyline for 7 d before testing (44). However, no differences in body weight were reported in the Gourley et al. (44) study. It is important to note that in that experiment, the authors used 4-pregnen-11 21-DIOL-3 20-DIONE 21-hemisuccinate CORT, which has a different rate of metabolism and clearance than the free-CORT, which we used in the present study; thus potentially altering the amount of exposure to CORT in the circulation. This difference, coupled with a treatment time almost 2 wk shorter than ours, may explain why the authors did not note a weight gain as shown in our study. However, the results of this study suggest that there could be longer-term effects of short-term CORT exposure.
Future directions
We believe that the model we present can serve as a starting point to ask mechanistic questions about how changing both the level, and timing, of plasma CORT can affect physiology and behavior. Using CORT in the drinking water in adrenally intact animals, rather than tonic replacement with pellets in adrenalectomized animals, more closely resembles the physiological realities of Cushing’s syndrome (15). Moreover, with intact adrenal glands, future questions can be asked of recovery, which cannot be addressed after adrenalectomy. Because this treatment results in a change in the pattern of CORT in the plasma, future work can be asked about how this treatment impacts circadian rhythmicity and physiological factors depending on CORT rhythms, because there are well-known influences of CORT on circadian “clock gene” expression throughout the brain (45,46) and body (47,48). How “reprogramming” clock gene expression (by altering the daily patterns of plasma CORT as we do here) affects physiology and behavior are still unknown, but is surely an important avenue for future research. Considering that animal models bearing genetically disrupted clocks have increased obesity and metabolic disruption, understanding the contribution of disruption of circadian clocks by disturbing normal CORT rhythms is also very important.
Conclusions
In conclusion, the present study has shown that chronic short-term (4 wk) exposure to CORT in the drinking water results in a phenotype that mimics the metabolic syndrome. Physiologically, we have shown that CORT treatment results in elevated nighttime plasma CORT, atrophy of thymus and adrenals, as well as increased weight gain and disruption of insulin and leptin hormone levels. Animals on high CORT also develop an impaired glucose tolerance. Behaviorally, CORT treatment results in hyperphagia and decreased home-cage activity. The model that we present can be an important tool in unraveling the connections between stress hormone-induced changes in endocrine function and physiology and the ramifications of exposures to high levels of CORT on the future functioning of the organism.
Supplementary Material
Acknowledgments
We thank Dr. Russell Romeo and Dr. Matthew Hill for helpful discussions on previous versions of this manuscript, Miss Rachel Lackert and Miss Madeline Ford for excellent technical assistance, and Tracey Frazier and Christopher Ariza for assistance with animal care.
Footnotes
This work was supported by a Canadian Institutes for Health Research postdoctoral fellowship (I.N.K.) and by the National Institutes of Health Grant 5RO1 MH41256 (to B.S.M.). B.S.M. was also supported by the Hope for Depression Research Foundation and by Sepracor, Inc.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 8, 2010
Abbreviations: CORT, Corticosterone; HPA, hypothalamic-pituitary-adrenal; POMC, proopiomelanocortin; RM, repeated measures; CORT, corticosterone.
References
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S 2003 Chronic stress and obesity: a new view of “comfort food.” Proc Natl Acad Sci USA 100:11696–11701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuizen AG, Rutters F 2008 The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol Behav 94:169–177 [DOI] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, Ho WS, Shi L, Patel S, Gorzalka BB, Hillard CJ 2008 Prolonged glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus 18:221–226 [DOI] [PubMed] [Google Scholar]
- Lussier AL, Caruncho HJ, Kalynchuk LE 2009 Repeated exposure to corticosterone, but not restraint, decreases the number of reelin-positive cells in the adult rat hippocampus. Neurosci Lett 460:170–174 [DOI] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS 1995 Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 69:89–98 [DOI] [PubMed] [Google Scholar]
- Björntorp P, Rosmond R 2000 Obesity and cortisol. Nutrition 16:924–936 [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R 2000 The metabolic syndrome–a neuroendocrine disorder? Br J Nutr 83(Suppl 1):S49–S57 [DOI] [PubMed] [Google Scholar]
- Minami I, Tateno T, Yoshimoto T, Doi M, Izumiyama H, Akashi T, Hirata Y 2006 Subclinical Cushings disease with amelioration of metabolic comorbidities after removal of pituitary tumor. Intern Med 45:1231–1235 [DOI] [PubMed] [Google Scholar]
- Schuff KG 2003 Issues in the diagnosis of Cushing’s syndrome for the primary care physician. Prim Care 30:791–799 [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Suthers K, Ferrucci L, Simonsick EM, Ble A, Schrager M, Bandinelli S, Lauretani F, Giannelli SV, Penninx BW 2007 Hypercortisolemic depression is associated with the metabolic syndrome in late-life. Psychoneuroendocrinology 32:151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzangs N, Penninx BW 2007 Cortisol and insulin in depression and metabolic syndrome. Psychoneuroendocrinology 32:856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhtz C, Zyriax BC, Klähn T, Windler E, Otte C 2009 Depressive symptoms and metabolic risk: effects of cortisol and gender. Psychoneuroendocrinology 34:1004–1011 [DOI] [PubMed] [Google Scholar]
- Rosenzweig JL, Ferrannini E, Grundy SM, Haffner SM, Heine RJ, Horton ES, Kawamori R 2008 Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93:3671–3689 [DOI] [PubMed] [Google Scholar]
- Grundy SM, Brewer Jr HB, Cleeman JI, Smith Jr SC, Lenfant C 2004 Definition of metabolic syndrome: report of the national heart, lung, and blood institute/american heart association conference on scientific issues related to definition. Circulation 109:433–438 [DOI] [PubMed] [Google Scholar]
- Yaneva M, Mosnier-Pudar H, Dugué MA, Grabar S, Fulla Y, Bertagna X 2004 Midnight salivary cortisol for the initial diagnosis of Cushing’s syndrome of various causes. J Clin Endocrinol Metab 89:3345–3351 [DOI] [PubMed] [Google Scholar]
- Warne JP, Akana SF, Ginsberg AB, Horneman HF, Pecoraro NC, Dallman MF 2009 Disengaging insulin from corticosterone: roles of each on energy intake and disposition. Am J Physiol Regul Integr Comp Physiol 296:R1366–R1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fleur SE, Akana SF, Manalo SL, Dallman MF 2004 Interaction between corticosterone and insulin in obesity: regulation of lard intake and fat stores. Endocrinology 145:2174–2185 [DOI] [PubMed] [Google Scholar]
- Christ-Crain M, Kola B, Lolli F, Fekete C, Seboek D, Wittmann G, Feltrin D, Igreja SC, Ajodha S, Harvey-White J, Kunos G, Müller B, Pralong F, Aubert G, Arnaldi G, Giacchetti G, Boscaro M, Grossman AB, Korbonits M 2008 AMP-activated protein kinase mediates glucocorticoid-induced metabolic changes: a novel mechanism in Cushing’s syndrome. FASEB J 22:1672–1683 [DOI] [PubMed] [Google Scholar]
- Banks AS, Davis SM, Bates SH, Myers Jr MG 2000 Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275:14563–14572 [DOI] [PubMed] [Google Scholar]
- Wolf G 2002 Glucocorticoids in adipocytes stimulate visceral obesity. Nutr Rev 60:148–151 [DOI] [PubMed] [Google Scholar]
- Sjögren J, Weck M, Nilsson A, Ottosson M, Björntorp P 1994 Glucocorticoid hormone binding to rat adipocytes. Biochim Biophys Acta 1224:17–21 [DOI] [PubMed] [Google Scholar]
- Rebuffé-Scrive M, Walsh UA, McEwen B, Rodin J 1992 Effect of chronic stress and exogenous glucocorticoids on regional fat distribution and metabolism. Physiol Behav 52:583–590 [DOI] [PubMed] [Google Scholar]
- Strack AM, Bradbury MJ, Dallman MF 1995 Corticosterone decreases nonshivering thermogenesis and increases lipid storage in brown adipose tissue. Am J Physiol 268:R183–R191 [DOI] [PubMed] [Google Scholar]
- Vaisse C, Halaas JL, Horvath CM, Darnell Jr JE, Stoffel M, Friedman JM 1996 Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14:95–97 [DOI] [PubMed] [Google Scholar]
- Friedman JM 1997 Leptin, leptin receptors and the control of body weight. Eur J Med Res 2:7–13 [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM 1995 Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269:543–546 [DOI] [PubMed] [Google Scholar]
- Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Alemán JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR 2008 Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab 7:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JT, Biddinger SB 2009 Dissecting the role of insulin resistance in the metabolic syndrome. Curr Opin Lipidol 20:206–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H, Ahrén B 1996 Short-term dexamethasone treatment increases plasma leptin independently of changes in insulin sensitivity in healthy women. J Clin Endocrinol Metab 81:4428–4432 [DOI] [PubMed] [Google Scholar]
- Miell JP, Englaro P, Blum WF 1996 Dexamethasone induces an acute and sustained rise in circulating leptin levels in normal human subjects. Horm Metab Res 28:704–707 [DOI] [PubMed] [Google Scholar]
- Russell CD, Petersen RN, Rao SP, Ricci MR, Prasad A, Zhang Y, Brolin RE, Fried SK 1998 Leptin expression in adipose tissue from obese humans: depot-specific regulation by insulin and dexamethasone. Am J Physiol 275:E507–E515 [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Strack AM, Scribner KS, Pecoraro N, La Fleur SE, Houshyar H, Gomez F 2004 Chronic stress-induced effects of corticosterone on brain: direct and indirect. Ann NY Acad Sci 1018:141–150 [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Pecoraro NC, Warne JP, la Fleur SE, Foster MT 2007 Glucocorticoids, the etiology of obesity and the metabolic syndrome. Curr Alzheimer Res 4:199–204 [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Strack AM, Hanson ES, Sebastian RJ 1995 The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons. Ann NY Acad Sci 771:730–742 [DOI] [PubMed] [Google Scholar]
- Bell ME, Bhatnagar S, Liang J, Soriano L, Nagy TR, Dallman MF 2000 Voluntary sucrose ingestion, like corticosterone replacement, prevents the metabolic deficits of adrenalectomy. J Neuroendocrinol 12:461–470 [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Bell ME, Liang J, Soriano L, Nagy TR, Dallman MF 2000 Corticosterone facilitates saccharin intake in adrenalectomized rats: does corticosterone increase stimulus salience? J Neuroendocrinol 12:453–460 [DOI] [PubMed] [Google Scholar]
- Kolaczynski JW, Goldstein BJ, Considine RV 1997 Dexamethasone, OB gene, and leptin in humans; effect of exogenous hyperinsulinemia. J Clin Endocrinol Metab 82:3895–3897 [DOI] [PubMed] [Google Scholar]
- Coll AP, Challis BG, López M, Piper S, Yeo GS, O'Rahilly S 2005 Proopiomelanocortin-deficient mice are hypersensitive to the adverse metabolic effects of glucocorticoids. Diabetes 54:2269–2276 [DOI] [PubMed] [Google Scholar]
- Smart JL, Tolle V, Low MJ 2006 Glucocorticoids exacerbate obesity and insulin resistance in neuron-specific proopiomelanocortin-deficient mice. J Clin Invest 116:495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS 1995 Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience 69:83–88 [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR 1995 Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology 20:117–134 [DOI] [PubMed] [Google Scholar]
- Armario A, Ortiz R, Balasch J 1984 Effect of crowding on some physiological and behavioral variables in adult male rats. Physiol Behav 32:35–37 [DOI] [PubMed] [Google Scholar]
- Ottenweller JE, Servatius RJ, Natelson BH 1994 Repeated stress persistently elevates morning, but not evening, plasma corticosterone levels in male rats. Physiol Behav 55:337–340 [DOI] [PubMed] [Google Scholar]
- Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, Taylor JR 2008 Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry 63:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall LA, Perrin JS, Walker CD, Stewart J, Amir S 2006 Glucocorticoid rhythms control the rhythm of expression of the clock protein, Period2, in oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neuroscience 140:753–757 [DOI] [PubMed] [Google Scholar]
- Lamont EW, Robinson B, Stewart J, Amir S 2005 The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci USA 102:4180–4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U 2000 Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289:2344–2347 [DOI] [PubMed] [Google Scholar]
- Balsalobre A 2002 Clock genes in mammalian peripheral tissues. Cell Tissue Res 309:193–199 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.