Abstract
Background:
Little is known about the possible impact of the system of care on mortality during the first year after acute traumatic spinal cord injury (TSCI).
Objective:
To evaluate late mortality (ie, >7 days after trauma) during the first year after acute TSCI in 2 European Union (EU) regions, Thessaloniki in Greece and Stockholm in Sweden.
Methods:
This paper is part of the Stockholm Thessaloniki Acute Traumatic Spinal Cord Injury Study (STATSCIS), which is a prospective, population-based study. Incidence cohorts of TSCI cases were identified and followed up in both study regions through STATSCIS. Data from Thessaloniki region were collected through physical examination, medical records review, and interviews with TSCI individuals and the medical teams. Data from Stockholm were retrieved mainly from the Nordic Spinal Cord Injury Registry, as well as from direct contact with all intensive care facilities of the region.
Results:
The annual case mortality rate after acute TSCI was nearly 20% in Thessaloniki and 0% in Stockholm. The mean time of survival after trauma for the 12 mortality cases of Thessaloniki was 47 days (median = 24, SD ± 67, range = 8–228). Factors associated with mortality were higher age and presence of comorbid spinal disorders but also the inefficient transfer logistics, initially missed spinal instability, and unsuccessfully treated complications.
Conclusions:
The annual case mortality rate in Thessaloniki was dramatically higher than in Stockholm. The different approaches to care, one systematic and the other not, is postulated to be an important factor leading to such major discrepancies between the outcomes of these 2 EU regions.
Keywords: Spinal cord injuries, traumatic, complications, mortality; Outcomes; Greece; Sweden; Stockholm Thessaloniki Acute Traumatic Spinal Cord Injury Study; Nordic Spinal Cord Injury Registry
INTRODUCTION
Mortality after acute traumatic spinal cord injury (TSCI) has been assessed in several studies, mainly through a retrospective approach. Evaluation of trauma-related deaths has been widely used in evaluating the quality of trauma systems (1–4). Mullins et al (4) suggested that evaluating death as an outcome for hospitalized injured patients could be framed using 3 criteria: (a) type and severity of injury under study, (b) principal cause of death recorded on the death certificate, and (c) duration of follow up.
When considering TSCI mortality, exactly what is under study must be clarified: death due to TSCI or death with TSCI. Dying due to TSCI may involve (a) a high cervical level lesion causing respiratory insufficiency, (b) consequences of TSCI (eg, autonomic dysfunction), and (c) complications (eg, infections). On the other hand, dying with TSCI may involve (a) the presence of severe extraspinal injuries (eg, traumatic brain injury), (b) concomitant vascular injuries with excessive bleeding, and (c) the presence of severe comorbidities. Surveillance and analysis of factors related to mortality due to TSCI can be used to identify risk factors associated with prehospital, in-hospital, and outpatient management.
Furthermore, depending on which time frame is chosen, there are at least 4 different types of studied mortality after TSCI.
(a) Prehospital mortality (5): Systematic autopsies on all trauma cases are needed to adequately identify lesions of the spinal cord, because many of such deaths occur before any detailed clinical or radiologic examination is carried out. Clear guidelines for microscopic identification of TSCI would be necessary to assure specificity and sensitivity of the TSCI diagnosis, because the presence of spinal column injury alone is not adequate for inclusion. Due to the retrospective character of such studies, police reports, population-based trauma registries, death registries, and hospital records are important for case identification.
(b) In-hospital mortality (6–9): Systematic autopsies may be needed to identify cases with TSCI, because many of such cases, especially patients dying early after admission, are clinically concealed, for example, by coma and the presence of traumatic brain injury. A clear definition of what is defined as the in-hospital period and exactly when it ends is necessary, especially when a patient is treated in multiple health care facilities with a varying degree of available services and resources. Using such cut-off points as the end of acute care often seen in studies from the USA makes it difficult for international comparisons because of different criteria for, and timing of, discharge (1).
(c) First-year post-trauma mortality (10–12): Such studies focus on acute and postacute medical management, as well as early postdischarge outcome. They are usually restricted to mortality after hospital admission. It has been suggested that setting the cut-off at a specific time point after trauma (eg, 30 d after trauma) rather than at “discharge” would increase the validity of comparative studies (1).
(d) Long-term mortality (10,13–16): Typically, in these types of studies, data are retrieved from large TSCI registries, such as that of the Model Systems in the USA. These types of studies are greatly facilitated by the availability of national death registries and usually exclude mortality within the first year after trauma.
The present study belongs to category (c) and focuses on death occurring due to TSCI. Given the lack of systematic forensic data in early fatal trauma cases in Greece, we adopted a conservative strategy for defining late mortality attributable to acute TSCI by only including deaths occurring during the first year but at least 1 week after trauma. Within this time frame, the contribution of SCI and its consequences and complications associated with death can be assessed more reliably.
This paper is part of a comparative study on TSCI in Greece and Sweden known as the Stockholm Thessaloniki Acute Traumatic Spinal Cord Injury Study (STATSCIS). Its purpose is to evaluate prospectively the demographic and clinical characteristics, clinical process, and outcomes at 1 year after acute TSCI in a Northern (Stockholm, Sweden) and Southern (Thessaloniki, Greece) European Union region.
The 2 regions have radically different approaches to TSCI care: one region takes a system approach (Stockholm) and the other a “nonsystem” approach (Thessaloniki). The system approach to care has been defined during the last decades (17–20). This concept is mainly process related. The Stockholm region is a representative example of such a system with the following key features: case management and coordination of clinical process; specialization; centralization; long-term, planned follow ups; processing of cases according to specific medical and functional criteria. In contrast, a nonsystem approach, such as that of Thessaloniki, is haphazard and ad hoc. When it comes to skills and resources, however, both regions share the availability of modern diagnostic and therapeutic equipment and techniques. Thus, a comparison between these 2 regions provides an ideal setting for evaluating the impact of the process components, that is, the particular system of care.
The specific aim of this paper is to evaluate mortality during the first year after trauma for the incidence cohorts with TSCI in the greater Thessaloniki and Stockholm regions.
MATERIALS AND METHODS
Inclusion Criteria
All of the following criteria had to be satisfied for inclusion in STATSCIS: (a) acute TSCI or traumatic cauda equina injury, (b) injury occurring during the first 12 months of the study period (September 2006–September 2007), (c) age 16 years or older at the time of injury, (d) in-patient care in a hospital of the Thessaloniki or Stockholm regions, (e) survival for at least 7 days after the injury, (f) resident of the country of the respective region, and (g) informed consent for the STATSCIS given by the individual.
Settings and Case Identification
The greater Thessaloniki region (Thessaloniki) comprises Central and West Macedonia in Northern Greece, and the greater Stockholm region (Stockholm) comprises Stockholm and Gotland Counties in central Sweden. The 2 regions have a similar population size of approximately 2 million, with Thessaloniki being about 3 times larger area wise than Stockholm.
Within the greater Thessaloniki region, out of a total of 30 hospitals, 5 are at a tertiary level and thus, in principle, able to handle acute TSCI. However, 1 of these is a military hospital, unavailable to the public, and was therefore not included in the surveillance system. Of the secondary hospitals, the only 2 that could treat cases with acute TSCI were included in the surveillance system.
Within the greater Stockholm region, there is a comprehensive SCI system of care, consisting of 1 hospital-based spinal injury unit, 2 inpatient rehabilitation centers, and 1 outpatient clinic for lifelong follow up.
Acute TSCI cases in Thessaloniki were identified using a comprehensive active surveillance system (21) designed and implemented for the purposes of this study. The main investigator (A.D.) maintained a weekly personal contact with the 25 hospital wards of the 4 tertiary hospitals and regular telephone contact with the 4 wards of the 2 secondary level hospitals. All potential cases were evaluated as they occurred in accordance with the inclusion criteria of STATSCIS.
Acute TSCI cases in Stockholm were identified through a passive surveillance system (21) using the Nordic Spinal Cord Injury Registry since it includes all cases treated in the Stockholm SCI system of care. Additionally, an active surveillance component was added by contacting all intensive care units in the region that did not typically treat TSCI. This type of design was chosen because although the regional SCI system of care is highly centralized, severe multitrauma cases may occasionally receive acute treatment in other hospitals. Thus, a letter of inquiry was sent to all 7 intensive care units in the region; the units were asked whether they had hospitalized anyone with acute TSCI who had died during the study period.
Data Collection and Analysis
Data were obtained by physical examination before death. The International Standards for the Neurological Classification of SCI were applied; medical records, including death certificates, were reviewed; and there was personal communication with the attending physicians and staff and with a first-degree relative. Use of multiple sources of information was necessary, because, as also reported elsewhere (22), death certificates in Greece are typically too unspecific by themselves to allow for clarification of the course of events leading to death. Consequently, in accordance with suggested practice in injury surveillance (23), the information obtained by the official death certificate was supplemented by data from medical records. All authors (3 of whom specialize in neurology and/or rehabilitation medicine and 1 of whom is a registered nurse) reviewed the death certificates and medical records. Four of the authors have doctoral degrees in clinical SCI research and more than 20 years of clinical experience in SCI.
For the purpose of this study, only the incidence cases (ie, sustaining their injury from September 2006 through September 2007) were considered, whereas remaining STATSCIS cases injured outside of this timeframe were not included in the analyses. Thus, 64 cases in Thessaloniki and 32 cases in Stockholm were checked with regard to survival on a weekly basis during the first year after trauma. Nordic Spinal Cord Injury Registry (www.nscic.se) forms were used on admission and at 1 year after trauma. Clinical neurologic examination, including assessment according to the International Standards for the Neurological Classification of SCI, was performed in all cases. In Thessaloniki, the main investigator (A.D.) performed all such examinations that provided data for the present study. This strategy was followed because the International Standards for the Neurological Classification of SCI evaluation was not universally utilized in Greece. The main investigator has 8 years of clinical experience in SCI, has participated in an official workshop on the International Standards for the Neurological Classification of SCI, and has been using this assessment method for several years. Physicians and physiotherapists specialized in SCI performed the corresponding examinations in Stockholm. Clinical diagnosis of TSCI was confirmed by neuroimaging studies in all cases in both regions. Overall, for those 6 cases in Thessaloniki and 4 cases in Stockholm, in which a full classification according to ASIA on admission was prohibited by severe extraspinal injuries or injury occurring abroad, we could establish the neurologic level and whether the lesion was complete or incomplete based on the medical records.
In addition to the quantitative methods described above, we used the richer data source of the total medical records to create the clinical vignettes presented in Appendix 1. The case reports are intended to qualitatively clarify some uncontrolled variations in care provision, which most likely contributed to mortality.
With regard to “initially missed spinal instability,” we adopted the definition given by Clarke et al (24), who defined a “missed injury” as an injury that escaped detection during the primary and secondary survey (prehospital evaluation and management) and initial investigation (on arrival at hospital evaluation) or during operative exploration.
Quality assurance was performed in both regions, that is, by cross-checking data in the registry forms with medical records, in order to maximize validity and minimize missing data. All retrieved data were jointly reevaluated in detail by the authors for the purposes of medical accuracy and uniform interpretation as they relate to extra-spinal injuries and any secondary morbidity.
Statistical Analysis
Descriptive data are presented as n (%), mean, SD, median, and interquartile range (IQR). Statistical significance was set at P < 0.05. Differences in proportions between regions were examined by chisquare test and Fisher exact test. Statistical mean differences between regions were determined by independent Student's t test. In cases of non-normal distribution, the Mann-Whitney U test was used. All statistical analyses were performed with SPSS version 16.0 software.
Ethics
Ethical approvals for STATSCIS were obtained by the Human Ethics Committee at Karolinska Institutet, the Hellenic Data Protection Authority, the Nordic SCI Council, the Scientific Committee, and the Board of each participating hospital in Thessaloniki. Informed consent was necessary for inclusion in STATSCIS, even for the fatalities, and was given, according to the initial plan, either by the TSCI individual or by a first-degree relative.
RESULTS
Overall, 12 of the 64 cases in Thessaloniki and none of the 32 cases in Stockholm died during the first year after trauma. Further confirmation of the absence of deaths in Stockholm was provided by the negative responses received from all intensive care units. The 1-year case mortality rate was 18.8% in Thessaloniki and 0% in Stockholm, showing a significant difference of P = 0.007. Inclusion in STATSCIS was denied for 3 cases in Thessaloniki, 2 of which subsequently died, and for 2 cases in Stockholm.
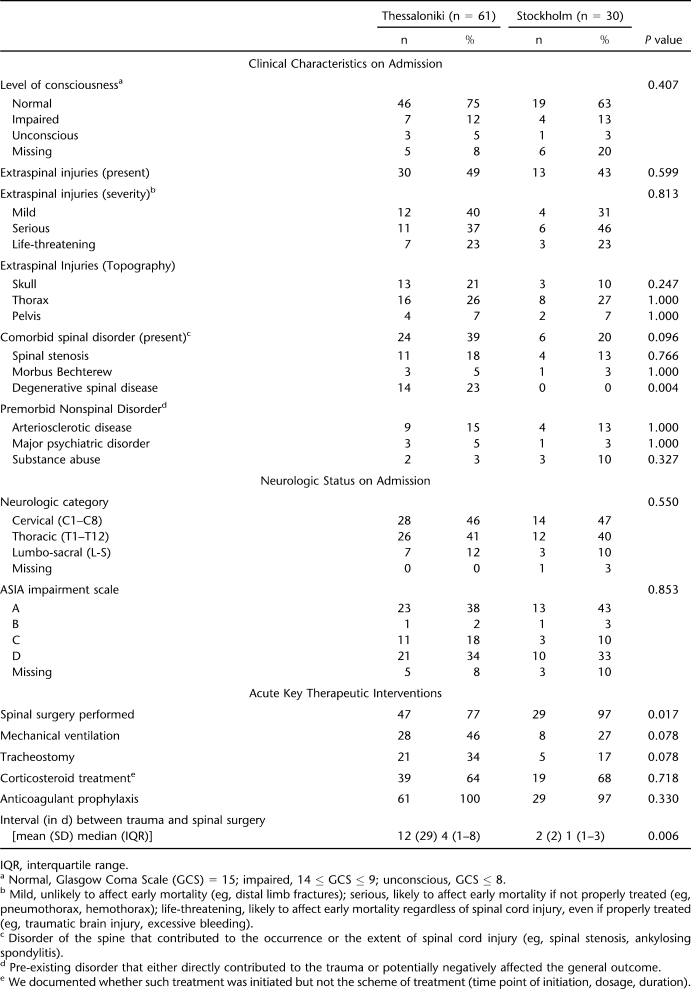
Table 1 summarizes the key characteristics of the 2 STATSCIS incidence cohorts of consented cases. As can be seen from the table, the 2 groups were very similar on admission but nevertheless received rather different acute key therapeutic interventions.
Table 1.
Characteristics of the Stockholm Thessaloniki Acute Traumatic Spinal Cord Injury Study Incidence Cohorts (Consented Cases)
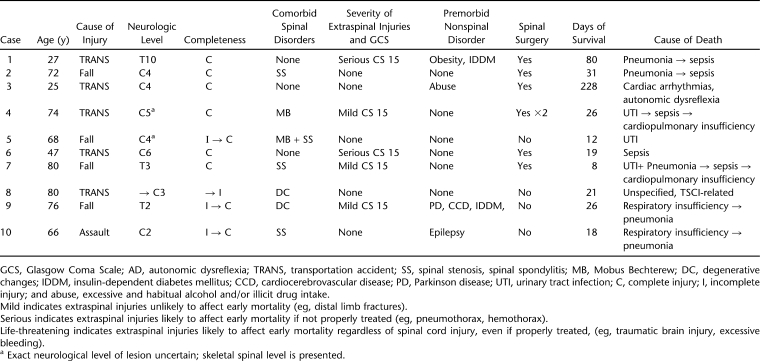
Overview characteristics of the 10 consented mortality cases, all of which occurred in Thessaloniki, can be found in Table 2 and show the age at injury to range from 25 to 80 years with a mean of 62 years (median = 70, SD ± 21). There were 9 men and 1 woman; 5 had sustained transportation-related injuries, 4 had fall-related injuries, and 1 had an assault-related injury. All but 1 case were initially transferred to smaller regional hospitals before being transferred to a tertiary level hospital.
Table 2.
Characteristics of Mortality Cases
With regard to the neurologic category of the lesion, 6 had cervical lesions, 3 had thoracic, and 1 had no initial neurologic lesion after trauma but did later develop C3 tetraplegia due to missed spinal column instability (Case II in Appendix 1). Four cases deteriorated neurologically during prehospital and/or during early in-hospital management. On first examination, 6 were found with complete lesions, 3 were found with incomplete lesions, and 1 was without any reported neurologic signs. The 3 initially incomplete lesions later progressed to complete, and the case without any initial neurologic signs subsequently progressed to an incomplete lesion.
Half of the cases had no extraspinal injuries, 3 had mild injuries, and 2 had serious such lesions. All cases were reported to be conscious on initial admission (or before intubation, if intubated during the prehospital phase) with a Glasgow Coma Scale score of 15. Seven of 10 cases had comorbid spinal disorders, with spinal stenosis being the most frequently seen, and 4 had at least 1 premorbid, nonspinal disorder.
Initially missed spinal instability was recorded in at least 3 cases, all of which had initially been admitted to a local hospital. Spinal surgery was offered to 7 of 10 cases, 1 out of which refused to be operated.
Mean time of survival after trauma was 47 days (median = 24, SD ± 67, range = 8–228). In 8 cases, death was attributed to infections; in 1 case, it was attributed to autonomic dysfunction and in another to unspecified TSCI-related reasons.
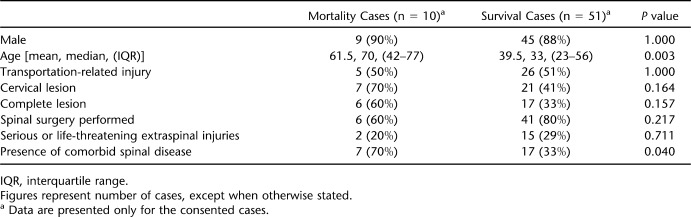
As shown in Table 3, such factors as higher age and the presence of comorbid spinal disorders were significantly associated with the mortality cases compared with those who survived in Thessaloniki. Appendix 1 presents case reports of 3 illustrative mortality cases in Thessaloniki, including “inefficient transfer logistics” in case I, “initially missed spinal instability” in case II, and “unsuccessfully treated complications” in case III.
Table 3.
Characteristics of the Incidence Cohort Cases in Thessaloniki, Greece
DISCUSSION
In the present study, the 1-year case mortality rate of individuals with acute TSCI who had survived the first week after trauma was nearly 20% in Thessaloniki and 0% in Stockholm. Both incidence cohorts and the total STATSCIS cohorts were fairly similar in their demographic profiles (21) (eg, age, gender, marital status, living conditions, vocational situation), as well as core clinical characteristics on admission (eg, neurologic level, completeness of lesion, ASIA total motor score, ASIA impairment scale grading, presence and severity of extraspinal injuries, level of consciousness, presence of premorbid spinal diseases) (25). The most obvious potential reason for varying mortality is a difference in early management, and this was indeed the case (eg, more and longer in duration interhospital transfers from the scene of trauma to a tertiary level hospital, more frequent invasive respiratory treatment, less frequent and more delayed spinal surgery in Thessaloniki than in Stockholm) (25). Additional factors to be analyzed as to their potential correlation with fatal outcome are presented in this paper.
Over time, most likely due to the advance of prehospital management, there has been a decrease in prehospital and a relative increase in in-hospital trauma mortality (26). With regard to acute TSCI, there are very few studies examining the early temporal distribution of mortality. In a retrospective study on acute TSCI from Portugal, Fernando Martins et al (5) reported that 40% of 398 consecutive acute TSCI cases had died during the first week after trauma. We could assume that several of these cases died with and not due to a TSCI.
As has been shown previously by others (26), pre- and early in-hospital deaths (occurring during the first week after trauma) were significantly associated with more severe injuries, compared with late in-hospital deaths. Furthermore, as reported by the Liverpool Hospital Trauma Death Peer Review Committee (27), a greater proportion of potentially avoidable trauma deaths occurred after and not during the first week of admission. Considering our inclusion criteria, and especially the 7-day survival cut-off, we could assume that the most severely injured who died were excluded from this study. Hence, by imposing such a time frame, survival for the included cases could perhaps be seen as an “expected” outcome and death as an adverse event.
Precise comparisons with other studies are hampered by methodologic differences that greatly affect the estimated mortality rates. In the population-based study from Portugal, 26% of those with acute TSCI who had survived the first week after trauma died during the following months (5). Other studies reported in-hospital mortality rates after acute TSCI to be 5.7% to 8% in Canada (6,8,28), 8.6% in Germany (7), and 11.9% in Teresina/Brazil (9). The 1-year TSCI mortality rate was reported to be 5.8% in Australia (11) and 3.6% in the US Model Spinal Cord Injury System (10). A more recent study from the US Model Spinal Cord Injury System revealed a 40% decline in mortality during the first 2 years after trauma since the 1970s (28).
In Thessaloniki, mortality was significantly associated with higher age and presence of comorbid spinal disorders. DeVivo et al (12) reported that ventilatory status, age, Frankel grade, and injury level were the strongest predictors of mortality during the first year after TSCI. Similarly, a recent study by Furlan et al (29) reported that older age, relevant pre-existing medical conditions, and motor complete SCI were major risk factors for in-hospital death after acute SCI. In the present study, although we could see a tendency towards association of cervical spinal injury and neurologically complete lesion with mortality, this failed to reach statistical significance due to the small sample size.
In the present study, unsuccessfully treated respiratory and/or urinary tract infections leading to sepsis and cardiopulmonary insufficiency were a common terminal sequence leading to death. Case III in Appendix 1 is one example. Sepsis was overall associated with 6 of the cases and pneumonia with 5. Such findings agree with other studies on late mortality after TSCI (6,7,9,10,12). The retrospective population-based study from Ontario, Canada, attributed death either directly to SCI or to associated head trauma and sepsis (6). In both studies from Germany (7) and Brazil (9), pulmonary and cardiac insufficiencies in addition to sepsis were the main causes of death. In the large retrospective study from the US Model Spinal Cord Injury System, DeVivo et al (12), examining a cohort of 28,239 individuals who had survived 24 hours after acute TSCI, reported that respiratory and heart-related causes jointly accounted for more than half of deaths that occurred during the first year after trauma. Autonomic dysfunction was only conclusively associated with the death of one person in our study. However, it is possible and also to be expected to have contributed to a fatal outcome in some more cases with high lesions.
Initially missed spinal instability could be recorded in at least 3 of the fatalities in Thessaloniki, all of them initially transferred to a local hospital. Case II in Appendix 1 is an illustrative example. Poonnoose et al (30) reported that correct diagnosis was initially missed in 52 (9.1%) of 569 TSCI patients in their study, with 6 of these cases ultimately dying. The authors reported different factors contributing to missed cases: circumstances surrounding the injury (eg, high energy impact, influence of drugs and alcohol), inadequate neurologic assessment, associated injuries, and radiographic errors (30).
In the present study, in the absence of a SCI system of care in Greece, long and/or repeated interfacility transfers of patients occurred very early after trauma in several cases, often in the presence of hemodynamic and spinal instability. Some instances were associated with further neurologic deterioration, possibly contributing to a fatal outcome. As Case I in Appendix 1 illustrates clearly, multiple interfacility transfers may occur throughout the first year because there is no designated facility to manage TSCI. Tator et al (8) reported a significant decrease in mortality rates after acute TSCI when the treatment was provided in a spinal injury unit as opposed to a general ward. Furthermore, DeVivo reported that acute TSCI cases admitted early to a spinal injury unit had greater chances for survival than those admitted at a later stage (31).
The rather small sample size and the design of this study by necessity impose some limitations with regard to the ability to conclusively show all interacting factors ultimately leading to a higher mortality rate in Thessaloniki. Nevertheless, the study has been able to display a number of severe drawbacks related to a nonsystem approach, even in the presence of contemporary medical resources. Although the inability to obtain reliable data on 1-week mortality rates could theoretically conceal a scenario of higher mortality in Stockholm than in Thessaloniki during this period, we believe that such a scenario has no clinical plausibility. Therefore, the 7-day cut-off should rather be seen as a methodologic strength of STATSCIS than a limitation, given the explorative and innovative nature of the study.
CONCLUSIONS
The present study reports a dramatically higher case mortality rate due to TSCI in Thessaloniki than in Stockholm, despite the 2 study groups' appearing largely similar in their demographic and core clinical characteristics on admission. Within the Thessaloniki cases, higher age and presence of comorbid spinal disorders were associated with mortality, because these cases usually are more vulnerable to a nonsystem approach. Factors that apparently affected mortality include inefficient transfer logistics, initially missed spinal instability, and unsuccessfully treated complications. Overall, the differing approaches to managing TSCI, one systematic and the other not, could be seen as the leading factor in such major discrepancies in mortality after acute TSCI between these 2 European Union regions.
Acknowledgments
We thank the Spinalis Foundation for providing financial support to STATSCIS. We also thank Sara Runesdotter for assistance with statistical analysis, Elizabeth Gustafsson for secretarial support, and all collaborators from both regions.
Collaborators: G.P.G. Papanikolaou Hospital; Bitzani, Lavrentieva (ICU); Kapravelos, Abatzidou (ICU); Mparoutas, Skoullios (Neurosurgical); Pournaras, Christodoulou (Orthopaedic); Christaki (Respiratory). Ahepa Univ. Hospital; Sofianos, Giala (ICU); Skourtis, Setzis, Ourailoglou (ICU); Harlaftis, Papaavramidis (ICU); Selviaridis, Ioannou, Stavrinou (Neurosurgical). G.P.G. Papageorgiou Hospital; Matamis, Sinnefaki (ICU); Kampelis, Alexiadou (Neurosurgical); Kapetanos, Likomitros (Orthopaedic); Kyriakidis, Moschoglou, Valanos (Orthopaedic). Ippokrateio Hospital; Gerogianni, Efthimiou, Papageorgiou (ICU); Mpallas (ICU); Tsitsopoulos, Marinopoulos, Tsitsopoulos, Tsitouras (Neurosurgical); Dimitriou, Boursinos (Orthopaedic). National Centre for Emergency Care; Mpoutlis, Matsikoudi. Karolinska Univ. Hospital; Hedman, Brofelth (SIU); Eriksson, Werhagen (Spinalis Clinic). Rehab Station Stockholm; Bjelak, Holmström, Lindgren. Stockholms Sjukhem; Kärvestedt, Aly, Westerlund. Nordic Spinal Cord Injury Council.
APPENDIX 1: REPORTS OF THREE MORTALITY CASES IN THESSALONIKI
CASE I Inefficient transfer logistics
A 25-year-old man with a history of substance abuse was injured as a front-seat passenger in a single car crash. He was then transported to the emergency department (ED) of a local hospital (A). After a 2.5-hour drive, the patient was transported by ambulance to the ED of a tertiary level hospital (B).
Investigations
Physical examination revealed a Glasgow Coma Scale of 15 and a C5 complete tetraplegia. Computed tomographic scans of the brain, thorax, and abdomen showed no pathologic signs, whereas magnetic resonance imaging examination of the cervical spine showed a C5–C6 posterior dislocation and a C6 vertebral fracture with a corresponding spinal cord edema.
Course of Hospitalization
On the first day post trauma (DPT), the patient underwent C5–C6 fusion and thereafter was transferred to the intensive care unit (ICU) due to hemodynamic instability. Corticosteroid treatment was not provided. On the second DPT, efforts at extubation failed due to hypoxemia, paradoxic breathing, and poor compliance, and the patient was reintubated. During the third DPT, the patient showed signs of hypoventilation of the left lower lobe and bradycardia. On the eighth DPT, the patient developed high fever, and a chest radiograph confirmed pneumonia, which was treated accordingly. On the 14th DPT, the patient underwent tracheostomy. On the 19th DPT, transient tachycardia and tachypnea were observed. By the 23rd DPT, sedatives were reduced and the patient was hemodynamically stable with satisfactory diuresis. On the 24th DPT, the patient appeared septic and hemodynamically unstable and was successfully treated. During the following weeks, the patient had recurrent urinary tract infections, which led to the replacement of the indwelling catheter by a suprapubic one. Up to the 50th DPT in ICU, several instances of hemodynamic instability occurred, one of which resulted in cardiac arrest. On the 50th DPT, the patient was transferred to the ICU of a smaller regional hospital (C). On the 63rd DPT, he was transferred to a regional rehabilitation center (D) while still on mechanical ventilation. On 74th DPT, he was urgently transferred to another regional hospital (E), where after staying for 10 days, he was readmitted to the regional rehabilitation center (D). On the 94th DPT, he was transferred to a university hospital (F) with urinary tract and respiratory infections, as well as severe ischial pressure sores. On the 151st DPT, the patient was transferred to the regional rehabilitation center (D). Subsequently, he was urgently transferred 3 times and spent short periods in the regional hospital (E) because of cardiac arrhythmias, likely due to autonomic dysreflexia. During his last stay at the regional hospital (E) on the 228th DPT, he died while being prepared to return to the rehabilitation center.
Cause of Death
Cardiac arrhythmias, hemodynamic instability, and possible recurrent episodes of autonomic dysreflexia, leading to death.
Comment
This patient had C5 complete tetraplegia with prominent cardiac and hemodynamic instability. From time of trauma until time of death, the patient was transferred between 6 facilities. He would have benefited from specialized and comprehensive treatment in one center.
CASE II Initially missed spinal instability
An 80-year-old man with chronic cervical and lumbar radiculopathy was injured as a car driver in a car crash and transported to a local hospital.
Investigations
Physical examination at the time of admission revealed a Glasgow Coma Scale of 15, and the patient reported dizziness and neck pain but had no neurologic, orthopedic, or respiratory findings. A computed tomographic scan of the brain showed some atrophy but no other pathologic signs. A computed tomographic scan of the cervical spine showed degenerative changes and straightening of the cervical lordosis, without any signs of fracture. On the first day post trauma (DPT), the patient was discharged home with a collar and scheduled for a follow-up outpatient examination a few days later. On the fourth DPT, the follow-up physical examination was normal, so the collar was removed and he was sent home. On the seventh DPT, the patient experienced a spell of dizziness while sitting on the toilet. He remained at home, and on the ninth DPT, he manifested gradual deterioration with weakness of the lower limbs and neck pain. On the 11th DPT, he was transferred to the tertiary hospital by ambulance. On admission, his upper limbs felt normal sensations and exhibited “next to normal” strength. Lower limb strength was three fifths and four fifths in all of the key muscles, and sensory impairment was present. Spine radiographs confirmed previous findings. A Philadelphia collar was placed, and the patient was then admitted to a neurosurgical ward.
Course of Hospitalization
On the same day, the patient manifested further deterioration in terms of sensorimotor function of the lower limbs. Corticosteroid treatment was then initiated, and a urethral catheter was placed. On the 12th DPT, a magnetic resonance image of the cervical spine showed spinal cord compression by a C6–C7 dislocation, as well as by a posterior osteophyte at the C7 level. Immediately after the examination, the patient reported further deterioration, and an evaluation showed C3 AISA C tetraplegia. The patient refused surgery. On the 15th DPT, the patient insisted on being discharged home, where he died on the 21st DPT.
Cause of Death
Unspecified, TSCI related. No death certificate available.
Comment
Neurologic deterioration was due to an initially undisclosed unstable lesion of the cervical spine. Because the patient declined surgical treatment and decided to return home with an untreated unstable lesion, further deterioration is likely to have occurred and might by itself have constituted the cause of death. The lesion remained undetected through several evaluations including computed tomographic scans. The delay in diagnosis led to neurological deterioration, which is something that may have contributed to a fatal outcome. Initial correct diagnosis would likely have occurred in a specialized facility.
CASE III Complications from unsuccessful treatments
A 27-year-old woman with severe obesity and insulin-dependent diabetes mellitus was transferred to the emergency department of a tertiary hospital after being injured as a front-seat passenger in a high-speed single car crash.
Investigations
Physical examination at the time of admission revealed a Glasgow Coma Scale of 15 and the presence of complete T10 paraplegia. Radiologic examination showed a T10 fracture dislocation, fractures of several ribs, hemopneumothorax, and mild liver contusions.
Course of Hospitalization
On the first day post trauma (DPT), Bülau drainage was placed and the patient underwent T10–T11 laminectomy and T9-L2 fusion. She was then admitted to the intensive care unit. On the second DPT, she was extubated, with a good saturation level and diuresis, and was transferred to a neurosurgery ward. On the seventh DPT, further Bülau drainage was placed due to dyspnea. Initial respiratory symptoms ceased until the eighth DPT, when she also experienced chest pain, oxygen desaturation, and fever. On the 10th DPT, she was readmitted to the intensive care unit with respiratory insufficiency, which was treated with hemodynamics and oxygen supply. On the 13th DPT, she was readmitted to the neurosurgery ward. On the 28th DPT, after a severe bradycardic episode combined with low oxygen saturation levels, she was urgently intubated and treated with adrenaline. On the 29th DPT, she was extubated with a Glasgow Coma Scale of 15 and good breathing pattern. On the 32nd DPT, she was reintubated due to tachypnea, hypoxemia, respiratory fatigue, inability to cough, and atelectasis. She showed signs of progressive deterioration of kidney function, and hemodialysis treatment was initiated. On the 36th DPT, she underwent tracheostomy, and by the 39th DPT, she had a satisfactory general condition on biphasic intermittent positive airway pressure ventilation but was febrile. On the 51st DPT, ventilator-associated pneumonia was diagnosed, and she was put under heavier sedation to improve mechanical ventilation. On the 63rd DPT, while under sedation and mechanical ventilation, she had a septic episode. On the 73rd DPT, she had another septic episode followed by hemodynamic unstability and severe deterioration of respiratory capacity; thereafter, she was hypoxemic and hemodynamically instable. On the 80th DPT, she died from polyorganic failure due to sepsis.
Cause of Death
Respiratory insufficiency, leading to pneumonia, leading to sepsis.
Comment
Although this patient was young and had low-level paraplegia, her risk for respiratory complications was high due to the combination of thoracic injuries, severe obesity, and diabetes. One major factor likely to have contributed to the ultimately fatal outcome was a lack of proactive preventative measures (eg, early mobilization) in combination with the lack of defined clinical protocols for management of respiratory complications.
References
- Skaga NO, Eken T, Jones JM, Steen PA. Different definitions of patient outcome: consequences for performance analysis in trauma. Injury. 2008;39(5):612–622. doi: 10.1016/j.injury.2007.11.426. [DOI] [PubMed] [Google Scholar]
- Liberman M, Mulder DS, Lavoie A, Sampalis JS. Implementation of a trauma care system: evolution through evaluation. J Trauma. 2004;56(6):1330–1335. doi: 10.1097/01.ta.0000071297.76727.8b. [DOI] [PubMed] [Google Scholar]
- Boyd CR, Tolson MA, Copes WS. Evaluating trauma care: the TRISS method. J Trauma. 1987;27(4):370–378. [PubMed] [Google Scholar]
- Mullins RJ, Mann NC, Hedges JR, et al. Adequacy of hospital discharge status as a measure of outcome among injured patients. JAMA. 1998;279(21):1727–1731. doi: 10.1001/jama.279.21.1727. [DOI] [PubMed] [Google Scholar]
- Martins F, Freitas F, Martins L, Dartigues JF, Barat M. Spinal cord injuries: epidemiology in Portugal's central region. Spinal Cord. 1998;36(8):574–578. doi: 10.1038/sj.sc.3100657. [DOI] [PubMed] [Google Scholar]
- Pickett GE, Campos-Benitez M, Keller JL, Duggal N. Epidemiology of traumatic spinal cord injury in Canada. Spine. 2006;31(7):799–805. doi: 10.1097/01.brs.0000207258.80129.03. [DOI] [PubMed] [Google Scholar]
- Botel U, Glaser E, Niedeggen A. The surgical treatment of acute spinal paralysed patients. Spinal Cord. 1997;35(7):420–428. doi: 10.1038/sj.sc.3100407. [DOI] [PubMed] [Google Scholar]
- Tator CH, Duncan EG, Edmonds VE, Lapczak LI, Andrews DF. Neurological recovery, mortality and length of stay after acute spinal-cord injury associated with changes in management. Paraplegia. 1995;33(5):254–262. doi: 10.1038/sc.1995.58. [DOI] [PubMed] [Google Scholar]
- Leal MB, Borges G, de Almeida BR, et al. Spinal cord injury: epidemiological study of 386 cases with emphasis on those patients admitted more than four hours after the trauma. Arq Neuropsiquiatr. 2008;66(2B):365–368. doi: 10.1590/s0004-282x2008000300016. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Black KJ, Stover SL. Causes of death during the 1st 12 years after spinal-cord injury. Arch Phys Med Rehabil. 1993;74(3):248–254. [PubMed] [Google Scholar]
- O'Connor PJ. Survival after spinal cord injury in Australia. Arch Phys Med Rehabil. 2005;86(1):37–47. [PubMed] [Google Scholar]
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80(11):1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- Frankel HL, Coll JR, Charlifue SW, et al. Long-term survival in spinal cord injury: a fifty year investigation. Spinal Cord. 1998;36(4):266–274. doi: 10.1038/sj.sc.3100638. [DOI] [PubMed] [Google Scholar]
- Krause JS, Sternberg M, Lottes S, Maides J. Mortality after spinal cord injury: an 11-year prospective study. Arch Phys Med Rehabil. 1997;78(8):815–821. doi: 10.1016/s0003-9993(97)90193-3. [DOI] [PubMed] [Google Scholar]
- Garshick E, Kelley A, Cohen S, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43(7):408–416. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soden RJ, Walsh J, Middleton JW, Craven ML, Rutkowski SB, Yeo JD. Causes of death after spinal cord injury. Spinal Cord. 2000;38(10):604–610. doi: 10.1038/sj.sc.3101080. [DOI] [PubMed] [Google Scholar]
- Domingo M. Organisation of an autonomous spinal injuries unit. Paraplegia. 1967;5(3):170–176. doi: 10.1038/sc.1967.26. [DOI] [PubMed] [Google Scholar]
- Frankel H. Spinal-cord injury units. Paraplegia. 1987;25(3):239–240. doi: 10.1038/sc.1987.42. [DOI] [PubMed] [Google Scholar]
- Illis LS. The case for specialist units. Spinal Cord. 2004;42(8):443–446. doi: 10.1038/sj.sc.3101633. [DOI] [PubMed] [Google Scholar]
- Consortium for Spinal Cord Medicine. Early acute management in adults with spinal cord injury: a clinical practice guideline for health-care professionals. J Spinal Cord Med. 2008;31(4):403–479. doi: 10.1043/1079-0268-31.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divanoglou A, Levi R. Incidence of traumatic spinal cord injury in Thessaloniki, Greece and Stockholm, Sweden: a prospective population-based study. Spinal Cord. 2009;47(11):796–801. doi: 10.1038/sc.2009.28. Published online 7 April 2009. [DOI] [PubMed] [Google Scholar]
- Papadopoulos IN, Papaefthymiou M, Roumeliotis L, Panagopoulos VG, Stefanidou A, Kostaki A. Status and perspectives of hospital mortality in a public urban Hellenic hospital, based on a five-year review. BMC Public Health. 2008;8(28):1–11. doi: 10.1186/1471-2458-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan JM, Mallonee S. Injury surveillance. Epidemiol Rev. 2003;25:24–42. doi: 10.1093/epirev/mxg010. [DOI] [PubMed] [Google Scholar]
- Clarke DL, Gouveia J, Thomson SR, Muckart DJ. Applying modern error theory to the problem of missed injuries in trauma. World J Surg. 2008;32(6):1176–1182. doi: 10.1007/s00268-008-9543-7. [DOI] [PubMed] [Google Scholar]
- Divanoglou A, Seiger Å, Levi R. Acute management of traumatic spinal cord injury in a Greek and a Swedish region: a prospective, population-based study. Spinal Cord. Advance online publication, December 22, 2009 (DOI: 10.1038/sc.2009.160). [DOI] [PubMed]
- Soreide K, Kruger AJ, Vardal AL, Ellingsen CL, Soreide E, Lossius HM. Epidemiology and contemporary patterns of trauma deaths: changing place, similar pace, older face. World J Surg. 2007;31(11):2092–2103. doi: 10.1007/s00268-007-9226-9. [DOI] [PubMed] [Google Scholar]
- Sugrue M, Caldwell E, D'Amours S, et al. Time for a change in injury and trauma care delivery: a trauma death review analysis. Anz J Surg. 2008;78(11):949–954. doi: 10.1111/j.1445-2197.2008.04711.x. [DOI] [PubMed] [Google Scholar]
- Strauss DJ, DeVivo MJ, Paculdo DR, Shavelle RM. Trends in life expectancy after spinal cord injury. Arch Phys Med Rehabil. 2006;87(8):1079–1085. doi: 10.1016/j.apmr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Furlan JC, Kattail D, Fehlings MG. The impact of co-morbidities on age-related differences in mortality after acute traumatic spinal cord injury. J Neurotrauma. 2009;26(8):1361–1367. doi: 10.1089/neu.2008.0764. [DOI] [PubMed] [Google Scholar]
- Poonnoose PM, Ravichandran G, McClelland MR. Missed and mismanaged injuries of the spinal cord. J Trauma. 2002;53(2):314–320. doi: 10.1097/00005373-200208000-00021. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Kartus PL, Stover SL, Fine PR. Benefits of early admission to an organized spinal-cord injury care system. Paraplegia. 1990;28(9):545–555. doi: 10.1038/sc.1990.74. [DOI] [PubMed] [Google Scholar]