Abstract
BACKGROUND
Injury of a spinal nerve or dorsal root ganglion (DRG) during selective spinal nerve blocks is a potentially serious complication that has not been adequately investigated. Our hypothesis was that local anesthetic injection into these structures may result in an inflammatory response and hyperalgesia.
METHODS
We evaluated inflammatory and behavioral responses after injection of 4 μL lidocaine or saline into the L5 spinal nerve or DRG of rats after partial laminectomy. Behavioral testing was performed before and after surgery to examine hyperalgesia in response to nociceptive mechanical stimulation of the foot. DRGs were harvested and stained, and rings of immunoreactive glial cells around neurons were counted.
RESULTS
Animals demonstrated hyperalgesia on the ipsilateral paw up to 4 days after lidocaine injection into the DRG but not after injection into the spinal nerve. The number of glial fibrillary acid protein immunopositive glial cell rings, which represent activation of satellite cells, significantly increased in DRGs after injection of lidocaine into either the DRG or the spinal nerve. The number of glial fibrillary acid protein-positive cells in the lidocaine-injected group was significantly larger than in the saline-injected group. Sporadic OX-42 immunopositive cells, which represent activated microglia, were also seen in lidocaine-injected DRGs. Testing for Pan-T expression, which labels activated T lymphocytes, showed no positive cells.
CONCLUSIONS
Lidocaine injection into the DRG may produce hyperalgesia, possibly due to activation of resident satellite glial cells. In a clinical setting, local anesthetic injection into the DRG should be avoided during selective spinal nerve blocks.
Selective spinal nerve blocks (SSNBs) have been used for diagnostic purposes in patients with chronic back pain, as well as to manage cancer and back pain.1–3 Local anesthetics are injected during SSNB and have been found to be neurotoxic after more peripheral nerve injections.4–6 In contrast, injection of saline into peripheral nerves produces minimal changes.7,8
During SSNB, a needle is inserted into the intervertebral foramen,2 where the dorsal root ganglion (DRG) and ventral root converge to form the spinal nerve. Therefore, both spinal nerve and DRG can be traumatized by the needle and be permeated with local anesthetic during SSNBs. In 12.8% of lumbar SSNBs, the needle tip is positioned in the mediocranial quadrant of the intervertebral foramen, where the DRG is the most frequently located.9 Even with the use of radiological imaging, injections within the DRG are common during clinical SSNB.10
Injections into nervous tissues can cause discomfort. However, there are reports with what seem to be direct nerve injections in patients who were lightly sedated, or not sedated at all, and the patients did not report any pain in the course of the local anesthetic injection.11–14 Absence of an acute reaction in injected individuals may prevent them from seeking help immediately and lead to development of later consequences.
DRG neurons convey information from peripheral sensory receptors to the central nervous system but, after peripheral nerve and DRG trauma or inflammation, they can also become an important source of increased nociceptive signaling through increased neuronal excitability and generation of ectopic discharges.15,16 It has been reported that topical application of local anesthetic to the DRG induces mechanical hyperalgesia in the rat,17 but possible morphological changes in the DRG have not been studied. Whereas the histopathological and behavioral changes after peripheral nerve injection injury have been well investigated, there is little known about the consequences of injection within the DRG or adjacent spinal nerve. Our study was motivated by the increasing use of SSNBs for diagnosis and treatment, and because there are important structural and functional differences between the DRG and peripheral nerves.18 For instance, the DRG lacks a functional barrier between its interior milieu and both the blood and surrounding tissue,19 which could alter the inflammatory response to injected materials. We therefore evaluated the neuroinflammatory response in rat DRGs after lidocaine or saline injection into nerve and DRG. We hypothesized that an inflammatory response may follow local anesthetic injection into the DRG and produce hyperalgesia.
METHODS
Experimental Animals
All experimental protocols were approved by the Ethics Committee of the University of Split, School of Medicine. Sixty-two Sprague-Dawley rats weighing 150−200 g were used. Rats were randomly assigned to a group that had no surgery (control, n = 14), and groups in which lidocaine was injected into the DRG (DRG lidocaine, n = 22) or spinal nerve (nerve lidocaine, n = 7). In additional groups, saline was injected into the DRG (DRG saline, n = 13) or spinal nerve (nerve saline, n = 6). In lidocaine and saline groups of DRG injections, rats were killed after the 5th (early response group, n = 10 for lidocaine and n = 4 for saline injection) or 16th postoperative day (late response group, n = 12 for lidocaine and n = 9 for saline injection).
Surgery
Animals were anesthetized with isoflurane (Forane®, Abbott laboratories, Queenborough, UK) (3% in oxygen for induction, 2% for maintenance). An incision was made along the midline of the back and the right paravertebral region was exposed. Right paraspinal muscles were separated from transverse process at the L5 spinal level until the right L5 intervertebral foramen could be identified. For DRG and nerve injection, the L5 nerve and DRG were exposed using a micro bone rongeurs. Where indicated, the L5 DRG or the L5 nerve 5 mm distal to the ganglion was injected with 4 μL of clinically used and commercially available lido-caine (Lidokain 2%, Belupo d.d., Koprivnica, Croatia; pH = 6.0−7.0, osmolality = 311 mOsmol/kg, preservative free—data obtained from manufacturer) or 0.9% saline which duplicates the physical properties of lidocaine solution (pH 6.7−7.3, osmolality approximately 300 mOsml/kg) using a 29-gauge needle (BD Micro-Fine™ Plus, Becton Dickinson Insulin Syringe, Dublin, Ireland) with a beveled tip. The DRG injection was performed with about a 60 degree angle relative to the spine. The injected dose was chosen based on the fact that 4 μL volume in a 1 mg rat DRG corresponds to the clinically relevant injection of the 4 mL intoa1ghuman DRG.
Behavioral Testing
Behavioral testing was performed preoperatively and 1, 4, 8, and 15 days after surgery, using a method we have validated for identification of rats with neuropathic pain.20 The plantar skin of each hindpaw of unrestrained rats was stimulated with a 22-gauge spinal anesthesia needle (Quincke tip) with enough pressure to indent but not puncture the skin. We tabulated the frequency of hyperalgesia-type responses (sustained shaking, licking, grooming, and/or chewing of the paw) to this noxious mechanical stimulation, which are clearly distinct from the normal quick reflexive withdrawal. The degree of hyperalgesia was expressed as the difference between the probability of hyperalgesia response of the right and left paw for each animal. Additional behavioral testing was performed immediately after lidocaine injection to validate accuracy and completeness of injection.
Immunohistochemistry
One day after sensory testing rats were anesthetized with isoflurane and killed. For immunohisto-chemistry, both right and left L5 ganglia, along with the contiguous part of the spinal nerve (approximately 5 mm), were harvested on 5th day or the 16th day. For paraffin sections, the DRGs were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (PBS, pH 7.4), embedded in paraffin wax and 5 μm thick serial sections were cut transversally.
We used primary antibodies for anti-glial fibrillary acid protein antibody (GFAP Rabbit Polyclonal Antibody, Chemicon International, Temecula, CA) diluted 1:250, OX-42 antibody (Mouse antirat CD11B Monoclonal Antibody, Chemicon International, Temecula, CA) in dilution 1:250, and antirat Pan-T cells CBL 1503 (Chemicon Europe, Hampshire, UK) diluted 1:100. For secondary detection, we used corresponding LSAB + System-HRP kits (Dako Cytomation, Glostrup, Denmark).
During quantification, the investigator was blind to the source of the tissue. A conservative analysis design was used in which each animal provided a single data point. Specifically, for each section examined (6−8 sections per DRG), 2−4 images were acquired by digital camera Olympus DP-71 (Olympus America, Mellville, NY) using the same magnification (40×) and display settings. On each micrograph, we then superimposed 1 square (200 × 200 μm) in which at least 80% of the surface was occupied by ganglion cells. For evaluation of GFAP staining, we counted the total number of neurons per square, as well as the number of neurons surrounded with rings of GFAP-immunoreactive satellite cells. We counted only those cells with more than 50% of circumference containing GFAP-positive satellite cells. The number of neurons surrounded with those rings was expressed as a percentage of all neurons. Using this methodology, we obtained between 120 and 177 data points for each rat. These values were averaged and used for statistical analysis to evaluate the between-rat main effect.
Statistical Analysis
Behavioral test scores and immunopositive cell counts were analyzed using analysis of variance with post hoc Newman-Keuls test (Statistica, StatSoft, Tulsa). Data are presented as mean ± sem. Statistical significance was set at P < 0.05.
RESULTS
DRG Lidocaine Injection Produces Pain-Related Behavior
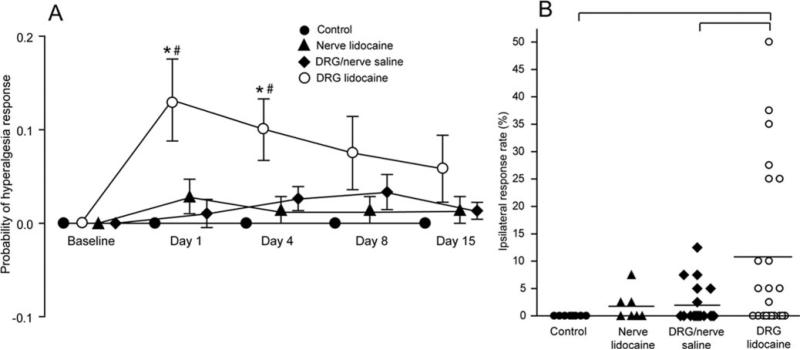
Analysis of the hyperalgesia-type response rate revealed a significant increase on the first two postoperative testing sessions in rats that had injections into the DRG with lidocaine in comparison with both control and saline-injected rats (F(4,80) = 3.09; P = 0.02) (Fig. 1). No other group showed any difference from control values. Since there were no differences in behavior between rats that had injections into the L5 DRG and nerve, we merged these groups.
Figure 1.
A, Postinjection probability of a hyperalgesia-type behavioral response to nociceptive mechanical stimulation. Each value represents the mean difference between the right and left paws (mean ± sem). *Difference from control value on corresponding day; #difference from saline-injected rats. B, Ipsilateral response rate represents the injury effect after subtracting the contralateral side and averaging four postoperative testing sessions. Bracket denotes statistically significant difference between groups.
All animals injected with saline showed normal sensory and motor behavior. The rats injected with lidocaine showed no responses to nociceptive mechanical stimulation immediately after DRG or spinal nerve injection, demonstrating accurate and complete delivery of lidocaine to the DRG and spinal nerve. Impaired motor behavior attributable to lidocaine injection was observed immediately after recovery from anesthesia, however, this resolved within 2 h, after which no signs of impairment could be found.
DRG Lidocaine Injection Produces Inflammation
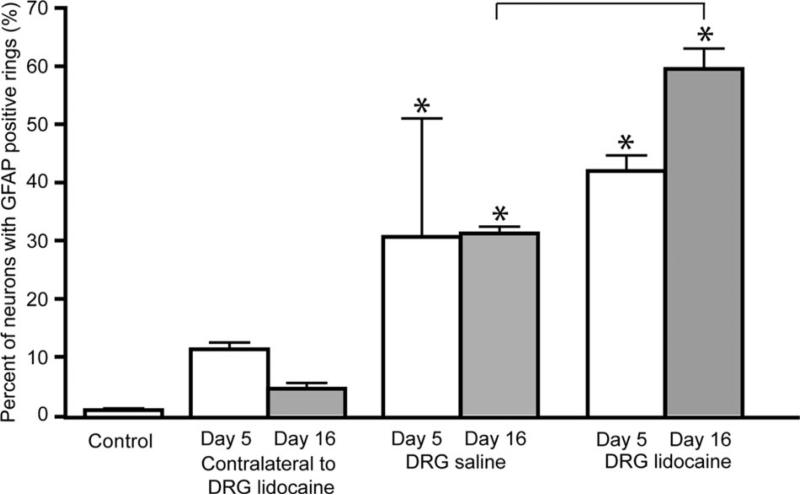
Perineuronal satellite glia in injected DRGs were immunoreactive for GFAP, which was exhibited as rings of stained satellite cells around neurons. These rings, which represent activation of satellite glial cells resident in the DRG,21 were present in statistically larger numbers in lidocaine-injected DRGs on day 5 (43% ± 2.6%) and day 16 (59.5% ± 3.5%) after surgery, compared to control (0.08% ± 0.04%; Newman-Keuls test, q = 5.4, P < 0.05 at day 5; q = 35.7, P < 0.001 at day 16) (Fig. 2). A comparison of the lidocaine-injected group with the saline-injected group (32% ± 3.5%, Newman-Keuls test, q = 15.7, P < 0.001) revealed significant differences only at 16 days (Fig. 2).
Figure 2.
Percent of dorsal root ganglion (DRG) neurons surrounded with rings of activated satellite cells in control and experimental groups after DRG injection. Data are presented as mean ± sem. *Asterisk denotes difference from control group; bracket—significant difference between saline and lidocaine-injected groups. Differences were evaluated by analysis of variance with post hoc Newman-Keuls test.
The appearance of activated satellite cell rings was similar in all DRGs with GFAP immunoreactivity (Figs. 3B and C). The ratio of DRG neurons surrounded with GFAP-positive satellite cell rings was not significantly increased in rats injected with saline. No differences in staining were observed for small versus large neurons.
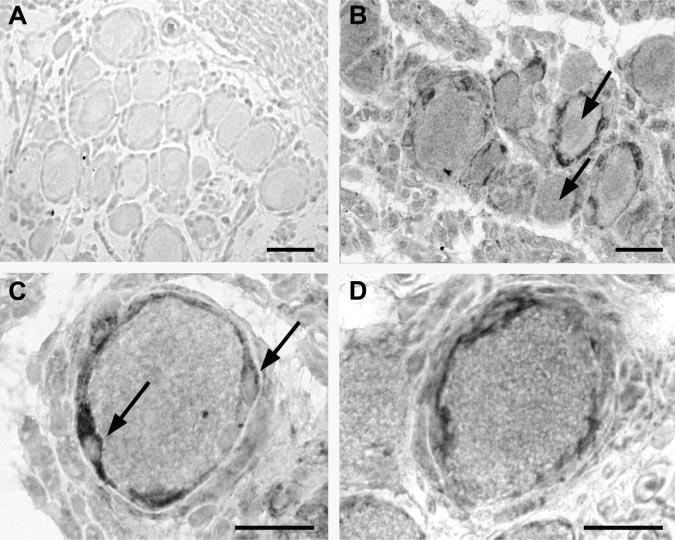
Figure 3.
Glial fibrillary acid protein (GFAP) and OX-42 immunoreactivity after lidocaine injection into the dorsal root ganglion (DRG). A, Micrograph of the GFAP-stained DRG tissue from an uninjected control animal; B, GFAP-stained section of a DRG after DRG lidocaine injection. Arrows mark one neuron with positive and one with negative GFAP ring; C, DRG from the lidocaine-injected DRG group, showing a neuron surrounded with a ring of GFAP-positive satellite cells. Arrows mark thickening of the satellite cells at the cell nucleus; D, OX-42 staining of the section from lidocaine-injected DRG. All microphotographs were contrasted using hemalaun dye. Scale bar in each panel, 50 μm.
Staining for OX-42, which represents activated microglia, was not sufficiently consistent to permit meaningful quantification, as we observed <10 immunopositive cells in all sections from lidocaine-injected DRG tissue (Fig. 3D). However, no OX-42 stained cells were observed in any control, contralateral or saline-injected DRG sections. After Pan-T staining, which labels activated T lymphocytes, no labeled cells were detected in sections of either the injected or the noninjected DRGs.
Lidocaine Injection into the Spinal Nerve Produces an Inflammation Reaction in DRG but no Behavioral Changes
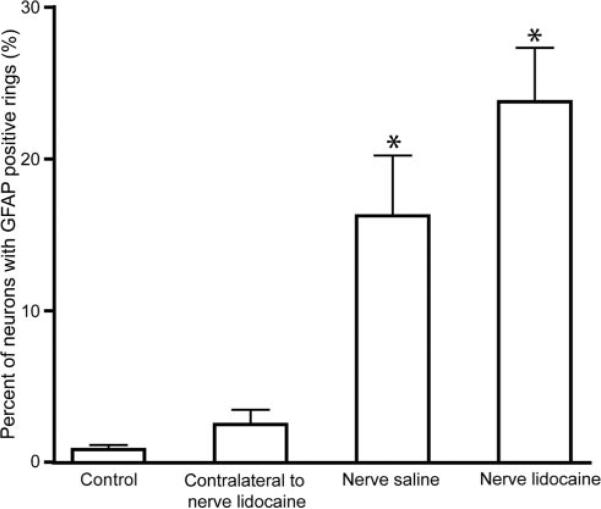
Although rats receiving injections of lidocaine or saline into the spinal nerve showed no hyperalgesia compared to control rats, injecting lidocaine and saline into the L5 nerve significantly increased activation of satellite glial cells around neurons in the connected DRG (Newman-Keuls test, q = 10, P < 0.001 or q = 6.7, P < 0.001, respectively; Fig. 4), however, at levels significantly below those seen after direct DRG injection with lidocaine (23.8% ± 3.6% positive after L5 nerve injection; 59.5% ± 3.5% positive after L5 DRG injection; P = 0.0008 by t-test). No differences in staining were observed for small versus large neurons. Neither OX-42 nor Pan-T staining was seen after spinal nerve injections.
Figure 4.
Percent of dorsal root ganglion (DRG) neurons surrounded with rings of activated satellite cells 15 days after injection into the L5 spinal nerve. Data are presented as mean ± sem. *Asterisk denotes difference from control group. Comparison between nerve-saline and nerve lido-caine injected groups revealed no significant difference. Differences were evaluated by analysis of variance with post hoc Newman-Keuls test.
DISCUSSION
We found that injection of clinically used concentrations of lidocaine into a DRG induced pain-related behavior and a marked inflammatory response in the DRG. This inflammation was a result of activation of resident and not hematogenous immunological cells. Although we also identified an inflammatory response in saline-injected rats, this was not accompanied with marked hyperalgesia, perhaps because this inflammatory response was significantly less than after lidocaine injection. Together, our findings raise the possibility that hyperalgesia after injection of lidocaine into the DRG may be attributed to an inflammatory response.
Our analyses revealed significantly more frequent hyperalgesia-type responses to nociceptive mechanical stimulation during the first two postoperative sessions in rats after injection of lidocaine into the DRG than in either noninjected controls or saline-injected rats. After injection into the spinal nerve, there was no significant difference in hyperalgesic responses among different experimental groups. These results indicate that injection of a local anesthetic in the DRG may cause neuropathic pain early after the injection, but that the injection into the spinal nerve will not have this consequence. The nerve injection site is further away from the DRG and injection into nerve produces a diminished inflammatory response compared to direct DRG injection, which may then be inadequate to provoke neuropathic pain.
These behavioral changes could not have been due to mechanical effects of the needle and pressure of injected liquid,7 since we observed no behavioral changes in the group that was injected with an identical volume of saline. We propose that pain behavior after local anesthetic injection is the result of the anesthetic, as has been previously described after topical bupivacaine application.17 Since the time pattern of GFAP activation and the peak behavioral response differ, activation of resident immunological cells is unlikely to be the only pathogenic factor, and additional mechanisms, perhaps abnormal neuronal activity, may contribute to the generation of hyperalgesia. However, a phenotypic switch induced by activation of satellite cells once initiated may persist for some time after the behavioral changes cease.
A substantial increase of GFAP immunoreactivity in the DRG is due to activation of resident immuno-logical cells, namely the satellite glial cells, which in addition to an increase in resident or hematogenous macrophages also occurs in the DRG after peripheral nerve injury.22 DRGs lack a functional blood-nerve barrier and may be readily invaded with leukocytes. Assuming that the same might happen after direct DRG injury, we stained DRGs with markers for non-resident immunological cells. There were no cells marked with Pan-T antibodies. However, OX-42 staining revealed sporadic immunopositive cells only in DRGs directly injected with lidocaine, as has been previously seen after peripheral nerve injury.23 Although the OX-42 staining duplicated the appearance of satellite cells, the OX-42 antibody is highly specific and it has been previously confirmed that there is no crossreactivity between OX-42 cells and GFAP positive cells.23 The reason for the similar appearance is the fact that OX-42 immunopositive cells can penetrate the glial sheath and end up lying close to the DRG cell, thus being indistinguishable from satellite cells in size and shape.22,23 As opposed to others,24,25 we did not find any differences in staining for small versus large neurons. These findings indicate that lidocaine injection into the DRG activates resident immunological cells, but it does not cause significant immigration of nonresident inflammatory cells into the DRG.
Our group has previously described neuroinflammation in DRGs and spinal nerves in animals that developed neuropathic pain after peripheral nerve injury, using the same markers of inflammation as in the present study.26 These findings, together with previous observations,23,27–29 indicate that the increase in the GFAP and OX-42 immunopositive cell counts in DRGs, spinal nerves or the dorsal horn of spinal cord may be associated with the onset of hyperalgesia after neural injury. The lack of a blood-nerve barrier in the DRG19 may predispose this organ to inflammation since tissue-resident and circulating immune cells may more freely interact.
In conclusion, a potential complication of SSNB is unintended needle entry into a spinal nerve or DRG. The present study shows that injection of lidocaine into a DRG may cause neuroinflammation and neuropathic pain. This argues for making efforts to minimize the risk of local anesthetic DRG injection in clinical settings.
Acknowledgments
Supported by the Ministry of Science, Education and Sports of the Republic of Croatia (grant No. 216-2160528-0522 and 2160528).
REFERENCES
- 1.Boswell MV, Trescot AM, Datta S, Schultz DM, Hansen HC, Abdi S, Sehgal N, Shah RV, Singh V, Benyamin RM, Patel VB, Buenaventura RM, Colson JD, Cordner HJ, Epter RS, Jasper JF, Dunbar EE, Atluri SL, Bowman RC, Deer TR, Swicegood JR, Staats PS, Smith HS, Burton AW, Kloth DS, Giordano J, Manchikanti L. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10:7–111. [PubMed] [Google Scholar]
- 2.Wolff AP, Groen GJ, Crul BJ. Diagnostic lumbosacral segmental nerve blocks with local anesthetics: a prospective double-blind study on the variability and interpretation of segmental effects. Reg Anesth Pain Med. 2001;26:147–55. doi: 10.1053/rapm.2001.21436. [DOI] [PubMed] [Google Scholar]
- 3.Vranken JH, Van Der Vegt MH, Ubags LH, Pijl AJ, Dzoljic M. Continuous sacral nerve root block in the management of neuropathic cancer pain. Anesth Analg. 2002;95:1724–5. doi: 10.1097/00000539-200212000-00047. table of contents. [DOI] [PubMed] [Google Scholar]
- 4.Selander D, Brattsand R, Lundborg G, Nordborg C, Olsson Y. Local anesthetics: importance of mode of application, concentration and adrenaline for the appearance of nerve lesions. An experimental study of axonal degeneration and barrier damage after intrafascicular injection or topical application of bupivacaine (Marcain). Acta Anaesthesiol Scand. 1979;23:127–36. doi: 10.1111/j.1399-6576.1979.tb01432.x. [DOI] [PubMed] [Google Scholar]
- 5.Barsa J, Batra M, Fink BR, Sumi SM. A comparative in vivo study of local neurotoxicity of lidocaine, bupivacaine, 2-chloroprocaine, and a mixture of 2-chloroprocaine and bupivacaine. Anesth Analg. 1982;61:961–7. [PubMed] [Google Scholar]
- 6.Powell HC, Kalichman MW, Garrett RS, Myers RR. Selective vulnerability of unmyelinated fiber Schwann cells in nerves exposed to local anesthetics. Lab Invest. 1988;59:271–80. [PubMed] [Google Scholar]
- 7.Kapur E, Vuckovic I, Dilberovic F, Zaciragic A, Cosovic E, Divanovic KA, Mornjakovic Z, Babic M, Borgeat A, Thys DM, Hadzic A. Neurologic and histologic outcome after intraneural injections of lidocaine in canine sciatic nerves. Acta Anaesthesiol Scand. 2007;51:101–7. doi: 10.1111/j.1399-6576.2006.01169.x. [DOI] [PubMed] [Google Scholar]
- 8.Gentili F, Hudson AR, Hunter D, Kline DG. Nerve injection injury with local anesthetic agents: a light and electron microscopic, fluorescent microscopic, and horseradish peroxidase study. Neurosurgery. 1980;6:263–72. [PubMed] [Google Scholar]
- 9.Wolff AP, Groen GJ, Wilder-Smith OH. Influence of needle position on lumbar segmental nerve root block selectivity. Reg Anesth Pain Med. 2006;31:523–30. doi: 10.1016/j.rapm.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Pfirrmann CW, Oberholzer PA, Zanetti M, Boos N, Trudell DJ, Resnick D, Hodler J. Selective nerve root blocks for the treatment of sciatica: evaluation of injection site and effectiveness–a study with patients and cadavers. Radiology. 2001;221:704–11. doi: 10.1148/radiol.2213001635. [DOI] [PubMed] [Google Scholar]
- 11.Bonner SM, Pridie AK. Sciatic nerve palsy following uneventful sciatic nerve block. Anaesthesia. 1997;52:1205–7. doi: 10.1111/j.1365-2044.1997.258-az0396.x. [DOI] [PubMed] [Google Scholar]
- 12.Sala-Blanch X, Pomes J, Matute P, Valls-Sole J, Carrera A, Tomas X, Garcia-Diez AI. Intraneural injection during anterior approach for sciatic nerve block. Anesthesiology. 2004;101:1027–30. doi: 10.1097/00000542-200410000-00033. [DOI] [PubMed] [Google Scholar]
- 13.Shah S, Hadzic A, Vloka JD, Cafferty MS, Moucha CS, Santos AC. Neurologic complication after anterior sciatic nerve block. Anesth Analg. 2005;100:1515–7. doi: 10.1213/01.ANE.0000150613.23987.92. table of contents. [DOI] [PubMed] [Google Scholar]
- 14.Bigeleisen PE. Nerve puncture and apparent intraneural injection during ultrasound-guided axillary block does not invariably result in neurologic injury. Anesthesiology. 2006;105:779–83. doi: 10.1097/00000542-200610000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Sapunar D, Ljubkovic M, Lirk P, McCallum JB, Hogan QH. Distinct membrane effects of spinal nerve ligation on injured and adjacent dorsal root ganglion neurons in rats. Anesthesiology. 2005;103:360–76. doi: 10.1097/00000542-200508000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142:809–22. doi: 10.1016/j.neuroscience.2006.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang JM, Homma Y, Ackerman WE, Brull SJ. Topical application of acidic bupivacaine to the lumbar ganglion induces mechanical hyperalgesia in the rat. Anesth Analg. 2001;93:466–71. doi: 10.1097/00000539-200108000-00045. [DOI] [PubMed] [Google Scholar]
- 18.Devor M. Unexplained peculiarities of the dorsal root ganglion. Pain. 1999;(Suppl 6):S27–S35. doi: 10.1016/S0304-3959(99)00135-9. [DOI] [PubMed] [Google Scholar]
- 19.Abram SE, Yi J, Fuchs A, Hogan QH. Permeability of injured and intact peripheral nerves and dorsal root ganglia. Anesthesiology. 2006;105:146–53. doi: 10.1097/00000542-200607000-00024. [DOI] [PubMed] [Google Scholar]
- 20.Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology. 2004;101:476–87. doi: 10.1097/00000542-200408000-00030. [DOI] [PubMed] [Google Scholar]
- 21.Woodham P, Anderson PN, Nadim W, Turmaine M. Satellite cells surrounding axotomised rat dorsal root ganglion cells increase expression of a GFAP-like protein. Neurosci Lett. 1989;98:8–12. doi: 10.1016/0304-3940(89)90364-9. [DOI] [PubMed] [Google Scholar]
- 22.Lu X, Richardson PM. Responses of macrophages in rat dorsal root ganglia following peripheral nerve injury. J Neurocytol. 1993;22:334–41. doi: 10.1007/BF01195557. [DOI] [PubMed] [Google Scholar]
- 23.Hu P, Bembrick AL, Keay KA, McLachlan EM. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav Immun. 2007;21:599–616. doi: 10.1016/j.bbi.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Janig W, McLachlan E. On the fate of sympathetic and sensory neurons projecting into a neuroma of the superficial peroneal nerve in the cat. J Comp Neurol. 1984;225:302–11. doi: 10.1002/cne.902250213. [DOI] [PubMed] [Google Scholar]
- 25.Lisney SJ. Regeneration of unmyelinated axons after injury of mammalian peripheral nerve. Q J Exp Physiol. 1989;74:757–84. doi: 10.1113/expphysiol.1989.sp003348. [Review] [DOI] [PubMed] [Google Scholar]
- 26.Znaor L, Lovric S, Hogan Q, Sapunar D. Association of neural inflammation with hyperalgesia following spinal nerve ligation. Croat Med J. 2007;48:35–42. [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue K, Tsuda M, Koizumi S. ATP- and adenosine-mediated signaling in the central nervous system: chronic pain and microglia: involvement of the ATP receptor P2X4. J Pharmacol Sci. 2004;94:112–4. doi: 10.1254/jphs.94.112. [DOI] [PubMed] [Google Scholar]
- 28.Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J Comp Neurol. 2001;439:127–39. [PubMed] [Google Scholar]
- 29.Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79:163–75. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]