Abstract
The role of γδ T cells in adaptive immunity remains uncertain. Recent studies have demonstrated that a unique subset of γδ T cells in primates can mount adaptive immune responses during mycobacterial infections. This Review discusses notable similarities and differences in adaptive immune responses between non-peptide-specific γδ T cells and peptide-specific αβ T cells, and discusses both the molecular basis for γδ T-cell responses and potential functions of these enigmatic cells.
γδ T cells are a minor T-cell population that express T-cell receptors (TCRs) comprised of γ and δ heterodimers. The γδ T-cell population can be separated into different subsets or subpopulations on the basis of their expression of particular Vγ or Vδ elements. These subsets can be further divided into two major groups according to tissue distribution and TCR diversity: resident γδ T cells with or without TCR diversity that take up residence in epithelia or mucosae of the organs, such as skin, lung, intestine, uterus, vagina and tongue; and circulating γδ T cells with substantial TCR diversity that are found in the blood and lymphoid tissues. Murine γδ T cells have important roles in immunity to infections, surveillance against tumors, immune regulation, modulating auto-immune responses and epithelial homeostasis [1–3]. By contrast, the roles of human γδ T cells in immune responses are poorly understood. Although human Vγ2Vδ2+ T cells recognize non-peptide phosphoantigens from bacteria and plants [4], little is known about the nature and functions of immune responses of antigen-specific γδ T cells. Recent studies have contributed considerably to our understanding of the immune biology and functions of resident and circulating γδ T cells. This Review discusses new hypotheses and discoveries concerning immunological features of γδ T cells and how they differ from αβ T cells.
Major γδ T-cell subsets in primates and their unique antigen specificity
The definition of antigens recognized by γδ T cells and the availability of appropriate animal models for their study are of central importance for elucidating the immune functions of human γδ T cells. Vγ2Vδ2+Tcells comprise the majority of circulating human γδ T cells and recognize non-peptide antigens from microbes and plants [4]. By contrast, Vδ1+ T cells are enriched in tissue mucosae and recognize self and foreign lipids presented by CD1 [5] and the stress-inducible MHC class I-related chains A and B (MICA and MICB) [6,7]. The non-peptide antigens recognized by Vγ2Vδ2+ T cells are prenyl pyrophosphates [4], bisphosphonates [8] and alkylamines [9]. Vγ2Vδ2+ T cells also recognize Staphylococcus enterotoxin A and canarypox antigens [4,10]. Interestingly, the canarypox-specific and Mycobacterium bovis Calmette–Guérin (BCG)-specific Vγ2Vδ2+ T cells have unique antigen specificity [4,10]. Despite the definition of these non-peptide antigens, studies of immune responses of Vγ2Vδ2+ T cells have been hindered by an absence of relevant animal models. Mice do not express a homologue of the Vγ2Vδ2+ TCR and there is no functional equivalent of these cells in rodents. Because non-human primates are genetically and biologically close to humans, macaques have been exploited as a model system in which to study the immune biology of γδ T cells. Non-human primates express human TCR homologs, including γδ TCR [11]. Macaque γ and δ genes encoding variable (V), joining (J) and constant (C) regions have been sequenced by us and others (Table 1) [12–15]. The macaque Vγ or Vδ elements can share ≤91% similarity in amino-acid sequences to their human counterparts, although the frequencies of Vγ+ and Vδ+ T-cell subsets are not the same as their human counterparts. Given the remarkable similarity in sequences between human and macaque γδ TCRs, it is predictable that macaque Vγ2Vδ2+ T cells can share antigen specificity with their human counterparts. In fact, macaque Vγ2Vδ2+Tcells can recognize prenyl pyrophosphonates, alkylamines and M. bovis BCG phosphoantigens as efficiently as human Vγ2Vδ2+ T cells [14–16]. It is important to note that only primate Vγ2Vδ2+ T cells, not γδ T cells from non-primate species, recognize the non-peptide antigens identified to date. Given their structural and functional similarities to human Vγ2Vδ2+ T cells, non-human primates should provide invaluable models to explore the diverse roles of microbe-specific γδ T cells in the immune response to infections as well as to test Vγ2Vδ2 T-cell vaccines.
Table 1.
γδ TCR genes in rhesus monkeysa
| γδ TCR genes in rhesus monkeys | % similarity in a.a. sequences to human counterparts | Cross-reactive human TCR antibodies (clone name) |
|---|---|---|
| Vδ1 | 84 | δTCS1; TS8-1E12 |
| Vδ2 | 87 | 15D |
| Vδ3 | 86 | P11.5B |
| Vδ4 | 84 (monkey Vδ4 is Vα6.2) | |
| Vδ5 | 83 (monkey Vδ5 is Vα21) | |
| Dδb | ||
| Jδ1 | >90 to J1 | |
| Jδ2 | >90 to J2 | |
| Jδ3 | >90 to J3 | |
| Cδ | 86 | anti-TCRδ1 |
| Vγ1.1;1.4 | 78 (Vγ1.1); 84 (Vγ1.4) | 23D12; 4A11(Vγ1.4) |
| Vγ2 (Vγ9) | 91 | 7A5 |
| VγIII (Vγ10)c | N/A | |
| Jγ1 | >75 to Jγ1.2 | |
| Jγ2 | >90 to Jγ2.3 | |
| Cγ | 93 |
Abbreviations: a.a., amino acid; TCR, T-cell receptor.
D and J gene segments are predicted from cDNA sequences.
Vγ10 is expressed only in non-human primates; it is inactivated in humans.
Adaptive immune responses of primate Vγ2Vδ2+ T cells
Unlike αβ T cells, the roles of γδ T cells in adaptive immunity remain unclear. It is attractive to suppose that Vγ2Vδ2+ T cells function as a bridge linking innate and adaptive immune responses, given that this subset of γδ T cells can recognize a broad spectrum of non-peptide antigens. It is also conceivable that Vγ2Vδ2+ T cells can mount adaptive immune responses during microbial infections because these unique γδ T cells resemble αβ T cells in their expression of diverse TCRs and have a conserved capacity to proliferate and expand following their TCR recognition of non-peptide antigens in culture [15]. To test this hypothesis, we have examined the role of the TCR in Vγ2Vδ2+ T cells in adaptive immunity using a Mycobacterium-infected macaque model. Following M. bovis BCG infection, macaque Vγ2Vδ2+ T cells expanded ≤25-fold in percentage and 200-fold in absolute numbers in the peripheral blood. Interestingly, a clear memory-type response of Vγ2Vδ2+ T cells was detected as early as 4–6 days after BCG re-infection and the magnitude of this expansion was 2–9-fold greater than that seen during primary BCG infection. The recall expansion of Vγ2Vδ2+ T cells persisted for as long as seven months after the second BCG inoculation. Importantly, some clonotypic Vδ2+ T cells exhibited clonal expansion during BCG infection and re-infection, suggesting that antigen-specific Vγ2Vδ2+ T cells participate in primary and memory immune responses [15]. Primary and recall expansions of Vγ2Vδ2+ T cells are also seen following Mycobacterium tuberculosis aerosol challenge of naïve and BCG-vaccinated macaques, respectively. The capacity to rapidly expand coincided with reduced M. tuberculosis burdens and immunity to fatal tuberculosis in BCG-vaccinated macaques [15]. These results provide evidence that Vγ2Vδ2+ T cells, as well as αβ+ T cells, contribute to the adaptive immune response in BCG and M. tuberculosis infections. Conventionally, the adaptive (memory) immune response of T cells is characterized by their antigen-specific persistence following an initial infection and the rapid and prolonged recall responses on re-infection or re-exposure to the same categories of antigens. The findings in monkey models demonstrate that Vγ2Vδ2+ T cells possess the functional memory of immune responses and suggest that Vγ2Vδ2+ T cells are involved in the immune control of mycobacterial infections.
The adaptive immune responses of Vγ2Vδ2+ T cells following mycobacterial infection of monkeys is similar to the γδ T-cell expansions detected during bacterial and parasitic infections in humans [17–36]. Some humans can exhibit an early and prolonged γδ T-cell expansion. It is difficult to determine in humans whether a γδ T-cell expansion identified during an infection represents a primary or recall response because a history of previous exposure of γδ T cells to non-peptide antigens or other infections is usually unknown. Given the typical course of primary and memory immune responses of macaque Vγ2Vδ2+ T cells in mycobacterial infections, an early and prolonged expansion of γδ T cells in humans might indeed represent a recall or memory response that results from a second exposure to non-peptide antigens during a particular infection. The kinetics and magnitude of the in vivo expansion of γδ T cells in primates with bacterial and parasitic infections appears to be similar to the enhanced ability of Vγ2Vδ2+ T cells of BCG-vaccinated humans to expand in vitro following stimulation with an M. tuberculosis lysate [37]. This marked in vitro expansion of Vγ2Vδ2+ T cells from BCG-vaccinated humans suggests that human γδ T cells can mount a memory-like response on exposure to non-peptide antigens [37]. The adaptive immune response of macaque Vγ2Vδ2+ T cells appears to be consistent with post-birth selection of predominant human Vγ2Vδ2+ T cells in the circulation. The dominance of human Vγ2Vδ2+ T cells is probably adapted by selection pressure derived from endogenous and exogenous environments. Phenotypic analyses of human γδ T cells also suggest that Vγ2Vδ2+ T cells can have effector or memory phenotypes based on their expression of the CD45RA and CD27 molecules. Central memory Vγ2Vδ2+ T cells are CD45RA−CD27+, whereas effector memory cells have lost the expression of CD27 costimulatory molecules and lack the proliferative potential of central memory cells [38]. Interestingly, the frequency of effector Vγ2Vδ2+ T cells are decreased in peripheral-blood mononuclear cells (PBMCs) of humans with pulmonary tuberculosis or active HIV-1 infection [38,39].
The magnitude of Vγ2Vδ2+T-cell expansions appears to be greater than that of αβ T cells during bacterial infections. The differences in the magnitudes of these expansions are particularly evident if the frequency of phosphoantigen-specific Vγ2Vδ2+ T cells is compared with those of CD4+ and CD8+ T cells specific for a single epitope or protein. During BCG infection or re-infection, interferon-γ (IFN-γ)-producing CD4+ or CD8+ T cells that are specific for a pool of overlapping 12mer peptides spanning Ag85B are usually less than 1–5/1000 T cells, shown by ELISpot or intracellular cytokine assays (Z.W. Chen et al., unpublished). By contrast, Vγ2Vδ2+ T cells comprise ≤30% of T cells in BCG-infected monkeys, ≤48% in patients with tularemia, samonellosis, and brucelosis [18–20] and 98% in those with ehrlichiosis [21]. In addition, the recall expansion of Vγ2Vδ2+ T cells can be seen as early as four days after BCG re-infection and lasts seven months to one year after resolution of active infection [15,40]. The longer duration of Vγ2Vδ2+ T-cell expansions compared with αβ T-cell expansions in individuals with bacterial infections suggests that these cells can more readily undergo expansions in response to repeated infections. The diverse TCR repertoire and capacity of Vγ2Vδ2 T cells to mount a memory response might indeed separate this γδ-cell subset from conventional innate immune cells. Finally, because phospholigands have been demonstrated in mycobacteria, Gram-negative rods, some Gram-positive cocci and parasitic protozoa, Vγ2Vδ2+ T cells might mount memory responses to a variety of pathogens after an initial infection with one of them. This notion is supported by the observation that humans pre-sensitized with BCG can exhibit a Vγ2Vδ2 T-cell expansion in response to in vitro stimulation with mycobacterial lysate containing phospholigands [37]. The remarkable breadth and magnitude of Vγ2Vδ2+ T-cell responses might, therefore, be unique in representing cross-reactive adaptive immunity to multiple microbes.
Migration of activated γδ T cells during adaptive immune responses
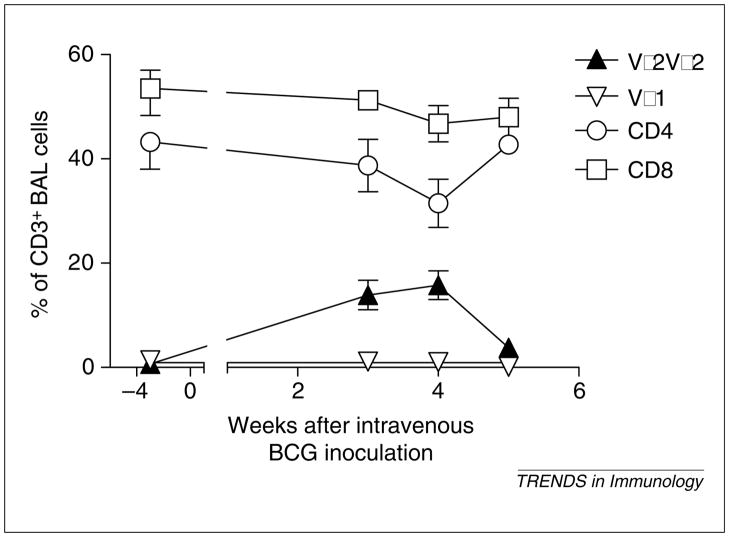
Most natural infections occur as a result of pathogen invasion through mucosae resulting from airborne, oral or sexually associated transmission. Recruiting immune cells to infected tissues is therefore an important defense mechanism for immune control of infection. In murine models of infectious diseases, the γδ T cells involved have been identified at local sites of infections but not in draining lymph nodes or spleen [1]. This contrasts with what has been seen for the homing of peptide-specific αβ T cells. Although the chemoattraction of leukocytes during inflammation has been studied, tissue trafficking and localization of antigen-specific γδ T cells in immune responses to infecting microbes are poorly characterized. We have recently demonstrated that rapid recall expansion of Vγ2Vδ2+ T cells is seen in bronchial alveolar lavage (BAL) fluid following M. tuberculosis aerosol challenge of BCG-vaccinated monkeys [15]. This expansion of Vγ2Vδ2+ T cells is associated with an inflammatory-cell response characterized by increased numbers of neutrophils and macrophages in BAL fluid. The accumulation of Vγ2Vδ2+ T cells in the lung is probably a result of the recruitment of these cells from the circulation as well as local clonal expansion after M. tuberculosis challenge. Interestingly, increases in the number of Vγ2Vδ2+ T cells are also apparent in pulmonary and intestinal mucosae when an expansion of these cells is seen in the blood of monkeys inoculated intravenously with BCG [15]. This increased number of Vγ2Vδ2+ T cells is particularly marked in the lung despite the fact that BCG loads in the lung are extremely low. In addition, no apparent inflammation can be seen in the pulmonary compartment following intravenous BCG inoculation (Z.W. Chen et al., unpublished). Surprisingly, larger increases in numbers of Vγ2Vδ2+ T cells than αβ T cells are evident in the lungs of the monkeys intravenously inoculated with BCG (Fig. 1). These results suggest that there might be a preferential migration of activated Vγ2Vδ2+ T cells to the lung from the circulation or lymphoid tissues after mycobacterial infection. We cannot exclude the possibility that local expansions of Vγ2Vδ2+ T cells also occur in the pulmonary compartment. Vγ2Vδ2+ T cells in the lung and blood of monkeys infected with M. tuberculosis or BCG express remarkably high levels of chemokine receptors CXCR3 and CCR5 (Z.W. Chen et al., unpublished). It is probable that the chemokine receptor-mediated trafficking in response to chemokines is responsible for transendothelial migration of immune cells to the lung from the circulation and/or lymphoid tissues. In fact, Glatzel et al. have recently shown that human Vδ2+ but not Vδ1+ or αβ TCR+ T cells express high levels of CCR5 and CXCR3 [41]. Cipriani et al. have confirmed the high expression of CCR5 and related CC chemokines in Vγ2Vδ2+ T cells [42]. Furthermore, the ligands for CCR5, such as macrophage inflammatory protein-1α (MIP-1α), MIP-1β and RANTES, have been shown to facilitate γδ T-cell migration in an in vitro migration system [43]. Because inflammation after mycobacterial infection is inevitably associated with an increased production of chemokines, these chemokines might have roles in transendothelial chemotaxis of Vγ2Vδ2+ T cells into the lung or other organs. Although the mechanisms responsible for this transendothelial migration have not been characterized, γδ T-cell-initiated migration pathways involving endothelial cells and/or stromal cells might regulate this process. Vδ2+ T cells can express the natural-killer (NK) receptor protein 1a (NKRP1a or CD161) and engagement of NKRP1a on Vδ2+ T cells results in activation of calcium calmodulin-dependent kinase II [44]. Interestingly, Vδ2+ T cells use NKRP1a for transmigration across endothelial monolayers in vitro and this transmigration relies on the calcium calmodulin-dependent kinase II pathway [44,45]. Interleukin-12 (IL-12) appears to enhance CD161 expression and transendothelial migration of Vγ2Vδ2 T cells [44]. The unique expression of chemokine receptors on γδ T cells might explain differences between αβ and γδ T cells in tissue trafficking and homing. Thus, these inflammatory cytokines, including the chemotaxis-associated chemokines, probably have a role in the tissue trafficking of γδ T cells during infections (Fig. 2).
Fig. 1.
Systemic Mycobacterium bovis Calmette–Guérin (BCG) infection introduced by intravenous inoculation can result in a preferential increase in Vγ2Vδ2+ T cells in bronchial alveolar lavage (BAL) fluid. Note the lack of increase in numbers of CD4+ and CD8+ T cells at the time the increase in Vγ2Vδ2+ T cells is identified. Shown are mean values with the error bars of standard error of the mean (SEM) from four animals.
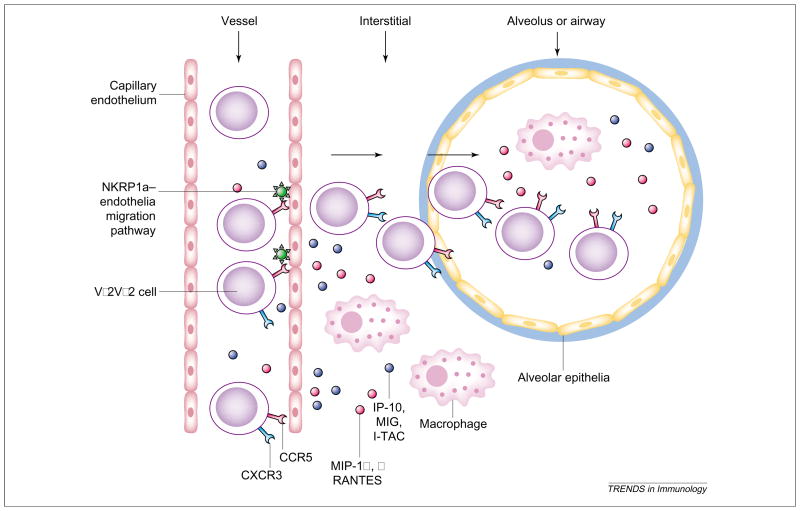
Fig. 2.
Proposed migration of Vγ2Vδ2 T cells to lungs or other epithelial compartments during infections. MIP-1α, MIP-1β and RANTES (ligands for CCR5) produced after infections in the pulmonary compartment might have a role in chemoattracting CCR5+ Vγ2Vδ2 T cells to the lung from the circulation or lymphoid tissues [41–43]. Because Vγ2Vδ2 T cells also express CXCR3, these cells are probably attracted by chemokines, such as MIG, IP-10 and I-TAC. The chemoattracting process and IL-12 might also involve the NKPR1a-mediated endothelia migration pathway [44–45]. It is also probable that chemokines produced in the blood by activated Vγ2Vδ2 T cells themselves or other immune cells initiate the transendothelial migration of these γδ T cells to the lung from the circulation during systemic infection or immune activation. Abbreviations: IL-12, interleukin-12; IP-10, interferon (IFN)-inducible protein 10; I-TAC, IFN-inducible T-cell α-chemoattractant; MIG, monokine induced by IFN-γ; MIP-1α, macrophage inflammatory protein-1α; NKRP1a, NK receptor protein 1a (CD161).
Distinct molecular basis of γδ T-cell responses
Differences between immune responses of Vγ2Vδ2+ T cells and αβ T cells during bacterial infections have already been described (Table 2) and distinct molecular characteristics might determine these differences. As discussed, these cell populations express different chemokine receptors and use different receptor-related signaling pathways. Hayes and Love have recently compared these cell populations through the components of their γδ TCR and their TCR-induced signal transduction [46]. They found that most γδ T cells but not αβ T cells express a γδ TCR complex that lacks a CD3δ unit. Moreover, following activation of γδ T cells, FcεR1 γ is expressed and included in the γδ TCR complex. Moreover, TCR-mediated signal transduction and proliferation for γδ T cells is superior to that seen in αβ T cells [46]. The efficient signal transduction initiated by γδ TCR engagement is consistent with the documented ability of Vγ2Vδ2+ T cells to expand rapidly in humans and monkeys following bacterial infection. Distinct signaling pathways of γδ T cells have also been suggested in the studies of transcriptional profiles of intestinal γδ intraepithelial lymphocytes (IELs) in mice.
Table 2.
Comparative biological features of Vγ2Vδ2, Vδ1–IELs and αβ T cells
| Vγ2Vδ2 T cells (in the circulation) | Human Vδ1 or murine γδ IELa | αβ T cells | Refs | |
|---|---|---|---|---|
| Development | Thymus | Thymus or extra-thymus | Thymus | [1,59] |
| TCR repertoire | Diverse | Not diverse for DECTs | Diverse | [1,2,15] |
| CD3 complex | No CD3δ for γδ PBL | [46] | ||
| Phenotypes | Similar to αβ T cells CD5+, CD28+, CD57− | Different from αβ T cells CD5−, CD28−, CD57+ | Typical T cells | [2,59,60] |
| Chemokine receptors (difference in expression) | CCR5+, CXCR3+ | CCR5−, CXCR1+ | CCR5−/+ | [41,42] |
| Migration-related molecules | CD161+, CD31− | CD161−, CD31+ | CD161 ±, CD31 ± | [44,45] |
| Innate function | Naïve expression of CTL-related genes and others | [47,48] | ||
| Production of cytokines or chemokines on activation | IFN-γ; TNF-α; RANTES; MIP-1α and β | Epithelial growth factor | IFN-γ, TNF-α, RANTES, MIP-1α and β | [38,42,53,54] |
| Antigen recognition | Non-peptide phosphoantigens from bacteria and plants | Self-reactive; CD1, MICA; ICB; TL | Peptide or lipid antigens | [4–7,60,61] |
| Antigen presentation | MHC-independent, antigen processing not required | MHC- or CD1-restricted, antigen processing required | [4] | |
| Precursor frequency for a single antigen | 0.5–5/100 CD3+ T cells | 1/2 × 105 CD8+ T cells | [62] | |
| Immune responses | Adaptive responses | No evidence of adaptive responses | Adaptive responses | [15,63] |
| Expansion magnitude in bacterial infections | 30–98% of CD3+ T cells | 0.1–0.5% for Ag85B-specific CD4+ T cells | [15,21] | |
| Expansion duration after resolution of infection | Can be longer than 7–12 months | Transient | [15,40] | |
| CTL effector function | Yes | Yes | [1,51,52] | |
| Non-immune function | ? | Wound repair by murine DETCs | [2] |
Abbreviations: CTL, cytotoxic T lymphocyte; CCR5, chemokine C receptor 5; DETCs, dendritic epidermal T cells; IELs, intraepithelial lymphocytes; IFN-γ, interferon-γ; MICA, stress-inducible MHC class I-related chains A; MIP, macrophage inflammation protein; PBL, peripheral-blood leukocytes; TCR, T-cell receptor; TL, thymus leukemia antigen; TNF-α, tumor necrosis factor-α.
Fahrer and colleagues [47] have recently reported that γδ IELs do not express transcripts for certain key signaling proteins used by αβ T cells. In addition, γδ IELs can constitutively express cytotoxic effector genes, such as granzymes A and B, and the inflammatory chemokine RANTES. By contrast, lymph node CD8+ αβ T cells must be activated to express those cytotoxic effector genes. Interestingly, NK inhibitory and activating genes are also constitutively expressed by γδ IELs [47]. Studies reported by Shires et al. show the naïve expression of high levels of granzymes and Fas ligand, chemokines, anti-proliferative genes Slfn2, Btg1 and Btg2, and some signaling-related genes, Rgs1 and Junb, in γδ IELs, although there appears to be no difference in expression of those genes between γδ IELs and αβ IELs in naïve mice [48]. These findings at both protein and transcriptional levels support the presumption that there are fundamental differences in some phenotypes and signal transduction machineries of γδ T cells and αβ T cells. Molecular differences might enable these two T-cell populations to function differently in innate and adaptive immune responses. In fact, engagement of NKG2D on Vγ2Vδ2+ T cells by MICA augments their effector function in response to antigens [49], although it is not known whether this also occurs in the setting of αβ T cells. Killer Ig-like receptors (KIRs) might also be involved in the immune regulation of Vγ2Vδ2+ T cells because KIRs expressed on different γδ or αβ T-cell subsets can regulate innate and adaptive immune responses of these cells [50]. It would be useful to compare the molecular differences in TCR complexes, NK receptors, signaling kinases and chemokine and cytokine production between non-peptide-specific Vγ2Vδ2+ T cells and peptide-specific αβ T cells during immune responses to microbial infections.
Potential roles of Vγ2Vδ2+ T cells in antimicrobial immunity
Vγ2Vδ2+ T cells that expand during bacterial or parasitic infections could contribute to adaptive immunity in various ways. Activated and expanded Vγ2Vδ2+ T cells might directly participate in antimicrobial immune responses. Vγ2Vδ2+ T cells kill bacteria-infected cells and bacteria [51,52], although this effector function detected in vitro might not reflect the in vivo function of these cells. On activation, Vγ2Vδ2+ T cells can produce a large amount of IFN-γ and tumor necrosis factor-α (TNF-α) [38,53,54], the cytokines important for controlling mycobacterial infection in mice. Through production of those Th1 cytokines, Vγ2Vδ2+ T cells could function as a link connecting innate and adaptive immune systems and facilitate the development of adaptive immune responses of antigen-specific αβ T cells. In fact, Vγ2Vδ2+ T cells activated by non-peptide antigens are able to react quickly and protect SCID (severe combined immunodeficiency) mice from bacterial infections by reducing bacterial numbers [55]. The impact of γδ T cells on innate immune cells, such as macrophages and NK cells, has also been reported in murine models [1]. Mice deficient in γδ T cells develop enhanced inflammation characterized by disruption of macrophage homeostasis and liver necrosis. In the absence of γδ T cells, IFN-γ production by NK cells is reduced, which leads to a delay in granuloma formation and an increase in bacterial growth [56]. The γδ-deficient mice lack the whole population of γδ T cells, including those subsets with ‘innate’ phenotypes and, therefore, the results observed in these animals might not represent the potential roles of Vγ2Vδ2+ T cells. However, human Vγ2Vδ2+ T cells might reserve some of those functions exerted by murine γδ T cells. Vγ2Vδ2+ T cells might also regulate immune functions of other immune cells, such as dendritic cells and B cells [57,58], given their ability to produce various cytokines. Finally, non-peptide antigen-specific γδ T cells could contribute to anti-inflammatory function or tissue repair during infection and disease. This possibility is supported by the recent novel observation that resident γδ T cells can have unique functions in epithelia or tissue homeostasis [2]. Murine γδ T cells in the skin can be activated at wound sites and produce cytokines, including keratinocyte growth factors (KGFs) that participate in wound repair. In the absence of skin γδ T cells, there are defects in keratinocyte proliferation and tissue re-epithelialization following tissue damage [2]. It is conceivable that Vγ2Vδ2+ T cells, once activated locally or recruited to tissue compartments, could participate in tissue repair or wound healing after tissue damage that occurs as a result of active infections and inflammation. The fact that the expansion of Vγ2Vδ2+Tcells is so prolonged after the resolution of bacterial infections in primates [15] suggests that these γδ T cells might contribute to anti-inflammatory functions rather than simply contributing to the clearance of microbes.
Concluding remarks
In conclusion, primate Vγ2Vδ2+ T cells, as well as αβ+ T cells, can contribute to adaptive immune responses in microbial infections, despite the fact that these two T-cell populations differ in many of their biological characteristics. The non-peptide phosphoantigen-specific Vγ2Vδ2+ T cells appear to differ from peptide-specific αβ T cells in their antigen recognition and the magnitude of their immune responses. The unique ability of Vγ2Vδ2+ T cells to expand during mycobacterial infections suggests that vaccine-elicited Vγ2Vδ2+ T-cell immunity might prove beneficial. Vγ2Vδ2+ T cells might broadly contribute to both innate and acquired immunity against microbial infections.
Acknowledgments
We acknowledge the financial support of the National Institute of Health (HL64560 and RR13601, to Z.W.C.), and technical assistance from members at the laboratory of Z.W.C.
References
- 1.Carding SR, Egan PJ. γδ T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 2.Jameson J, et al. A role for skin γδ T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 3.Born W, et al. Immunoregulatory functions of γδ T cells. Adv Immunol. 1999;71:77–144. [PubMed] [Google Scholar]
- 4.Morita CT, et al. Antigen recognition by human γδ T cells: pattern recognition by the adaptive immune system. Springer Semin Immunopathol. 2000;22:191–217. doi: 10.1007/s002810000042. [DOI] [PubMed] [Google Scholar]
- 5.Spada FM, et al. Self-recognition of CD1 by γδ T cells: implications for innate immunity. J Exp Med. 2000;191:937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groh V, et al. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, et al. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial γδ T cells. J Immunol. 2002;169:1236–1240. doi: 10.4049/jimmunol.169.3.1236. [DOI] [PubMed] [Google Scholar]
- 8.Kunzmann V, et al. γδ T-cell stimulation by pamidronate. N Engl J Med. 1999;340:737–738. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 9.Bukowski JF, et al. Human γδ T cells recognize alkylamines derived from microbes, edible plants and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 10.Worku S, et al. Canarypox vaccines induce antigen-specific human γδ T cells capable of interferon-γ production. J Infect Dis. 2001;184:525–532. doi: 10.1086/322792. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZW, et al. Conserved T-cell receptor repertoire in simian immunodeficiency virus-infected rhesus monkeys. J Immunol. 1993;151:2177–2187. [PubMed] [Google Scholar]
- 12.Rakasz E, et al. γδ T-cell receptor repertoire in blood and colonic mucosa of rhesus macaques. J Med Primatol. 2000;29:387–396. doi: 10.1111/j.1600-0684.2000.290602.x. [DOI] [PubMed] [Google Scholar]
- 13.MacDougall A, et al. Vγ2 TCR repertoire overlap in different anatomical compartments of healthy, unrelated rhesus macaques. J Immunol. 2001;166:2296–2302. doi: 10.4049/jimmunol.166.4.2296. [DOI] [PubMed] [Google Scholar]
- 14.Daubenberger CA, et al. Functional and structural similarity of Vγ9Vδ2 T cells in humans and aotus monkeys, a primate infection model for Plasmodium falciparum malaria. J Immunol. 2001;167:6421–6430. doi: 10.4049/jimmunol.167.11.6421. [DOI] [PubMed] [Google Scholar]
- 15.Shen Y, et al. Adaptive immune response of Vγ2Vδ2+ T cells during mycobacterial infections. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans PS, et al. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vγ2-Jγ1.2/Vδ2 T-cell receptors. Immunology. 2001;104:19–27. doi: 10.1046/j.0019-2805.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumida T, et al. Predominant expansion of Vγ9Vδ2 T cells in a tularemia patient. Infect Immun. 1992;60:2554–2558. doi: 10.1128/iai.60.6.2554-2558.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hara T, et al. Predominant activation and expansion of Vγ9-bearing γδ T cells in vivo as well as in vitro in Salmonella infection. J Clin Invest. 1992;90:204–210. doi: 10.1172/JCI115837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poquet Y, et al. Expansion of Vγ9Vδ2 T cells is triggered by Francisella tularensis-derived phosphoantigens in tularemia but not after tularemia vaccination. Infect Immun. 1998;66:2107–2114. doi: 10.1128/iai.66.5.2107-2114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertotto A, et al. Lymphocytes bearing the γδ T-cell receptor in acute Brucella melitensis infection. Eur J Immunol. 1993;23:1177–1180. doi: 10.1002/eji.1830230531. [DOI] [PubMed] [Google Scholar]
- 21.Caldwell CW, et al. Lymphocytosis of γδ T cells in human ehrlichiosis. Am J Clin Pathol. 1995;103:761–766. doi: 10.1093/ajcp/103.6.761. [DOI] [PubMed] [Google Scholar]
- 22.Modlin RL, et al. Lymphocytes bearing antigen-specific γδ T-cell receptors accumulate in human infectious disease lesions. Nature. 1989;339:544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- 23.Dieli F, et al. Predominance of Vγ9/Vδ2 T lymphocytes in the cerebrospinal fluid of children with tuberculous meningitis: reversal after chemotherapy. Mol Med. 1999;5:301–312. [PMC free article] [PubMed] [Google Scholar]
- 24.Ueta C, et al. Increase of γδ T cells in hospital workers who are in close contact with tuberculosis patients. Infect Immun. 1994;62:5434–5441. doi: 10.1128/iai.62.12.5434-5441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balbi B, et al. T-lymphocytes with γδ+ Vδ2+ antigen receptors are present in increased proportions in a fraction of patients with tuberculosis or with sarcoidosis. Am Rev Respir Dis. 1993;148:1685–1690. doi: 10.1164/ajrccm/148.6_Pt_1.1685. [DOI] [PubMed] [Google Scholar]
- 26.Ito M, et al. Increased proportions of peripheral blood γδ T cells in patients with pulmonary tuberculosis. Chest. 1992;102:195–197. doi: 10.1378/chest.102.1.195. [DOI] [PubMed] [Google Scholar]
- 27.Schneider T, et al. The number and proportion of Vγ9Vδ2 T cells rise significantly in the peripheral blood of patients after the onset of acute Coxiella burnetii infection. Clin Infect Dis. 1997;24:261–264. doi: 10.1093/clinids/24.2.261. [DOI] [PubMed] [Google Scholar]
- 28.Jouen-Beades F, et al. In vivo and in vitro activation and expansion of γδ T cells during Listeria monocytogenes infection in humans. Infect Immun. 1997;65:4267–4272. doi: 10.1128/iai.65.10.4267-4272.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raziuddin S, et al. γδ T lymphocytes and proinflammatory cytokines in bacterial meningitis. J Allergy Clin Immunol. 1994;93:793–798. doi: 10.1016/0091-6749(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 30.Russo DM, et al. Antigen-reactive γδ T cells in human leishmaniasis. J Immunol. 1993;151:3712–3718. [PubMed] [Google Scholar]
- 31.Ho M, et al. Increased γδ T cells in acute Plasmodium falciparum malaria. Immunol Lett. 1990;25:139–141. doi: 10.1016/0165-2478(90)90105-y. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz E, et al. Delayed expansion of Vδ2+ and Vδ1+ γδ T cells after acute Plasmodium falciparum and Plasmodium vivax malaria. J Allergy Clin Immunol. 1996;97:1387–1392. doi: 10.1016/s0091-6749(96)70208-7. [DOI] [PubMed] [Google Scholar]
- 33.Roussilhon C, et al. T lymphocytes bearing the γδ T-cell receptor in patients with acute Plasmodium falciparum malaria. J Infect Dis. 1990;162:283–285. doi: 10.1093/infdis/162.1.283-a. [DOI] [PubMed] [Google Scholar]
- 34.Raziuddin S, et al. γδ T cells and the immune response in visceral leishmaniasis. Eur J Immunol. 1992;22:1143–1148. doi: 10.1002/eji.1830220506. [DOI] [PubMed] [Google Scholar]
- 35.Scalise F, et al. Lymphocytes bearing the γδ T-cell receptor in acute toxoplasmosis. Immunology. 1992;76:668–670. [PMC free article] [PubMed] [Google Scholar]
- 36.Perera MK, et al. Transient increase in circulating γδ T cells during Plasmodium vivax malarial paroxysms. J Exp Med. 1994;179:311–315. doi: 10.1084/jem.179.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoft DF, et al. Bacille Calmette-Guerin vaccination enhances human γδ T-cell responsiveness to Mycobacteria suggestive of a memory-like phenotype. J Immunol. 1998;161:1045–1054. [PubMed] [Google Scholar]
- 38.Gioia C, et al. Lack of CD27−CD45RA−Vγ9Vδ2+ T-cell effectors in immunocompromised hosts and during active pulmonary tuberculosis. J Immunol. 2002;168:1484–1489. doi: 10.4049/jimmunol.168.3.1484. [DOI] [PubMed] [Google Scholar]
- 39.Martini F, et al. Acute human immunodeficiency virus replication causes a rapid and persistent impairment of Vγ9Vδ2 T cells in chronically infected patients undergoing structured treatment interruption. J Infect Dis. 2002;186:847–850. doi: 10.1086/342410. [DOI] [PubMed] [Google Scholar]
- 40.Kroca M, et al. The proportion of circulating γδ T cells increases after the first week of onset of tularaemia and remains elevated for more than a year. Clin Exp Immunol. 2000;120:280–284. doi: 10.1046/j.1365-2249.2000.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glatzel A, et al. Patterns of chemokine receptor expression on peripheral-blood γδ T lymphocytes: strong expression of CCR5 is a selective feature of Vδ2Vγ9 γδ T cells. J Immunol. 2002;168:4920–4929. doi: 10.4049/jimmunol.168.10.4920. [DOI] [PubMed] [Google Scholar]
- 42.Cipriani B, et al. Activation of C–C β-chemokines in human peripheral-blood γδ T cells by isopentenyl pyrophosphate and regulation by cytokines. Blood. 2000;95:39–47. [PubMed] [Google Scholar]
- 43.Roth SJ, et al. Transendothelial chemotaxis of human αβ and γδ T lymphocytes to chemokines. Eur J Immunol. 1998;28:104–113. doi: 10.1002/(SICI)1521-4141(199801)28:01<104::AID-IMMU104>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 44.Poggi A, et al. Transendothelial migratory pathways of Vδ1+TCR γδ+ and Vδ2+TCR γδ+ T lymphocytes from healthy donors and multiple sclerosis patients: involvement of phosphatidylinositol 3 kinase and calcium calmodulin-dependent kinase II. J Immunol. 2002;168:6071–6077. doi: 10.4049/jimmunol.168.12.6071. [DOI] [PubMed] [Google Scholar]
- 45.Poggi A, et al. IL-12-mediated NKRP1A upregulation and consequent enhancement of endothelial transmigration of Vδ2+ TCR γδ+ T lymphocytes from healthy donors and multiple sclerosis patients. J Immunol. 1999;162:4349–4354. [PubMed] [Google Scholar]
- 46.Hayes SM, Love PE. Distinct structure and signaling potential of the γδ TCR complex. Immunity. 2002;16:827–838. doi: 10.1016/s1074-7613(02)00320-5. [DOI] [PubMed] [Google Scholar]
- 47.Fahrer AM, et al. Attributes of γδ intraepithelial lymphocytes as suggested by their transcriptional profile. Proc Natl Acad Sci U S A. 2001;98:10261–10266. doi: 10.1073/pnas.171320798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shires J, et al. Biological insights into TCR γδ+ and TCR αβ+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE) Immunity. 2001;15:419–434. doi: 10.1016/s1074-7613(01)00192-3. [DOI] [PubMed] [Google Scholar]
- 49.Das H, et al. MICA engagement by human Vγ2Vδ2 T cells enhances their antigen-dependent effector function. Immunity. 2001;15:83–93. doi: 10.1016/s1074-7613(01)00168-6. [DOI] [PubMed] [Google Scholar]
- 50.Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol. 2002;20:217–251. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- 51.Dieli F, et al. Vγ9/Vδ2 T lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. Eur J Immunol. 2000;30:1512–1519. doi: 10.1002/(SICI)1521-4141(200005)30:5<1512::AID-IMMU1512>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 52.Ottones F, et al. V γ9V δ2 T cells impair intracellular multiplication of Brucella suis in autologous monocytes through soluble factor release and contact-dependent cytotoxic effect. J Immunol. 2000;165:7133–7139. doi: 10.4049/jimmunol.165.12.7133. [DOI] [PubMed] [Google Scholar]
- 53.Garcia VE, et al. Single-cell cytokine analysis of γδ T-cell responses to non-peptide mycobacterial antigens. J Immunol. 1997;159:1328–1335. [PubMed] [Google Scholar]
- 54.Wang L, et al. Human Vγ2Vδ2 T cells produce IFN-γ and TNF-α with an on/off/on cycling pattern in response to live bacterial products. J Immunol. 2001;167:6195–6201. doi: 10.4049/jimmunol.167.11.6195. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, et al. Antibacterial effect of human Vγ2Vδ2 T cells in vivo. J Clin Invest. 2001;108:1349–1357. doi: 10.1172/JCI13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ladel CH, et al. Control of natural killer cell-mediated innate resistance against the intracellular pathogen Listeria monocytogenes by γδ T lymphocytes. Infect Immun. 1996;64:1744–1749. doi: 10.1128/iai.64.5.1744-1749.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moretto M, et al. γδ T-cell-deficient mice have a downregulated CD8+ T-cell immune response against Encephalitozoon cuniculi infection. J Immunol. 2001;166:7389–7397. doi: 10.4049/jimmunol.166.12.7389. [DOI] [PubMed] [Google Scholar]
- 58.Rhodes SG, et al. Antigen recognition and immunomodulation by γδ T cells in bovine tuberculosis. J Immunol. 2001;166:5604–5610. doi: 10.4049/jimmunol.166.9.5604. [DOI] [PubMed] [Google Scholar]
- 59.De Rosa SC, et al. Vδ1 and Vδ2 γδ T cells express distinct surface markers and might be developmentally distinct lineages. J Leukoc Biol. 2001;70:518–526. [PubMed] [Google Scholar]
- 60.Hayday A, et al. Intraepithelial lymphocytes: exploring the third way in immunology. Nat Immun. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 61.Crowley MP, et al. A population of murine γδ T cells that recognize an inducible MHC class Ib molecule. Science. 2000;287:314–316. doi: 10.1126/science.287.5451.314. [DOI] [PubMed] [Google Scholar]
- 62.Blattman JN, et al. Estimating the precursor frequency of naïve antigen-specific CD8 T cells. J Exp Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith AL, Hayday AC. An αβ T-cell-independent immunoprotective response towards gut coccidia is supported by γδ cells. Immunology. 2000;101:325–332. doi: 10.1046/j.1365-2567.2000.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]