Abstract
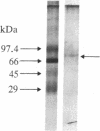
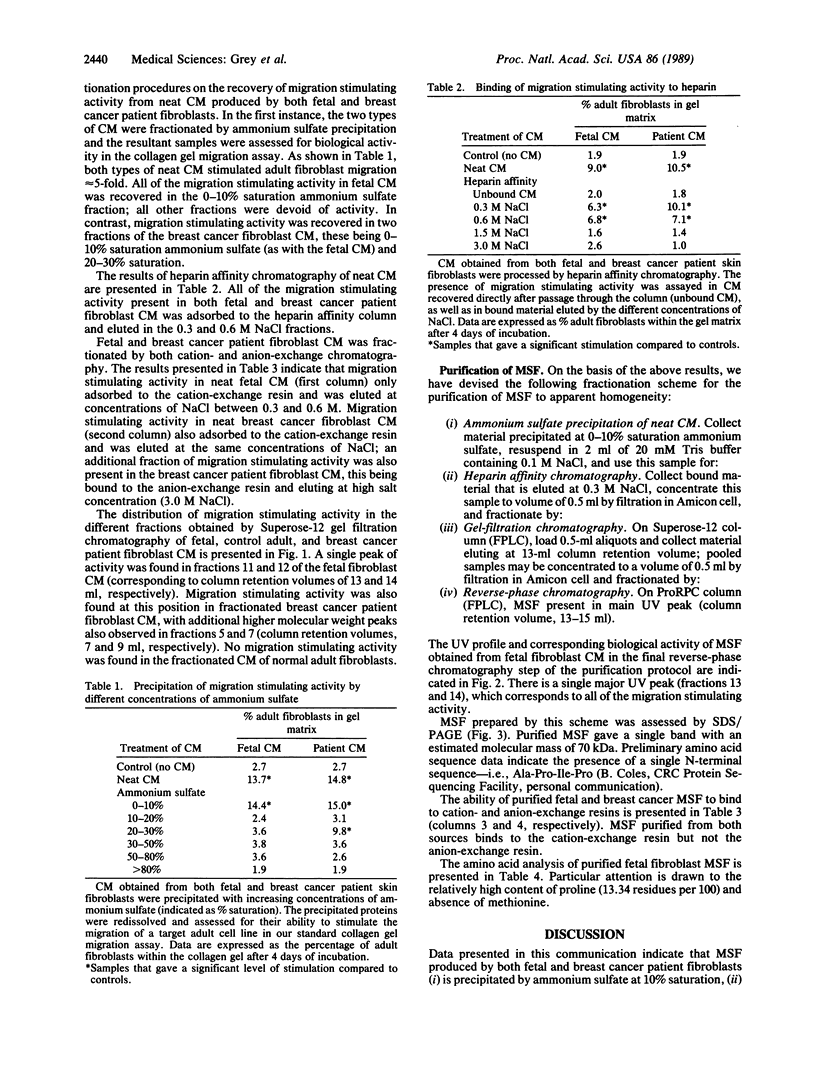
We have previously shown that (i) human skin fibroblasts of fetal and adult origin display distinctive migratory phenotypes, (ii) this difference in cell behavior results from the production of a soluble "migration stimulating factor" (MSF) by fetal cells, and (iii) skin fibroblasts from breast cancer patients commonly resemble fetal fibroblasts both in migratory phenotype and in production of MSF. Data are now presented indicating that MSF present in the conditioned medium of fetal and cancer patient fibroblasts is precipitated at 10% saturation ammonium sulfate and binds to heparin and cation-exchange resins. Based on this information, we have devised a scheme for the purification of MSF involving the sequential application of ammonium sulfate precipitation, heparin affinity, gel filtration, and reverse-phase chromatography. Purified MSF has an estimated molecular mass of 70 kDa; amino acid analysis reveals a relatively high level of proline (13.34 residues per 100). Our results further suggest that skin fibroblasts from breast cancer patients produce an additional factor with migration stimulating activity; this factor is precipitated at higher concentrations of ammonium sulfate and binds to anion-exchange resins. We have previously discussed the possible direct involvement of fetal-like fibroblasts in cancer pathogenesis. The availability of MSF obtained from cancer patient fibroblasts provides a potential means with which to examine the complex cellular interactions contributing to this process as well as develop a screening regime for identifying individuals at elevated risk of developing cancer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antecol M. H., Darveau A., Sonenberg N., Mukherjee B. B. Altered biochemical properties of actin in normal skin fibroblasts from individuals predisposed to dominantly inherited cancers. Cancer Res. 1986 Apr;46(4 Pt 1):1867–1873. [PubMed] [Google Scholar]
- Azzarone B., Mareel M., Billard C., Scemama P., Chaponnier C., Macieira-Coelho A. Abnormal properties of skin fibroblasts from patients with breast cancer. Int J Cancer. 1984 Jun 15;33(6):759–764. doi: 10.1002/ijc.2910330608. [DOI] [PubMed] [Google Scholar]
- Caplan A. I., Fiszman M. Y., Eppenberger H. M. Molecular and cell isoforms during development. Science. 1983 Sep 2;221(4614):921–927. doi: 10.1126/science.6348946. [DOI] [PubMed] [Google Scholar]
- Castellani P., Siri A., Rosellini C., Infusini E., Borsi L., Zardi L. Transformed human cells release different fibronectin variants than do normal cells. J Cell Biol. 1986 Nov;103(5):1671–1677. doi: 10.1083/jcb.103.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodak G. W., Shing Y., Borge M., Judge S. M., Klagsbrun M. Presence of heparin binding growth factor in mouse bladder tumors and urine from mice with bladder cancer. Cancer Res. 1986 Nov;46(11):5507–5510. [PubMed] [Google Scholar]
- Deuel T. F. Polypeptide growth factors: roles in normal and abnormal cell growth. Annu Rev Cell Biol. 1987;3:443–492. doi: 10.1146/annurev.cb.03.110187.002303. [DOI] [PubMed] [Google Scholar]
- Durning P., Schor S. L., Sellwood R. A. Fibroblasts from patients with breast cancer show abnormal migratory behaviour in vitro. Lancet. 1984 Oct 20;2(8408):890–892. doi: 10.1016/s0140-6736(84)90653-6. [DOI] [PubMed] [Google Scholar]
- Haggie J. A., Sellwood R. A., Howell A., Birch J. M., Schor S. L. Fibroblasts from relatives of patients with hereditary breast cancer show fetal-like behaviour in vitro. Lancet. 1987 Jun 27;1(8548):1455–1457. doi: 10.1016/s0140-6736(87)92206-9. [DOI] [PubMed] [Google Scholar]
- Kopelovich L., Conlon S., Pollack R. Defective organization of actin in cultured skin fibroblasts from patients with inherited adenocarcinoma. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3019–3022. doi: 10.1073/pnas.74.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A., Pircher R., Krycève-Martinerie C., Jullien P. Normal embryo fibroblasts release transforming growth factors in a latent form. J Cell Physiol. 1984 Oct;121(1):184–188. doi: 10.1002/jcp.1041210123. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Mandler R., Murano G., Katz D. A., Gordon R. K., Chiang P. K., Schiffmann E. Tumor cell autocrine motility factor. Proc Natl Acad Sci U S A. 1986 May;83(10):3302–3306. doi: 10.1073/pnas.83.10.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura H., Hakomori S. The oncofetal domain of fibronectin defined by monoclonal antibody FDC-6: its presence in fibronectins from fetal and tumor tissues and its absence in those from normal adult tissues and plasma. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6517–6521. doi: 10.1073/pnas.82.19.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nakano S., Ts'o P. O. Cellular differentiation and neoplasia: characterization of subpopulations of cells that have neoplasia-related growth properties in Syrian hamster embryo cell cultures. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4995–4999. doi: 10.1073/pnas.78.8.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor S. L. Cell proliferation and migration on collagen substrata in vitro. J Cell Sci. 1980 Feb;41:159–175. doi: 10.1242/jcs.41.1.159. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Haggie J. A., Durning P., Howell A., Smith L., Sellwood R. A., Crowther D. Occurrence of a fetal fibroblast phenotype in familial breast cancer. Int J Cancer. 1986 Jun 15;37(6):831–836. doi: 10.1002/ijc.2910370606. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Schor A. M. Clonal heterogeneity in fibroblast phenotype: implications for the control of epithelial-mesenchymal interactions. Bioessays. 1987 Nov;7(5):200–204. doi: 10.1002/bies.950070503. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Schor A. M., Durning P., Rushton G. Skin fibroblasts obtained from cancer patients display foetal-like migratory behaviour on collagen gels. J Cell Sci. 1985 Feb;73:235–244. doi: 10.1242/jcs.73.1.235. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Schor A. M. Foetal-to-adult transitions in fibroblast phenotype: their possible relevance to the pathogenesis of cancer. J Cell Sci Suppl. 1987;8:165–180. doi: 10.1242/jcs.1987.supplement_8.9. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Schor A. M., Grey A. M., Rushton G. Foetal and cancer patient fibroblasts produce an autocrine migration-stimulating factor not made by normal adult cells. J Cell Sci. 1988 Jul;90(Pt 3):391–399. doi: 10.1242/jcs.90.3.391. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Schor A. M., Howell A., Crowther D. Hypothesis: persistent expression of fetal phenotypic characteristics by fibroblasts is associated with an increased susceptibility to neoplastic disease. Exp Cell Biol. 1987;55(1):11–17. doi: 10.1159/000163389. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Schor A. M., Rushton G. Fibroblasts from cancer patients display a mixture of both foetal and adult-like phenotypic characteristics. J Cell Sci. 1988 Jul;90(Pt 3):401–407. doi: 10.1242/jcs.90.3.401. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Schor A. M., Rushton G., Smith L. Adult, foetal and transformed fibroblasts display different migratory phenotypes on collagen gels: evidence for an isoformic transition during foetal development. J Cell Sci. 1985 Feb;73:221–234. doi: 10.1242/jcs.73.1.221. [DOI] [PubMed] [Google Scholar]
- Stoker M., Gherardi E., Perryman M., Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987 May 21;327(6119):239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]