Abstract
The heptahelical G protein–coupled receptors (GPCRs) belong to the largest family of cell surface signaling receptors encoded in the human genome. GPCRs signal to diverse extracellular stimuli and control a vast number of physiological responses, making this receptor class the target of nearly half the drugs currently in use. In addition to rapid desensitization, receptor trafficking is crucial for the temporal and spatial control of GPCR signaling. Sorting signals present in the intracytosolic domains of GPCRs regulate trafficking through the endosomal-lysosomal system. GPCR internalization is mediated by serine and threonine phosphorylation and arrestin binding. Short, linear peptide sequences including tyrosine- and dileucine-based motifs, and PDZ ligands that are recognized by distinct endocytic adaptor proteins also mediate internalization and endosomal sorting of GPCRs. We present new data from bioinformatic searches that reveal the presence of these types of sorting signals in the cytoplasmic tails of many known GPCRs. Several recent studies also indicate that the covalent modification of GPCRs with ubiquitin serves as a signal for internalization and lysosomal sorting, expanding the diversity of mechanisms that control trafficking of mammalian GPCRs.
Keywords: GPCR, arrestin, ubiquitin, trafficking, clathrin, PDZ, bioinformatic
INTRODUCTION
Seven-transmembrane domain GPCRs (G protein–coupled receptors) comprise the largest family of cell surface signaling receptors encoded in the human genome with ~900 members. GPCRs are the targets of nearly half the drugs currently in use and for the development of new therapeutics that can be used for the treatment of a wide range of human diseases, including neurodegenerative, psychiatric and immune disorders, cardiovascular, gastrointestinal, renal, and pulmonary diseases, and cancer. Upon activation, GPCRs undergo conformational changes that facilitate activation of heterotrimeric G proteins and signaling effectors at the plasma membrane. In addition, several internalized GPCRs can signal from endosomal compartments through non–G protein–dependent effectors. Thus, diverse mechanisms must exist to precisely regulate the magnitude, duration, and spatial aspects of GPCR signaling.
Most activated GPCRs are rapidly desensitized at the cell surface by phosphorylation and arrestin binding. GPCRs are then internalized but some receptors can continue to signal independent of G proteins from such intracellular compartments. Within endosomes, GPCRs are dephosphorylated and efficiently recycled back to the cell surface in a resensitized state in which the receptors are competent to signal again. By contrast, other GPCRs are sorted from endosomes to lysosomes and degraded, a process important for signal termination. Thus, GPCR trafficking has critical functions in signal termination and propagation as well as receptor resensitization. The rates of GPCR internalization, recycling, and lysosomal sorting differ widely among receptors, suggesting that different mechanisms control trafficking of distinct receptors. Indeed, several studies have revealed diverse mechanisms that regulate trafficking of distinct GPCRs within the endosomal-lysosomal system (7, 43, 56, 78).
The transport of GPCRs within the endosomal-lysosomal system is initiated through a series of events that leads to deformation of select regions of the plasma membrane (Figure 1). Clathrin-coated pits (CCPs) form at plasma membrane sites enriched in PIP2 [phosphatidylinositol (4,5)-bisphosphate] and serve as the major pathway for GPCR internalization. Clathrin, adaptor proteins, and dozens of regulatory proteins coordinate the assembly and invagination of CCPs, which are released from the plasma membrane by the GTPase dynamin. Clathrin adaptor proteins recruit GPCRs to CCPs by recognizing short linear peptide sequences as well as phosphorylated and ubiquitinated receptors (a topic recently reviewed in Reference 1). Once internalized, GPCRs are then sorted within an early endosomal tubulo-vesicular compartment to either a recycling or a lysosomal degradative pathway. Sorting of GPCRs to a recycling pathway occurs through a default pathway similar to bulk membrane flow or through a regulated process. One lysosomal sorting pathway for GPCRs involves the ubiquitin-dependent ESCRT (endosomal-sorting complex required for transport) machinery, which is comprised of a complex network of proteins that function coordinately to sort ubiquitinated cargo to a degradative pathway (2). In addition, several GPCRs appear to target to lysosomes for degradation independent of ubiquitination and some components of the ESCRT machinery, suggesting that additional pathways exist (56, 94, 98). The molecular mechanisms that regulate trafficking of mammalian GPCRs have yet to be fully elucidated and are vital to our understanding of receptor signaling. Here, we discuss the diverse mechanisms that regulate GPCR trafficking through the endosomal-lysosomal system.
Figure 1.
Model of GPCR recruitment to clathrin-coated pits (CCPs). The clathrin adaptor AP-2 is first recruited to the plasma membrane at sites enriched in PIP2 and facilitates the recruitment of more AP-2, clathrin, and alternate clathrin adaptors. Many activated GPCRs are phosphorylated and bind arrestins, which facilitates recruitment into the nascent CCPs. Other GPCRs harbor short linear peptide sequences or are modified with ubiquitin and recognized by alternate clathrin adaptors. The newly formed CCPs gradually invaginate and pinch off from the plasma membrane through the actions of the GTPase dynamin to form a vesicle. Many other accessory proteins and alternate clathrin adaptors are also present in the nascent and forming CCPs.
PHOSPHORYLATION, ARRESTINS, AND GPCR TRAFFICKING
GPCR phosphorylation occurs predominantly on serine (Ser) and threonine (Thr) residues within the C-tail and the third intracellular loop, but rarely on tyrosine (Tyr) residues. Agonist-activated GPCRs are rapidly phosphorylated by GRKs (G protein–coupled receptor kinases) and bind arrestins, processes that facilitate receptor uncoupling from G proteins and receptor internalization.
The mechanism by which phosphorylation functions in GPCR trafficking is best characterized for β2AR (β2-adrenergic receptor) internalization (Figure 2). GRK2-dependent phosphorylation of the activated β2AR induces translocation of arrestins to the plasma membrane where the receptor preferentially binds arrestin-3 rather than arrestin-2 (also known as β-arrestin-2 and β-arrestin-1, respectively) (3). Once phosphorylated, activated GPCRs bind arrestins through multiple interactions. Arrestins are comprised of distinct N and C domains linked by a twelve-residue polar core (4), which engages receptor-associated phosphates. A conformational change in arrestin is induced upon binding to GPCRs, exposing the C-terminal domain, which interacts with clathrin and the β2-adaptin subunit of the clathrin adaptor AP-2 (adaptor protein complex-2), components of the endocytic machinery (5, 6). The β2AR-arrestin complex is then internalized through CCPs, and arrestin rapidly dissociates from the receptor. Interestingly, the endocytic activity of arrestin is subject to dynamic regulation by dephosphorylation and ubiquitination, the latter of which occurs upon binding to activated β2AR, as discussed below (7). Recent work also indicates that under certain conditions GRK2 activity and its ability to phosphorylate β2AR is regulated by S-nitrosylation, the covalent attachment of nitric oxide, or S-nitrosothiols to cysteine (Cys) residues (8), providing another level of regulation for GPCR phosphorylation.
Figure 2.
G protein–coupled receptor trafficking within the endosomal-lysosomal system. Several studies indicate that distinct mechanisms control trafficking of different mammalian GPCRs. (a) PAR1 displays constitutive and agonist-induced internalization that occur independent of arrestins and is differentially regulated by ubiquitination. Constitutive internalization of PAR1 requires AP-2 and is negatively regulated by ubiquitination. Activated PAR1 is phosphorylated, rapidly internalized, and sorted from endosomes to lysosomes through a SNX1-dependent pathway, which is independent of HRS and TSG101, a component of the ESCRT-I machinery. Whether PAR1 enters MVBs (multivesicular bodies) or sorts directly to lysosomes is not known. (b) Activated CXCR4 is ubiquitinated at the plasma membrane by the E3 ubiquitin ligase AIP4. Ubiquitination functions as an endosomal sorting signal and is not required for CXCR4 internalization. Ubiquitinated CXCR4 is concentrated on HRS-positive microdomains together with AIP4. AIP4 mediates ubiquitination of HRS following CXCR4 activation, which is critical for MVB sorting. CISK phosphorylates and inhibits AIP4 activity and thereby inhibits endosomal sorting of CXCR4. VPS4 also regulates the ubiquitination status of CXCR4 and MVB sorting. (c) Agonist induces rapid phosphorylation and ubiquitination of the β2AR. Arrestin-3 is ubiquitinated by MDM2 and recruited to activated β2AR, a process required for β2AR internalization. Once internalized, β2AR are dephosphorylated and rapidly recycled back to the plasma membrane through a pathway that involves EBP50/NHERF, NSF, and HRS. Whether deubiquitination of β2AR is involved in the regulated mode of receptor recycling is not known. After prolonged agonist exposure, activated β2AR sorts from endosome to lysosomes and are degraded through an ubiquitin-dependent mechanism.
In contrast to the β2AR, GPCRs that are phosphorylated on multiple Ser and Thr residues positioned at the end of the C-tail bind and cointernalize with arrestins. GPCR-associated phosphates are thought to stabilize arrestin binding by inducing a conformational change that promotes high-affinity binding to activated receptors (9). The AT1AR (angiotensin II type 1A receptor), V2R (vasopressin 2 receptor), and NK1R (neurokinin-1 receptor) have C-tail Ser and Thr clusters and have been shown to internalize together with arrestins (10, 11). Our bioinformatic searches have revealed several other human GPCRs that contain clusters of Ser and Thr residues within their C-tails (Table 1). Although arrestins engage activated and phosphorylated GPCRs through multiple interactions, the presence of Ser and Thr clusters in certain GPCRs may stabilize the interaction with arrestins to promote important cellular responses. The ubiquitination status of arrestin following receptor activation also correlates with the nature of its interaction with some GPCRs. The activation of the β2AR results in transient ubiquitination and arrestin association, whereas other GPCRs, such as AT1AR form stable complexes with arrestins and induce prolonged arrestin ubiquitination following agonist exposure (12). The biological consequences of transient versus prolonged arrestin association with GPCRs are varied. In some cases, such as the V2R, stable association with arrestin slows receptor dephosphorylation and recycling and consequently delays resensitization (13). The AT1AR, V2R, and NK1R bind and co-internalize together with arrestins, which facilitates arrestins’ ability to function as a scaffold to sustain ERK-1, 2 (extracellular signal-regulated kinase-1, 2) signaling in the cytoplasm (14, 15). Arrestins also cointernalize with PAR2 (protease-activated receptor-2), a GPCR that does not recycle, and promotes sustained ERK-1, 2 signaling independently of G protein activation (16, 17). Arrestins are required for internalization of most activated GPCRs that form stable complexes with these proteins. However, arrestins are not essential for N-formyl peptide receptor (FPR) endocytosis but appear to regulate recycling of the FPR, suggesting a distinct endocytic function for arrestins in receptor recycling (18). The ability of arrestins to control different aspects of GPCR signaling and trafficking probably involves distinctly modified arrestin forms derived from differential phosphorylation and/or ubiquitination, which have yet to be fully characterized.
Table 1.
Alignment of human GPCR C-tail sequences containing serine and threonine clusters
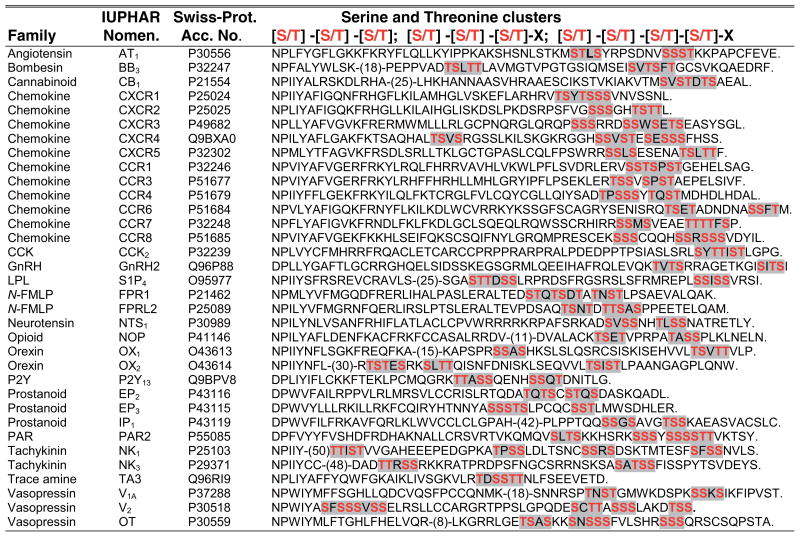 |
See Supplemental Appendix 1 for a description of how the list of human GPCR C-tails with serine and threonine clusters in this table and other motifs in other tables were acquired and amino acid designations (follow the Supplemental Material link from the Annual Reviews home page at http://www.annualreviews.org). The serine and threonine clusters are shaded in grey and critical residues are shown in red. Abbreviations used: CCK, cholecystokinin; GnRH, gonadotropin-releasing hormone; LPL, lysophospholipid; N-FMLP, N-Formylpeptide; PAR, protease-activated receptor.
In addition to the receptor, phosphorylation of critical components of the endocytic machinery regulates GPCR trafficking. The p38 MAP (mitogen-activated protein) kinase promotes endocytosis of the MOR (μ-opioid receptor) by directly phosphorylating EEA-1 (early endosome antigen-1) and Rabenosyn-5, rather than the receptor (19). EEA1 and Rabenosyn-5 are effectors of the monomeric small GTP binding protein Rab5 and are involved in tethering and fusion of early endosomes (20). Rab5 activation has also been shown to regulate trafficking of several other GPCRs, including the β2AR and the D2 dopamine receptor (21, 22), although how this occurs mechanistically is not known. Another study recently reported that phosphorylation of the transport machinery negatively regulates GPCR trafficking (23). CISK (cytokine-independent survival kinase), a Ser and Thr kinase, inhibits sorting of the chemokine receptor CXCR4 from endosomes to lysosomes by directly phosphorylating AIP4 (atrophin-interacting protein 4), an E3 ubiquitin ligase required for CXCR4 ubiquitination and lysosomal sorting (Figure 2) (23), as discussed further below. Thus, multiple kinases can control diverse aspects of GPCR trafficking by phosphorylating the receptor and/or components of the transport machinery.
SHORT PEPTIDE SORTING SIGNALS
Increasing evidence suggests that the cytoplasmic carboxyl tails (C-tails) of GPCRs harbor structural determinants and/or posttranslational modifications that are recognized by distinct endocytic adaptor proteins that facilitate transport through the endocytic pathway. Short, linear peptide sequences can mediate sorting of GPCRs within the endosomal-lysosomal system. Some GPCRs harbor Tyr- and dileucine(Leu)-based motifs that are recognized by adaptor-protein (AP) complexes associated with the cytosolic face of endocytic membranes. PDZ [postsynaptic density 95-kDa protein (PSD-95), Drosophila discs large protein (DLG), and zonula occludens-1 protein (ZO-1)] ligands are also present in the C-tail of many GPCRs and appear to regulate critical aspects of receptor signaling and endocytic sorting (38–40).
Tyrosine- and Dileucine-Based Motifs
Several mammalian GPCRs contain C-tail Tyr- and di-Leu-based motifs that mediate receptor trafficking (1). The clathrin adaptor AP-2 directly binds to Tyr-based motifs and regulates mammalian GPCR internalization through CCPs. AP-2 is composed of α, β2, μ2, and σ2 subunits; the μ2 subunit recognizes Tyr-based Y-X-X-Ø motifs (where Y denotes Tyr, X is any amino acid, and Ø is a bulky hydrophobic residue) localized within the cytosolic regions of cargo proteins (25). The activity of this type of motif requires that the critical Tyr remain unphosphorylated. Recent work indicates that AP-2, rather than arrestins, is required for constitutive internalization of PAR1 (protease-activated receptor-1), a GPCR activated by thrombin (Figure 2) (24). In the absence of agonist, PAR1 internalizes constitutively through CCPs independently of phosphorylation and arrestin binding (26, 27). Surface plasmon resonance was used to show that the μ2-subunit of AP-2 directly binds to a distal Tyr-based motif Y420KKL423 within the C-tail of PAR1 (24). Moreover, the expression of a PAR1 tyrosine mutant or depletion of AP-2 by siRNA significantly inhibited PAR1 constitutive internalization. In addition to canonical Tyr-based motifs, some GPCRs contain noncanonical sequences that appear to be regulated by AP-2. The TPβ (thromboxane-A2β) receptor contains a related Y-X-X-X-Ø motif that functions in arrestin-independent constitutive internalization (28). The binding pocket of the μ2-subunit of AP-2 can accommodate related Y-X-X-G-Ø motifs (29), suggesting that it can function in TPβ endocytosis, although this remains to be determined. AP-2 may also directly regulate activation-dependent GPCR internalization, presumably by recruiting receptors to CCPs. The α1BAR (α1B-adrenergic receptor) binds the μ2-subunit of AP-2 through an unusual stretch of Arg residues within the C-tail (30). Deletion of this region ablates μ2 binding and agonist-induced internalization, although α1BAR internalization is also dependent on arrestins (31).
Similar to AP-2, other heterotetrameric clathrin AP complexes recognize Tyr-based motifs but have distinct functions within the endosomal-lysosomal system (25). AP-2 is abundant at plasma membrane CCPs and mediates endocytosis. AP-1 is associated with the trans-Golgi network and endosomal clathrin coats. AP-3 is also found on endosomes, often with clathrin. The role of these other AP complexes in GPCR trafficking remains relatively unexplored. However, we have identified several human GPCRs that contain canonical Tyr-based motifs within their C-tails (Table 2), suggesting that regulation of GPCR trafficking by AP complexes may be more common than previously appreciated. Interestingly, mutation of a critical Tyr-459 residue found within a canonical Tyr-based motif of the M2 muscarinic cholinergic receptor C-tail impairs downregulation following prolonged agonist exposure but does not perturb receptor internalization (32). Similarly, mutation of the PAR1 C-tail proximal Tyr-based Y383SIL386 motif inhibits receptor degradation but has minimal effects on receptor endocytosis (33). PAR1 has two C-tail Tyr-based motifs that appear to mediate distinct trafficking events: The distal Y420KKL423 motif controls constitutive internalization, whereas the proximal Y383SIL386 motif regulates lysosomal sorting. These findings suggest that multiple motifs of the same sorting signal within the C-tail may confer distinct endocytic functions.
Table 2.
Alignment of human GPCR C-tail sequences containing tyrosine-based motifs
 |
A search for human GPCR C-tail sequences containing tyrosine-based motifs that conformed to Y-X-X-Ø, where Y denotes tyrosine, X is any amino acid, and Ø is a bulky hydrophobic residue (W, L, I, F, V, M) was completed as described in Supplemental Appendix 1. The acidic di-leucine-based motif is shaded in grey and the critical residues are shown in red or green. Abbreviations used: 5-HT, 5-hydroxytryptamine; GnRH, gonadotropin-releasing hormone; GLYC-H, glycoprotein-hormone; PAR, protease-activated receptor; TRH, thyrotropin-releasing hormone.
In addition to Tyr-based motifs, AP complexes can recognize di-Leu motifs, which have been implicated in mediating endocytosis of several mammalian GPCRs, including β2AR (34), and chemokine receptors CXCR4 and CXCR2 (35, 36). A CXCR2 di-Leu mutant interacts with arrestin but fails to bind AP-2 and to internalize in response to agonist, suggesting a role for AP-2 in di-Leu motif recognition (36). Arrestins also bind AP-2 and are likely to function cooperatively to promote endocytosis of many GPCRs containing di-Leu motifs. However, di-Leu-based motifs may function independent of arrestins to control endocytic sorting of other GPCRs. Interestingly, the AP complexes also recognize acidic di-Leu [D/E]-X-X-X-L-[L/I] motifs through α/σ2-subunit and possibly β-subunit interactions (25). Our search of human GPCR C-tail sequences revealed several receptors that harbor acidic di-Leu motifs that conform to both [D/E]-X-X-X-L-[L/I] and [D/E]-X-X-L-[L/I] types (Table 3), suggesting a function in GPCR trafficking. Indeed, a recent study identified an acidic di-Leu motif within the C-tail of the β2AR that appears to be important for controlling a regulated form of recycling (37, see Reference 38a for further discussion).
Table 3.
Alignment of human GPCR C-tails sequences containing acidic di-leucine based motifs
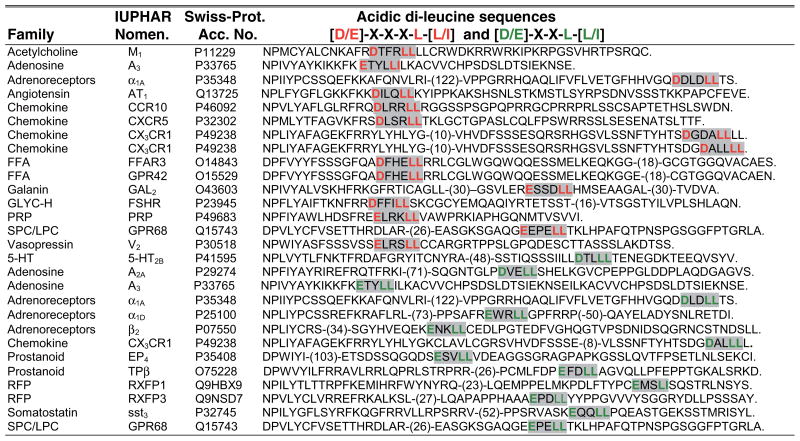 |
A search for human GPCR C-tail sequences containing acidic di-leucine based motifs that conform to [D/E]-X-X-X-L-[L/I] or [D/E]-X X-L-[L/I] was completed as described in Supplemental Appendix 1. The acidic di-leucine-based motif is shaded in grey and the critical residues are shown in red or green. Abbreviations used: FFA, free fatty acid; GLYC-H, glycoprotein-hormone; RFP, relaxin family peptide; PRP, prolactin-releasing peptide; SPC/LPC, (lyso)Phospholipid mediators; 5-HT, 5-hydroxytryptamine.
PDZ Ligands
PDZ domains are protein-protein recognition modules that bind to C-terminal short, linear PDZ ligand sequences that conform to type I (X-[S/T]-X-Ø), type II (X- Ø-X-Ø), or type III (X-[D/E]-X-Ø) sequences, but they can also recognize internal sequences that structurally mimic the C terminus (38). Although PDZ domain-containing proteins have established roles in localization and assembly of signaling complexes, recent studies suggest that they also function to control GPCR trafficking. In addition to the β2AR and mGluR (metabotropic glutamate receptor), our bioinformatic searches revealed several other human GPCRs with C-terminal type I, type II, and type III PDZ ligands (Table 4). One of the best-characterized roles for PDZ ligands in GPCR trafficking has been demonstrated for β2AR recycling (Figure 2) (see also 38a). Briefly, the β2AR has a C-terminal DSLL motif that conforms to a type I PDZ ligand, which controls receptor recycling (39). The β2AR PDZ ligand binds to NHERF (Na+/H+ exchanger regulatory factor)/EBP50 (ezrin/radixin/moesin-binding protein 50-kDa) family proteins (39, 40), and to NSF (N-ethylmaleimide-sensitive factor), although NSF lacks a PDZ domain (41). A type I PDZ ligand is also found in the β1AR and is capable of promoting PDZ domain interactions and receptor recycling (42). The β2AR displays a regulated form of recycling that not only requires the PDZ ligand, but is also dependent on HRS (hepatocyte growth factor–regulated tyrosine kinase substrate) (43). HRS is a modular protein that contains an N-terminal VHS domain, an FYVE domain important for PtdIns(3)P (phosphatidylinositol-3-phosphate) binding and endosomal localization, and an UIM (ubiquitin-interacting motif) that binds ubiquitin (44, 45). A recent study reported that a distinct type of acidic di-Leu (E371KENLL377) motif present in the β2AR C-tail is important for both HRS- and PDZ ligand-mediated recycling of the β2AR that requires the VHS domain (37), but how this occurs is not known. A subset of VHS domains have been shown to bind to acidic di-Leu motifs; however a direct interaction between the VHS domain of HRS and the acidic di-Leu motif of the β2AR is unlikely because the VHS domain lacks the critical residues important for binding to acidic di-Leu D-X-X-L-L motifs (46, 47). In addition to C-terminal PDZ ligands, one study has indicated that an internal PDZ ligand present in the ETA endothelin receptor C-tail can regulate receptor recycling (48), but the endocytic sorting machinery that mediates this process has not been identified. Interestingly, internal PDZ ligand sequences have also been identified in several other human GPCRs (48).
Table 4.
Alignment of human GPCR C-tail sequences containing PDZ ligands
 |
A search for human GPCR C-tail sequences containing PDZ ligands that conform to Type I X-[S/T]-X-[φ]; Type II X-[φ]-X- [φ]; Type III X-[D/E]-X-[φ], where X is any amino acid and φ is a bulky hydrophobic residue (W, L, I, F, V, M) was completed as described in Table 1. Of these several sequences were confirmed to be known GPCRs (excluding sensory and orphan receptors and psuedogenes) and aligned starting with the [N/D]PX2-3Y-like motif. The mGluR C-tail sequences were added separately. The PDZ ligand sequence is shaded in grey and the critical residues are shown in color. In some cases, numbers of amino acids were omitted either before or after the motif and are shown in parentheses. The period indicates the end of the C-tail sequence. The list includes the GPCR family name and nomenclature according to the International Union of Pharmacology (IUPHAR) (Foord et al. Pharmacol Rev. 2003, 55:587-9) and the Swiss-Protein accession number (http://ca.expasy.org/sprot/). Abbreviations used: 5-HT, 5-hydroxytryptamine; FFA, free fatty acid; LT/LX, leukotriene and lipoxin; LPL, lysophospholipid; MCH, melanin-concentrating hormone; PRP, prolactin-releasing peptide; mGluR, metabotropic-glutamate receptor.
In addition to recycling, PDZ ligands have been implicated in regulating endocytosis of certain GPCRs. A recent study showed that GPCR internalization is regulated by PDZ ligand interactions with the actin cytoskeleton. A δ-opioid receptor chimera with a non-native PDZ ligand sequence, as well as the β1AR and β2AR which naturally contain a PDZ ligand, displayed prolonged cell surface resident times and reduced rates of internalization compared to non-PDZ ligand containing GPCRs as measured by TIRF (total internal reflection fluorescence) microscopy (49). Moreover, linking GPCRs to cortical actin by fusion of the actin-binding domain of ezrin slowed internalization. The 5-HT2A (serotonin 5-hydroxytryptamine-2A) receptor C-tail PDZ ligand has also been shown to mediate interaction with PSD-95 (50). Binding of 5-HT2A to PSD-95 enhanced signaling and slowed receptor internalization, suggesting a role in GPCR endocytosis. Another recent study reported that the P2Y1 and P2Y12 purinergic GPCRs, which both possess type I PDZ ligands (Table 4), segregate from each other and internalize through distinct populations of CCPs (51). Although CCPs are known to form at preferential sites on the plasma membrane and are not likely to be induced by overexpressed cargo (52, 53), further studies are important to determine whether endogenous GPCRs behave similarly to their ectopically expressed counterparts.
UBIQUITIN AS AN ENDOCYTIC SORTING SIGNAL
Several studies have demonstrated diverse roles for ubiquitination in regulation of mammalian GPCR trafficking. An essential role for ubiquitination in lysosomal sorting of some mammalian GPCRs has been previously demonstrated (7, 54); in these cases ubiquitination is not required for internalization. However, ubiquitination has been shown to function indirectly to control endocytosis of other mammalian GPCRs (7). In yeast, ubiquitination of the Ste2 receptor promotes internalization (55). Interestingly, a recent study has revealed a novel function for ubiquitination in internalization of a mammalian GPCR (56).
The Ubiquitin Machinery
Modification of proteins by ubiquitin has important functions in proteasomal degradation, transcriptional regulation, signal transduction, and trafficking within the endocytic and biosynthetic pathways. Ubiquitin, a 76–amino acid protein, is covalently attached to proteins through the formation of an isopeptide bond between the C-terminus of ubiquitin and the ε-amino group of Lys residues on target proteins (Figure 3) (57). Ubiquitin contains seven Lys residues that can serve as acceptor sites for additional ubiquitin molecules. The fate of ubiquitinated proteins is determined in part by the length of the ubiquitin chain and on the configuration of ubiquitin-ubiquitin linkages. Chains of four or more ubiquitins, in which the C-terminus of one ubiquitin is attached to Lys-48 of the adjacent ubiquitin, are efficiently targeted to the 26S proteasome for degradation. By contrast, attachment of a single ubiquitin (mono-ubiquitination) to a target protein or attachment of ubiquitin to Lys-63 of an adjacent ubiquitin can have distinct cellular consequences, including endocytic sorting, but not proteasomal degradation.
Figure 3.
The ubiquitin modification of proteins. Ubiquitin (Ub) is attached to its target protein by the sequential action of E1, E2, and E3 enzymes. The E1 (activating enzyme) first activates ubiquitin in an ATP-dependent reaction by forming a thioester bond at its active-site cysteine with the COOH-terminus of ubiquitin. Ubiquitin is then transferred to the active site cysteine of the E2 (conjugating enzyme). The last step is catalyzed by either an E2 with the help of an E3 (RING-finger) or directly by an E3 (HECT-domain), leading to the transfer of ubiquitin to an epsilon amino group of a lysine residue on the target protein forming an isopeptide bond with the C-terminal glycine of ubiquitin. A single ubiquitin can be attached to proteins (Mono Ub) at a single and/or multiple lysine residues on the target protein. Alternatively multiple ubiquitins can be attached to one another forming poly Ub chains (Poly Ub), typically via lysine 48 or 63 linkages. Ubiquitin is removed from target proteins by the action of deubiquitinating enzymes (DUBs).
The attachment of ubiquitin to substrate proteins is carried out by an ATP-dependent mechanism that requires the sequential activity of three enzymes: E1 (ubiquitin-activating enzyme), E2 (ubiquitin-conjugating enzyme), and E3 (ubiquitin ligase) (Figure 3). The E3 ligase recognizes the substrate protein and provides specificity to the reaction, although a recent study showed that an E2 may also function in target recognition (58). E3 ubiquitin ligases can be divided into two major classes that contain either a HECT (homologous to E6-AP carboxyl terminus) domain or a RING (really interesting new gene) finger domain and differ in the mechanism by which ubiquitin is transferred to the target protein. A notable family of HECT E3 ubiquitin ligases involved in endocytic trafficking includes the Nedd4-like E3 family, which is comprised of nine family members (59). Rsp5, the only Nedd4 ortholog found in yeast Saccharomyces cerevisiae, is essential for endocytosis of several transmembrane proteins but has functions in other cellular processes (60). In contrast to the HECT domain E3s, the RING finger E3 ubiquitin ligases lack intrinsic catalytic activity and do not form a direct thiolester intermediate with ubiquitin but transfer ubiquitin to the target protein indirectly via the E2 (Figure 3) (61). The RING finger E3 ligase family is comprised of several hundred members that exist as single subunit E3s, including the MDM2 (mouse double minute-2) and Cbl (casitas B-cell lineage) proteins as well as multi-subunit E3s, including the SCF (Skp1-Cul1-F-box) proteins (62). E3 ligases in both classes mediate the ubiquitination of a diverse set of proteins involved in endosomal-lysosomal sorting of membrane proteins (63).
Ubiquitin-conjugation of proteins is a highly dynamic and reversible process. DUBs (deubiquitinating enzymes) are proteases that specifically cleave the isopeptide bond that links ubiquitin chains and ubiquitin protein conjugates. A large and diverse family of DUBs, comprised of ~90 functional members, are encoded in the human genome (64). In general, deubiquitination of substrate proteins by UCHs (ubiquitin carboxyl terminal hydrolases) is important for recycling ubiquitin from substrate proteins before degradation. In contrast, USPs (ubiquitin-specific proteases) are likely to recognize specific substrate proteins and serve important functions in modulating activity of proteins, some of which are directly involved in protein trafficking. A Drosophila deubiquitinating enzyme Fat facets/USP9X has been shown to regulate Delta/Notch receptor internalization by deubiquitinating Liquid facets, a homologue of epsin, a component of the clathrin-endocytic machinery (65). Two other deubiquitinating enzymes, UBPY/USP8 and AMSH (associated-molecule with the SH3 domain of STAM), interact with STAM (signal transducing adaptor molecule), an endosomal adaptor protein that binds to HRS to control the rate of EGFR lysosomal sorting and degradation (66, 67). Moreover, some DUBs are complexed with E3 ubiquitin ligases and appear to regulate E3 activity and stability in addition to substrate proteins, adding another level of complexity to ubiquitin-dependent regulation of protein trafficking (68, 69).
Ubiquitin As an Internalization Signal
In yeast, GPCR internalization is mediated by direct ubiquitination. Upon activation, Ste2 and Ste3 receptors are phosphorylated and rapidly ubiquitinated (55, 70). Studies using yeast strains that lack specific ubiquitin-conjugating enzymes and ubiquitin defective-Ste2 mutants indicate that ubiquitination is both necessary and sufficient for constitutive and agonist-induced receptor internalization (55, 71). Although a single ubiquitin moiety appears to be sufficient to drive Ste2 internalization (71), the attachment of short ubiquitin chains to Lys-63 of an adjacent ubiquitin facilitates endocytosis of many integral membrane proteins (72). The ubiquitin moiety on receptors serves as an internalization signal by binding to UBDs (ubiquitin-binding domains) of endocytic adaptor proteins and thereby facilitates transport through the endocytic pathway (for more information on UBDs, see the sidebar Ubiquitin-Binding Domains). Yeast do not express arrestin proteins. Instead, the clathrin adaptors Ent1 and Ent2 (epsin homologs) and Ede1 (eps15 homolog) harbor UBDs that recognize the ubiquitinated Ste2 receptor and facilitate internalization (73).
UBIQUITIN-BINDING DOMAINS
Ubiquitin-binding domains (UBDs) are a large class of protein modules that bind noncovalently to ubiquitin or polyubiquitin chains and include UIM, CUE, UBA, VHS, GAT, NZF, PAZ, UEV, and GLUE domains (115). UBDs are structurally diverse and vary in length from 20 to 150 amino acids; however, most bind to a critical hydrophobic patch on ubiquitin, which centers on the Ile-44 (115). UBDs are found in hundreds of proteins and many proteins carry multiple copies of UBDs. The binding of UBDs to ubiquitin is generally moderate to weak, with apparent dissociation constants in the micromolar range, but it is biologically relevant and allows for dynamic assembly and disassembly of protein complexes. Several proteins involved in endocytic sorting contain UBDs and are themselves ubiquitinated, including HRS, TSG101, epsin, and eps15, thereby providing a complex level of regulation in the transport process. HRS is the only ubiquitinated adaptor protein that contains UBDs and functions in mammalian GPCR trafficking (43, 78, 116), but others await discovery.
Recent work has revealed a direct role for ubiquitination in the regulation of mammalian GPCR internalization (56). PAR1 appears to be ubiquitinated under basal conditions and deubiquitinated following activation (Figure 2). Constitutive internalization of an ubiquitin-deficient PAR1 lysine-less mutant is enhanced, whereas fusion of ubiquitin to the C-tail of ubiquitin-deficient PAR1 inhibited constitutive internalization. These findings suggest that ubiquitination negatively regulates PAR1 constitutive internalization. Moreover, the major sites of PAR1 ubiquitination occur at the highly conserved Lys-421 and Lys-422 residues localized within the C-tail Tyr-based motif, the critical binding site of the μ2 subunit of AP-2 (24, 56). These findings suggest that ubiquitination of PAR1 may preclude AP-2 binding. These studies further demonstrate that ubiquitination of PAR1 specifies a distinct clathrin adaptor requirement for activated receptor internalization that functions independent of arrestins and AP-2.
Ubiquitin-Dependent Targeting to Lysosomes
Sorting of ubiquitinated cargo from early endosomes to lysosomes involves transit through a unique endosomal compartment that contains intralumenal vesicles, termed the MVBs (multivesicular bodies). MVBs fuse with lysosomes, resulting in the degradation of lipids and proteins (Figure 2). The ESCRT machinery that mediates sorting of ubiquitinated transmembrane proteins was originally discovered in Saccharomyces cerevisiae using genetic screens to identify proteins involved in sorting of biosynthetic and endocytic cargo to the vacuole/lysosome (2). The ESCRT machinery is conserved in mammalian cells, although mammalian cells have a greater diversity and specialization in endocytic sorting pathways. HRS is recruited to endosomes in part via its FYVE domain, which binds to PtdIns(3)P, a lipid enriched on endosomal membranes. HRS recruits clathrin to discrete microdomains that form flat clathrin lattices on endosomal membranes where proteins destined for degradation are concentrated (45, 74). HRS binds directly to ubiquitinated cargo on endosomes and to TSG101, a component of ESCRT-I. ESCRT-II and -III complexes are then recruited sequentially to endosomes and act downstream of ESCRT-I (a topic recently reviewed in 75). The ESCRT complexes function together to coordinate the recruitment and sorting of ubiquitinated cargo and MVB formation. Cargo is then deubiquitinated before entry into MVBs. The activity of VPS4 (vacuolar protein sorting), an AAA-ATPase that catalyzes the disassembly of the ESCRT components, is also important for sorting cargo into the MVB and for the formation of intralumenal vesicles (76, 77). In addition to transmembrane proteins in yeast, several mammalian GPCRs are targeted to lysosomes through an ubiquitin- and ESCRT-dependent mechanism.
The β2AR was the first mammalian GPCR shown to undergo agonist-dependent ubiquitination and receptor degradation (Figure 2) (7). A β2AR mutant receptor in which all Lys residues were converted to Arg internalized normally but failed to downregulate, as measured by a loss in the number of receptor binding sites following prolonged agonist exposure. Interestingly, activation of the β2AR results in rapid ubiquitination, which peaks within 1 h but is considerably diminished by 2 h, although a significant loss in receptor binding sites is not detectable until much later. The mechanistic basis for the delay in β2AR degradation following ubiquitination is not known. Agonist-induced ubiquitination of the β2AR requires phosphorylation and arrestin binding. A mutant β2AR defective in PKC and GRK phosphorylation sites, which does not bind arrestins, fails to undergo agonist-promoted ubiquitination and degradation, suggesting that arrestins are critical for activated receptor ubiquitination (7). Indeed, agonist-induced β2AR ubiquitination is lost in arrestin-3 null mouse embryonic fibroblasts and restored upon arrestin-3, but not arrestin-2, expression (7). Interestingly, ubiquitination of arrestin-3 by the E3 ubiquitin ligase MDM2 is essential for β2AR internalization; however, MDM2 is not responsible for β2AR ubiquitination. These studies establish an important role for arrestin-3 in β2AR ubiquitination and degradation but the E3 ubiquitin ligase and endocytic sorting machinery that mediate ubiquitin-dependent lysosomal degradation of the β2AR have yet to be identified.
The mechanism by which ubiquitination regulates lysosomal sorting and degradation of a mammalian GPCR is best understood for CXCR4. Activated CXCR4 is rapidly ubiquitinated, sorted to lysosomes and degraded within hours (Figure 2) (54). Sorting of activated CXCR4 to lysosomes is mediated by ubiquitination of Lys residues within a C-tail 324SSLKILSKGK333 degradation motif. The HECT domain Nedd4-like E3 ubiquitin ligase AIP4, also known as Itch, colocalizes with CXCR4 at the plasma membrane and mediates receptor ubiquitination upon agonist stimulation (78). Interestingly, mutation of Ser residues within the putative degradation motif attenuates agonist-promoted CXCR4 degradation, suggesting that phosphorylation functions in receptor ubiquitination.
Although ubiquitination of CXCR4 occurs at the plasma membrane, it functions as an endosomal sorting signal to facilitate CXCR4 transport to MVBs and degradation (23, 78). Activated CXCR4 colocalizes with HRS and the E3 ligase AIP4 on endosomal microdomains (78). Interestingly, AIP4 not only ubiquitinates CXCR4 but also mediates HRS ubiquitination following agonist exposure, suggesting that ubiquitination of HRS is important for CXCR4 endosomal sorting. Moreover, the endosomal function of AIP4 is negatively regulated by phosphorylation mediated by CISK (23), a Ser/Thr kinase activated downstream of PI(phosphoinositide)-3-kinase. CISK colocalizes with AIP4 on endosomal membranes and phosphorylates specific residues within the WW domains of AIP4 (23). The mechanism by which phosphorylation impairs the ability of AIP4 to facilitate CXCR4 lysosomal sorting is not known, but may involve disruption of the capacity of AIP4 to recognize, and ubiquitinate target proteins (23). Finally, CXCR4 deubiquitination and activity of the ATPase of VPS4 is required for efficient lysosomal sorting and degradation (78). A VPS4 mutant that prevents disassembly and release of the ESCRT complexes from endosomal membranes blocks CXCR4 degradation (78). Moreover, the abundance of ubiquitinated CXCR4 and HRS species are increased considerably when VPS4 activity is disrupted, suggesting that deubiquitination of CXCR4 and HRS is important for efficient lysosomal sorting and degradation. Consistent with this observation, the deubiquitinating enzyme AMSH directly binds to the CaR (calcium-sensing receptor) C-tail and enhances agonist-induced receptor degradation (79), suggesting that the deubiquitination of CaR and/or an adaptor protein is important for lysosomal degradation.
In addition to the β2AR and CXCR4, many other GPCRs are modified with ubiquitin and degraded in an agonist-dependent manner, suggesting a more general role for ubiquitination in GPCR trafficking. Indeed, activation induces ubiquitination and degradation of the V2R (80), sst3 somatostatin receptor (81), PAR2 (82), NK1R (83), and the S1P (sphingosine 1-phosphate) receptor (84). Although the PAFR (platelet-activating factor receptor) is basally ubiquitinated, agonist-promoted PAFR degradation may require ubiquitination (85). PAR2 ubiquitination and lysosomal sorting appears to be regulated by c-Cbl, an E3 RING finger ubiquitin ligase (82). A deletion mutant of c-Cbl lacking the RING finger domain acts as a dominant-negative and inhibits activated PAR2 ubiquitination and degradation. Ubiquitination of activated PAR2 by c-Cbl appears to be mediated by a Src-dependent mechanism, but whether PAR2 directly binds to c-Cbl or requires an intermediate protein is not known.
UBIQUITIN AND TRAFFICKING THROUGH THE BIOSYNTHETIC PATHWAY
GPCR ubiquitination also has an important role in quality control during biosynthesis and functions to sort misfolded receptors to the ERAD [endoplasmic reticulum (ER)-associated protein degradation] pathway that involves degradation by the proteasome, a large multiprotein complex localized in the cytoplasm or nucleus. Polyubiquitination and degradation of misfolded GPCRs by the proteasome has been demonstrated for the δ-opioid receptor (86), rhodopsin (87, 88), TRHR (thyrotropin-releasing hormone receptor) (89), and the CaR (90). The E3 ubiquitin ligase dorfin (double-RING finger protein) binds to the C-tail of the CaR to possibly mediate its ubiquitination and degradation by the proteasome during biosynthesis. Siah1A (seven in absentia homolog 1A), a RING finger E3 ubiquitin ligase, mediates ubiquitination and degradation of the long splice forms of mGluR1 and mGluR5 (91), but whether this occurs through a proteasomal or lysosomal pathway has not been clearly established. Ubiquitination of the FSHR (follitropin receptor) appears to regulate cell surface expression but does not affect the rate of FSHR internalization (92), suggesting a function in trafficking through the biosynthetic pathway. In addition to ubiquitination, deubiquitination of GPCRs during biosynthesis regulates ER quality control and increases receptor surface expression. The A2A adenosine receptor binds directly to USP4, a deubiquitinating enzyme, which results in enhanced cell surface expression of functionally active receptor (93).
UBIQUITIN AND ESCRT-INDEPENDENT LYSOSOMAL SORTING
Several mammalian GPCRs have been reported to sort through the endosomal-lysosomal system independent of ubiquitination and some components of the ESCRT machinery. A mutant DOR (δ-opioid receptor) in which all intracellular Lys were changed to Arg undergoes agonist-promoted degradation similar to the wild-type receptor, suggesting that DOR ubiquitination is not required for efficient lysosomal sorting (94). Although degradation of activated DOR is not dependent on ubiquitination, lysosomal sorting occurs through an HRS- and VPS4-dependent pathway, similar to CXCR4. However, degradation of DOR does not require TSG101, a component of the ESCRT-I complex, which binds and sorts ubiquitinated cargo. Interestingly, lysosomal sorting of DOR is mediated by direct interaction with GASP (GPCR-associated sorting protein) (95). GASP is a large 1395–amino acid cytoplasmic protein that lacks any known protein-protein interaction domains but contains 22 stretches of 15 acidic residues in the N-terminal region and a conserved C-terminal domain that is found in 10 related genes (96). GASP binds to many GPCRs, including those that internalize and efficiently recycle and/or sort through an ubiquitin-dependent lysosomal degradation pathway, such as the β2AR (95–97). Interestingly, a recent study indicates that GASP binds to a conserved region within the putative eighth α-helix of the DOR and β1AR (96), but how GASP functions precisely to regulate GPCR sorting within the endosomal-lysosomal system is not known (see Reference 38a for further discussion).
PAR1 also sorts from endosomes to lysosomes independent of ubiquitination, HRS, and TSG101 (56, 98), suggesting that PAR1 is targeted to lysosomes through an ESCRT-independent pathway. A recent study showed that PAR1 is deubiquitinated following activation and an ubiquitin-defective mutant is degraded comparably to the wild-type receptor (56). These findings indicate that deubiquitinated rather than ubiquitinated PAR1 transits through the endosomal-lysosomal system. The sorting of activated PAR1 to lysosomes is dependent, however, on SNX1 (sorting nexin-1), a protein that localizes to early endosomes and is known to function in membrane trafficking (99) (Figure 2). Agonist-induced PAR1 lysosomal degradation was impaired by ablation of endogenous SNX1 expression and disruption of SNX1 localization (98, 98a). In addition to PAR1, an in vitro protein-protein interaction screen using SNX1 and a library of 59 GPCR C-tails revealed that 10 different GPCRs are capable of interacting with SNX1 (97). SNX1 contains a PX (phox homology) domain that binds phosphoinositides and a C-terminal BAR (Bin/Amphiphysin/Rvs) domain that allows SNX1 to dimerize and to sense membrane curvature (99). SNX1 is likely to regulate sorting of PAR1 early in the endocytic pathway. SNX1 binds to the tubular portion of early endosomes and forms oligomers that facilitate the pinching off of endosomal tubules through a process that likely involves other as-yet unidentified endocytic proteins (99). The identities of other endocytic adaptor proteins that mediate ubiquitin-independent lysosomal sorting of PAR1 remain to be determined.
PALMITOYLATION AND GPCR TRAFFICKING
Palmitoylation is a regulated and reversible process that has been reported to affect GPCR signaling and trafficking. S-palmitoylation of GPCRs occurs through the covalent attachment of a C16 fatty-acid chain via a thiolester linkage to Cys residues localized within the C-tail of a GPCR and was first reported for the rhodopsin receptor (100). The atomic resolution structure of bovine rhodopsin revealed that palmitoylation contributes to the formation of a fourth intracellular loop that adopts an α-helical structure (101). A large number of GPCRs appear to be modified with palmitoylation, suggesting a general posttranslational modification. A mutant β2AR in which Cys-341 was mutated to Gly is not palmitoylated and displays hyperphosphorylation, constitutive desensitization, and internalization (102), suggesting that depalmitoylation promotes receptor phosphorylation. Similar findings were observed with the A3 adenosine receptor and luteinizing hormone receptor, but this is clearly not the case for all GPCRs (103). Indeed, a mutant form of the chemokine CCR5 receptor that is not palmitoylated shows a decrease in agonist-promoted phosphorylation and internalization (104). Moreover, palmitoylation appears to regulate GPCR stability. An A1 adenosine receptor mutant defective in palmitoylation displayed an increase in receptor degradation during biogenesis but showed minimal effects on agonist-promoted G-protein activation, internalization or downregulation (105). A palmitoylation-defective CCR5 receptor mutant was fully functional but showed decreased surface expression as a result of increased degradation that appears to be mediated through a lysosomal degradation pathway (106), but whether this involves trafficking from the cell surface or via the trans-Golgi network to lysosomes was not determined. Recent studies examining the protein stability of SNAREs (soluble N-ethylmaleimide sensitive factor attachment protein receptors) in yeast (107) and the anthrax toxin in mammalian cells (108) indicate that palmitoylation protects these proteins from ubiquitination and subsequent degradation. The function of palmitoylation in regulation of GPCR ubiquitination, or vice versa, has not been explored and deserves further investigation.
DYSREGULATED GPCR TRAFFICKING AND DISEASE
Dysregulated GPCR trafficking is associated with several human diseases. Patients with nephrogenetic diabetes insipidus show reduced or complete loss of renal epithelial V2R surface expression that has been attributed to multiple V2R mutations that impair trafficking through the biosynthetic pathway (see review in 109). Similarly, mutations in the GnRH (gonadotropin-releasing hormone receptor) and rhodopsin result in defective trafficking to the cell surface and associated human pathophysiological diseases. In addition, many GPCRs are overexpressed in human cancers and contribute to tumor progression (a topic recently reviewed in 110). Recent work has revealed that dysregulated trafficking of CXCR4 and PAR1 within the endosomal-lysosomal system contributes to increased surface expression in breast cancer cells and cancer progression. A recent study showed that ErbB2 (HER2), an oncogenic tyrosine kinase, which is overexpressed in ~30% of breast cancers, impairs CXCR4 degradation and thereby increases its surface expression (111). ErbB2 enhances CXCR4 expression by increasing protein synthesis and by impairing CXCR4 ubiquitination and lysosomal degradation through a mechanism that may involve PI-3-kinase activity. As discussed above, CISK is activated by PI-3-kinase signaling and blocks AIP4-mediated lysosomal sorting and degradation of CXCR4 (23), raising the possibility that CISK may contribute to elevated CXCR4 expression in breast cancer. PAR1 is also overexpressed in malignant breast cancer (112), and can promote tumor progression through multiple mechanisms (see recent review in 113). In addition to PAR1 overexpression, tumor cells display aberrant PAR1 trafficking, which causes persistent signaling and cellular invasion (114). These defects appear to be specific to breast cancer cells, because PAR1 in normal human mammary epithelial cells displays proper trafficking and signal termination. However, the mechanisms responsible for defective PAR1 trafficking in breast carcinoma remain to be defined.
CONCLUSIONS AND FUTURE PERSPECTIVES
The GPCR family transduces signals of diverse extracellular stimuli and controls a large number of physiological responses. Rapid desensitization and receptor trafficking tightly control the temporal and spatial regulation of GPCR signaling. Given the vast number and diversity of GPCRs, multiple complex mechanisms likely function to control sorting of distinct GPCRs within the endosomal-lysosomal system. Many studies have advanced our understanding of the molecular mechanisms that control GPCR internalization, recycling, and lysosomal sorting. The discovery that certain GPCRs are recruited to CCPs and internalize independent of arrestins led to the suggestion that there are other clathrin adaptor proteins that have critical functions in receptor endocytosis. The presence of short, linear peptide sequences that are recognized by distinct adaptor proteins within the C-tails of many GPCRs further suggests that diverse pathways exist for endocytic sorting of certain GPCRs. Indeed, recent studies have shown a function for C-tail PDZ ligand sequences in GPCR recycling and internalization. Despite many advances in understanding the function of sorting signals present in the C-tail of GPCRs, fundamental questions remain regarding the endocytic sorting machinery and regulatory mechanisms that control trafficking of most GPCRs.
The discovery that mammalian GPCRs are ubiquitinated has added a new challenge to understanding the mechanisms involved in regulation of GPCR signaling and trafficking. One function of GPCR ubiquitination is to regulate receptor trafficking to lysosomes, but more questions remain regarding the nature of the ubiquitin-dependent sorting machinery and how these dynamic processes are regulated. Recent studies have identified a novel function for ubiquitination in negative regulation of GPCR internalization, suggesting additional functions for ubiquitination in the control of GPCR trafficking. GPCR ubiquitination is also likely to regulate multiple protein-protein interactions and to impact diverse GPCR functions. Future studies that assess how ubiquitin functions and the mechanisms controlling GPCR ubiquitination should continue to yield novel insights. In addition to ubiquitin, posttranslational modification of GPCRs with ubiquitin-like proteins (Ubls), such as sumoylation, is known to occur (118). The extent GPCR modification with Ubls is not known but is likely to occur and to profoundly effect receptor function (for more information on Ubls, see the sidebar Ubiquitin-Like Proteins).
UBIQUITIN-LIKE PROTEINS
In addition to ubiquitin, a number of ubiquitin-like proteins (Ubls), including SUMO, ISG15, Nedd8, and Atg8, have been found to function in protein modifications (117). Although ubiquitin and Ubls do not share much primary sequence similarity, all Ubls possess essentially the same three-dimensional structure, the ubiquitin or β-grasp fold. All Ubls appear to be covalently linked to substrates via related enzymatic reactions as described for ubiquitination above. The SUMO consensus site [I/V/L]-K-X-[D/E] is a binding site for the E2 SUMO-conjugating enzyme Ubc9. SUMO E2 and E1 are sufficient for sumoylation of target proteins in vitro without an E3, but whether this occurs in vivo is not known. Unlike ubiquitin modification of GPCRs, the function of Ubls in GPCR signaling and trafficking remains relatively unexplored. Sumoylation is known to regulate transcriptional activity and recent findings suggest a broader role in cell functions outside the nucleus. Pias1, a SUMO E3 ligase, was found to bind to the mGluR8 C-tail region and mGluR8 was shown to be sumoylated at Lys-882 within a consensus sequence, but the mechanism and function of mGluR8 sumoylation remains to be determined (118).
Given the importance of GPCR trafficking in the temporal and spatial control of signaling, it is critical to understand. Identifying the molecular machinery that regulates endocytic sorting of distinct GPCRs will enable the development of new strategies to manipulate receptor signaling and will provide novel targets for the development of drugs that can be used in the prevention and treatment of a wide range of human diseases. In addition, GPCRs are allosteric proteins that adopt many distinct conformations, some of which promote receptor internalization independent of G-protein activation (118a). The identification of new drugs (termed allosteric modulators) that promote GPCR internalization independent of cellular signaling could be potentially useful therapeutics for certain disease scenarios. It will be important to determine how some current drugs modulate GPCR trafficking to provide a better understanding of their beneficial and untoward effects.
SUMMARY POINTS
The sorting of activated GPCRs to lysosomes for degradation occurs through ubiquitin-dependent and -independent pathways.
The recycling of GPCRs occurs through bulk membrane flow or through a regulated process that involves PDZ ligand sequences.
The sorting of GPCRs on endosomes is a highly regulated process involving many proteins and it controls the amount of receptor present on the cell surface and hormone responsiveness.
Short, linear peptide sequences and/or posttranslational modifications determine the endocytic pathway followed by GPCRs.
Posttranslational modifications of GPCRs regulate the quality control and trafficking within the early biosynthetic pathway.
Supplementary Material
Acknowledgments
We apologize to those colleagues whose original and important work could not be cited owing to space limitations. We thank Dayle Houston and members of our laboratories for critical readings of this manuscript. This work is supported by NIH HL073328, an American Heart Association Established Investigator Award and a Susan G. Komen Breast Cancer Foundation Award (J.T.), and an American Heart Association Scientist Development Grant and NIH GM075159 (A.M.). Brenda R.S. Temple is a member of the R. J. Juliano Structural Bioinformatics Core Facility.
- Clathrin-coated pits (CCPs)
150-nm structures that occupy ~2% of the plasma membrane and serve as the major route for internalization in animal cells
- Endosomal-sorting complex required for transport (ESCRT)
endosomal protein complexes originally identified in yeast that support transport of ubiquitinated cargo into intraluminal vesicles of multivesicular bodies
- Adaptor protein complex-2 (AP-2)
a clathrin adaptor complex composed of α, β2, μ2, and σ2 subunits, which recognize short linear peptide sequences
- CISK
cytokine-independent survival kinase
- AIP4
atrophin-interacting protein-4
- Downregulation
a process in which the cell decreases the number of receptors to a given hormone to reduce its sensitivity to this molecule
- NSF
N-ethylmaleimide sensitive factor
- HRS
hepatocyte growth factor-regulated tyrosine kinase substrate
- Total internal reflection fluorescence (TIRF) microscopy
a microscopy technique that increases the spatial resolution of specimens located at or within 100 nm of the plasma membrane
- DUB
deubiquitinating enzyme
- AMSH
associated-molecule with the SH3 domain of STAM
- STAM
signal transducing adaptor molecule
- Multivesicular bodies (MVBs)
a distinct endosomal compartment containing intralumenal vesicles that fuse with lysosomes resulting in degradation of vesicles and transmembrane proteins
- TSG101
tumor suppressor gene product 101
- GASP
GPCR-associated sorting protein
- SNX1
sorting nexin-1
- Desensitization
a process that results in the loss of responsiveness of a signaling system to the continuing or increasing dose of a hormone or drug
- SNARE
soluble N-ethylmaleimide sensitive factor attachment protein receptor
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8:1–9. doi: 10.1111/j.1600-0854.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 2.Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–55. doi: 10.1016/s0092-8674(01)00434-2. Excellent comprehensive review on the ubiquitin-dependent endosomal sorting required for transport (ESCRT) machinery. [DOI] [PubMed] [Google Scholar]
- 3.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–17. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch JA, Schubert C, Gurevich V, Sigler PA. The 2.8 A crystal structure of visual arrestin: a model for arrestin’s regulation. Cell. 1999;97:257–69. doi: 10.1016/s0092-8674(00)80735-7. [DOI] [PubMed] [Google Scholar]
- 5.Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, et al. β-arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature. 1996;383:447–50. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 6.Laporte S, Oakley RH, Zhang J, Holt JA, Ferguson SSG, et al. The β2-adrenergic receptor/β-arrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA. 1999;96:3712–17. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-arrestin. Science. 2001;294:1307–13. doi: 10.1126/science.1063866. First demonstration that ubiquitination mediates downregulation of a mammalian GPCR and that the endocytic function of arrestins is regulated by transient ubiquitination. [DOI] [PubMed] [Google Scholar]
- 8.Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, et al. Regulation of β-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase-2. Cell. 2007;129:511–22. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 9.Gurevich VV, Gurevich EV. The structural basis of arrestin-mediated regulation of G protein-coupled receptors. Pharmacol Ther. 2006;110:465–502. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, β-arrestin1, and β-arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–10. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 11.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-β-arrestin complexes after receptor endocytosis. J Biol Chem. 2001;276:19452–60. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- 12.Shenoy SK, Lefkowitz RJ. Trafficking patterns of β-arrestin and G protein-coupled receptors determine the kinetics of β-arrestin deubiquitination. J Biol Chem. 2003;278:14498–506. doi: 10.1074/jbc.M209626200. [DOI] [PubMed] [Google Scholar]
- 13.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of β-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248–57. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 14.Tohgo A, Choy EW, Getsy-Palmer D, Pierce KL, Laporte SA, et al. The stability of the G protein-coupled receptor-β-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem. 2003;278:6258–67. doi: 10.1074/jbc.M212231200. [DOI] [PubMed] [Google Scholar]
- 15.Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. β-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem. 2002;277:9429–36. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- 16.DeFea KA, Zalevski J, Thoma MS, Dery O, Mullins RD, Bunnett NW. β-arrestin-dependent endocytosis of proteinase-activated receptor-2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–81. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stalheim L, Ding Y, Gullapalli A, Paing MM, Wolfe BL, et al. Multiple independent functions of arrestins in regulation of protease-activated receptor-2 signaling and trafficking. Mol Pharm. 2005;67:1–10. doi: 10.1124/mol.104.006072. [DOI] [PubMed] [Google Scholar]
- 18.Vines CM, Revankar CM, Maestas DC, LaRusch LL, Cimino DF, et al. N-formyl peptide receptors internalize but do not recycle in the absence of arrestins. J Biol Chem. 2003;278:41581–84. doi: 10.1074/jbc.C300291200. [DOI] [PubMed] [Google Scholar]
- 19.Mace G, Miaczynska M, Zerial M, Nebreda AR. Phosphorylation of EEA1 by p38 MAP kinase regulates μ opioid receptor endocytosis. EMBO J. 2005;24:3235–46. doi: 10.1038/sj.emboj.7600799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christoforidis S, McBride H, Burgoyne R, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–25. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- 21.Seachrist JL, Anborgh PH, Ferguson SSG. β2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling by Rab GTPases. J Biol Chem. 2000;275:27221–28. doi: 10.1074/jbc.M003657200. [DOI] [PubMed] [Google Scholar]
- 22.Iwata K, Ito K, Fukazaki A, Inaki K, Haga T. Dynamin and rab5 regulate GRK2-dependent internalization of dopamine D2 receptors. Eur J Biochem. 1999;283:596–602. doi: 10.1046/j.1432-1327.1999.00549.x. [DOI] [PubMed] [Google Scholar]
- 23.Slagsvold T, Marchese A, Brech A, Stenmark H. CISK attenuates degradation of the chemokine receptor CXCR4 via the ubiquitin ligase AIP4. EMBO J. 2006;25:3738–46. doi: 10.1038/sj.emboj.7601267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paing MM, Johnston CA, Siderovski DP, Trejo J. Clathrin adaptor AP2 regulates thrombin receptor constitutive internalization and endothelial cell resensitization. Mol Cell Biol. 2006;28:3221–42. doi: 10.1128/MCB.26.8.3231-3242.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 26.Paing MM, Stutts AB, Kohout TA, Lefkowitz RJ, Trejo J. β-arrestins regulate protease-activated receptor-1 desensitization but not internalization or down-regulation. J Biol Chem. 2002;277:1292–300. doi: 10.1074/jbc.M109160200. [DOI] [PubMed] [Google Scholar]
- 27.Hein L, Ishii K, Coughlin SR, Kobilka BK. Intracellular targeting and trafficking of thrombin receptors: a novel mechanism for resensitization of a G protein-coupled receptor. J Biol Chem. 1994;269:27719–26. [PubMed] [Google Scholar]
- 28.Parent JL, Lebrecque P, Rochdi MD, Benovic JL. Role of the differentially spliced carboxyl terminus in thromboxane A2 receptor trafficking. J Biol Chem. 2001;276:7079–85. doi: 10.1074/jbc.M009375200. [DOI] [PubMed] [Google Scholar]
- 29.Royle SJ, Qureshi OS, Bobanovic LK, Evans PR, Owen D, Murrell-Lagnado RD. Non-canonical YXXGΦ endocytic motifs: recognition by AP2 and preferential utilization in P2×4 receptors. J Cell Sci. 2005;118:3073–80. doi: 10.1242/jcs.02451. [DOI] [PubMed] [Google Scholar]
- 30.Diviani D, Lattion A-L, Abuin L, Staub O, Cotecchia S. The adaptor complex 2 directly interacts with the α1b-adrenergic receptor and plays a role in receptor endocytosis. J Biol Chem. 2003;278:19331–40. doi: 10.1074/jbc.M302110200. [DOI] [PubMed] [Google Scholar]
- 31.Diviani D, Lattion AL, Larbi N, Kunapuli P, Pronin A, et al. Effect of different G protein-coupled receptor kinases on phosphorylation and desensitization of the α1B-adrenergic receptor. J Biol Chem. 1996;271:5049–58. doi: 10.1074/jbc.271.9.5049. [DOI] [PubMed] [Google Scholar]
- 32.Goldman PS, Nathanson NM. Differential role of the carboxyl-terminal tyrosine in down-regulation and sequestration of the m2 muscarinic acetylcholine receptor. J Biol Chem. 1994;269:15640–45. [PubMed] [Google Scholar]
- 33.Paing MM, Temple BRS, Trejo J. A tyrosine-based sorting signal regulates intracellular trafficking of protease-activated receptor-1: multiple regulatory mechanisms for agonist-induced G protein-coupled receptor internalization. J Biol Chem. 2004;279:21938–47. doi: 10.1074/jbc.M401672200. [DOI] [PubMed] [Google Scholar]
- 34.Gabilondo AM, Hegler J, Krasel C, Boivin-Jahns V, Hein L, Lohse MJ. A dileucine motif in the C-terminus of the β2-adrenergic receptor is involved in receptor internalization. Proc Natl Acad Sci USA. 1997;94:12285–90. doi: 10.1073/pnas.94.23.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orsini MJ, Parent JL, Mundell SJ, Marchese A, Benovic JL. Trafficking of the HIV coreceptor CXCR4: role of arrestins and identification of residues in the C-terminal tail that mediate receptor internalization. J Biol Chem. 2000;274:31076–86. doi: 10.1074/jbc.274.43.31076. [DOI] [PubMed] [Google Scholar]
- 36.Fan G-H, Yang W, Wang X-J, Qian Q, Richmond A. Identification of a motif in the carboxyl terminus of CXCR2 that is involved in adaptin 2 binding and receptor internalization. Biochemistry. 2001;40:791–800. doi: 10.1021/bi001661b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanyaloglu AC, von Zastrow M. A novel sorting sequence in the β2-adrenergic receptor switches recycling from default to the Hrs-dependent mechanism. J Biol Chem. 2007;282:3095–104. doi: 10.1074/jbc.M605398200. [DOI] [PubMed] [Google Scholar]
- 38.Hillier BJ, Christopherson KS, Prehoda KE, Bredt DS, Lim WA. Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS-syntrophin complex. Science. 1999;284:812–15. [PubMed] [Google Scholar]
- 38a.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48 doi: 10.1146/annurev.pharmtox.48.113006.094830. In press. [DOI] [PubMed] [Google Scholar]
- 39.Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β 2-adrenergic receptor. Nature. 1999;401:286–90. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- 40.Hall RA, Premont RT, Chow C-W, Blitzer JT, Pitcher JA, et al. The β2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998;392:626–30. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- 41.Cong M, Perry SJ, Hu LA, Hanson PI, Claing A, Lefkowitz RJ. Binding of the β2 adrenergic receptor to N-ethylmaleimide-sensitive factor regulates receptor recycling. J Biol Chem. 2001;276:45145–52. doi: 10.1074/jbc.M106087200. [DOI] [PubMed] [Google Scholar]
- 42.Gage RM, Matveeva EA, Whiteheart SW, von Zastrow M. Type I PDZ ligands are sufficient to promote rapid recycling of G protein-coupled receptors independent of binding to NSF. J Biol Chem. 2004;280:3305–13. doi: 10.1074/jbc.M406934200. [DOI] [PubMed] [Google Scholar]
- 43.Hanyaloglu AC, McCullagh E, von Zastrow M. Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling. EMBO J. 2005;24:2265–83. doi: 10.1038/sj.emboj.7600688. Demonstrates that HRS, an endosomal protein typically involved in ubiquitin-dependent sorting into the MVB, also mediates recycling of the β2AR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raiborg C, Bache KG, Gilooly DJ, Madshus IH, Stang E, Stenmark H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol. 2002;4:394–98. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- 45.Raiborg C, Bremnes B, Mehlum A, Gillooly DJ, D’Arrigo A, et al. FYVE and coiled-coil domains determine the specific localisation of Hrs to early endosomes. J Cell Sci. 2001;114:2255–63. doi: 10.1242/jcs.114.12.2255. [DOI] [PubMed] [Google Scholar]
- 46.Misra S, Puertollano R, Kato Y, Bonifacino JS, Hurley JH. Structural basis for acidic-cluster-dileucine sorting signal recognition by VHS domains. Nature. 2002;415:933–37. doi: 10.1038/415933a. [DOI] [PubMed] [Google Scholar]
- 47.Shiba T, Takatsu H, Nogi T, Matsugaki N, Kawasaki M, et al. Structural basis for recognition of acidic-cluster dileucine sequence by GGA1. Nature. 2002;415:937–41. doi: 10.1038/415937a. [DOI] [PubMed] [Google Scholar]
- 48.Paasche JD, Attramadal T, Kristiansen K, Oksvold MP, Johansen HK, et al. Subtype-specific sorting of the ETA endothelin receptor by a novel endocytic recycling signal for G protein-coupled receptors. Mol Pharm. 2005;67:1581–90. doi: 10.1124/mol.104.007013. [DOI] [PubMed] [Google Scholar]
- 49.Puthenveedu MA, von Zastrow M. Cargo regulates clathrin-coated pit dynamics. Cell. 2006;127:113–24. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 50.Xia Z, Gray JA, Compton-Toth BA, Roth BL. A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal transduction. J Biol Chem. 2003;278:21901–8. doi: 10.1074/jbc.M301905200. [DOI] [PubMed] [Google Scholar]
- 51.Mundell SJ, Luo J, Benovic JL, Conley PB, Poole AW. Distinct clathrin-coated pits sort different G protein-coupled receptor cargo. Traffic. 2006;7:1–12. doi: 10.1111/j.1600-0854.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 52.Santini F, Marks MS, Keen JH. Endocytic clathrin-coated pit formation is independent of receptor internalization signal levels. Mol Biol Cell. 1998;9:1177–94. doi: 10.1091/mbc.9.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaidarov I, Santini F, Warren RA, Keen JH. Spatial control of coated-pit dynamics in living cells. Nat Cell Biol. 1999;1:1–7. doi: 10.1038/8971. [DOI] [PubMed] [Google Scholar]
- 54.Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509–12. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- 55.Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–87. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- 56.Wolfe BL, Marchese A, Trejo J. Ubiquitination differentially regulates clathrin-dependent internalization of protease-activated receptor-1. J Cell Biol. 2007;177:905–16. doi: 10.1083/jcb.200610154. Reveals for the first time a direct and novel function for ubiquitination in internalization of a mammalian GPCR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 58.Somesh BP, Sigurdsson S, Saeki H, Erdjument-Bromage H, Tempst P, Svejstrup JQ. Communication between distant sites in RNA polymerase II through ubiquitylation factors and the polymerase CTD. Cell. 2007;129:57–68. doi: 10.1016/j.cell.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 59.Ingham RJ, Gish G, Pawson T. The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene. 2004;23:1972–84. doi: 10.1038/sj.onc.1207436. [DOI] [PubMed] [Google Scholar]
- 60.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–72. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 61.Joazeiro CA, Weissman AM. RING finger proteins: mediators of ubiquitin ligase activity. Cell. 2000;102:549–52. doi: 10.1016/s0092-8674(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 62.Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:534–39. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- 63.d’ Azzo A, Bongiovanni A, Nastasi T. E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic. 2005;6:429–41. doi: 10.1111/j.1600-0854.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- 64.Nijman SMB, Luna-Vargas MPA, Velds A, Brummelkamp TR, Dirac AMG, et al. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–86. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Chen X, Zhang B, Fischer JA. A specific protein substrate for a deubiquitination enzyme: liquid facets is the substrate of Fat facets. Genes Dev. 2002;16:289–94. doi: 10.1101/gad.961502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCullough J, Clague MJ, Urbe S. AMSH is an endosome-associated ubiquitin isopeptidase. J Cell Biol. 2004;166:487–92. doi: 10.1083/jcb.200401141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Row PE, Prior IA, McCullough J, Clague MJ, Urbe S. The ubiquitin isopeptidase UBPY regulates endosomal ubiquitin dynamics and is essential for receptor down-regulation. J Biol Chem. 2006;281:12618–24. doi: 10.1074/jbc.M512615200. [DOI] [PubMed] [Google Scholar]
- 68.Mouchantaf R, Azakir BA, McPherson PS, Millard SM, Wood SA, Angers A. The ubiquitin ligase itch is auto-ubiquitylated in vivo and in vitro but is protected from degradation by interacting with the deubiquitylating enzyme FAM/USP9X. J Biol Chem. 2006;281:38738–47. doi: 10.1074/jbc.M605959200. [DOI] [PubMed] [Google Scholar]
- 69.Kee Y, Munoz W, Lyon N, Huibregtse JM. The deubiquitinating enzyme Ubp2 modulates Rsp5-dependent Lys63-linked polyubiquitin conjugates in Saccharomyces cerevisiae. J Biol Chem. 2006;281:36724–31. doi: 10.1074/jbc.M608756200. [DOI] [PubMed] [Google Scholar]
- 70.Roth AF, Davis NG. Ubiquitination of the yeast a-factor receptor. J Cell Biol. 1996;134:661–74. doi: 10.1083/jcb.134.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Terrell J, Shih S, Dunn R, Hicke L. A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol Cell. 1998;1:193–202. doi: 10.1016/s1097-2765(00)80020-9. [DOI] [PubMed] [Google Scholar]
- 72.Galan J-M, Haguenauer-Tsapis R. Ubiquitin Lys63 is involved in ubiquitination of a yeast plasma membrane protein. EMBO J. 1997;16:5847–54. doi: 10.1093/emboj/16.19.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shih SC, Katzmann DJ, Schnell JD, Sutano M, Emr SD, Hicke L. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat Cell Biol. 2003;4:389–93. doi: 10.1038/ncb790. [DOI] [PubMed] [Google Scholar]
- 74.Raiborg C, Bache KG, Mehlum A, Stang E, Stenmark H. Hrs recruits clathrin to early endosomes. EMBO J. 2001;20:5008–21. doi: 10.1093/emboj/20.17.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol. 2007;8:355–68. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- 76.Babst M, Wendland B, Estepa EJ, Emr SD. The Vps4 AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 1998;17:2982–93. doi: 10.1093/emboj/17.11.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Babst M, Sato TK, Banta LM, Emr SD. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4. EMBO J. 1997;16:1820–31. doi: 10.1093/emboj/16.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marchese A, Raiborg C, Santini F, Keen JT, Stenmark H, Benovic JL. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev Cell. 2003;5:709–22. doi: 10.1016/s1534-5807(03)00321-6. First to identify an E3 ubiquitin ligase that mediates ubiquitination, endosomal sorting, and downregulation of a mammalian GPCR. [DOI] [PubMed] [Google Scholar]
- 79.Reyes-Ibarra AP, García-Regalado A, Ramírez-Rangel I, Esparza-Silva AL, Valadez-Sánchez M, et al. Calcium-sensing receptor endocytosis links extracellular calcium signaling to PTHrP secretion via a Rab11a-dependent and AMSH-sensitive mechanism. Mol Endocrinol. 2007;21:1394–407. doi: 10.1210/me.2006-0523. [DOI] [PubMed] [Google Scholar]
- 80.Martin NP, Lefkowitz RJ, Shenoy SK. Regulation of V2 vasopressin receptor degradation by agonist-promoted ubiquitination. J Biol Chem. 2003;278:45954–59. doi: 10.1074/jbc.M308285200. [DOI] [PubMed] [Google Scholar]
- 81.Tulipano G, Stumm R, Pfeiffer M, Kreienkamp H-J, Höllt V, Schulz S. Differential β-arrestin trafficking and endosomal sorting of somatostatin receptor subtypes. J Biol Chem. 2004;279:21374–82. doi: 10.1074/jbc.M313522200. [DOI] [PubMed] [Google Scholar]
- 82.Jacob C, Cottrell GS, Gehringer D, Schmidlin F, Grady EF, Bunnett NW. c-Cbl mediates ubiquitination, degradation, and down-regulation of human protease-activated receptor 2. J Biol Chem. 2005;280:16076–87. doi: 10.1074/jbc.M500109200. [DOI] [PubMed] [Google Scholar]
- 83.Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, et al. Ubiquitin-dependent down-regulation of the Neurokinin-1 receptor. J Biol Chem. 2006;281:27773–83. doi: 10.1074/jbc.M603369200. [DOI] [PubMed] [Google Scholar]
- 84.Oo ML, Thangada S, Wu M-T, Liu CH, MacDonald TL, et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–89. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 85.Dupre DJ, Chen Z, Le Gouill C, Theriault C, Parent J-L, et al. Trafficking, ubiquitination, and down-regulation of the human platelet-activating factor receptor. J Biol Chem. 2003;278:48228–35. doi: 10.1074/jbc.M304082200. [DOI] [PubMed] [Google Scholar]
- 86.Petaja-Repo UE, Hogue M, Laperriere A, Bhalla S, Walker P, Bouvier M. Newly synthesized human opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J Biol Chem. 2001;276:4416–23. doi: 10.1074/jbc.M007151200. [DOI] [PubMed] [Google Scholar]
- 87.Saliba RS, Munro PM, Luthert PJ, Cheetman ME. The cellular fate of mutant rhodopsin: quality control, degradation and aggressome formation. J Cell Sci. 2002;115:2907–18. doi: 10.1242/jcs.115.14.2907. [DOI] [PubMed] [Google Scholar]
- 88.Illing ME, Rajan RS, Bence NF, Kopito RR. A rhodopsin mutant linked to autosomal dominant-retinitis pigmentosa is prone to aggregate and interacts with the ubiquitin proteasome system. J Biol Chem. 2002;277:34150–60. doi: 10.1074/jbc.M204955200. [DOI] [PubMed] [Google Scholar]
- 89.Cook LB, Zhu C-C, Hinkle PM. Thyrotropin-releasing hormone receptor processing: Role of ubiquitination and proteasomal degradation. Mol Endocrinol. 2003;17:1777–91. doi: 10.1210/me.2003-0073. [DOI] [PubMed] [Google Scholar]
- 90.Huang Y, Niwa J-I, Sobue G, Breitwieser GE. Calcium-sensing receptor ubiquitination and degradation mediated by the E3 ubiquitin ligase dorfin. J Biol Chem. 2006;281:11610–17. doi: 10.1074/jbc.M513552200. [DOI] [PubMed] [Google Scholar]
- 91.Moriyoshi K, Iijima K, Fujii H, Ito H, Cho Y, Nakanishi S. Seven in absentia homolog 1A mediates ubiquitination and degradation of group 1 metabotropic glutamate receptors. Proc Natl Acad Sci USA. 2004;101:8614–19. doi: 10.1073/pnas.0403042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cohen BD, Bariteau JT, Magenis LM, Dias JA. Regulation of follitropin receptor cell surface residency by the ubiquitin-proteasome pathway. Endocrinology. 2003;144:4393–402. doi: 10.1210/en.2002-0063. [DOI] [PubMed] [Google Scholar]
- 93.Milojevic T, Reiterer V, Stefan E, Korkhov VM, Dorostkar MM, et al. The ubiquitin-specific protease Usp4 regulates the cell surface level of the A2a receptor. Mol Pharm. 2006;69:1083–94. doi: 10.1124/mol.105.015818. [DOI] [PubMed] [Google Scholar]
- 94.Tanowitz M, von Zastrow M. Ubiquitination-independent trafficking of G protein-coupled receptors to lysosomes. J Biol Chem. 2002;277:50219–22. doi: 10.1074/jbc.C200536200. [DOI] [PubMed] [Google Scholar]
- 95.Whistler JL, Enquist J, Marley A, Fong J, Gladher F, et al. Modulation of postendocytic sorting of G protein-coupled receptors. Science. 2002;297:615–20. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- 96.Simonin F, Karcher P, Boeuf JJ-M, Matifas A, Kieffer BL. Identification of a novel family of G-protein-coupled receptor associated sorting proteins. J Neurochem. 2004;89:766–75. doi: 10.1111/j.1471-4159.2004.02411.x. [DOI] [PubMed] [Google Scholar]
- 97.Heydorn A, Sondergaard BP, Ersboll B, Holst B, Nielsen FC, et al. A library of 7TM receptor C-terminal tails. J Biol Chem. 2004;279:54291–303. doi: 10.1074/jbc.M406169200. [DOI] [PubMed] [Google Scholar]
- 98.Gullapalli A, Wolfe BL, Griffin CT, Magnuson T, Trejo J. An essential role for SNX1 in lysosomal sorting protease-activated receptor-1: Evidence for retromer, Hrs and Tsg101 independent functions of sorting nexins. Mol Biol Cell. 2006;17:1228–38. doi: 10.1091/mbc.E05-09-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98a.Wang Y, Zhou Y, Szabo K, Haft CR, Trejo J. Down-regulation of protease-activated receptor-1 is regulated by sorting nexin 1. Mol Biol Cell. 2002;13:1965–76. doi: 10.1091/mbc.E01-11-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carlton J, Bujny M, Peter BJ, Oorschot VMJ, Rutherford A, et al. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high curvature membranes and 3-phosphoinositides. Curr Biol. 2004;14:1791–800. doi: 10.1016/j.cub.2004.09.077. [DOI] [PubMed] [Google Scholar]
- 100.O’Brien PJ, Zatz M. Acylation of bovine rhodopsin by [3H]palmitic acid. J Biol Chem. 1984;259:5054–57. [PubMed] [Google Scholar]
- 101.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–45. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 102.Moffett S, Mouillac B, Bonin H, Bouvier M. Altered phosphorylation and desensitization patterns of a human β 2-adrenergic receptor lacking palmitoylated Cys341. EMBO J. 1993;12:349–56. doi: 10.1002/j.1460-2075.1993.tb05663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Torrecilla I, Tobin AB. Co-ordinated covalent modification of G-protein coupled receptors. Curr Pharm Des. 2006;12:1797–808. doi: 10.2174/138161206776873716. [DOI] [PubMed] [Google Scholar]
- 104.Kraft K, Olbrich H, Majoul I, Mack M, Proudfoot A, Oppenmann M. Characterization of sequence determinants within the carboxyl-terminal domain of chemokine receptor CCR5 that regulate signaling and receptor internalization. J Biol Chem. 2001;276:34408–18. doi: 10.1074/jbc.M102782200. [DOI] [PubMed] [Google Scholar]
- 105.Gao Z, Ni Y, Szabo G, Linden J. Palmitoylation of the recombinant human A1 adenosine receptor: enhanced proteolysis of palmitoylation-deficient mutant receptors. Biochem J. 1999;342:387–95. [PMC free article] [PubMed] [Google Scholar]
- 106.Percherancier Y, Planchenault T, Valenzuela-Fernandez A, Virelizier J-L, Arenzana-Seisdedos F, Bachelerie F. Palmitoylation-dependent control of degradation, life span, and membrane expression of the CCR5 receptor. J Biol Chem. 2001;276:31936–44. doi: 10.1074/jbc.M104013200. [DOI] [PubMed] [Google Scholar]
- 107.Valdez-Taubas J, Pelham H. Swf1-dependent palmitoylation of the SNARE Tlg1 prevents its ubiquitination and degradation. EMBO J. 2005;24:2524–32. doi: 10.1038/sj.emboj.7600724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abrami L, Leppla SH, van der Groot FG. Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J Cell Biol. 2006;172:309–20. doi: 10.1083/jcb.200507067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bernier V, Lagace M, Bichet DG, Bouvier M. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol Metab. 2004;15:222–28. doi: 10.1016/j.tem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 110.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. This is an excellent comprehensive review on the role and mechanisms by which GPCRs contribute to cancer progression. [DOI] [PubMed] [Google Scholar]
- 111.Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–69. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 112.Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, et al. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med. 1998;4:909–14. doi: 10.1038/nm0898-909. [DOI] [PubMed] [Google Scholar]
- 113.Arora P, Ricks TK, Trejo J. Protease-activated receptor signalling, endocytic sorting and dysregulation in cancer. J Cell Sci. 2007;120:921–28. doi: 10.1242/jcs.03409. [DOI] [PubMed] [Google Scholar]
- 114.Booden MA, Ekert L, Der CJ, Trejo J. Persistent signaling by dysregulated thrombin receptor trafficking promotes breast carcinoma cell invasion. Mol Cell Biol. 2004;24:1990–99. doi: 10.1128/MCB.24.5.1990-1999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hicke L, Schubert HL, Hill CP. Ubiquitin-binding domains. Nat Rev Mol Cell Biol. 2005;6:610–21. doi: 10.1038/nrm1701. [DOI] [PubMed] [Google Scholar]
- 116.Hislop JN, Marley A, von Zastrow M. Role of mammalian vacuolar protein-sorting proteins in endocytic trafficking of a non-ubiquitinated G protein-coupled receptor to lysosomes. J Biol Chem. 2004;279:22522–31. doi: 10.1074/jbc.M311062200. [DOI] [PubMed] [Google Scholar]
- 117.Kerscher O, Felberbaum R, Hochstrasser M. Modifications of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–80. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 118.Tang Z, El Far O, Betz H, Scheschonka A. Pias1 interaction and sumoylation of metabotropic glutamate receptor 8. J Biol Chem. 2005;280:38153–59. doi: 10.1074/jbc.M508168200. [DOI] [PubMed] [Google Scholar]
- 118a.Kenakin T. Collateral efficacy in drug discovery: taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol Sci. 2007;26:407–415. doi: 10.1016/j.tips.2007.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





