Abstract
Background
Several reports show evidence for the existence of high levels of prolactin (PRL) in alcoholic men and women. Previously we have shown that ethanol increases PRL release both in vivo and in vitro. How ethanol increases PRL release is not well understood.
Methods
In this study, we determined the effects of ethanol in the presence and absence of estradiol-17β on PRL messenger RNA (mRNA) levels, dopamine D2 receptor mRNA splicing, and the PRL-inhibitory response of a dopaminergic agent, bromocriptine, in the pituitary of Fischer-344 rats and in primary cultures of anterior pituitary cells. Real-time reverse transcriptase-polymerase chain reaction was used for mRNA detection, and radioimmunoassay was used for hormone detection.
Results
Estradiol and ethanol alone increased PRL mRNA expression in the pituitary gland. Ethanol also potentiated estradiol action on PRL mRNA expression in the pituitary. Determination of the D2 receptor splicing, by determining the changes in the percentage of D2 receptor mRNA expressed as its long form (D2L) and as its short form (D2S), revealed that both ethanol and estradiol altered D2 receptor splicing. Ethanol and estradiol, alone and together, increased the percentage of the D2L receptor but decreased the D2S receptor percentage. Similarly, ethanol and estradiol alone and in combination increased D2L, but decreased the D2S receptor percentage in primary cultures of pituitary cells. Evaluation of bromocriptine’s inhibition of PRL release in primary cultures of pituitary cells indicated that ethanol reduced the ability of this D2 receptor agonist to inhibit PRL release.
Conclusions
These results confirm estradiol’s inhibition of D2 function and provide novel evidence that ethanol, like estradiol, reduces dopamine’s ability to inhibit PRL release by modifying alternative splicing of the dopamine D2 receptor in the pituitary.
Keywords: Hyperprolactinemia, Estradiol, Ethanol, D2 Receptors, Dopamine Regulation of Prolactin
Chronic drinking of alcohol has been shown to increase blood levels of prolactin (PRL), which leads to hyperprolactinemia and various reproductive dysfunctions in both humans and animals. Using Fischer-344 female rats as an animal model, we have shown that ethanol increases and potentiates estradiol’s stimulatory action on plasma levels of PRL and lactotrophic cell proliferation (De et al., 1995). Furthermore, ethanol stimulates both basal and estradiol-induced PRL secretion and lactotrophic cell proliferation in primary cultures of rat pituitary cells (De et al., 2002). However, the mechanism by which ethanol induces hyperprolactinemia is not well understood.
Dopamine secreted from the hypothalamus into hypophysial portal vessels is the major inhibitor of PRL expression and secretion (Ben-Jonathan and Hnasko, 2001). Dopamine’s inhibitory action of PRL is mediated by the dopamine D2 receptor, which belongs to the pertussis toxin-sensitive Gi/Go protein-coupled receptor family (Missale et al., 1998). Recent studies have provided some indirect evidence of alcohol’s effect on dopaminergic neurotransmission (Robbins and Everitt, 1999). Dopamine D2 receptors in the brain are decreased in alcoholic patients (Blum et al., 1991; Hietala et al., 1994; Tupala et al., 2001; Volkow et al., 1996). These reports suggest that dopamine D2 receptors in the brain may be a target for ethanol’s action. However, little is known about the action of alcohol on the D2 receptor system that regulates the function of the pituitary lactotropes.
The dopamine D2 receptor exists as two alternatively spliced isoforms, short (D2S) and long (D2L). The D2L isoform has an insertion of 29 amino acids in the third intercellular loop. Both isoforms of the dopamine D2 receptor are expressed in lactotropes (Missale et al., 1998). It has been reported that the D2L receptor displays a lower affinity than the D2S receptor (Dal Toso et al., 1989). In addition, each receptor isoform individually couples to a specific Gα protein because the third intercellular loop, which has a deletion in D2S, seems to play a central role in G-protein coupling (Albert, 2002; Montmayeur et al., 1993; Senogles, 1994; Wolfe and Morris, 1999). A D2S receptor-specific signaling pathway has also been reported (Senogles, 2000). Hence, each isoform of the dopamine D2 receptor may have its own specific physiologic function.
The D2L receptor is predominant in the pituitary gland and in the striatum, but it is no more abundant than D2S in the substantia nigra and the hypothalamus (Guivarc’h et al., 1995). These observations suggest that a tissue-specific factor could modulate the messenger RNA (mRNA) splicing. In addition, it was observed that changes in the physiologic concentrations of sex steroid hormones (estradiol, progesterone, or testosterone) were able to modify the ratio of these two D2 receptor isoform expressions (Guivarc’h et al., 1995). Such an effect was also observed in primary cultures of pituitary cells and lactotrophic tumor-derived MMQ cells. The relative amounts of each isoform were modified by estradiol, progesterone, and testosterone. In particular, estradiol increased the D2L:D2S ratio in primary cultures of pituitary cells and the MMQ cell line (Guivarc’h et al., 1998). Furthermore, estrogen treatment alters the lactotropes’ responsiveness to dopamine and reduces dopamine’s inhibitory action on PRL secretion (Livingstone et al., 1998). This evidence suggests that alteration of the ratio of the two D2 receptor isoforms impairs the inhibitory effect of dopamine on PRL secretion.
In this study, we tested whether ethanol alters the expression and splicing of the dopamine D2 receptor by determining the D2 receptor expression and alternative splicing in vivo and in vitro, using real-time reverse transcriptase-polymerase chain reaction (RT-PCR) techniques. In addition, we examined ethanol’s effect on dopamine’s inhibitory action of PRL secretion. We show here that ethanol administration increased the D2L receptor percentage but decreased the D2S receptor percentage in vivo and in vitro and reduced the dopaminergic agent’s inhibition of PRL release.
METHODS
Animals
Female rats of the Fischer-344 strain (160–200 g body weight) were obtained from Simonsen Laboratories (Gilroy, CA), housed in a controlled environment (temperature 22°C, lights on 0700–1900 hr), and provided with certified Rodent Chow meal (Purina Mills, Inc., St. Louis, MO) and water ad libitum. Rats were ovariectomized under sodium pentobarbital anesthesia (40 mg/kg intraperitoneally) and subcutaneously implanted with an estradiol-17β-filled (Sigma, St. Louis, MO) 1-cm Silastic (Dow Corning, Midland, MI) capsule. The estradiol capsule maintained plasma levels of estradiol-17β between 120 and 150 pg/ml (De et al., 1995). Animal surgery and care were performed in accordance with institutional guidelines and complied with NIH policy.
Ethanol Administration
We have previously described the procedure for ethanol administration with a liquid diet (De et al., 2002). Briefly, the rats were either pair-fed an isocaloric liquid diet or fed an ethanol-containing liquid diet (Bio-Serv, Frenchtown, NJ) for 2 weeks. Previously, we found that alcohol feeding with a liquid-diet paradigm significantly increased pituitary weight in cyclic and estradiol-treated ovariectomized rats as compared with pair-fed and ad libitum-fed rats (De et al., 1995, 2002). In these studies, we showed that rats fed with isocaloric liquid diet (pair-fed rats) and ad libitum fed with rat chow had similar pituitary weight and lactotrophic cell growth response to estradiol. Hence, we did not include the ad libitum-fed control rats in this study. We gradually introduced rats for ethanol administration to the ethanol diets by providing them only one third and two thirds of the total ethanol volume for the first 2 days of administration. On the third day, the rats received the full supplement of ethanol. After 2 weeks of ethanol feeding, animals were removed from the home cage and immediately killed by rapid decapitation, and the anterior pituitary glands were obtained. Rats were under the influence of ethanol at the time of death. We have previously shown that the blood alcohol concentrations of Fischer rats range between 90 and 123 mg/dl after 2 weeks of an ethanol-containing liquid diet (De et al., 1995, 2002).
Primary Cultures of Anterior Pituitary Cells
Pituitary-derived cells were enzymatically dissociated and cultured by using the methods described previously (Pastorcic et al., 1995). The cells were grown on poly-L-lysine-coated coverslips and maintained in Dulbecco’s minimum essential medium (DMEM) and Ham’s F-12 mixture (1:1, DMEM:F-12; Sigma; containing 100 units/ml of penicillin and 100 mg/ml of streptomycin) with 10% fetal calf serum (HyClone Laboratories, Inc., Logan, UT) for 1 day and then in medium containing 2.5% fetal calf serum and 10% horse serum (HyClone Laboratories) for another 2 days. Cultures were then maintained in a serum-free DMEM/F-12-containing serum supplement (100 μM human transferrin, 5 μM insulin, 1 μM putrescine, and 30 nM sodium selenite) during experimentation.
Treatment of Primary Cultures of Primary Cells
Cultures were treated with a DMEM/F-12-containing serum supplement. The estrogen used was estradiol-17β (Sigma; water-soluble) at a 10 nM concentration. The 10 nM dose of estradiol was chosen because this dose has been shown produce a maximal effect on PRL secretion from primary cultures of pituitary cells (Chun et al., 1998). Bromocriptine was obtained from Sigma. To determine D2 receptor mRNA levels in the cells, ethanol or estradiol treatment time was 48 hr. The medium and treatment were changed at 12-hr intervals. Previously we determined ethanol levels in primary cultures of hypothalamic cells and found that ethanol concentration is maintained for 12 hr in the conditions maintained in this study (Boyadjieva and Sarkar, 1994). For the PRL-secretion study, cultures were treated with ethanol (0 and 50 mM) at 12-hr intervals for 48 hr, then the medium was changed, and cultures were treated with bromocriptine (0, 0.01, 1.0, and 10.0 μM) for 3 hr.
Measurement of Gene Expression Levels by Real-Time RT-PCR
Expression levels of the D2 receptor, D2L, and PRL in rat anterior pituitaries were evaluated by quantitative RT-PCR (TaqMan assay, Applied Biosystems, Roche Molecular Systems, Inc., Branchburg, NJ) by using an ABI PRISM 7700 sequence detector (PerkinElmer Applied Biosystems, Foster City, CA), as described previously (Heid et al., 1996). This assay is based on the 5′ nuclease activity of Taq DNA polymerase for fragmentation of a dual-labeled fluorogenic hybridization probe and was performed by following the protocols provided by the manufacturer. Total RNA from each pituitary gland or from cultured cells was isolated with an RNeasy kit (Qiagen, Valencia, CA). Total RNA (1 μg) was subjected to the first-strand complementary DNA (cDNA) synthesis by using a Superscript II first-strand synthesis system for RT-PCR (Invitrogen, Carlsbad, CA). The sequences of primers and probes were as follows: 5′ 6 carboxy-fluorescein (FAM)—CCA CCA GCT CAC TCT CCC TGA TCC ATC-6 carboxy-tetramethyl rhodamine (TAMRA) as a TaqMan probe for the dopamine D2 receptor, CCC AGA GAG GAC CCG GTA TAG as a 5′ sense primer for the dopamine D2 receptor, and CTG GTT TGG CAG GAC TGT CA as a 3′ antisense primer for the dopamine D2 receptor; 5′ FAM—CCC TGA GGA CAT GAA ACT CTG CAC CGT TAT-TAMRA as a TaqMan probe for the dopamine D2L receptor, AGC AGT CGA GCT TTC AGA GCC as a 5′ sense primer for D2L, and ATT CTC CGC CTG TTC ACT GG as a 3′ antisense primer for D2L; 5′ FAM—TTT TGA ACC TGA TCC TCA GTTT GGT GCA CTC-TAMRA as a TaqMan probe for PRL, CAG AAA GTC CCT CCG GAA GTT as a 5′ sense primer for PRL, and AGG AGC TTC ATG GAT TCC ACC as a 3′ antisense primer for PRL. Quantitation of cDNA was performed by relating the PCR threshold cycle obtained from tissue samples to amplicon-specific standard curves. Serial dilutions of the plasmid containing cDNA for specific genes were performed in duplicate PCR reactions for a standard curve. Measurement of 18S ribosomal RNA levels, as an internal standard for calibration, was performed with a ribosomal RNA control reagent (PerkinElmer Applied Biosystems). Amplification was performed for 1 cycle of a sequential incubation at 50°C for 2 min and 95°C for 10 min, and subsequent 60 cycles of a consecutive incubations were performed at 95°C for 15 sec and 60°C for 1 min.
Radioimmunoassay for PRL
The radioimmunoassay method for PRL, using NIDDK radioimmuno-assay kits, has been previously described (Gottschall et al., 1986). The standard used in this assay was rPRL-RP-3. The minimum detectable level for this assay was 11 pg of PRL per tube. The intra-assay coefficient of variation was 5% at 180 pg of PRL per tube. Tissue levels of protein were measured with bicinohoninic acid (BCA) reagents (Pierce, Rockford, IL). PRL levels in the medium were normalized to micrograms of protein per milliliter of medium.
Statistics
The data shown in the text and the figures are mean ± SEM. Data were analyzed with one-way ANOVA or Student’s t test when only two treatment groups were compared. Post hoc analyses after ANOVA used the Student-Newman-Keuls test. A value of p < 0.05 was considered significant.
RESULTS
Effect of Estradiol and Chronic Alcohol Administration on PRL Gene Expression In Vivo
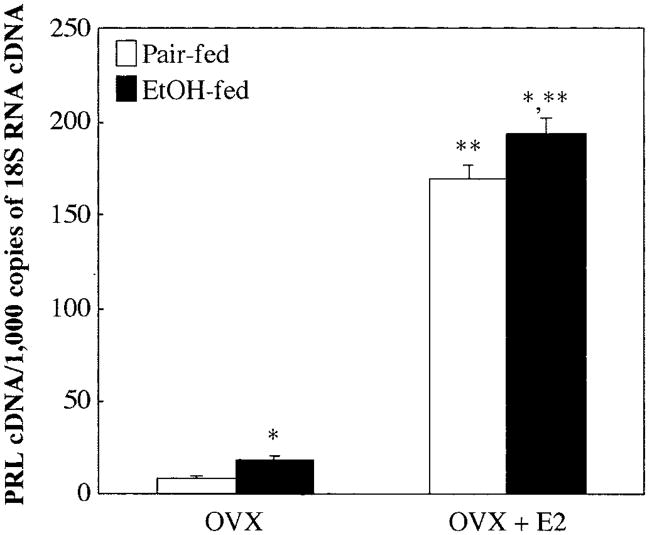
Previously it has been shown that ethanol potentiates estrogen-induced serum PRL levels (De et al., 1995). We determined ethanol’s action with or without estradiol on PRL mRNA levels in the rat pituitary gland. As shown in Fig. 1, both ethanol and estradiol alone enhanced PRL mRNA expression, although estradiol produced a stronger effect than ethanol. Ethanol also potentiated estrogen-induced PRL mRNA expression. Thus, ethanol and estradiol increased PRL mRNA expression and, in combination, produced an additive effect.
Fig. 1.
Effect of ethanol and estradiol on PRL mRNA levels in the anterior pituitary of Fischer-344 rats. Ovariectomized (OVX) and estradiol (E2)-treated ovariectomized rats were fed a liquid diet containing ethanol or pair-fed a diet containing sucrose as a substitution for ethanol for 2 weeks. Total RNA was isolated from the anterior pituitary and reverse-transcribed. Expression levels of PRL were measured with quantitative real-time RT-PCR. The level of PRL mRNA was normalized by 18S RNA. Data are mean ± SEM values of six or seven rats. *p < 0.05, significantly different from the pair-fed group; **p < 0.05, significantly different from the OVX group in each treatment.
Effect of Estradiol and Alcohol Administration on D2 Receptor Gene Alternative Splicing In Vivo
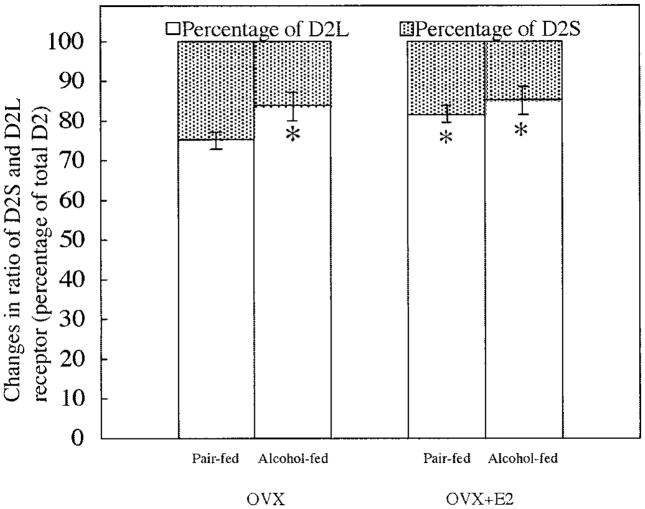
To determine the effect of ethanol with or without estradiol on dopamine D2 receptor alternative splicing, we measured total D2 receptor and D2L receptor mRNA transcripts and then calculated what percentage of D2 receptor is D2L or D2S (total D2 minus D2L divided by total D2). Because it was difficult to prepare a D2S-specific primer, we used this indirect method for measuring the level of D2S mRNA. Previously it has been shown that estradiol increases the level of the low-affinity D2L receptor and reduces the level of the high-affinity D2S receptor (Dal Toso et al., 1989; Livingstone et al., 1998). In this study, we found a similar effect of estradiol on D2L and D2S mRNA splicing (Fig. 2). Like estradiol, ethanol increased D2L mRNA and decreased D2S mRNA. Ethanol also moderately (not significantly) increased estradiol’s effect on the D2L and D2S mRNA splicing. These results showed that like estradiol, ethanol impaired the alternative splicing of the D2 receptor.
Fig. 2.
Effect of ethanol and estradiol on the percentage of D2 receptor isoforms in the anterior pituitary of Fischer-344 rats. Ovariectomized (OVX) and estradiol (E2)-treated ovariectomized rats were fed a liquid diet containing ethanol or pair-fed a diet containing sucrose as a substitute for ethanol for 2 weeks. The levels of total D2 receptor and D2L receptor mRNA in the anterior pituitary were measured with quantitative real-time PCR. The level of gene expression was normalized by 18S RNA. The percentages of the two D2 receptor isoforms (D2S and D2L) were calculated and are presented in this figure. Data are mean ± SEM values of five or six rats. *p < 0.05, significantly different from the pair-fed OVX group.
Effect of Estradiol and Alcohol Administration on D2 Receptor Gene Alternative Splicing In Vitro
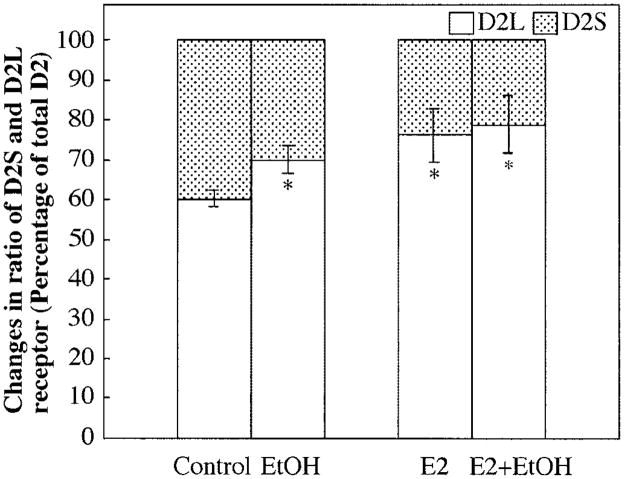
To determine the direct effect of ethanol on the alteration of D2 receptor splicing, percentages of D2L and D2S receptor mRNA were determined by real-time PCR. Both ethanol (50 mM) and estradiol (10 nM) increased the D2L:D2S ratio (Fig. 3). In combination, ethanol and estradiol also moderately, but not significantly, increased the percentage of the D2L receptor mRNA and decreased the percentage of the D2S receptor mRNA as compared with ethanol or estradiol alone.
Fig. 3.
Effect of ethanol (EtOH) and estradiol (E2) on the percentage of each D2 receptor isoform in primary cultured anterior pituitary cells. Primary cultures of anterior pituitary cells were plated on culture plates at a density of 2.5 × 105/ml. Cells were plated in culture for 4 days and then were incubated with or without ethanol (50 mM) and estradiol (10 nM) for an additional 2 days in serum-free defined medium. The levels of total D2 receptor and D2L receptor mRNA in the anterior pituitary were measured with quantitative real-time PCR. The level of gene expression was normalized by 18S RNA. The percentages of the two D2 receptor isoforms (D2S and D2L) were calculated and are presented in this figure. Data are mean ± SEM values of five or six cultures. *p < 0.05, significantly different from the control group.
Effect of Ethanol on Bromocriptine’s Inhibition of PRL Release From Pituitary Cells in Primary Culture
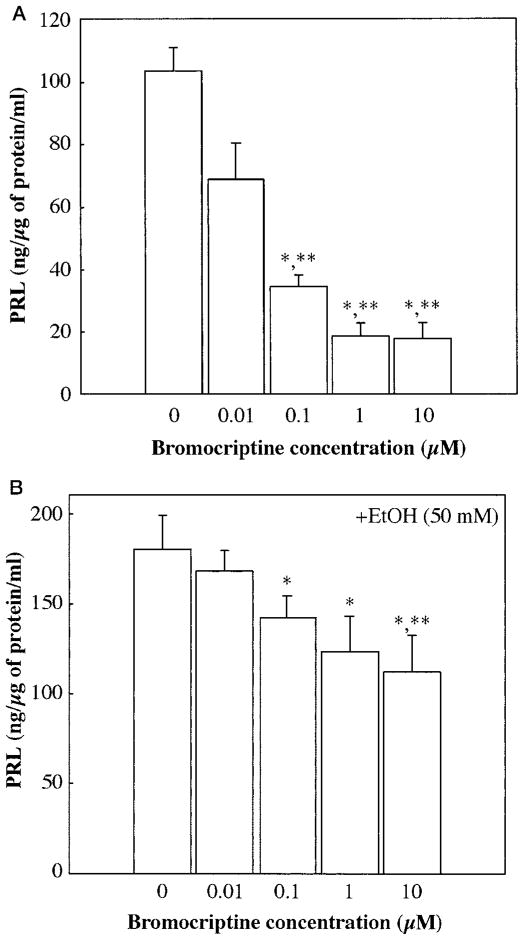
To determine whether ethanol’s effect on D2 receptor splicing is reflected in an altered response of pituitary cells to a dopaminergic agent, the PRL-inhibitory response to bromocriptine in the presence and absence of ethanol was determined. As a D2 receptor-specific dopamine analog, bromocriptine inhibited PRL secretion from pituitary cells in a dose-dependent manner (Fig. 4A). Ethanol treatment significantly decreased the PRL-release response to bromocriptine (Fig. 4B) in primary cultures of pituitary cells.
Fig. 4.
Prolactin (PRL) response to bromocriptine after exposure of pituitary cells in culture to ethanol (EtOH). Pituitary cells were pretreated (A) without ethanol or (B) with ethanol (50 mM) for 48 hr. Cells were then treated for 3 hr with various doses of bromocriptine (0, 0.01, 1.0, or 10.0 μM). The media samples were used to measure levels of PRL by radioimmunoassay. Data are mean ± SEM of five cultures. *p < 0.05, significantly different from the control group; **p < 0.05, significantly different from the 0.01 μM bromocriptine dose group.
DISCUSSION
Data presented in this study show that ethanol alters the ratio of D2 receptor alternative splicing in the normal rat anterior pituitary gland and in primary cultured pituitary cells. In addition, ethanol diminished bromocriptine’s inhibition of PRL secretion in primary cultured pituitary cells. A clinical study has been reported in which the dopamine-induced PRL decrement was significantly smaller in alcoholics than in controls, although the PRL response to thyrotropin-releasing hormone (TRH) was similar in those groups (Marchesi et al., 1997). Furthermore, the plasma PRL level in alcoholic patients was increased by the administration of haloperidol, a dopamine antagonist, but was significantly lower than in the haloperidol-administered control group. Interestingly, the low responsiveness to dopamine in alcoholic patients was recovered after alcohol detoxification with diazepam and a vitamin B1/B6/B12 combination (Markianos et al., 2000). These clinical reports are consistent with our finding that ethanol reduces bromocriptine’s ability to reduce PRL secretion. Furthermore, our data showed that ethanol alters the D2L and D2S receptor splicing in pituitary cells in culture and in vivo in the pituitary gland. The D2L receptor displays a lower affinity than the D2S receptor (Dal Toso et al., 1989). Hence, it could be hypothesized that the reduced dopamine action after ethanol might be partially due to alteration in the expression of D2 receptor isoforms.
It has been reported that estradiol treatment increases the D2L:D2S ratio in a lactotrophic tumor cell line, MMQ cells (Guivarc’h et al., 1998), and reduces dopamine’s inhibitory action on PRL secretion (Livingstone et al., 1998). We demonstrated similar effects of ethanol in this study. In addition, we have previously shown that ethanol increases estrogen-induced PRL secretion and lactotrophic cell proliferation in ovariectomized female rats (De et al., 1995). Furthermore, in this study ethanol enhanced estradiol-induced PRL mRNA expression. These data indicate that there are some interactions between ethanol’s and estradiol’s actions in the anterior pituitary gland. Although the molecular mechanisms of interactions between ethanol and estrogen are poorly understood, it has been recently reported that ethanol causes a dose-dependent increase in the transcriptional activity of the ligand-bound, but not the non-ligand-bound, estrogen receptor-α level in MCF-7 human breast cancer cells (Fan et al., 2000). Estradiol regulates the growth and differentiation of both mammary epithelial cells and lactotropes. Hence, it could be hypothesized that ethanol may also alter estrogen receptor levels to regulate the lactotropes’ function.
Our data showed that ethanol regulates the alternative splicing of the dopamine D2 receptor in the rat anterior pituitary gland. Ethanol also regulates alternative splicing of the N-methyl-D-aspartate receptor in the rat brain (Winkler et al., 1999). Although the molecular mechanisms of ethanol-induced alternative splicing are not clear, recent studies show that the positive exon cis-acting elements, known as exonic splicing enhancers (ESEs), and serine-arginine (SR) protein, which can be associated with ESE sequences, play important roles for the regulation of alternative splicing (Akker et al., 2001). SR proteins form bridging complexes between the ESE and the 3′ intron site, as well as between the 5′ and 3′ intron sites (Wu and Maniatis, 1993). In the former example, SR proteins have been shown to be associated with ESE sequences in the downstream exon. In addition, ESEs are specifically recognized by one or more SR proteins (Schaal and Maniatis, 1999). Hence, SR proteins are expressed at different levels in different tissues, and their expression also seems to be regulated by alternative splicing. In some cases, hormone-or growth factor-induced alternative splicing is regulated by splicing factor-2/alternative splicing factor (SF-2/ASF), one of the SR protein family (Akker et al., 2001). The exonic sequence motif of SF-2/ASF has been identified as SR-SASGA (S represents G or C, and R represents purine; Liu et al., 1998). We observed the SF-2/ASF exonic sequence motif GAGAGGA in the downstream exon of the alternative splicing site of the D2 receptor in rats. This motif was also observed in the mouse and the human, both of which have already been identified as having alternative splicing of D2 receptors. These observations support the hypothesis that alternative splicing of the D2 receptor involves SF-2/ASF. Further studies are necessary to determine whether SF-2/ASF regulates alternative splicing of D2 receptors, and whether ethanol alters SF-2/ASF production and activity.
In conclusion, the data presented here provide evidence that ethanol and estradiol alone or in combination increase PRL release partly by reducing dopamine’s ability to inhibit PRL secretion. Ethanol and estradiol reduce the dopamine D2 receptor action on lactotropes by modifying alternative splicing of the neurotransmitter receptor and subsequently increasing expression of low-affinity D2L receptors and decreasing expression of high-affinity D2S receptors. Furthermore, these data provide the first evidence that ethanol and estradiol have similar modes of action on dopamine-regulated PRL release.
Acknowledgments
Supported by NIH Grants AA11591, CA77500, and AA00220.
References
- Akker SA, Smith PJ, Chew SL. Nuclear post-transcriptional control of gene expression. J Mol Endocrinol. 2001;27:123–131. doi: 10.1677/jme.0.0270123. [DOI] [PubMed] [Google Scholar]
- Albert PR. G protein preferences for dopamine D2 inhibition of prolactin secretion and DNA synthesis in GH(4) pituitary cells. Mol Endocrinol. 2002;16:1903–1911. doi: 10.1210/me.2001-0329. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22:724–763. doi: 10.1210/edrv.22.6.0451. [DOI] [PubMed] [Google Scholar]
- Blum K, Noble EP, Sheridan PJ, Finley O, Montgomery A, Ritchie T, Ozkaragoz T, Fitch RJ, Sadlack F, Sheffield D. Association of the A1 allele of the D2 dopamine receptor gene with severe alcoholism. Alcohol. 1991;8:409–416. doi: 10.1016/0741-8329(91)90693-q. [DOI] [PubMed] [Google Scholar]
- Boyadjieva N, Sarkar DK. Effects of chronic alcohol on β-endorphin secretion from hypothalamic neurons in primary cultures: evidence for alcohol tolerance, withdrawal and sensitization responses. Alcohol Clin Exp Res. 1994;18:1497–1501. doi: 10.1111/j.1530-0277.1994.tb01456.x. [DOI] [PubMed] [Google Scholar]
- Chun T-Y, Gregg D, Sarkar DK, Gorski J. Differential regulation by estrogens of growth and prolactin synthesis in pituitary cells suggests that only a small pool of estrogen receptors is required for growth. Proc Natl Acad Sci USA. 1998;95:2325–2330. doi: 10.1073/pnas.95.5.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Toso R, Sommer B, Ewert M, Herb A, Pritchett DB, Bach A, Shivers BD, Seeburg PH. The dopamine D2 receptor: two molecular forms generated by alternative splicing. EMBO J. 1989;8:4025–4034. doi: 10.1002/j.1460-2075.1989.tb08585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De A, Boyadjieva N, Oomizu S, Sarkar DK. Ethanol induces hyperprolactinemia by increasing prolactin release and lactotrope growth in female rats. Alcohol Clin Exp Res. 2002;26:1420–1429. doi: 10.1097/01.ALC.0000030621.35354.E0. [DOI] [PubMed] [Google Scholar]
- De A, Boyadjieva N, Pastorcic MG, Sarkar DK. Potentiation of the mitogenic effect of estrogen on the pituitary gland by alcohol consumption. Int J Oncol. 1995;7:643–648. doi: 10.3892/ijo.7.3.643. [DOI] [PubMed] [Google Scholar]
- Fan S, Meng Q, Gao B, Grossman J, Yadegari M, Goldberg ID, Rosen EM. Alcohol stimulates estrogen receptor signaling in human breast cancer cell lines. Cancer Res. 2000;60:5635–5639. [PubMed] [Google Scholar]
- Gottschall PE, Sarkar DK, Meites J. Persistence of low hypothalamic dopaminergic activity after removal of chronic estrogen treatment. Proc Soc Exp Biol Med. 1986;181:78–86. doi: 10.3181/00379727-181-42227. [DOI] [PubMed] [Google Scholar]
- Guivarc’h D, Vernier P, Vincent JD. Sex steroid hormones change the differential distribution of the isoforms of the D2 dopamine receptor messenger RNA in the rat brain. Neuroscience. 1995;69:159–166. doi: 10.1016/0306-4522(95)00228-b. [DOI] [PubMed] [Google Scholar]
- Guivarc’h D, Vincent JD, Vernier P. Alternative splicing of the D2 dopamine receptor messenger ribonucleic acid is modulated by activated sex steroid receptors in the MMQ prolactin cell line. Endocrinology. 1998;139:4213–4221. doi: 10.1210/endo.139.10.6246. [DOI] [PubMed] [Google Scholar]
- Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- Hietala J, West C, Syvalahti E, Nagren K, Lehikoinen P, Sonninen P, Ruotsalainen U. Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology (Berl) 1994;116:285–290. doi: 10.1007/BF02245330. [DOI] [PubMed] [Google Scholar]
- Liu HX, Zhang M, Krainer AR. Identification of functional exonic splicing enhancer motifs recognized by individual SR proteins. Genes Dev. 1998;12:1998–2012. doi: 10.1101/gad.12.13.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone JD, Lerant A, Freeman ME. Ovarian steroids modulate responsiveness to dopamine and expression of G-proteins in lacto-tropes. Neuroendocrinology. 1998;68:172–179. doi: 10.1159/000054363. [DOI] [PubMed] [Google Scholar]
- Marchesi C, Ampollini P, Chiodera P, Volpi R, Coiro V. Alteration in dopaminergic function in abstinent alcoholics. Neuropsychobiology. 1997;36:1–4. doi: 10.1159/000119350. [DOI] [PubMed] [Google Scholar]
- Markianos M, Moussas G, Lykouras L, Hatzimanolis J. Dopamine receptor responsivity in alcoholic patients before and after detoxification. Drug Alcohol Depend. 2000;57:261–265. doi: 10.1016/s0376-8716(99)00056-3. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Montmayeur JP, Guiramand J, Borrelli E. Preferential coupling between dopamine D2 receptors and G-proteins. Mol Endocrinol. 1993;7:161–170. doi: 10.1210/mend.7.2.7682286. [DOI] [PubMed] [Google Scholar]
- Pastorcic M, De A, Boyadjieva N, Vale W, Sarkar DK. Reduction in the expression and action of transforming growth factor beta 1 on lactotropes during estrogen-induced tumorigenesis in the anterior pituitary. Cancer Res. 1995;55:4892–4898. [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- Schaal TD, Maniatis T. Selection and characterization of pre-mRNA splicing enhancers: identification of novel SR protein-specific enhancer sequences. Mol Cell Biol. 1999;19:1705–1719. doi: 10.1128/mcb.19.3.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senogles SE. The D2 dopamine receptor isoforms signal through distinct Gi alpha proteins to inhibit adenylyl cyclase. A study with site-directed mutant Gi alpha proteins. J Biol Chem. 1994;269:23120–23127. [PubMed] [Google Scholar]
- Senogles SE. The D2s dopamine receptor stimulates phospholipase D activity: a novel signaling pathway for dopamine. Mol Pharmacol. 2000;58:455–462. doi: 10.1124/mol.58.2.455. [DOI] [PubMed] [Google Scholar]
- Tupala E, Hall H, Bergstrom K, Sarkioja T, Rasanen P, Mantere T, Callaway J, Hiltunen J, Tiihonen J. Dopamine D-2/D-3-receptor and transporter densities in nucleus accumbens and amygdala of type 1 and 2 alcoholics. Mol Psychiatry. 2001;6:261–267. doi: 10.1038/sj.mp.4000859. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Winkler A, Mahal B, Kiianmaa K, Zieglgansberger W, Spanagel R. Effects of chronic alcohol consumption on the expression of different NR1 splice variants in the brain of AA and ANA lines of rats. Brain Res Mol Brain Res. 1999;72:166–175. doi: 10.1016/s0169-328x(99)00218-1. [DOI] [PubMed] [Google Scholar]
- Wolfe SE, Morris SJ. Dopamine D2 receptor isoforms expressed in AtT20 cells differentially couple to G proteins to acutely inhibit high voltage-activated calcium channels. J Neurochem. 1999;73:2375–2382. doi: 10.1046/j.1471-4159.1999.0732375.x. [DOI] [PubMed] [Google Scholar]
- Wu JY, Maniatis T. Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell. 1993;75:1061–1070. doi: 10.1016/0092-8674(93)90316-i. [DOI] [PubMed] [Google Scholar]