Abstract
This study describes a novel gel based vehicle for the delivery of acetylcholine (ACh) during quantitative sudomotor axon reflex testing (QSART). A dose and current response study were undertaken on 20 healthy control participants to characterize the efficiency of a gel based vehicle for the delivery of ACh. Values obtained for total sweat volume and latency to sweat onset with gel iontophoresis of ACh during QSART were comparable to previously published normative data using solution based vehicles. Patient discomfort, utilizing the gel based vehicle during the QSART procedure, was minimal. Improvement in iontophoresis using the gel formulation as a vehicle for ACh delivery has the potential to lower the voltage required to overcome skin resistance during QSART and may result in improved patient comfort during the procedure.
Keywords: Acetylcholine, QSART, Iontophoresis, Gel, Solution, Vehicle
1. Introduction
Well validated tests of autonomic function are routinely used in clinical practice (Low, 2003). The routine autonomic evaluation includes QSART (quantitative sudomotor axon reflex testing), a sensitive measure of postganglionic sympathetic axon integrity (Low et al., 1983). QSART has been shown to be useful in detection of various neuropathies related to diabetes, idiopathic small-fiber loss, autonomic dysfunction, etc. (Kihara et al., 1998; Singer et al., 2004; Low et al., 2006). At the test site, a multi-compartment sweat cell is applied to the skin, acetylcholine (ACh) is iontophoresed in one compartment and the sweat response is recorded in a second compartment (Low et al., 1992). The sweat response is measured as time to onset of sweating after the stimulus is applied (latency) and total sweat volume (area under the curve).
A major limitation of the test is the inefficiency of iontophoresis. In a dose–response study using ionic solution preparations, a concentration of one molar acetylcholine was required to generate a maximal response to iontophoresis of 2 mA of constant current for 5 min (Low et al., 1992). Due to the inefficiency of iontophoresis, the test does result in modest patient discomfort with occasional mild skin irritation, presumably related to higher current densities.
The most common vehicle used for iontophoresis of ACh is a 10% (wt/v) ionic solution of acetylcholine chloride (Sletten et al., 2005). However, reagents for iontophoresis suspended in agarose gel preparations are commonly used to perform diagnostic testing, such as the Pilogel® Iontophoretic Discs used in the diagnosis of cystic fibrosis (Losty et al., 2006). Gel formulations, by ensuring greater surface area contact, can increase the efficiency of iontophoresis thereby decreasing the perceived level of discomfort by patients. Furthermore, the potential for improved iontophoresis with gel preparations could also reduce the latency to sweat onset and increase the total sweat volume during QSART.
The objective of this study was to describe the use of a novel gel based vehicle for delivery of ACh during QSART. A dose/current response study measuring varying concentrations and current levels during QSART was undertaken to characterize the efficiency of a gel based vehicle for the delivery of ACh.
2. Methods
2.1. Study participants
Prior to starting the study, informed consent was obtained for each participant in compliance with institutional review board guidelines. Studies were conducted on 20 healthy control participants, ages 18–70 years (median age, 50 years), with an equal male to female ratio. Median values for height, weight, and BMI of study participants were 169.5 cm (range 157 to 187), 70.5 kg (range 53 to 99), and 24.5 kg/m2 (range 19 to 32), respectively. No food, caffeine, or nicotine was permitted for 8 h prior to the study. All participants were medication free 24 h prior to testing and remained medication free for the total study period; exceptions were made for participants taking daily vitamins and oral birth control tablets.
2.2. Sudomotor recordings
Quantitative sudomotor axon reflex testing (QSART) was performed to evaluate the postganglionic sympathetic sudomotor axon as previously described (Low et al., 1983). All studies were performed in the Autonomic Disorders Center, Mayo Clinic (Rochester) using the Mayo-built Sudorometer, constant current generator, and multi-compartmental sweat cells. Room temperature and humidity were held constant at 23 °C and 25–35%, respectively. The participants’ skin was prepped according to standard clinical protocol, which included the removal of any excess hair, followed by a four-step cleaning process (acetone, alcohol, water and dry gauze). Skin temperature at each test site was maintained between 31 and 34 °C using a heat lamp.
The effect of ACh concentration and current strength was assessed using the gel vehicle formulation (see Table 1, Fig. 1). A randomized dose/current response study using 0.0055 M ACh (0.1% wt/v), 0.055 M ACh (1% wt/v), and 0.55 M ACh (10% wt/v) concentrations at 1 mA and 2 mA of constant current was performed. Bilateral forearm sites were studied over three consecutive days using non-identical test sites. Constant current stimulus was applied for 5 min. Sweat responses were recorded for an additional 5 min after discontinuation of the stimulus. Measurements of total sweat volume in microliters (over 10 min) and time to onset of sweating in minutes (latency) were recorded and calculated for all participants. Participants were asked to rate their level of discomfort during the stimulation period using an 11-point Visual Analog Scale (VAS); where 0 = no pain or discomfort and 10 = most severe pain or discomfort.
Table 1.
0.55 M acetylcholine–agarose gel formulation.
| Supplier |
| Sigma-Aldrich Corp., St. Louis MO, phone: 1-800-325-3010 |
| Materials |
| Low melt point agarose: product # A0169 |
| Acetylcholine chloride: product # A2661 |
| Distilled water |
| Procedure |
| A. Water bath preparation |
| 1. Heat water bath to 47 °C. |
| 2. Add 150 mL distilled water into a clean 600 mL beaker. Place in water bath. |
| 3. Place closed Falcon tube in 600 mL beaker to warm. |
| 4. Place a 5 mL-pipette tip inside a 50 mL Falcon tube. Place Falcon tube in the 600 mL beaker inside water bath. |
| B. Agarose preparation (3.0% w/v) |
| 1. Weigh 1.8 g of agarose into a 250 mL beaker. |
| 2. Measure 60 mL of distilled water with a graduated cylinder and add to the agarose in the beaker. |
| 3. Cover the beaker with parafilm. |
| 4. Place beaker in microwave oven and melt agarose using 10–15 s bursts until completely melted. Ensure solution does not over boil. Approximate heating time is 1 min depending on the microwave oven used. Note: Solution will thicken and change from a cloudy appearance to a clear or transparent appearance. Some bubbles may be present. |
| 5. Allow the beaker to cool at room temperature for about 5 min before placing the beaker in the pre-heated water bath at 47 °C. This is done to keep the agarose melted. |
| C. Acetylcholine preparation (50% w/v) |
| 1. Weigh 7.5 g of acetylcholine into weighing boat. |
| 2. Pour acetylcholine into the pre-heated 50 mL falcon tube. |
| 3. Using safety wash bottle, add sufficient distilled water to fill 50 mL Falcon tube to 15 mL line. |
| 4. Invert the tube until acetylcholine is fully dissolved. |
| 5. Place the Falcon tube in the water bath at 47 °C to bring to agarose temperature. Typically this is about 5 min. |
| D. Preparing acetylcholine–agarose mixture and iontophoresis chamber preparation |
| 1. Heat stirring hot plate to 47 °C. |
| 2. Pour acetylcholine solution into 250 mL beaker with the agarose within the 47 °C water bath. |
| 3. Insert the stirring bar into 250 mL beaker and place on stirring hot plate. |
| 4. Slowly mix the solution for 3–5 min. |
| 5. Replace the 250 mL beaker with acetylcholine–agarose solution into water bath. |
| 6. Set the adjustable pipette to 3 mL. |
| 7. Deliver 1.5–2.0 mL of acetylcholine–agarose solution into each iontophoresis electrode, until solution is slightly convex. |
| 8. Allow electrodes with solution to completely cool at room temperature. As the solution cools it will thicken up and becomes the gel. Approximate time: 30 min. |
| 9. Place each electrode in plastic bag after gel has completely set. |
| 10. Apply a label to the bag of each electrode, stating the following: Product name, expiration date, along with any institutional requirements Example: ACh Gel, exp. 12/12/2002, IRB #xxx–yyy |
| 11. Store the electrodes in a labeled damp container or plastic bag at 4 °C. This will help keep the gel from drying out. |
| 12. Gel expiration: Theoretically the gels should be good up to 3 months. Realistically the gels will be good for about 1 month depending on storage environment. Before using the gels perform a visual inspection to be sure no mold or corrosion build up has occurred, if it has do not use the gels. Also, be sure the gels will make full contact with the skin, as the gel dries out they shrink. |
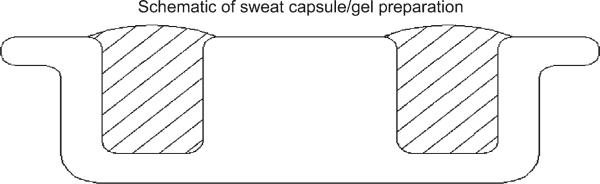
Fig. 1.
Cross section of electrode: hatch area represents where gel is poured. Note the convex of the gel on the side of the electrode that makes contact with the skin. This slight convex assures proper contact with the skin.
2.3. Statistical analysis
Data from the dose/current response study was analyzed using a two-way random effects ANOVA model with a current effect, a concentration effect, and a random subject-specific intercept which accounts for the correlation among measurements taken from the same subject. As a result of skewness and increased variability at higher volumes, the volume data was analyzed by taking the log10(x + 1) transformation. This transformation accommodated the two subjects whose volumes were not detectible and recorded as zero.
3. Results
The formula for the production of the gel vehicle is outlined in Table 1. A further cross section schematic of the gel preparation within the sweat capsule is shown in Fig. 1. The estimated dose/current response curves are shown in Fig. 2 and the observed values are summarized in Table 2. For analysis, a random effects model with current, concentration, and their interaction was first fit to the data. Since the interactions were not significant for total sweat volume (p = 0.61), latency to sweat onset (p = 0.70), or perceived patient discomfort (p = 0.12), these terms were removed from the models. The lack of significance of the interaction term can be interpreted as evidence that the effect of increased current is the same across concentration levels.
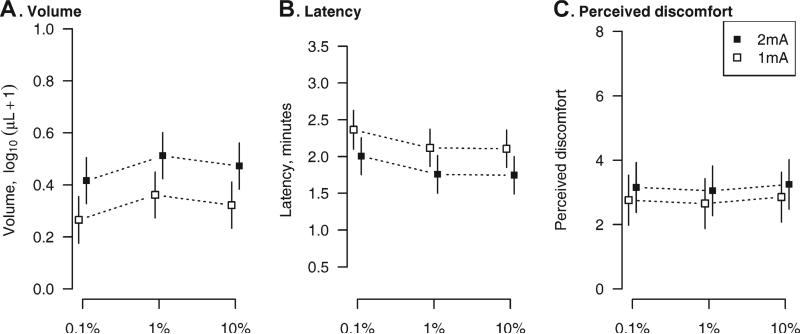
Fig. 2.
Estimated mean (boxes) and 95% confidence intervals (vertical lines) for forearm total sweat volume (A), latency to sweat onset (B), and perceived discomfort on the 11-point VAS scale (C) at each reagent concentration and current level based on the two-way random effects ANOVA estimates. The vertical axes are scaled to cover the range of the observed data. To avoid overlap, the means are shifted along the x-axis.
Table 2.
Median (range) values at each ACh concentration and current strength using the gel preparation during quantitative sudomotor axon reflex testing at the forearm.
| Treatment | Volume, μL | Latency, min | Perceived discomfort, 0 to 10 |
|---|---|---|---|
| 0.1% | |||
| 1 mA | 0.5 (0.0, 4.0) | 2.5 (0.6, 3.7) | 2.0 (0.0, 7.0) |
| 2 mA | 1.8 (0.0, 5.3) | 1.8 (1.5, 3.5) | 3.0 (0.0, 7.0) |
| 1% | |||
| 1 mA | 1.4 (0.1, 4.5) | 2.0 (1.3, 3.2) | 2.5 (1.0, 6.0) |
| 2 mA | 2.5 (0.0, 8.8) | 1.7 (1.1, 3.1) | 3.0 (0.0, 6.0) |
| 10% | |||
| 1 mA | 0.8 (0.0, 4.2) | 2.2 (0.3, 3.4) | 2.5 (0.0, 6.0) |
| 2 mA | 2.3 (0.1, 6.9) | 1.6 (0.2, 3.6) | 3.0 (0.0, 8.0) |
Overall, when analyzed on the log scale, total sweat volume increased with the higher current level (p < 0.001) and differed by concentration (p = 0.007). The volume at 1% did not differ significantly from the volume at 10% (p = 0.19), while the volume at 0.1% was reduced relative to the 1% ACh concentration (p = 0.002) but not the 10% ACh concentration (p = 0.07).
The higher current (2 mA) reduced latency (p < 0.001) while increasing discomfort (p = 0.008). These effects were small. Across concentration levels, latency was found to be on average 22 s shorter at 2 mA and discomfort was on average 0.40 points higher. There was some evidence that latency differed by concentration. The lowest ACh concentration (0.1%) resulted in latencies that were on average approximately 15 s longer compared to the 1% (p = 0.04) and 10% (p = 0.03) ACh concentrations. ACh concentration had no effect on patient discomfort (p = 0.54).
4. Discussion
Iontophoresis is currently in use for treatment of hyperhydrosis, transdermal drug delivery (i.e., lidocaine) and in non-invasive monitoring of glucose concentrations in human subjects (Wang et al., 2005). Specifically, iontophoresis with ACh is a useful technique for assessment of vasodilation in the peripheral microcirculation and for assessment of postganglionic sudomotor function in QSART (Cracowski et al., 2006; Low et al., 1983). All these techniques have relied on ionic solutions or deionized water as the primary vehicle for drug/reagent delivery during iontophoresis. Losty et al. (2006) have previously described a gel based system for iontophoresis of pilocarpine in the diagnosis of cystic fibrosis. In this study we present for the first time the use of a gel based vehicle for the iontophoresis of ACh during QSART.
Methods using ionic solutions for the delivery of ACh are not without difficulty. Iontophoresis, while safe and effective, does result in minor discomfort and occasional irritation to patients’ skin. Certain patients require higher voltage levels to overcome the skin resistance for transdermal delivery of ACh, likely the potential cause of discomfort and irritation. Studies using various concentrations of NaCl in solution have shown no significant change in electrical resistance during iontophoresis compared with deionized water (Abou-Elenin et al., 2002; Khan et al., 2004). The use of a 0.5% NaCl solution does appear to reduce some effects related to iontophoresis such as hyperemia versus deionized water (Ferrell et al., 2002). Gel preparations have the advantage of greater surface area contact and the potential to reduce areas of high current density (i.e., air pocket within the stimulus compartment of the sweat cell) thereby potentially reducing the voltage required and decreasing the perceived discomfort during testing. Another advantage is the reduction of leakage from the stimulus chamber holding the ACh solution into the recording chamber; reducing the number of technical issues encountered during testing. Study patients had low levels (2–3) of discomfort during testing based on the 11-point VAS scale for pain across both current levels (1–2 mA). These values are at least comparable to discomfort during QSART using solution based vehicles but this has not been directly studied.
As expected higher current levels (2 mA) increased sweat volume, decreased latency and resulted in a minimal increase in patient discomfort. Values obtained for total sweat volume and latency with iontophoresis using the gel vehicle during QSART were comparable to previously published normative data using solution based vehicles (Low et al., 1983, 1997; Low and Sletten 2008). There was an interesting trend toward increased sweat volume and reduced latency with 1% ACh in gel compared to the 10% concentration (Table 2; Fig. 2). Although this difference was nonsignificant, increased efficacy using the gel vehicle at a 1% ACh concentration may be the cause for these findings. Previous plateau effects with increasing ACh concentrations have been noted in assessing vasodilatation in the microcirculation (Christen et al., 2004).
Increased blood flow in the microcirculation during iontophoresis with ACh in deionized water has been observed compared to NaCl in solution (Khan et al., 2004). However, other studies indicate that the effects on vasodilation within the microcirculation using either deionized water or a NaCl solution as a vehicle for ACh are minimal (Abou-Elenin et al., 2002). This has lead to debate as to whether ionic solutions may decrease the effectiveness of the procedure by competing with ACh during iontophoresis. Direct comparisons of total sweat volume, latency to sweat onset and patient discomfort using either gel or solution based vehicles during QSART are currently underway in the Low laboratory.
5. Conclusions
This study describes a novel gel based vehicle for the delivery of ACh during QSART. Dose/current response studies reveal that the gel formulation is an efficient vehicle for ACh delivery during QSART testing. Furthermore improvement in iontophoresis using this gel formulation as a vehicle for ACh delivery has the potential to lower voltage levels required during QSART and result in improved patient comfort during the procedure. Further studies comparing the efficiency of gel versus conventional solutions as a vehicle for iontophoresis of ACh are currently underway.
Acknowledgments
We would like to thank Dr. Arnold Lindall and Terri Martin for their expertise in helping develop the gel formulation. We would like to also thank Toni Gehrking and Jade Gehrking for their technical assistance. This work was supported by NIH grants (NS3 2352, NS4 4233, NS4 3364, UL1 RR24150) and Mayo Clinic. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Abbreviations
- ACh
Acetylcholine
- QSART
Quantitative sudomotor axon reflex testing
- VAS
Visual Analog Scale
References
- Abou-Elenin K, Xydakis A, Hamdy O, Economides PA, Horton ES, Veves A. The effect of aspirin and various iontophoresis solution vehicles on skin microvascular reactivity. Microvasc. Res. 2002;63:91–95. doi: 10.1006/mvre.2001.2369. [DOI] [PubMed] [Google Scholar]
- Christen S, Delachaux A, Dischl B, Golay S, Liaudet L, Feihl F, Waeber B. Dose-dependent vasodilatory effects of acetylcholine and local warming on skin microcirculation. J. Cardiovasc. Pharmacol. 2004;44:659–664. doi: 10.1097/00005344-200412000-00006. [DOI] [PubMed] [Google Scholar]
- Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol. Sci. 2006;27:503–508. doi: 10.1016/j.tips.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Ferrell WR, Ramsay JE, Brooks N, Lockhart JC, Dickson S, McNeece GM, Greer IA, Sattar N. Elimination of electrically induced iontophoretic artefacts: implications for non-invasive assessment of peripheral microvascular function. J. Vasc. Res. 2002;39:447–455. doi: 10.1159/000064515. [DOI] [PubMed] [Google Scholar]
- Khan F, Newton DJ, Smyth EC, Belch JJ. Influence of vehicle resistance on transdermal iontophoretic delivery of acetylcholine and sodium nitroprusside in humans. J. Appl. Physiol. 2004;97:883–887. doi: 10.1152/japplphysiol.00373.2004. [DOI] [PubMed] [Google Scholar]
- Kihara M, Mitsui M, Nishikawa S, Nishimoto K, Takahashi M. Comparison of electrophysiologic and autonomic tests in sensory diabetic neuropathy. Clinic. Auton. Res. 1998;8:213–220. doi: 10.1007/BF02267784. [DOI] [PubMed] [Google Scholar]
- Losty HC, Wheatley H, Doull I. The evaluation of a novel conductometric device for the diagnosis of cystic fibrosis. Ann. Clin. Biochem. 2006;43:375–381. doi: 10.1258/000456306778520025. [DOI] [PubMed] [Google Scholar]
- Low PA. Testing the autonomic nervous system. Semin. Neurol. 2003;23:407–421. doi: 10.1055/s-2004-817725. [DOI] [PubMed] [Google Scholar]
- Low PA, Sletten DM. Laboratory evaluation of autonomic function. In: Low PA, Benarroch EE, editors. Clinical Autonomic Disorders. Lippincott, Williams and Wilkins; Philadelphia: 2008. pp. 130–163. [Google Scholar]
- Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann. Neurol. 1983;14:573–580. doi: 10.1002/ana.410140513. [DOI] [PubMed] [Google Scholar]
- Low PA, Opfer-Gehrking TL, Kihara M. In vivo studies on receptor pharmacology of the human eccrine sweat gland. Clinic. Auton. Res. 1992;2:29–34. doi: 10.1007/BF01824208. [DOI] [PubMed] [Google Scholar]
- Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O'Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve. 1997;20:1561–1568. doi: 10.1002/(sici)1097-4598(199712)20:12<1561::aid-mus11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Low VA, Sandroni P, Fealey RD, Low PA. Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve. 2006;34:57–61. doi: 10.1002/mus.20551. [DOI] [PubMed] [Google Scholar]
- Singer W, Spies JM, McArthur J, Low J, Griffin JW, Nickander KK, Gordon V, Low PA. Prospective evaluation of somatic and autonomic small fibers in selected autonomic neuropathies. Neurology. 2004;62:612–618. doi: 10.1212/01.wnl.0000110313.39239.82. [DOI] [PubMed] [Google Scholar]
- Sletten DM, Nickander KK, Low PA. Stability of acetylcholine chloride solution in autonomic testing. J. Neurol. Sci. 2005;234:1–3. doi: 10.1016/j.jns.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Thakur R, Fan Q, Michniak B. Transdermal iontophoresis: combination strategies to improve transdermal iontophoretic drug delivery. Eur. J. Pharm. Biopharm. 2005;60:179–191. doi: 10.1016/j.ejpb.2004.12.008. [DOI] [PubMed] [Google Scholar]