Abstract
Background
The management of slow or non-healing ulcerations constitutes an increasing clinical challenge in the developed world because of the ageing of the population and the pandemic rise in type II diabetes. Recent studies suggest that molecular circuitries deployed by tumor cells to promote cancerous growth may also contribute to tissue regeneration. Here, we exploited this emerging information to search for novel molecular targets to accelerate wound healing.
Methodology/Principal Findings
We found that the activation of the PI3K-Akt-mTOR pathway, whose aberrant function is a frequent event in human neoplasia, represents an integral component of the normal wound healing process. By the use of genetically defined approaches, including the epithelial-specific ablation of Pten and Tsc1, we show that mTOR activation can dramatically increase epithelial cell proliferation, migration, and cutaneous wound healing, while pharmacological inhibition of mTOR with rapamycin delays wound closure.
Conclusions/Significance
Overall, our findings indicate that the transient pharmacologic activation of the PI3K-Akt-mTOR signaling axis may represent a novel clinical intervention strategy to accelerate the healing of debilitating and life-threatening wounds.
Introduction
Humans display a remarkable capacity to heal injured tissues after external trauma to the body. This capacity, however, may be compromised by the large size of the injury, by underlying medical conditions, or by advanced age. For example, critical-size burn victims, patients who received chemotherapy and/or radiotherapy, elderly, and diabetic patients often present with slow or non-healing ulcers that constitute a significant clinical challenge, and the public health impact of non-healing wounds is likely to increase within the coming decades, due to the ageing of the world population and the pandemic rise in type II diabetes [1], [2], [3].
Certain molecular pathways that are exploited by tumor cells to promote cancer progression have been recently shown to play also a primary physiological role in the regeneration of injured tissues [4], [5], [6]. In this regard, the phosphatidylinositol 3 kinase (PI3K)-Akt pathway is one of the most frequently aberrantly activated intracellular signaling routes in cancer [7], [8], [9], [10]. The persistent activation of growth factor receptors by mutations or autocrine and paracrine mechanisms, gene amplification resulting in overexpression of PI3K and/or mutations in its coding sequence rendering this lipid kinase constitutively active, as well as decreased activity of the lipid phosphatase PTEN by multiple genetic or epigenetic events, all converge to promote the aberrant accumulation phosphatidylinositol-3,4,5-triphosphate (PIP3) in cancer cells, thereby causing the activation of the serine-theronine kinase Akt and its downstream targets, including the mammalian target of rapamycin (mTOR) [7], [8], [11]. These observations prompted us to explore whether the PI3K-Akt pathway participates in wound repair, as well as whether the targeted activation of this signaling pathway represents a suitable approach to accelerate wound healing. We show here that PI3K-Akt activation promotes cutaneous wound repair via mTor. More importantly, we demonstrate that elevating mTor activity dramatically accelerates the healing process, suggesting a novel strategy for the clinical management of slow or non-healing ulcerations.
Results
The Akt/mTOR signaling pathway is up regulated in wound healing
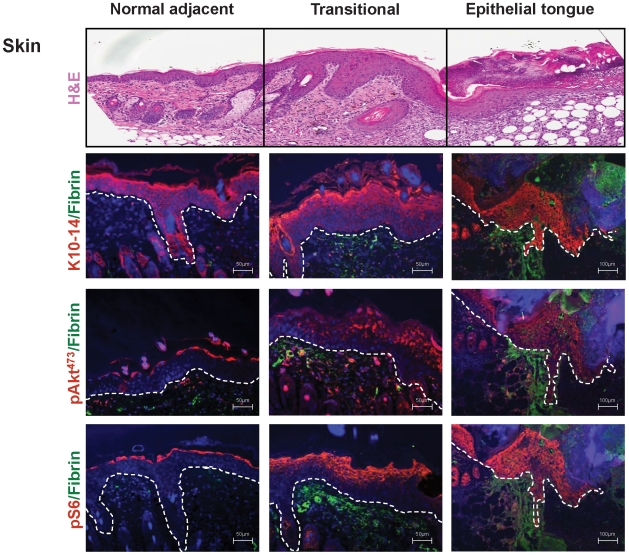
To begin addressing whether the PI3K/Akt pathway is activated during normal wound healing, we performed incisional skin wounds in mice and harvested tissues for molecular analysis. The stratified squamous epithelium adjacent to the wound site (Figure 1, normal) is organized into the basal layer that progressively differentiates into the spinous layer, granular layer and finally the stratum corneum. These four layers express distinct cytokeratins. For example, cytokeratin (K) 14 is expressed on the basal layer while and K10 is expressed in the suprabasal layers. During wound healing, migrating keratinocytes form a thin wedge-shape epithelial tongue of a few cell layers which establish the leading wound edge (figure 1- Epithelial tongue). There is also an increase in the thickness of the spinous layer adjacent to the wound and a rapid proliferation of epithelial cells immediately next to the migrating epithelial tongue (figure 1- Transitional)[1]. During the healing process, proliferation and migration are intimately related, with hyperproliferative cells in the transitional tissue feeding the migrating epithelial sheaths that form the epithelial tongue, as part of an interdependent process [1], [12].
Figure 1. The Akt-mTOR pathway is upregulated during wound healing.
(Upper panel). Representative histological sections of skin after incisional cutaneous wounds in mice. H&E stained section displaying the histological characteristics of the unwounded epithelium (normal adjacent) followed by an increase in the thickness of the spinous layer (acanthosis) (transitional), and the presence of migrating epithelial cells that form an epithelial tongue (epithelial tongue) at the wound edge. (second panel from top). Immunofluorescence for Cytokeratins 10 and 14 (K10-14, red) reveals the epidermal layer, which is delineated from the dermis by a white dotted line. Note the expansion of pAkt473 and pS6 (red) during wound healing from a single cell layer in the granular layer to multiple cell layers in the transitional epithelium, followed by expression in all cell layers including the basal layer in the migrating epithelial tongue (lower two panels). Cell nuclei were stained with DAPI (blue) and fibrin(ogen) with a FITC-conjugated antibody (green) in all panels. Scale bar, 50 µm and 100 µm, as indicated.
We next analyzed the status of activation of the PI3K/Akt pathway during wound healing. In normal skin, phosphorylated forms of the S6 ribosomal protein, pS6, and serine 473 Akt, pAkt473, which represent downstream targets of mTOR complex 1 and mTOR complex 2 respectively [13], [14], accumulate in the granular layer, consistent with our recent findings in postnatal skin development [15]. During wound healing, however, there is an expansion of the cell layers exhibiting pAkt473 and pS6 in the spinous layer of the transitional epithelium, and a remarkable phosphorylation of these molecules in all layers in the migrating epithelial tongue. The most striking was the presence of pAkt473 and pS6 in the basal layer of the epithelial tongue, which was always absent from the normal epithelium (Figure 1).
Pten excision accelerates wound closure
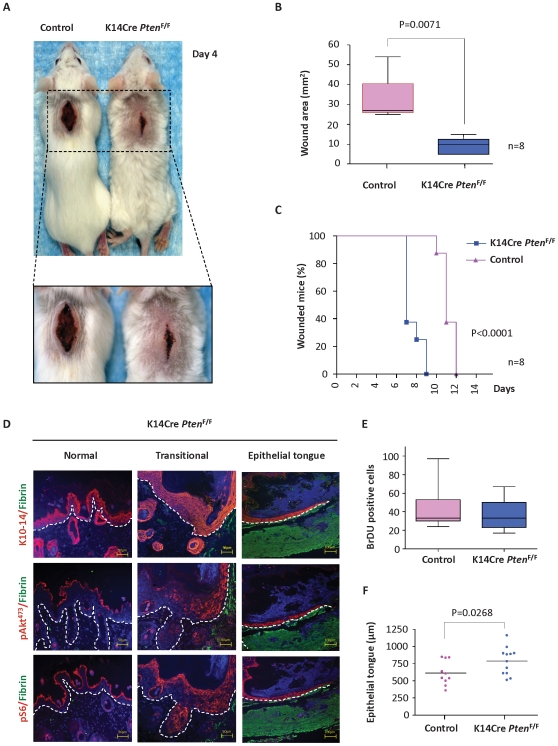
To begin exploring the contribution of the PI3K/Akt/mTOR pathway to wound healing, we first perturbed this pathway by excising Pten, a key negative regulator of this signaling axis [11]. We specifically removed Pten from the epithelial compartment of the skin by crossing mice harboring a floxed Pten allele (Pten F/F) with mice expressing the Cre recombinase under the control of the K14 promoter (K14Cre). Incisional skin wounds were performed in control and Pten epithelial-specific conditional knockout mice (K14Cre Pten F/F). A clearly faster rate of healing upon Pten excision from the skin mice was apparent as early as 4 days after wounding (Figure 2A), which was confirmed by quantification of the wound area (p = 0.0071, Figure 2B). Analysis of wound closure also indicated that the Pten epithelial-specific conditional knockout mice display a significant increase in the rate of wound healing, with half of the K14Cre Pten F/F mice healing by day 7, compared to the control mice that displayed a median wound closure of 11 days (p<0.0001, Figure 2C).
Figure 2. Accelerated wound closure upon epidermal Pten deletion: Enhanced mTor activation and re-epithelization.
(A) Representative example of accelerated wound closure in Pten-deficient (K14Cre Pten F/F) mice compared to control mice 4 days after skin wounding. (B) Quantification of the wound area is represented by the box-and-whiskers graphic (horizontal line, median value; n = 13 for each group; **p = 0.0071). (C) Percentage of mice exhibiting open wounds after surgical incision in control mice (triangles, n = 8) and K14Cre Pten F/F mice (squares, n = 8) at the indicated days is depicted. There was a significant increase in the rate of wound closure (***p<0.0001). (D) Representative examples of K10 and K14 (red-upper panel), pAkt473 (red-middle panel), and pS6 (red–lower panel) expression in K14Cre Pten F/F wounds as determined by immunofluorescence. DAPI (blue) and fibrin(ogen) (green). Note the accumulation of pAkt473 and pS6 in the transitional zone and epithelial tongue at the wound edge (transitional and epithelial tongue), when compared to the adjacent epithelium (Normal). (E) Quantification of BrDU positive cells in histological slides at the transitional and wound edge of control and K14Cre Pten F/F mice (n = 10 for each group; *p = 0.0331). (F) Quantification of epithelial migration and re-epithelization. The length of the epithelial tongue was measured in histological slides of wounded control (n = 10) and K14Cre Pten F/F mice (n = 12) (mean; *p = 0.0268).
Pten excision leads to upregulation of Akt/mTor pathway and enhanced epithelial migration
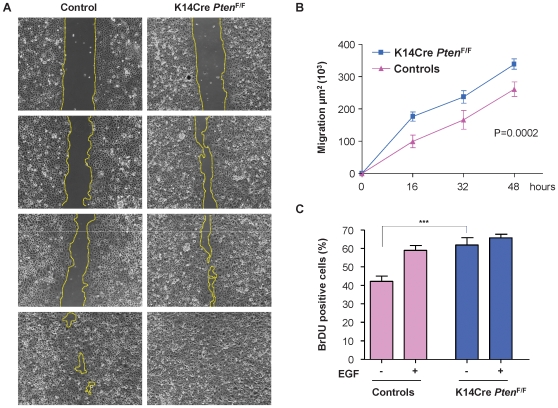
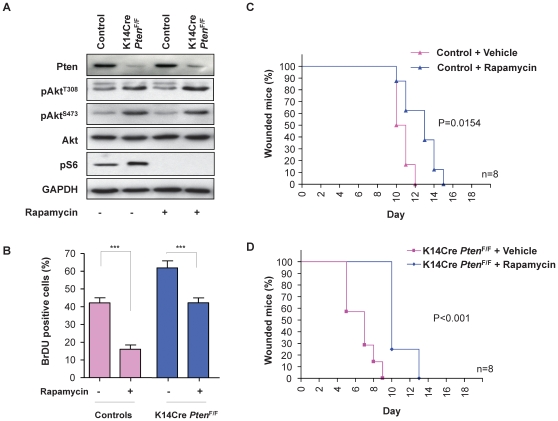
To test whether accelerated wound healing correlated with Akt and mTor activation, tissue samples were harvest at day 4 post wounding. Immunohistochemical staining of wound samples showed that the levels of pAkt473 and pS6 were enhanced in the suprabasal, spinous, and granular cell layers in the transitional skin of Pten conditional knockout mice (Figure 2D-transition) as compared to non-wounded epithelium (Figure 2D, normal). Pten excision enhanced epidermal proliferation as reflected by the number of BrDU-positive keratinocytes in the transitional compartment adjacent to the wound edge (Figure 2E, p = 0.0331). In addition, the wounds of K14Cre Pten F/F mice were characterized by the presence of an elongated migrating epithelial tongue that displayed high levels of expression of pAkt473 and pS6 (Figure 2D). In line with this observation, the Pten epithelial-specific conditional knockout mice showed accelerated re-epithelization as judged by the direct quantification of the length of the epithelial migrating tongue (p = 0.0268, Figure 2F). This increased migratory behavior could be recapitulated in primary keratinocyte cultures from control and K14Cre Pten F/F mice (Figure 3A–3B). As expected, Pten was almost absent in keratinocytes isolated from K14Cre Pten F/F mice (Figure 4A, top panel). Using a scratch wound assay, we observed that excision of Pten accelerated keratinocyte migration into the denuded area (p = 0.0002, Figure 3A–3B). These results are aligned with in vitro studies linking Pten with cell polarization and directional migration [16], [17]. In addition, the lack of Pten expression led to an enhanced proliferation of the primary keratinocytes (p<0.001, Figure 3C). The addition of EGF, a growth and pro-migratory factor that stimulates the PI3K-Akt pathway, served as an internal control. While EGF promoted the proliferation of control keratinocytes to nearly the same levels than Pten deficient cells, this growth factor failed to increase the proliferation of keratinocytes lacking Pten, likely because they may have already achieved a maximal proliferative capacity due to the persistent activation of Akt in the absence of Pten. Indeed, elevated levels of pAkt308, pAkt473, and pS6 were also observed in primary cultures of keratinocytes isolated from K14Cre Pten F/F mice (Figure 4A), albeit primary keratinocyte cultures from control mice often have demonstrable basal levels of pS6. Rapamycin treatment, which inhibits mTOR [14], [18], led to a complete ablation of pS6 phosphorylation, although it had only a limited impact on pAktSer473 levels (Figure 4A), aligned with a direct effect of rapamycin on the mTORC1 complex after short time treatment [19], [20]. Rapamycin caused a decreased proliferation of control and Pten knock out keratinocytes (Figure 4B), supporting a role for mTOR in epithelial cell growth in wild type cells as well as downstream from Pten.
Figure 3. Keratinocyte migration in vitro is enhanced upon Pten excision.
(A) Scratch wound assays in primary cultures of keratinocytes from control mice and K14Cre Pten F/F mice. Wounds were generated after cell confluence. In vitro cell migration and wound closure were assessed every 16 h. Areas of migration were measured in duplicates wells using keratinocytes from three distinct mice for each group (dotted line) and represented in (B) as mean ± s.e.m. (p = 0.0002) (C) Graphics shows cell proliferation represented as the percentage of BrDU incorporation by the primary keratinocytes. EGF was added where indicated (***p≤0.001).
Figure 4. Rapamycin regulates the Akt-mTOR network in Pten-deficient keratinocytes and delays wound healing.
Primary cultures of keratinocytes isolated from control and K14Cre Pten F/F mice were treated with vehicle or rapamycin (50 nM), as indicated. Data are representative of three independent experiments performed in triplicate. (A) Western blot analysis showing very low Pten levels in K14Cre Pten F/F keratinocytes, which presented increased levels of both pAktThr308 and pAktSer473. Rapamycin effectively ablates S6 phosphorylation, decreases pAktS473, and increases pAktThr308 in K14Cre Pten F/F keratinocytes when compared to control keratinocytes. GAPDH provided loading controls. (B) Bar chart represents the percentage of BrDU positive cells in the primary culture of keratinocytes (***p≤0.001). (C and D) The graphic represents the percentage of animals with open wounds in the control mice (C) and K14Cre Pten F/F mice group (D) at each indicated day after cutaneous wounding. Rapamycin and vehicle administration was initiated 48 h before the wounding (see methods for details) and wound closure was used as endpoint; n = 8 for each genotype and time point; p values are indicated.
Rapamycin delays healing in vivo
Accumulated experience in the clinic suggests that prolonged rapamycin treatment may result in delayed wound healing [21], [22]. This was reflected in a significant delay in wound closure upon rapamycin treatment of control mice when compared to mice treated with vehicle (p = 0.0154, Figure 4C). We also analyzed the consequences of chronic and long term use of rapamycin by performing incisional wounds on mice treated for more than 6 months with rapamycin, which delayed wound closure to an extent similar to that caused by rapamycin administration during the acute wound healing studies (data not shown). Using this pharmacological approach, we next investigated whether mTor contributes to the acceleration of wound healing caused by Pten deletion. K14Cre Pten F/F mice treated with rapamycin presented a delayed wound closure when compared to vehicle injected K14Cre Pten F/F mice (p<0.0001, Figure 4D). These results showed that inhibition of mTOR in vivo abrogates the accelerated wound healing caused by Pten deletion in the epithelial compartment, thus suggesting an important role for mTor in the wound healing process downstream from PI3K and Pten.
Activation of mTor in the epithelial compartment accelerates wound closure
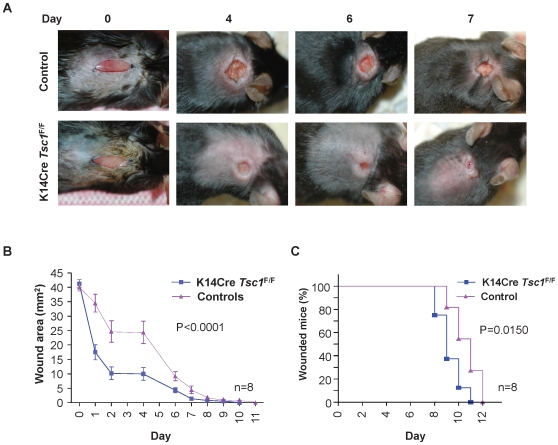
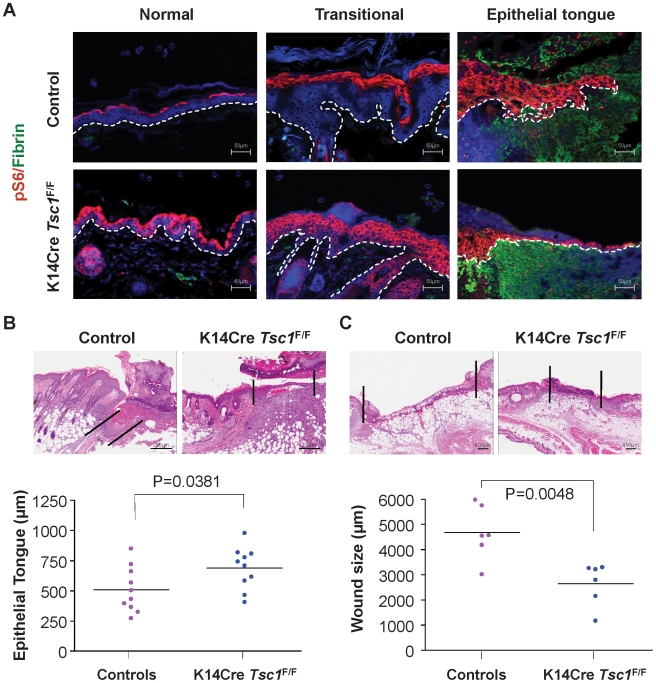
As rapamycin may exert multiple effects upon mTOR inhibition in the epithelial and stromal compartments of the wound area, we sought to examine the direct consequences on wound healing of the conditional activation of mTOR in the epithelial cells. The pathway linking PI3K-Akt to mTOR is initiated by phosphorylation and inactivation of the tumor-suppressor protein, tuberous sclerosis complex protein 2 (TSC2) by Akt [23]. TSC2 associates with a second tumor-suppressor protein, tuberous sclerosis complex protein 1 (TSC1), acting together as a GTPase activating protein (GAP) for the small GTPase Rheb1. The inactivation of TSC2/TSC1 complex by Akt leads to the accumulation of the GTP-bound (active) form of Rheb1, which in turn promotes the direct activation of mTOR (reviewed in [23]. Thus, as a genetically defined approach to further examine the role of epithelial mTOR in accelerated wound healing, we conditionally excised Tsc1 from keratinocytes thereby activating mTOR by perturbing a negative regulatory step acting immediately downstream from PI3K and Akt. After wounding, the area of the wounds was measured daily (Figures 5A and 5B) and the time to wound closure was determined. Epithelial specific excision of Tsc1 accelerates wound healing in vivo (figure 5A, B, & C), which is evident throughout the healing process as early as day 1 (p<0.0001, Figure 5B). Indeed, the median wound closure time in K14Cre Tsc1 F/F mice is 9 days, as compared to day 11 in littermate control mice (p = 0.0150, Figure 5C). Histological analysis of the wounds confirmed the upregulation of mTOR signaling on the K14Cre Tsc1 F/F mice, which displayed an overexpression of pS6 in the normal, transitional and the elongated migrating epithelial tongue of the skin epithelium when compared to the control mice (Figure 6A). Moreover, ablation of Tsc1 in the skin compartment resulted in the increased re-epithelization in the K14Cre Tsc1 F/F mice as judged by the length of the epithelial tongue (p = 0.0381, Figure 6B). Quantification of the wound size confirmed that K14Cre Tsc1 F/F mice displayed accelerated wound closure (p = 0.0048, Figure 6C).
Figure 5. Accelerated wound closure in upon epithelial Tsc1 excision.
Representative photographs of control and K14Cre Tsc1 F/F mice at the indicated days after wounding. (B) Quantification of the wound area. Results represent the mean ± s.e.m; n = 8 for each time point and genotype. (***p<0.0001) (C) Graphic representation of the percentage of animals displaying open wounds in control mice (A) and K14Cre Tsc1 F/F mice (B) at each depicted time (days) (*p = 0.0150).
Figure 6. Acceleration of wound healing upon ablation of Tsc1 in the skin.
(A) Representative examples of immunofluorescence in histological sections of incisional skin wounds in mice; pS6 (red), fibrin(ogen) (green), DAPI (blue), as above. The white dotted line delineates the epithelial layer from the underlining dermis. Note the expansion of pS6 (red) expression in the normal, transitional and elongated migrating tongue of the epithelial compartment of the K14Cre Tsc1 F/F mice when compared to control mice. Scale bar, 50 µm. (B) Representative H&E stained sections showing enhanced re-epithelization of the wound area as reflected by the length of the migrating epithelial tongue in the K14Cre Tsc1 F/F mice when compared to the control mice. The black lines delineate the epithelial migrating tongue. Quantification of the epithelial tongue from each genotype is represented by the dot plot (n = 10 for each genotype; scale bar, 200 µm; *p = 0.0381). (C) Representative histological sections of wounded skin from control and K14Cre Tsc1 F/F mice. The black line indicates the wound width, and the scatter plot represents the quantification of the wound width from control and K14Cre Tsc1 F/F mice (n = 6 mice per genotype; scale bar, 400 µm; **p = 0.0048).
Discussion
There is an urgent need to develop effective approaches to accelerate the healing of cutaneous wounds. In this regard, whereas tumoral growth has been often referred to as wounds that do not heal [24], we can now begin to take advantage of the emerging information on growth promoting pathways in cancer cells to accelerate the healing of injured tissues. We observed here that the activation of the PI3K-Akt-mTOR pathway, whose aberrant function is one of the most frequent events in human neoplasia, represents an integral component of the normal cutaneous healing process. Most importantly, we demonstrate that the targeted activation of this signaling pathway can dramatically accelerate wound healing rates. Indeed, by the use of pharmacological and genetically-defined approaches we found that the activation of the PI3K-Akt pathway enhances the rate of wound closure dependent on the activation of mTOR, and that the activation of mTOR in epithelial cells by genetic ablation of either of two of its upstream regulators, Pten and Tsc1, suffices to accelerate cutaneous wound healing.
An interesting observation in this study is that the activation of mTOR in normal epidermis always occurs in granular layers, which represent differentiated non-proliferative cells. This may explain why mTOR inhibitors do not exert a widespread cytostatic effect on normal epithelial cells in vivo, nor do they affect normal skin function, as suggested by clinical studies and our recently published reports [15], [25], [26], together suggesting that the basal keratinocytes may not strictly depend on mTOR activity to proliferate. However, during wound healing, mTOR activation is observed in the basal, subrabasal, and spinous layers of the stratified epithelium adjacent to the wound wedge and in the migrating epithelial tongue, suggesting that both the proliferative capacity and migratory activity of these cells may be controlled by mTOR. In addition, the formation of the epithelial tongue and wound closure involves of the interaction between the proliferating and migrating keratinocytes and the stromal cells. Thus, it is possible that mTOR activity in the epithelial cells may also regulate the expression of cytokines such as TGFβ, TGFα, FGF, and VEGF, which are known to contribute to the epithelial/stromal interactions during wound healing (reviewed in [27], [28], [29]). In this context, it is possible that mTOR activity in the epithelial cell compartment may facilitate wound closure by regulating epithelial cell proliferation and migration, together with its ability to regulate paracrine mechanisms involved in cellular communication processes that contribute to the wound microenvironment.
mTOR inhibitors such as rapamycin (serolimus) have been used in combination with cyclosporine or tacrolimus in the clinic since 1999 for decreasing the risk of acute allograft rejection episodes, as it helps preserve the long-term renal function better than high doses of cyclosporine (CsA) alone [26], [30]. The need to activate mTOR as part of the normal cutaneous healing process may now explain why mTOR inhibitors can cause a delay in wound closure as recently reported [21], [26]. This potential undesirable side effect could be controlled with countermeasure therapies or by the interruption of the treatment with mTOR inhibitors during the healing process [31].
On the other hand, the PI3K-Akt-mTOR pathway may be amenable for activation by chemical approaches by the use of small synthetic molecules in addition to genetically defined strategies. This could be achieved, for example, by the use of PTEN inhibitors, some of which have been recently described [32], [33]. In this regard, small molecules interfering with PTEN function can enhance wound closure and rapidly restore lung epithelial monolayer integrity following injury in relevant models in vitro [34], [35]. Although their current off-target toxicities may prevent their clinical application, future studies may provide opportunities for the development of therapeutically relevant agents inhibiting PTEN and other molecules on its signaling pathway as a molecular-targeted approach to accelerate wound healing upon local delivery.
Indeed, based on our current results, we can expect that the transient and localized activation of mTOR by molecules interfering with the activity of TSC1 and or TSC2 may represent a direct approach to trigger mTOR activation with limited interference with other intracellular signaling routes, thereby reducing unwanted side effects. In particular, whereas genetic defects in TSC1 and TSC2 may lead to hamartomas, a non-neoplastic proliferative disorder [36], [37], the deletion of Tsc1 did not have a demonstrable impact on normal biology of the skin, nor did we observe spontaneous tumor formation in mice with conditional Tsc1 deletion in the skin after more than one year of observation (not shown). These findings may provide a rationale for the future development of small molecule inhibitors of TSC1/TSC2 and/or other molecules suppressing the mTOR pathway for local delivery to accelerate wound healing. Whereas the cancer risk of this approach should be carefully examined, we have recently observed that epithelial stem cells are endowed with protective mechanisms triggering stem cell differentiation or senescence upon aberrant activation of mTOR [15]. Thus, pharmacological or genetic strategies aimed at the local activation of mTOR in the epidermis may accelerate the ability to epithelial cells to migrate and repopulate damaged skin areas hence accelerating wound closure without resulting in the aberrant growth of basal keratinocytes and their stem cells.
Overall, our present findings indicate that the transient pharmacologic activation of the PI3K/Akt/mTOR pathway may represent a novel clinical intervention strategy to accelerate the healing of critical sized, debilitating chronic, and life-threatening wounds in patients.
Materials and Methods
Wound healing assay and experimental mice
This study was approved by the Animal Care and User Committee (ACUC), according to NIH animal study protocols approved by the ACUC, protocol 06-408, National Institute of Dental and Craniofacial Research (NIDCR), in compliance with the “Guide for the Care and Use of Laboratory Animals”. Animals were housed on 12-h light/dark cycles and received food, standard rodent chow, and water ad libitum in compliance with AAALAC guidelines. The animals were observed daily by the investigators and animal care staff. Any animals displaying signs of discomfort, wasting, ruffled hair coat, hunching, or other signs indicative of distress were treated appropriately to alleviate discomfort or euthanized if recommended by animal care staff or the facility veterinary. Epithelial-specific Pten and Tsc1 knockouts were obtained by crossing mice carrying the floxed Pten [38] and Tsc1 alleles [39] with mice expressing Cre recombinase under the control of cytokeratin 14 promoter (K14-Cre) [40] as previously described [25]. Mice were anesthetized and dorsal surfaces were shaved and cleaned with 10% povidone-iodine topical solution. Fifteen millimeters full-thickness incisional skin wounds were made in the mid-dorsal area. The wound field was excised and fixed in aqueous buffered zinc formalin for 24 h, transferred to 70% ethanol, paraffin embedded and sectioned. The wound sites were monitored and measured daily utilizing wound closure as endpoint as previously described [41].
Rapamycin administration and Bromodeoxyuridine (BrDU) incorporation in vivo
Freshly prepared 5-bromo-2′-deoxyuridine (BrDU) was injected intraperitoneally (i.p.) in a concentration of 100 µg/g body weight, 2 h before sacrificing the animals. Rapamycin (LC Laboratories, MA) was reconstituted in absolute ethanol at 10 mg ml−1 and stored at −20°C. Rapamycin was diluted in 5.2% Tween-80 (Sigma, MO) and 5.2% polyethyleneglycol (PEG-400, Hampton Research, CA), and injected intraperitoneally (i.p.) 1 mg/kg every other day, as reported [25].
Culture of primary keratinocytes, in vitro wound closure (scratch) assay, BrDU incorporation, and Western blotting
Epidermis of new born mice from control and specific epidermal knockout mice were used to isolate primary murine keratinocytes as previous described [42]. Briefly, the mouse skin was peeled off, stretched out with the dermis faced down in a 60 mm culture dish, and floated on the surface of 0.25% trypsin at 4°C for 18 h. The epidermis was then separated from the dermis, minced in keratinocyte growth medium (KGM, Gibco, Carlsbad, CA) supplemented with high Ca2+. The undigested cornified fragments were removed by filtering the cell suspension through a sterile nylon cell strainer. The cells were collected by centrifugation, resuspended in fresh KGM Ca2+ supplemented with 10% FBS, and plated. After 24 h, attached keratinocytes were rinsed with PBS and incubated with fresh KGM in low Ca2+. Primary keratinocytes were assay with BrDU incorporation. After 24 hours starvation, cells were left untreated or treated for 24 h with 10 ng/ml EGF and 50 nM Rapamycin where indicated; and 10 µM BrDU was added for the last 2 h. Cells were fixed with Carnoy's fixative and then treated with 2 N HCl. BrDU was detected by immunofluorescence with anti-BrDU antibody (Axyll-Accurate Chemical & Scientific Corporation, Westbury, NY). Images were taken using Zeiss Axio Imager Z1 microscope equipped with an Apotome device (Carl Zeiss, Thornwood, NY). Quantitative analyses were performed by counting the total number of cells and cells expressing nuclear BrDU stain. Scratch assays were performed with keratinocytes grown to confluence on fibronectin coated wells and starved overnight. Scratches were made with a plastic pipette tip across the diameter of each well. Quantitative analysis of the scratch area of closure was performed using the Axiovision Rel. 4.7 (Carl Zeiss, Thornwood, NY). Cells were also lysed and 30 µg of cellular proteins were separated on 10% SDS-PAGE, transferred to nitrocellulose membranes, and analyzed by Western blot using primary antibodies against Akt, phospho-specific Akt (pAktSer473 and pAktThr308) and S6 (pS6) (Cell Signaling Technology, MA), PTEN (Cascade Bioscience, MA), and GAPDH (Santa Cruz Biotechnology, CA). Results are representative of three independent experiments performed in triplicate.
Histology and Immunofluorescence
Haematoxylin-Eosin (H&E) staining was performed on formalin-fixed and paraffin-embedded 4 µm serial sections according to standard procedures. Immunofluorescence was performed on cells and tissue sections after antigen-retrieval using antibodies developed against Cytokeratin 10 (K10) and Cytokeratin 14 (K14) (Covance, Princeton, NJ), Fibrin(ogen) (Dako, CA); pAktSer473 and pS6 (Cell Signaling Technology, MA), and BrDU (Axyll-Accurate Chemical & Scientific Corporation, Westbury, NY), and rhodamine-X or FITC-conjugated secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA) as described previously[15]. For nuclear staining, propidium iodide or mounting media with DAPI (Vector Laboratories, Burlingame, CA) was used. Images were taken using Zeiss Axio Imager Z1 microscope equipped with an Apotome device (Carl Zeiss, Thornwood, NY) and ScanScope (Aperio, CA). Quantitative analysis of the migrating epithelial tongue and wound size were done with ImageScope (Aperio, CA) and Axiovision Rel. 4.6 (Carl Zeiss, Thornwood, NY). H&E stain and immunofluorescence were performed in all the samples from every control and transgenic mice.
Statistical analysis
Statistical analyses were performed by ANOVA analysis of variance test, followed by the Bonferroni's multiple comparison. The Kaplan-Meier analysis followed by log rank test was performed to analyze time to wound closure. T-test was used for comparisons of histological wounded size and area, migration of the epithelial tongue, and BrDU incorporation using GraphPad Prism 4.03 (GraphPad Software, San Diego, CA). Asterisks denote statistic significance (NS, P>0.05; * P<0.05; ** P<0.01; and *** P<0.001).
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by the Intramural Research Program of NIH, National Institute of Dental and Craniofacial Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Ehrenreich M, Ruszczak Z. Update on tissue-engineered biological dressings. Tissue Eng. 2006;12:2407–2424. doi: 10.1089/ten.2006.12.2407. [DOI] [PubMed] [Google Scholar]
- 3.Ashcroft GS, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerontology. 2002;3:337–345. doi: 10.1023/a:1021399228395. [DOI] [PubMed] [Google Scholar]
- 4.Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds LE, Conti FJ, Lucas M, Grose R, Robinson S, et al. Accelerated re-epithelialization in beta3-integrin-deficient- mice is associated with enhanced TGF-beta1 signaling. Nat Med. 2005;11:167–174. doi: 10.1038/nm1165. [DOI] [PubMed] [Google Scholar]
- 6.Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 7.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 8.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 9.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 10.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 11.Keniry M, Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27:5477–5485. doi: 10.1038/onc.2008.248. [DOI] [PubMed] [Google Scholar]
- 12.Sarret Y, Woodley DT, Grigsby K, Wynn K, O'Keefe EJ. Human keratinocyte locomotion: the effect of selected cytokines. J Invest Dermatol. 1992;98:12–16. doi: 10.1111/1523-1747.ep12493517. [DOI] [PubMed] [Google Scholar]
- 13.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 14.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 15.Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009;5:279–289. doi: 10.1016/j.stem.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- 17.Comer FI, Parent CA. Phosphoinositides specify polarity during epithelial organ development. Cell. 2007;128:239–240. doi: 10.1016/j.cell.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Kunz J, Henriquez R, Schneider U, Deuter-Reinhard M, Movva NR, et al. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993;73:585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- 19.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, et al. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 21.Knight RJ, Villa M, Laskey R, Benavides C, Schoenberg L, et al. Risk factors for impaired wound healing in sirolimus-treated renal transplant recipients. Clin Transplant. 2007;21:460–465. doi: 10.1111/j.1399-0012.2007.00668.x. [DOI] [PubMed] [Google Scholar]
- 22.Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet. 2000;356:194–202. doi: 10.1016/s0140-6736(00)02480-6. [DOI] [PubMed] [Google Scholar]
- 23.Inoki K, Guan KL. Tuberous sclerosis complex, implication from a rare genetic disease to common cancer treatment. Hum Mol Genet. 2009;18:R94–100. doi: 10.1093/hmg/ddp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 25.Squarize CH, Castilho RM, Gutkind JS. Chemoprevention and treatment of experimental Cowden's disease by mTOR inhibition with rapamycin. Cancer Res. 2008;68:7066–7072. doi: 10.1158/0008-5472.CAN-08-0922. [DOI] [PubMed] [Google Scholar]
- 26.Kahan BD. Fifteen years of clinical studies and clinical practice in renal transplantation: reviewing outcomes with de novo use of sirolimus in combination with cyclosporine. Transplant Proc. 2008;40:S17–20. doi: 10.1016/j.transproceed.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Raja, Sivamani K, Garcia MS, Isseroff RR. Wound re-epithelialization: modulating keratinocyte migration in wound healing. Front Biosci. 2007;12:2849–2868. doi: 10.2741/2277. [DOI] [PubMed] [Google Scholar]
- 28.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 29.Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathew TH, Van Buren C, Kahan BD, Butt K, Hariharan S, et al. A comparative study of sirolimus tablet versus oral solution for prophylaxis of acute renal allograft rejection. J Clin Pharmacol. 2006;46:76–87. doi: 10.1177/0091270005282628. [DOI] [PubMed] [Google Scholar]
- 31.Campistol JM, Cockwell P, Diekmann F, Donati D, Guirado L, et al. Practical recommendations for the early use of m-TOR inhibitors (sirolimus) in renal transplantation. Transpl Int. 2009;22:681–687. doi: 10.1111/j.1432-2277.2009.00858.x. [DOI] [PubMed] [Google Scholar]
- 32.Schmid AC, Woscholski R. Phosphatases as small-molecule targets: inhibiting the endogenous inhibitors of kinases. Biochem Soc Trans. 2004;32:348–349. doi: 10.1042/bst0320348. [DOI] [PubMed] [Google Scholar]
- 33.Rosivatz E, Matthews JG, McDonald NQ, Mulet X, Ho KK, et al. A small molecule inhibitor for phosphatase and tensin homologue deleted on chromosome 10 (PTEN). ACS Chem Biol. 2006;1:780–790. doi: 10.1021/cb600352f. [DOI] [PubMed] [Google Scholar]
- 34.Zhao M. PTEN: a promising pharmacological target to enhance epithelial wound healing. Br J Pharmacol. 2007;152:1141–1144. doi: 10.1038/sj.bjp.0707503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai JP, Dalton JT, Knoell DL. Phosphatase and tensin homologue deleted on chromosome ten (PTEN) as a molecular target in lung epithelial wound repair. Br J Pharmacol. 2007;152:1172–1184. doi: 10.1038/sj.bjp.0707501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 37.Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372:657–668. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- 38.Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 39.Meikle L, McMullen JR, Sherwood MC, Lader AS, Walker V, et al. A mouse model of cardiac rhabdomyoma generated by loss of Tsc1 in ventricular myocytes. Hum Mol Genet. 2005;14:429–435. doi: 10.1093/hmg/ddi039. [DOI] [PubMed] [Google Scholar]
- 40.Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- 41.Romer J, Bugge TH, Pyke C, Lund LR, Flick MJ, et al. Plasminogen and wound healing. Nat Med. 1996;2:725. doi: 10.1038/nm0796-725a. [DOI] [PubMed] [Google Scholar]
- 42.Castilho RM, Squarize CH, Patel V, Millar SE, Zheng Y, et al. Requirement of Rac1 distinguishes follicular from interfollicular epithelial stem cells. Oncogene. 2007;26:5078–5085. doi: 10.1038/sj.onc.1210322. [DOI] [PubMed] [Google Scholar]