Abstract
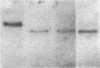
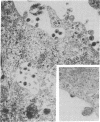
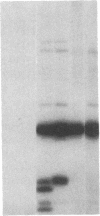
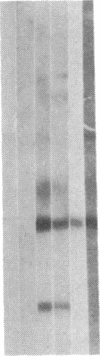
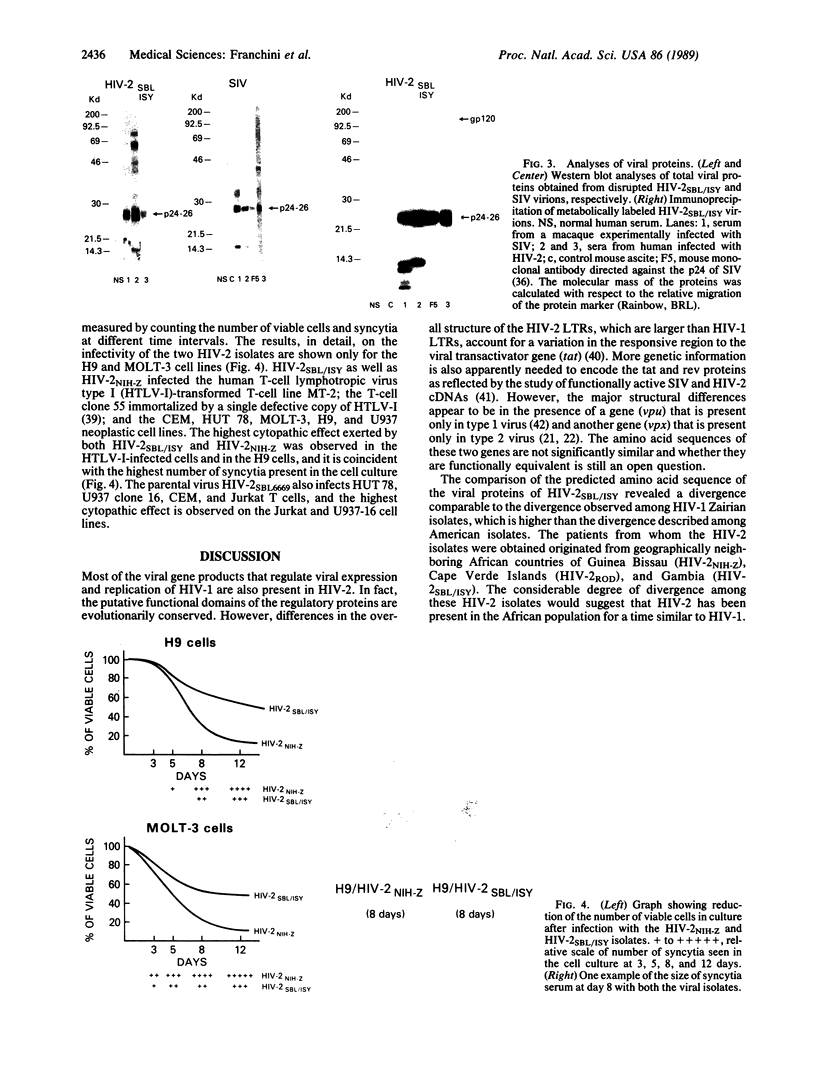
We obtained complete genomic clones of human immunodeficiency virus type 2 (HIV-2) from the DNA of the neoplastic human cell line HUT 78 freshly infected with a HIV-2 isolate, strain SBL6669. The recombinant phage DNA was transfected into the lymphocytes of CD4-positive HUT 78 cell line to test the replication competence of the proviral DNA. One genomic clone, designated HIV-2SBL/ISY, yielded retroviral particles after a few weeks of culture of the transfected cells. The HIV-2SBL/ISY clone contained a complete provirus and cellular flanking sequence. We obtained the DNA sequence of the provirus and compared it with the published sequence of two other HIV-2 isolates. The degree of variability among HIV-2 isolates is comparable to that observed among African HIV-1 isolates sequenced to date. Immunologically, HIV-2SBL/ISY is similar to the parental virus (HIV-2SBL6669) but differs in the envelope transmembrane protein that is truncated (gp32-34) in the parental virus and not in HIV-2SBL/ISY (gp41). Both the parental and the cloned viruses are infectious and cytopathic for some human T-cell lines, induce syncytia, and infect a human macrophage cell line (U937) in vitro. The availability of a biologically active HIV-2 clone provides the means to study the role and interaction of HIV-2 genes in vitro as well as to assess the functional similarities among HIV-1 and HIV-2 genes. Since HIV-2SBL/ISY cloned virus infects fresh peripheral blood T cells from Rhesus macaques in vitro and infects the same animal in vivo, its use in animals may represent a model for functional study of viral genes in vivo as well as for development of experimental approaches to prevent and cure retroviral infection in humans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert J., Bredberg U., Chiodi F., Böttiger B., Fenyö E. M., Norrby E., Biberfeld G. A new human retrovirus isolate of West African origin (SBL-6669) and its relationship to HTLV-IV, LAV-II, and HTLV-IIIB. AIDS Res Hum Retroviruses. 1987 Spring;3(1):3–10. doi: 10.1089/aid.1987.3.3. [DOI] [PubMed] [Google Scholar]
- Aldovini A., De Rossi A., Feinberg M. B., Wong-Staal F., Franchini G. Molecular analysis of a deletion mutant provirus of type I human T-cell lymphotropic virus: evidence for a doubly spliced x-lor mRNA. Proc Natl Acad Sci U S A. 1986 Jan;83(1):38–42. doi: 10.1073/pnas.83.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizon M., Wain-Hobson S., Montagnier L., Sonigo P. Genetic variability of the AIDS virus: nucleotide sequence analysis of two isolates from African patients. Cell. 1986 Jul 4;46(1):63–74. doi: 10.1016/0092-8674(86)90860-3. [DOI] [PubMed] [Google Scholar]
- Alter H. J., Eichberg J. W., Masur H., Saxinger W. C., Gallo R., Macher A. M., Lane H. C., Fauci A. S. Transmission of HTLV-III infection from human plasma to chimpanzees: an animal model for AIDS. Science. 1984 Nov 2;226(4674):549–552. doi: 10.1126/science.6093251. [DOI] [PubMed] [Google Scholar]
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Arya S. K. Human and simian immunodeficiency retroviruses: activation and differential transactivation of gene expression. AIDS Res Hum Retroviruses. 1988 Jun;4(3):175–186. doi: 10.1089/aid.1988.4.175. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Arthur L. O., Tsai C. C., Sowder R., Copeland T. D., Henderson L. E., Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J Virol. 1986 Nov;60(2):483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberfeld P., Kramarsky B., Salahuddin S. Z., Gallo R. C. Ultrastructural characterization of a new human B lymphotropic DNA virus (human herpesvirus 6) isolated from patients with lymphoproliferative disease. J Natl Cancer Inst. 1987 Nov;79(5):933–941. [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Clavel F., Guétard D., Brun-Vézinet F., Chamaret S., Rey M. A., Santos-Ferreira M. O., Laurent A. G., Dauguet C., Katlama C., Rouzioux C. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986 Jul 18;233(4761):343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- Cohen E. A., Terwilliger E. F., Sodroski J. G., Haseltine W. A. Identification of a protein encoded by the vpu gene of HIV-1. Nature. 1988 Aug 11;334(6182):532–534. doi: 10.1038/334532a0. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Feinberg M. B., Jarrett R. F., Aldovini A., Gallo R. C., Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986 Sep 12;46(6):807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Ensoli B., Ivanoff L., Chamberlain M., Petteway S., Ratner L., Gallo R. C., Wong-Staal F. The sor gene of HIV-1 is required for efficient virus transmission in vitro. Science. 1987 Aug 21;237(4817):888–893. doi: 10.1126/science.3497453. [DOI] [PubMed] [Google Scholar]
- Franchini G., Collalti E., Arya S. K., Fenyö E. M., Biberfeld G., Zagury J. F., Kanki P. J., Wong-Staal F., Gallo R. C. Genetic analysis of a new subgroup of human and simian T-lymphotropic retroviruses: HTLV-IV, LAV-2, SBL-6669, and STLV-IIIAGM. AIDS Res Hum Retroviruses. 1987 Spring;3(1):11–17. doi: 10.1089/aid.1987.3.11. [DOI] [PubMed] [Google Scholar]
- Franchini G., Gurgo C., Guo H. G., Gallo R. C., Collalti E., Fargnoli K. A., Hall L. F., Wong-Staal F., Reitz M. S., Jr Sequence of simian immunodeficiency virus and its relationship to the human immunodeficiency viruses. Nature. 1987 Aug 6;328(6130):539–543. doi: 10.1038/328539a0. [DOI] [PubMed] [Google Scholar]
- Franchini G., Kanki P. J., Bosch M. L., Fargnoli K., Wong-Staal F. The simian immunodeficiency virus envelope open reading frame located after the termination codon is expressed in vivo in infected animals. AIDS Res Hum Retroviruses. 1988 Aug;4(4):251–258. doi: 10.1089/aid.1988.4.251. [DOI] [PubMed] [Google Scholar]
- Franchini G., Rusche J. R., O'Keeffe T. J., Wong-Staal F. The human immunodeficiency virus type 2 (HIV-2) contains a novel gene encoding a 16 kD protein associated with mature virions. AIDS Res Hum Retroviruses. 1988 Aug;4(4):243–250. doi: 10.1089/aid.1988.4.243. [DOI] [PubMed] [Google Scholar]
- Fukasawa M., Miura T., Hasegawa A., Morikawa S., Tsujimoto H., Miki K., Kitamura T., Hayami M. Sequence of simian immunodeficiency virus from African green monkey, a new member of the HIV/SIV group. Nature. 1988 Jun 2;333(6172):457–461. doi: 10.1038/333457a0. [DOI] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Anderson D. C., Swenson R. B., Anand R., Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys). Proc Natl Acad Sci U S A. 1986 Jul;83(14):5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gallo R. C. The AIDS virus. Sci Am. 1987 Jan;256(1):46–56. [PubMed] [Google Scholar]
- Gottlieb M. S., Schroff R., Schanker H. M., Weisman J. D., Fan P. T., Wolf R. A., Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981 Dec 10;305(24):1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R. C., Copeland T. D., Benveniste R. E., Oroszlan S. Isolation and characterization of a novel protein (X-ORF product) from SIV and HIV-2. Science. 1988 Jul 8;241(4862):199–201. doi: 10.1126/science.3388031. [DOI] [PubMed] [Google Scholar]
- Knight D. M., Flomerfelt F. A., Ghrayeb J. Expression of the art/trs protein of HIV and study of its role in viral envelope synthesis. Science. 1987 May 15;236(4803):837–840. doi: 10.1126/science.3033827. [DOI] [PubMed] [Google Scholar]
- Looney D. J., Fisher A. G., Putney S. D., Rusche J. R., Redfield R. R., Burke D. S., Gallo R. C., Wong-Staal F. Type-restricted neutralization of molecular clones of human immunodeficiency virus. Science. 1988 Jul 15;241(4863):357–359. doi: 10.1126/science.3388046. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey-Corb M., Martin L. N., Rangan S. R., Baskin G. B., Gormus B. J., Wolf R. H., Andes W. A., West M., Montelaro R. C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986 May 22;321(6068):435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. The effect of helper virus on Abelson virus-induced transformation of lymphoid cells. J Exp Med. 1978 Apr 1;147(4):1126–1141. doi: 10.1084/jem.147.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarngadharan M. G., Popovic M., Bruch L., Schüpbach J., Gallo R. C. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984 May 4;224(4648):506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Dayton A., Terwilliger E., Haseltine W. A second post-transcriptional trans-activator gene required for HTLV-III replication. Nature. 1986 May 22;321(6068):412–417. doi: 10.1038/321412a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strebel K., Daugherty D., Clouse K., Cohen D., Folks T., Martin M. A. The HIV 'A' (sor) gene product is essential for virus infectivity. Nature. 1987 Aug 20;328(6132):728–730. doi: 10.1038/328728a0. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Chanda P. K., Ghrayeb J. Human immunodeficiency virus: the eighth gene. AIDS Res Hum Retroviruses. 1987 Spring;3(1):33–39. doi: 10.1089/aid.1987.3.33. [DOI] [PubMed] [Google Scholar]
- Zagury J. F., Franchini G., Reitz M., Collalti E., Starcich B., Hall L., Fargnoli K., Jagodzinski L., Guo H. G., Laure F. Genetic variability between isolates of human immunodeficiency virus (HIV) type 2 is comparable to the variability among HIV type 1. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5941–5945. doi: 10.1073/pnas.85.16.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]