The dynamics of actin and microtubules are coordinated in many cellular processes, but little is known about molecules mediating cross-talk. We describe intracellular dynamics of Shot in a structure-function analysis of its role as a cross-linker. Shot interacts with microtubules two ways through EB1 and along microtubule lattices by the GAS2 domain.
Abstract
The dynamics of actin and microtubules are coordinated in a variety of cellular and morphogenetic processes; however, little is known about the molecules mediating this cytoskeletal cross-talk. We are studying Short stop (Shot), the sole Drosophila spectraplakin, as a model actin–microtubule cross-linking protein. Spectraplakins are an ancient family of giant cytoskeletal proteins that are essential for a diverse set of cellular functions; yet, we know little about the dynamics of spectraplakins and how they bridge actin filaments and microtubules. In this study we describe the intracellular dynamics of Shot and a structure–function analysis of its role as a cytoskeletal cross-linker. We find that Shot interacts with microtubules using two different mechanisms. In the cell interior, Shot binds growing plus ends through an interaction with EB1. In the cell periphery, Shot associates with the microtubule lattice via its GAS2 domain, and this pool of Shot is actively engaged as a cross-linker via its NH2-terminal actin-binding calponin homology domains. This cross-linking maintains microtubule organization by resisting forces that produce lateral microtubule movements in the cytoplasm. Our results provide the first description of the dynamics of these important proteins and provide key insight about how they function during cytoskeletal cross-talk.
INTRODUCTION
The actin and microtubule cytoskeletal networks are often studied as distinct systems with independent behaviors and regulatory pathways. However, careful observation of actin filament and microtubule dynamics in living cells has revealed a level of coordination between these two systems that can only result from complex patterns of mechanical cross-talk (Waterman-Storer and Salmon, 1997; Waterman-Storer et al., 2000; Salmon et al., 2002; Schaefer et al., 2002; Mandato and Bement, 2003). Actin–microtubule interactions have now been implicated in a diverse set of cellular processes, including cell migration, mitosis, cortical flow, and tissue morphogenesis (Rodriguez et al., 2003). Factors known to mediate actin–microtubule cross-linking are either multivalent proteins with discrete actin- and microtubule-binding sites or protein complexes with distinct subunits (sometimes motor proteins) that each bind to a different type of filament (Lantz and Miller, 1998; Goode et al., 1999; McCartney et al., 2001; Fukata et al., 2002; Kuriyama et al., 2002; Goriounov et al., 2003; Hwang et al., 2003; Rothenberg et al., 2003). Although the list of known cross-linking factors continues to grow, we lack a deep understanding about how actin–microtubule cross-talk is regulated temporally and spatially within the cell and how these molecules contribute to cytoskeletal architecture.
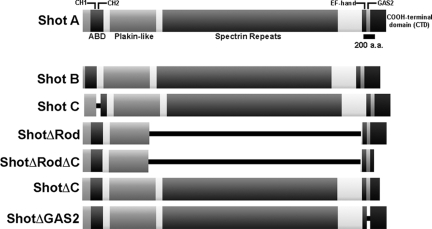
Spectraplakins are a family of giant cytoskeletal cross-linking proteins that have been highly conserved throughout animal evolution (Röper et al., 2002; Sonnenberg and Liem, 2007). These proteins exhibit a characteristic multidomain protein structure, and their transcripts are alternatively spliced to generate a wide diversity of isoforms (Bernier et al., 1996; Leung et al., 1999; Gong et al., 2001; Röper and Brown, 2003). All spectraplakins possess a series of plakin domains that are structurally similar to spectrin repeats. Many also have one or two NH2-terminal calponin homology (CH) domains, a COOH-terminal region containing one or more EF-hand motifs, and a GAS2-like domain (Figure 1). These NH2- and COOH-terminal domains are often separated by a three helix coiled-coil domain encompassing the plakin domains, multiple plectin-like repeats, and a long rod-like domain composed of spectrin repeats. When present in two tandem copies, the CH domains are able to bind tightly to actin filaments, the plectin repeats bind to cytoplasmic intermediate filaments, but only in mammals, and the GAS2 domain interacts with microtubules (Leung et al., 1999; Karakesisoglou et al., 2000; Sun et al., 2001; Lee and Kolodziej, 2002a). Thus, spectraplakins are thought to be elongated, rod-like molecules with cytoskeletal-binding sites on either end. This organization suggests that these molecules are able to act as multivalent cytoskeletal cross-linkers, and this hypothesis is supported by several functional studies in fly and mouse cells.
Figure 1.
Schematic Representation of Shot, its isoforms, and deletion constructs. (A) Schematic diagram of Shot A is given. The Shot A, B, and C isoforms vary at their NH2 terminus while having identical central and COOH-terminal domains. The Shot C isoform differs having only one CH domain. ShotΔRod, ShotΔRodΔC, ShotΔC, and ShotΔGAS2 are derived from Shot A. All constructs are COOH EGFP-tagged unless otherwise noted.
Although spectraplakins have been evolutionarily conserved across animal genomes, they have been studied in greatest detail in Drosophila, in which the sole homologue is named Short stop (Shot), and mouse, which has two paralogues (dystonin/BPAG1 and ACF7/MACF2) (Tamai et al., 1994; Bernier et al., 1996; Gregory and Brown, 1998; Leung et al., 1999). Loss-of-function studies of spectraplakins have revealed several important functions for these proteins. First, they are important for maintaining both cell–cell (via cadherins) and cell–matrix (via integrins) adhesions, and mutant cells exhibit defects in microtubule-activated turnover of focal adhesions (Gregory and Brown, 1998; Röper and Brown, 2003; Wu et al., 2008). Second, they play a role in neuronal cell motility and mutants exhibit defects in axon outgrowth, as well as neurodegeneration (Bernier et al., 1996; Prokop et al., 1998; Lee et al., 2000; Sanchez-Soriano et al., 2009). Cell biological studies also revealed that spectraplakins may have a more general role in motility because loss of ACF7 renders cells unable to polarize, and they crawl aberrantly in a wound-healing assay (Kodama et al., 2003; Drabek et al., 2006). Third, there is evidence that spectraplakins may act to organize plasma membrane subdomains and speculation that, given their size, they may act as scaffolding proteins to organize the assembly of higher order protein complexes (Strumpf and Volk, 1998). Fourth, genetic evidence suggests that Drosophila Shot is able to indirectly regulate localized protein synthesis during axon outgrowth (Lee et al., 2007). Given the wide range of biological activities in which these proteins function, additional studies into spectraplakin dynamics and mechanism are likely to provide insight into many basic cellular functions.
Many of the cellular processes in which spectraplakins participate are temporally and spatially regulated; yet, we know very little about spectraplakin dynamics or how they are regulated in living cells. In this study, we characterized the behavior of Shot in cultured Drosophila cells and conducted a structure–function analysis of Shot's activity as a cytoskeletal cross-linker. We found that Shot is able to interact with microtubules using two distinct protein domains. In the central part of the cell, Shot selectively interacted with growing microtubule plus ends. This behavior was mediated by an interaction between EB1 and the COOH-terminal ∼160 residues of Shot. In the actin-rich cell periphery, Shot interacted with the microtubule lattice and this localization was mediated by the protein's GAS2 domain. Shot's association with the microtubule lattice also required the NH2-terminal actin-binding CH domains, as well as filamentous actin, indicating that this pool of Shot actively cross-linked actin and microtubules. To determine whether Shot-mediated cytoskeletal cross-linking affected microtubule dynamics in S2 cells, we used RNA interference (RNAi) to deplete Shot and imaged microtubule dynamics using live cell microscopy. After Shot depletion, microtubules exhibited a pronounced lateral “fish-tailing” movement that was rarely observed in control cells. This lateral motion was produced by microtubule-based motor proteins and could be inhibited using RNAi to deplete kinesin and dynein. This study represents the first in vivo description of spectraplakin dynamics and reveals novel mechanisms for how this class of cross-linker regulates microtubule organization through interactions with the actin cytoskeleton.
MATERIALS AND METHODS
Cell Culture and RNAi
Drosophila S2 cell culture and RNAi were performed as described in Rogers and Rogers (2008). In brief, S2 cells were cultured in either Schneider's media (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen) or SF900II serum-free media (Invitrogen). RNAi was administered in six-well plates with cells (50–90% confluence) treated with 10 μg of double-stranded (ds)RNA in 1 ml of media, which was replenished every day with fresh media/RNA for 7 d. Actin was depolymerized with 125–250 nM final concentration of latrunculin A (Lat A) (Sigma-Aldrich, St. Louis, MO).
Immunofluorescence and Live Cell Microscopy
Localization of endogenous Shot was performed on S2 cells plated on concanavalin A (Con A)-coated coverslips and fixed with cold methanol as described in Rogers et al. (2003). Antibodies used in this study include anti-Rod (Short-stop, provided by the Developmental Hybridoma Bank, Department of Biology, University of Iowa, Iowa City, IA) and anti-tubulin DM1-α (Sigma-Aldrich) diluted to 1:200–1:500 in phosphate-buffered solution and 0.1% Triton X-100 (PBST). Secondary antibodies (Cy2 and Rhodamine Red; Jackson ImmunoResearch Laboratories, West Grove, PA) were used at final dilutions of 1:100. For live cell imaging, S2 cells were plated on 0.5 mg/ml Con A coated glass-bottomed dishes (MatTek, Ashland, MA) for at least 1 h before observation. Cells were imaged with a 100× numerical aperture 1.45 PlanApochromat objective by using a spinning-disk confocal (Yokogawa; PerkinElmer Life and Analytical Sciences, Boston, MA) mounted on a microscope (Eclipse TE300; Nikon, Tokyo, Japan) by using an interline transfer-cooled charge-coupled device camera (ORCA-ER; Hamamatsu Photonics, Bridgewater, NJ), a z-focus motor (Prior Scientific, Rockland, MA), an excitation and emission wheel controlled by the Lambda 10-2 controller (Sutter Instrument, Novato, CA), and emission filters (Semrock, Rochester, NY). All microscope hardware was controlled by MetaMorph (MDS Analytical Technologies, Sunnyvale, CA). All images were processed for brightness and contrast and prepared for publication using Photoshop (CS version 8.0; Adobe Systems, Mountain View, CA).
Constructs and Transfections
The pUAST-ShotA-EGFP, pUAST-ShotB-EGFP, pUAST-ShotC-EGFP, pUAST- ShotΔGAS2-EGFP, pUAST-ShotΔEF-hand-EGFP, pUAST-ShotΔRod1-EGFP, and pUAST-GAS2-EGFP plasmids were first described in Lee and Kolodziej 2002a. For expression in S2 cells, pUAST-plasmids were cotransfected with a pMT-Gal4 plasmid. The pMT-COOH-terminal domain (CTD) plasmid was constructed using standard polymerase chain reaction (PCR) procedures using the CTD forward primer and the CTD reverse primer (see Supplemental Table 1 for all specific primers sequences). The pMT CTD α, β, and γ green fluorescent protein (GFP)-fusions were cloned by 5′ overlapping extension using the following primers: CTDα-1, CTDα-2, CTDα-3, CTDβ-1, CTDβ-2, CTDβ-3, CTDγ-1, CTDγ-3, CTDγ-3, and EGFPrev. The pMT CTDdelta-mCherry fragment was cloned using CTDdelta Fwd and CTDdelta Rev primers. The pMT-ΔRodΔC-TagRFP and pMT-ShotΔC-TagRFP (TagRFP was a generous gift from R. Tsien, University of San Diego, San Diego, CA) constructs were constructed by standard PCR techniques using ΔRodΔC Fwd and ΔRodΔC Rev primers, respectively. Expression of pMT vectors (Invitrogen) was achieved with ∼25–50 μM final concentration of copper sulfate (Thermo Fisher Scientific, Waltham, MA) added directly to the media for induction of metallothionein promoter. Transient transfections were performed using the Nucleofector II (Amaxa Biosystems, Gaithersburg, MD) apparatus.
Immunoblotting
S2 whole cell extracts were generated by resuspending cell pellets in PBST. A small amount was removed to measure protein concentration. SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer was added to the remainder of the resuspended cell extracts and boiled for 5 min. The efficiency of RNAi was determined by Western blotting the lysates where equal protein amounts were loaded, and bands were normalized to antibodies against α-tubulin. Anti-bodies used in this study were diluted to 1:100–1:500 and include anti-α-tubulin DM1α (Sigma-Aldrich), anti-Rod (Short-Stop; Developmental Hybridoma Bank), anti-GFP JL8 (Clontech, Mountain View, CA), anti-dynein heavy chain (provided by J. Scholey, University of California, Davis, Davis, CA) and anti-EB1 (described in Rogers et al., 2002).
Immunoprecipitation
Transfected S2 cells were induced to express the various Shot-enhanced green fluorescent protein (EGFP) constructs with 500 μM CuSO4 for 24 h. Cells were then lysed in cell lysis buffer (CLB; 50 mM Tris, 150 mM NaCl, 0.5 mM EDTA, 1 mM dithiothreitol, 0.5% Triton X-100, and 2.5 mM phenylmethylsulfonyl fluoride), precleared, and lysates were diluted twofold in CLB. Lysates were mixed with polyclonal anti-GFP antibodies (Clontech) for 2 h at 4°C and then protein A-Sepharose beads (Sigma-Aldrich) were incubated with the antibody–lysate mixture for 2 h at 4°C. The beads were washed three times with 0.5 ml of CLB, boiled in SDS-PAGE sample buffer, and then proteins were analyzed by monoclonal anti-GFP (Clontech) and polyclonal anti-EB1 immunoblots.
RESULTS
Short Stop (Shot) Exhibits Two Distinct Modes of Interaction with Microtubules
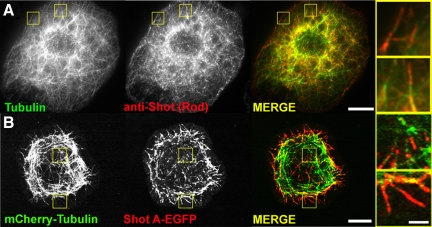
In previous work, we examined the subcellular distribution of Shot by immunofluorescence in S2 cells and found that it localized to microtubules and was enriched at plus ends where it partially colocalized with the microtubule plus end-interacting protein (+TIP) EB1 (Slep et al., 2005). We also performed time-lapse imaging of an EGFP fusion of the long 600-kDa isoform B (Shot B) and observed comet-like movements throughout the cell that were similar to EB1, as well as segments of microtubules that were decorated along their length. To begin our current study, we reexamined Shot localization by immunostaining Drosophila S2 cells with an antibody raised against the central Rod domain and an antibody against α-tubulin (Figure 2A). We observed a population of endogenous at the cell interior highly reminiscent of EB1 comets and a population of Shot that localized along the length of microtubules at the cell periphery. In comparing the endogenous localization of Shot to that of EGFP-Shot A and mCherry-Tubulin we observed similar distributions (Figure 2, A and B).
Figure 2.
Shot's localization by immunostaining and live cell imaging. (A) An S2 cell fixed and stained for endogenous α-tubulin (left, green in merged image) and Shot (middle, red in merged image). Regions shown at higher magnification (far right) are indicated by yellow boxes in low-magnification images. (B) An S2 cell expressing mCherry-Tubulin (left, green in merged image) and Shot A-EGFP (middle, red in merged image). Regions shown at higher magnification (far right) are indicated by yellow boxes in low magnification images. Bars, 10 μm in low-magnification images and 2 μm in high-magnification images.
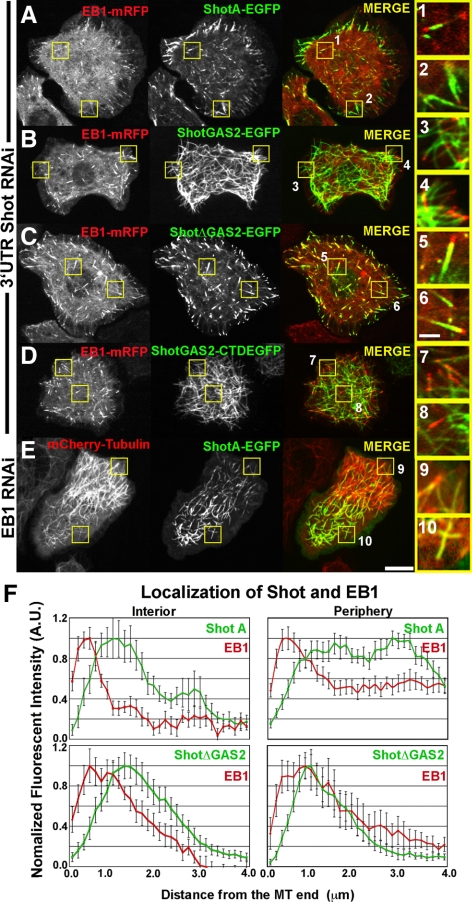
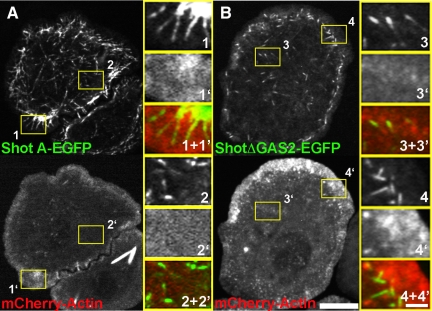
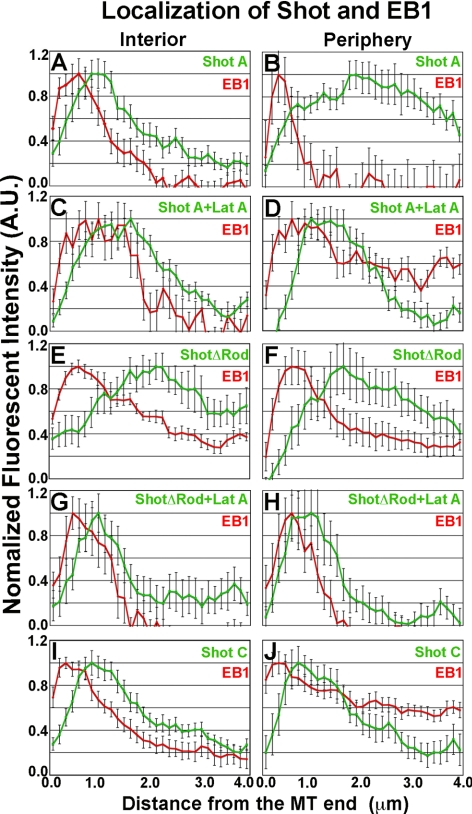
To examine Shot dynamics with respect to microtubules in living cells, we used a strategy in which endogenous Shot was depleted from S2 cells for a period of 7 d by using dsRNA that specifically targeted the 3′-untranslated region (3′-UTR) of the endogenous transcript and transfected EGFP-tagged versions of Shot (Supplemental Figure 1A). We coexpressed EB1-monomeric red fluorescent protein (mRFP) as a marker for plus ends and either Shot A-EGFP or Shot B-EGFP under the control of a copper-inducible promoter in S2 cells, and visualized cells by spinning disk confocal microscopy (Figure 3A and Supplemental Movie 1). Shot isoform A is identical to isoform B with respect to its domain structure except that it has a unique 143 residue sequence at its NH2 terminus; these two isoforms are also genetically redundant during embryonic development (Lee and Kolodziej, 2002a,b; Lee et al., 2007) (Figure 1). We used fluorescence intensity line scan measurements to assess the degree of colocalization between EB1-mRFP and Shot A-EGFP along microtubules in living cells (Figure 3F). In the cell interior, Shot A and B-EGFP dynamics resembled the behavior of EB1-mRFP and distributions of the two proteins were closely superimposable. We observed Shot's distribution extending <2 μm from the microtubule plus end (Figure 3F). At the cell periphery, however, Shot A and B-EGFP exhibited longer comet tails, with association with the microtubule lattice extending further away from the growing plus end. In this case, we observed Shot's distribution extending 4 μm away from the microtubule plus end (Figure 3F). We defined the cell periphery as a zone extending ∼5 μm from the cell margin (Iwasa and Mullins, 2007).
Figure 3.
Shot has two distinct modes of interacting with microtubules. (A) An S2 cell cotransfected with EB1-mRFP (left, red in merged image) and full-length Shot A (middle, green in merged image) after depletion of endogenous Shot using RNAi designed against the 3′-UTR of Shot. High-magnification images to the far right highlight Shot A and EB1 in the interior (1) and perimeter (2). (B) An S2 cell expressing EB1-mRFP (left, red in merged image) and the GAS2 domain of Shot (middle, green in merged image) after RNAi depletion of endogenous Shot. High-magnification images (3 and 4, far right) demonstrate that the GAS2 binds along the lattice of microtubules and does not plus end track. (C) EB1-mRFP (left, red in merged image) and ShotAΔGAS2 (middle, green in merged image) coexpressed in an S2 cell after depletion of endogenous Shot by RNAi against 3′-UTR of Shot. Higher magnification images (5 and 6, far right) demonstrate that ShotAΔGAS2 plus end tracks and does not bind to the lattice. (D) An S2 cell coexpressing EB1-mRFP (left, red in merged image) and ShotGAS2-CTD (middle, green in merged image) after depletion of endogenous Shot. Higher magnification images (7 and 8 far right) indicate that despite having the EB1 binding CTD, the GAS2 domain targets this fusion to the microtubule lattice. (E) An S2 cells expressing mCherry-Tubulin (left, red in merged image) and Shot A after RNAi depletion of EB1. High-magnification images (9 and 10, far right) indicate that ShotA maintains lattice association despite loss of +TIP association. (F) Graphical representations of line scans plotting the distribution of Shot (green) and EB1 (red) at the cell interior (top left), Shot A and EB1 at the cell periphery (top right), and ShotΔGAS2 and EB1 in the cell interior (bottom left) and at the cell periphery (bottom right), with their distributions being very similar at the two locations. For each graph, the average fluorescence intensity of 10–15 individual microtubules was plotted against the distance from the microtubule end. Two populations where measured, in the cell interior and at the cell periphery (1–5 μm in from the cell margin). Bar 10 μm (low-magnification images) and 2 μm (high-magnification images) (far right).
Because Shot is a large protein composed of multiple domains, we hypothesized that these two different modes of microtubule association were mediated by distinct domains of the molecule. The GAS2-like domains from spectraplakin family members possess a microtubule binding activity both in vivo and biochemically (Figure 1) (Leung et al., 1999; Karakesisoglou et al., 2000; Sun et al., 2001; Lee and Kolodziej, 2002a). Thus, we examined the role of this domain in the dynamics of Shot. We expressed the GAS2-like domain of Shot fused to a COOH-terminal EGFP (GAS2-EGFP) in S2 cells. This protein localized along the length of microtubules (Figure 3B and Supplemental Movie 2). Time-lapse confocal imaging revealed that GAS2-EGFP localized exclusively to the microtubule lattice and never exhibited enrichment at microtubule plus ends (n = 30 cells). Overexpression of some +TIPs causes them to localize along the lengths of microtubules and obscures tip-tracking behavior. To ensure that +TIP activity was not obscured by protein overexpression, we examined a range of GAS2-EGFP–expressing cells and found that the protein localized along the lengths of microtubules even at very low expression levels (Supplemental Figure 1). These experiments demonstrate that the Shot GAS2 domain is sufficient for microtubule lattice association.
We next tested the hypothesis that the GAS2 domain is necessary for targeting Shot to the lattice by transfecting S2 cells with a version of Shot A-EGFP in which the GAS2 domain was deleted (Figure 1). In contrast to full-length Shot A-EGFP, ShotΔGAS2-EGFP localized exclusively to microtubule plus ends and was never observed to associate with the lattice (n = 50 cells; Supplemental Figure 2). To exclude the possibility that endogenous Shot contributed to the dynamics we observed, we also transfected ShotΔGAS2-EGFP into S2 cells that had been depleted of endogenous Shot (Supplemental Figure 1A). In the absence of endogenous Shot, ShotΔGAS2-EGFP still localized exclusively to microtubule plus ends (Figure 3, C and F, and Supplemental Movie 3). Again, we observed ShotΔGAS2- EGFP comets extending <2 μm from the microtubule plus end regardless of whether the tip was in the cell interior or cell periphery (Figure 3F). Furthermore, when we expressed a fragment of Shot encompassing the GAS2 domain fused to the CTD of Shot, which is thought to be critical to Shot's association with EB1, we observed lattice association as opposed to +TIP tracking (Figure 3D). This suggests that Shot's lattice association is the default mode of microtubule interaction and that lattice binding must then be inhibited in the cell interior where we see strong tip tracking by Shot A (Figure 3, A and F).
Our previous work implicated EB1 in Shot recruitment to microtubule plus ends; however, Shot potentially could interact with microtubules independently of EB1 via its GAS2 domain. To test this, we used live cell imaging to simultaneously observe Shot A- or Shot B-EGFP and mCherry-Tubulin in cells depleted of EB1 by using RNAi (Figure 3E). Microtubule dynamic instability was dramatically reduced in these cells, and most microtubules neither grew nor shrank, confirming the efficiency of EB1 depletion (Rogers et al., 2002). Under these conditions, the Shot-EGFP constructs did not exhibit recruitment to plus ends but rather exhibited association with the microtubule lattice exclusively (Figure 3E and Supplemental Movie 4). From these experiments, we conclude that Shot can interact with microtubules in two ways: at growing plus ends, via EB1, and along the lattice, via the GAS2 domain.
Shot Has Multiple EB1-binding Sites at Its COOH Terminus
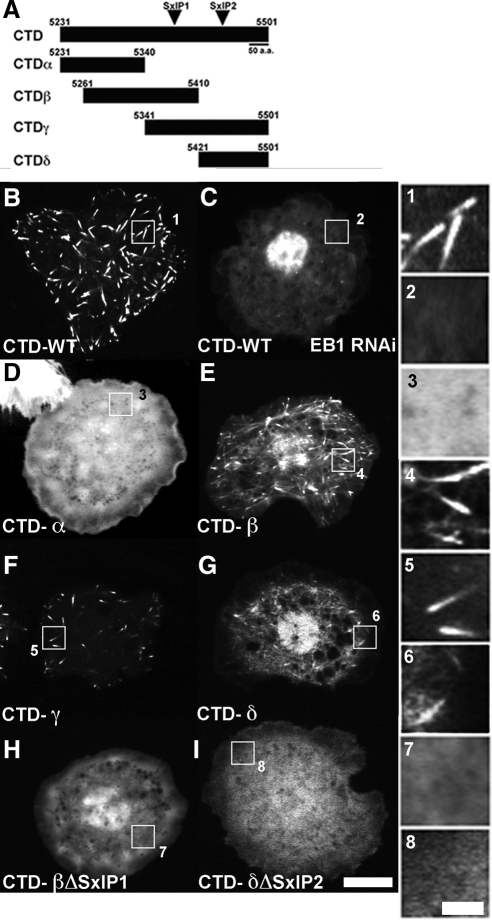
To characterize the mechanism by which EB1 targets Shot to the microtubule plus end, we set out to identify the minimal domains of Shot necessary for association with EB1 and plus end tracking. The CTD is highly conserved in most characterized isoforms of the protein (Röper and Brown, 2003) (Figures 1A and 4A). Previous work showed that this region could weakly associate with microtubules when overexpressed in NIH3T3 cells as a GFP fusion (C293-EGFP) (Lee and Kolodziej, 2002a). The CTD also contains a motif similar to the EB1-binding site of adenomatous polyposis coli (APC) (Slep et al., 2005). Therefore, we generated a fusion protein with the final 270 amino acids (residues 5231-5501) fused to EGFP (CTD-WT-EGFP) (Figure 4, A and B) to test the hypothesis that this was the EB1 interaction site. As predicted for an EB1-interacting factor, CTD-WT-EGFP exhibited robust comet-like movement in association with the plus ends of growing microtubules, and this was abolished after EB1 RNAi (Figure 4, B and C, and Supplemental Movie 5). These data demonstrate that the CTD of Shot is sufficient for microtubule plus end tracking.
Figure 4.
The COOH-terminal domain contains tandem SxIP motifs required for plus end tracking. (A) Schematic representation of the various fusion proteins we generated in order to isolate the SxIP motifs. (B–I) S2 cells transfected with the various EGFP or mCherry fusion proteins outlined in B. (B) CTD-WT (EGFP) expressed in an S2 cell. (C) CTD-WT (EGFP) expressed in an S2 cell after depletion of EB1, under these conditions the CTD of Shot does not plus end track. (D) CTD-α (EGFP). (E) CTD-β (EGFP). (F) CTD-γ (EGFP). (G) CTD-δ (mCherry). (H) CTD-β (EGFP) containing a deletion of the first SxIP motif (residues 5377-5380). (I) CTD-δ (mCherry) containing a deletion in the second SxIP motif (residues 5438-5445). (1–8) Higher magnification images corresponding to the lower magnification images pictured in B-I. Bar, 10 μm (low-magnification images) and 2 μm (high-magnification images).
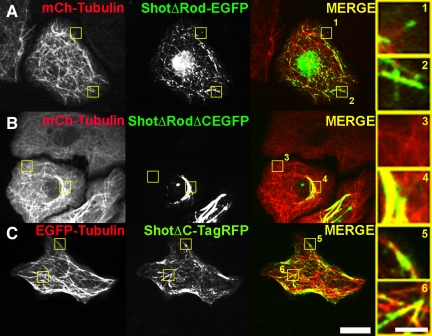
We next tested whether the CTD was necessary to target Shot to microtubule plus ends. For simplicity, we used a truncated form of Shot (ShotΔRod-EGFP) that lacks the central spectrin and plakin domains that comprise the central portion of the molecule (Figure 1). Ectopic expression of ShotΔRod-EGFP rescues many of the in vivo phenotypes in Shot mutants (Lee and Kolodziej, 2002a); however, its dynamics and localization have never been compared with full-length Shot. Unlike Shot A- or B-EGFP, ShotΔRod-EGFP exhibited both plus end tracking as well as lattice association throughout the cell, suggesting that the central rod domain confers some spatial specificity to the full-length molecule (Figure 5A). To test the role of the CTD in Shot dynamics, we deleted the last 160 COOH-terminal residues from ShotΔRod and fused it to red fluorescent TagRFP (ShotΔRodΔC-TagRFP, Figure 1). When expressed in S2 cells, this protein exclusively associated with microtubules along their lattice and never exhibited microtubule plus end localization, demonstrating that the CTD of Shot is necessary for microtubule plus end tracking (Figure 5B). Furthermore, when we made the analogous deletion to full-length Shot and C-terminally tagged this molecule with TagRFP (ShotΔC-TagRFP), we again observed weak microtubule lattice association as assessed by coexpression EGFP-Tubulin and failed to see plus-end tracking dynamics (Figure 5C and Supplemental Movie 6). Thus, the dynamics of ShotΔC-TagRFP were very similar to what we observed in S2 cells transfected with full-length Shot after depletion of EB1 by RNAi (Figure 3E).
Figure 5.
Partial deletion of the CTD ablates plus end tracking. (A) Shown in low-magnification images, an S2 cell cotransfected with mCherry-Tubulin (left, red in merge) and ShotΔRod-EGFP (middle, green in merge). Regions 1 and 2, shown at higher magnification (far right), are indicated by yellow boxes in the lower magnification image. ShotΔRod-EGFP both binds to the lattice of MT as well as plus end tracks. (B) Lower magnification images show mCherry-Tubulin (left, red in merged image) and ShotΔRodΔC-EGFP (middle, green in merged image). Regions 3 and 4 are shown at higher magnification, far right. ShotΔRodΔC-EGFP fails to plus in track but does associate with MT lattice. (C) Shown lower magnification an S2 cell coexpressing EGFP-Tubulin (left, red in merged image) and ShotΔC-TagRFP. Far right, higher magnification images (5 and 6, indicated by yellow boxes in low magnification images) demonstrates that ShotΔC, similar to ShotΔRodΔC, is unable to plus end track but retains residual MT lattice binding. Bars, 10 μm (low-magnification images) and 2 μm (high-magnification images).
To identify the plus end-targeting site with greater resolution, we subdivided the CTD into three overlapping fragments designated CTD-α (residues 5231-5430), CTD-β (residues 5261-5411), and CTD-γ (residues 5341-5501) and examined their dynamics in living cells as COOH-terminal EGFP-fusions (Figure 4A and D–F and Supplemental Movie 7). CTD-α-EGFP did not exhibit plus end tracking or association with microtubules; instead it was diffuse throughout the cytoplasm (Figure 4D). Furthermore, residues 5231-5430, which comprise CTD-α, are identical to the remaining residues in the C-terminal deletion constructs (ShotΔRodΔC and ShotΔC), confirming the lack plus end tracking we observed in these larger constructs. In contrast, CTD-β-EGFP and CTD-γ-EGFP exhibited plus-end tracking (Figure 4, E and F). CTD-γ-EGFP exhibited a more robust localization to the plus end than the CTD-β-EGFP, which was often diffuse and not associated with microtubules. When we examined the CTD for the presence of SxIP motifs, protein–protein interaction motifs described for other EB1-dependent plus end tracking proteins (Honnappa et al., 2009), we found that CTD-β and CTD-γ each contained putative SxIP motifs (Figure 4, A and B). We isolated an additional fragment encompassing the second SxIP motif and the final 90 residues of the CTD (residues 5411-5501), designated CTD-δ (Figure 4A). As predicted, CTD-δ-mCherry exhibited plus end tracking behavior (Figure 4G). These data indicate that there are at least two independent EB1-binding sites within the CTD of Shot, suggesting a mechanism of EB1-dependent plus end tracking similar to what has been reported for human CLASP2 and artificially dimerized portions of human microtubule actin cross-linking factor (MACF) and APC (Honnappa et al., 2009).
To map the exact residues that are required for EB1-dependent plus end tracking, we deleted the potential SxIP motifs in the isolated GFP fragments. When we deleted SxIP1 (residues 5377-5380) from CTD-β (Figure 4A), this was sufficient to ablate plus end tracking in this fragment (Figure 4H and Supplemental Movie 8). We next deleted the second putative SxIP (SxIP2, residues 5438-5445) from CTD-δ; this also abrogated plus end tracking (Figure 4I and Supplemental Movie 8). These results demonstrate that these two SxIP motif are required for plus end tracking of these smaller CTD-fusions and indicate that like other EB1-dependent plus end tracking proteins, Shot uses multiple motifs as well as several basic residues with in the CTD to bolster its interaction with EB1 (Figure 4A) (Honnappa et al., 2009).
To further characterize CTD–EB1 interactions, we examined whether EB1 could be immunoprecipitated with the CTD-EGFP-fusion fragments (Supplemental Figure 2B). We transfected CTD-wild type (WT), -α, -β, and -γ-EGFP into S2 cells and immunoprecipitated endogenous EB1 by using anti-GFP antibodies. In addition, we attempted to immunoprecipitate EB1 with anti-GFP antibodies in cells transfected with GAS2-EGFP (Supplemental Figure 1B). As expected, GAS2-EGFP failed to pull-down EB1, further supporting the notion that Shot is able to interact with microtubules via to separate mechanisms. In agreement with the live cell microscopy data; however, EB1 coimmunoprecipitated with CTD-β and CTD-γ. Interestingly, we also immmunoprecipitated EB1 in cells transfected with CTD-α. Thus, despite the lack of tip tracking, it is able to interact with EB1. It has been demonstrated through glutathione transferase-pull downs that substitution of Ser-Ser dipeptides at the Ile-Pro positions of the SxIP motif ablates tip tracking without abolishing the interaction with EB1. Thus, it is possible that CTD-α contains a cryptic SxIP motif that is not capable of plus end tracking once isolated but retains EB1 binding (Honnappa et al., 2009).
Interaction of Shot with the Microtubule Lattice Requires Actin
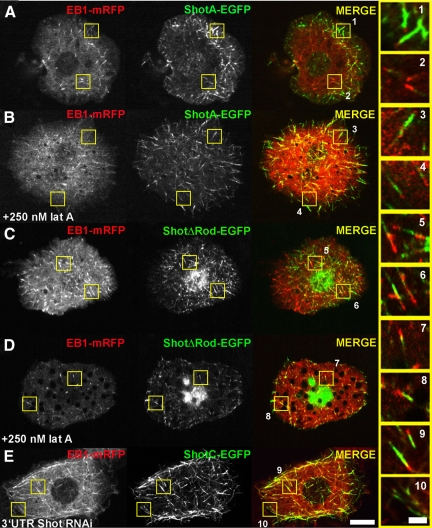
Our results indicate that the interactions between Shot and microtubules are differentially regulated between the cell interior and periphery. When plated on concanavalin A, S2 cells build a circumferential actin-rich lamella with the highest density of actin at the cell periphery (Rogers et al., 2003) (Figure 6). The position of this actin meshwork spatially correlates with the shift in Shot localization from plus end associated to microtubule lattice associated, with the latter being predominant in the lamella (Figure 6A). Because the Shot A and B isoforms both possess two tandem actin-binding CH domains at their NH2 termini (Figure 1), we hypothesized that the change in Shot's association with microtubules may reflect differences in its interaction with the actin cytoskeleton in the cell interior versus the periphery (Figures 7A and 8, A and B). To test this possibility, we cotransfected S2 cells with Shot A-EGFP and EB1-mRFP and imaged cells before and after perfusion with the drug Lat A, an actin monomer-binding drug that potently depolymerizes cellular F-actin within minutes (Figure 7B). Visualization of mCherry-Actin confirmed that the dense actin network in the lamellae exhibited the characteristic organization and retrograde flow we previously described and Lat A perfusion caused an immediate cessation of retrograde flow and an loss of actin structure within minutes of application (data not shown; Rogers et al., 2003). Perfusion of Lat A also led to a dramatic relocalization of Shot A-EGFP such that the lattice-bound pool redistributed exclusively to microtubule plus ends throughout the entire area of the cell (Figures 7B and 8, C and D). Lat A treatment also altered localization of truncated ShotΔRod-EGFP, redistributing it exclusively to plus ends after treatment (Figures 7, C and D, and 8, E–H).
Figure 6.
Shot's lattice binding corresponds to peripheral actin-rich regions of the cell. (A) An S2 cell cotransfected with Shot A-EGFP (top) and mCherry-Actin (bottom). Regions shown at higher magnification (1–1′ and 2–2′) are indicated by the yellow boxes in lower magnification images. At the cell periphery (right, 1 and 1′), Shot is localizing along the length of the microtubules that have entered a region of increased actin fluorescent intensity. In contrast, in the cell interior (2 and 2′) Shot is restricted to the tips of microtubules where the actin fluorescent intensity is low. (B) An S2 cell transfected with ShotΔGAS2-EGFP (top) and mCherry-Actin (bottom). Regions shown at higher magnification are indicated with yellow boxes (3–3′ and 4–4′). ShotΔGAS2- EGFP fails to decorate along the length of microtubules in the cell interior (3 and 3′) or at the cell periphery despite the increased actin fluorescent intensity (4 and 4′). Bars, 10 μm (low-magnification images) and 2 μm (high-magnification images).
Figure 7.
Shot's lattice binding is actin-dependent. (A–E) S2 cells coexpressing EB1-mRFP (left panels, red in merged images, right) and full-length Shot A-EGFP (A and B), ShotΔRod-EGFP (C and D), or Shot C-EGFP (E) (middle, green in merged images, right). (A) Shot preferentially binds along the lattice at the actin-rich periphery, but displays +TIP dynamics in the cell interior. (B) After perfusion with Lat A, which depolymerizes actin, Shot's localization shifts relative to EB1 and the lattice bound population is decreased. (C) ShotΔRod-EGFP demonstrates both lattice and +TIP behavior everywhere in the cell. (D) ShotΔRod also shifts to a more +TIP-like distribution after perfusion of Lat A. (E) The C isoform of Shot contains a single CH domain and binds actin much more weakly than the A isoform as a result the ShotC isoform's localization is more similar to that of EB1. Bar, 10 μm. (1–10) Higher magnification images of regions specified in lower magnification images (A–E). Bar, 2 μm.
Figure 8.
Quantification of Shot's localization in the cell interior or at the cell periphery. Graphical representations of line scans of EB1(red) and Shot derivative fusion proteins (green) from images represented in Figure 7. For each graph, the average fluorescence intensity of 10–15 individual microtubules was plotted against the distance from the microtubule end. Two populations where measured, in the cell interior and at the cell periphery (1–5 μm in from the cell margin). (A–D) The distribution of full-length Shot and EB1 are quantified in the cell interior (A) and at the cell periphery (B) as well the resulting shift in distribution after perfusion with 250 nM Lat A (C and D). (E–H) Quantification of the distribution of EB1 and ShotΔRod before perfusion Lat A (E and F) and after the treatment (G and H). Quantified in I and J are the distributions of EB1 and the ShotC isoform in the cell interior (I) and periphery (J) after depletion of endogenous Shot by RNAi.
We next tested the hypothesis that Shot's ability to bind to actin through its CH domains regulates its mode of interaction with microtubules. The Shot A isoform possesses a pair of tandem CH domains that, together, bind to actin filaments in cosedimentation assays, with a Kd ∼0.022 μM (Lee and Koloziej, 2002a). In contrast, the Shot C isoform possesses only one CH domain at its NH2 terminus and does not appreciably bind to F-actin (Lee et al., 2000; Lee and Kolodziej, 2002a) (Figure 1). We depleted endogenous Shot from S2 cells and cotransfected cultures with Shot C-EGFP and EB1-mRFP under the control of inducible promoters to observe their dynamics using confocal microscopy (Figure 7E). Shot C-EGFP colocalized with EB1-mRFP at microtubule tips throughout the cell and did not exhibit the peripheral, lattice-bound interaction observed with Shot A and B (Figures 7E and 8, I and J). These data suggest that Shot's actin-binding CH domains regulate its interactions with microtubules, probably due to a direct interaction with F-actin. Collectively, these data demonstrate that association of Shot with the microtubule lattice requires an interaction with the actin cytoskeleton via the NH2-terminal CH domains.
Shot Suppresses Lateral Microtubule Movements Driven by Motor Proteins
The interphase microtubule cytoskeleton in Drosophila S2 cells is acentrosomal and lacks radial polarity (Rogers et al., 2008). Acentrosomal microtubule arrays are not unique to cultured cells as many tissues within the developing Drosophila embryo also lack a clearly defined microtubule organizing center. Drosophila also lack intermediate filaments which often serve as a second mechanical scaffolding network for microtubules in other animals. Thus, the connection between actin and microtubules is even more crucial for the organization of microtubules in flies, and Shot has been implicated in this connection during development (Subramanian et al., 2003).
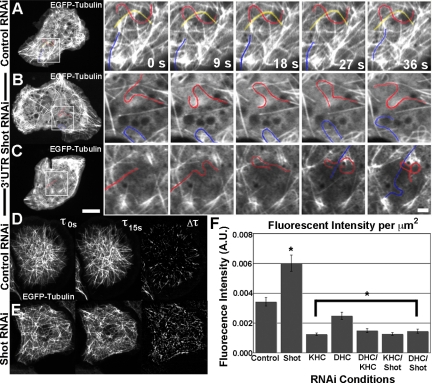
Our data demonstrate that Shot has a role in linking microtubules that grow into the lamellipodium to the actin cytoskeleton. However, we also observed a more global consequence of Shot depletion on microtubule dynamics. In control cells, microtubules rarely exhibited lateral “side-to-side” movements (Figure 9A, high-resolution panels, right, and Supplemental Movie 9). In contrast, microtubules in Shot RNAi-treated S2 cells exhibited a dramatic increase in the occurrence of lateral sliding movements, resulting in fish-tailing (Figure 9, B and C). We hypothesized that microtubule fish-tailing is produced by forces generated by motor proteins, such as kinesin and dynein, that displace microtubules lacking Shot-mediated cross-links to the actin cytoskeleton (Kulic et al., 2008). To quantify this phenotype we used sequential subtraction image analysis of microtubule dynamics in control and Shot-depleted S2 cells (Figure 9, D and E). In this technique, sequential images from a time-lapse series are subtracted from each other (Vorobjev et al., 1999). The resulting images produce a uniform field on which microtubule translocations appear as a white streak on a black background. We then quantified the extent of lateral movements by measuring the difference in tubulin fluorescence intensity between images acquired at 15-s intervals, normalizing the fluorescence intensity by the area measured to compensate for heterogeneity in S2 cell shape and size (Figure 9F). We observed a substantial and statistically significant increase in the fluorescence intensity unit per area in Shot depleted cells versus control RNAi cells, indicating that microtubules exhibited a higher degree of lateral movement after Shot depletion.
Figure 9.
Shot depletion leads to microtubule fish-tailing. (A–C) Time-lapsed stills of S2 cells transfected with EGFP-Tubulin after depletion by control RNAi (A) or 3′-UTR (B and C) of Shot. In lower magnification images (left), the region of higher magnification is indicated by white box. In higher magnification images (sequences to the right), individual microtubules are highlighted and followed over time. Bar, 10 μm (lower magnification images) and 2 μm (higher magnification images). (D and E) Method of quantification of the phenotype. In brief, S2 cells expressing mCherry-Tubulin were subtracted at 15-s intervals. The resulting fluorescence is an indicator of microtubule movement over the specified time period. This fluorescence was measured and normalized by size of the region of interest and quantified in F. (F) Quantification of fluorescence intensity per square micrometer after RNAi treatments. Asterisks indicate statistical significance as compared with control RNAi treated samples (p < 0.03, Student's t test) For each condition, five to 20 different cells were measured (a minimum of 80 data points); error bars represent SE.
To test the hypothesis that these movements were driven by microtubule-associated motor proteins, we used RNAi to deplete conventional kinesin, cytoplasmic dynein, or both motors simultaneously, and we measured the extent of lateral microtubule motion. Depletion of both motors resulted in a significant reduction in the extent of lateral microtubule movements as compared with control cells. To test the hypothesis that kinesin and dynein drive the exaggerated movements, we observed in the absence of Shot, we simultaneously depleted Shot together with kinesin or dynein and again measured the extent of microtubule displacement (Figure 9F and Supplemental Figure 1C). In both cases, lateral movements were suppressed to the same extent as depletion of the motors alone, suggesting that Shot immobilizes the microtubule network within the cytoplasm, perhaps by cross-linking to the actin cytoskeleton, and in its absence, microtubules are deformed by motor-mediated forces.
DISCUSSION
Physical cross-links between the actin and microtubule cytoskeletal networks are important in many cellular contexts; however, we have a poor understanding of when and where these interactions occur within the cell. Spectraplakins are a family of large cross-linking molecules that possess NH2-terminal actin-binding and COOH-terminal microtubule-binding domains and thus are prime candidates for the analysis of cytoskeletal cross-talk. In this study, we investigated the role of the single Drosophila spectraplakin family member, Shot, as a cytoskeletal cross-linker. Our analyses revealed that Shot is able to interact with microtubules via two distinct mechanisms, association with the microtubule lattice mediated by the GAS2 domain of Shot, and an interaction with growing plus ends that is mediated by EB1, through multiple EB1-interacting motifs at the COOH terminus of Shot. The interaction of Shot with the microtubule lattice, but not with the plus end, requires an intact network of F-actin and a functional actin-binding domain at its NH2 terminus. Our data also demonstrate that the actin- and microtubule-binding domains cooperate to regulate microtubule dynamics by cross-linking the two cytoskeletal networks. Shot cross-linking couples microtubules to actin in order to resist motor-mediated lateral sliding movements. Together, these results provide the first in vivo description of spectraplakin dynamics and provide important insight into how this family of proteins acts on a mechanistic level.
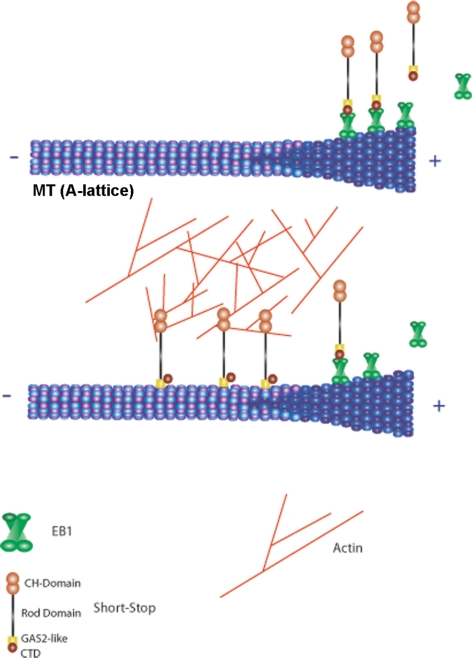
Our data suggest that Shot can exist in at least two different states. In the first state, observed after actin depolymerization or with the C isoform that exhibits low affinity for actin, Shot preferentially interacts with microtubule plus ends. In the second state, observed with the A, B, or Shot-ΔRod isoforms in the actin-rich region of the peripheral lamella, Shot is bound to the microtubule lattice via the GAS2 domain (Figure 10). Our results suggest that the pool of Shot that is actively serving as a cross-linker is the lattice-bound population. We speculate that the interconversion between these two states represents a regulated conformational change, so that the timing and placement of Shot-mediated cross-linking is precisely controlled within the cell. Understanding how Shot is regulated will be an important next step, but several potential mechanisms immediately present themselves. First, it is interesting to note that the Shot-GAS2-CTD construct decorated the microtubule lattice despite the presence of the EB1-interaction motif. This result suggests that GAS2 exerts a dominant effect when present in the same molecule and further suggests that it must be actively repressed when Shot is at microtubule plus ends. We favor the hypothesis that Shot is regulated by an intramolecular interaction that simultaneously represses the GAS2 and actin-binding CH domains during tip tracking. On activation, the molecule opens and is able to bind the lattice and actin. Second, the EF-hand motifs are closely positioned to the GAS2 domain, suggesting that calcium may participate in regulation of microtubule lattice binding. Third, there is evidence that Shot may act downstream of the small GTPase Rho; perhaps activation of this pathway leads to activation of the cross-linking activity (Lee and Kolodziej, 2002b). Fourth, binding of the CH domains to actin may itself be sufficient to trigger the transition from tip-tracker to lattice-binder. Understanding when and where Shot is activated to cross-link will be key to understanding its cellular functions.
Figure 10.
Shot's conversion from +Tip tracking to lattice binding/cross-linking is dependent on actin binding. (Top) Shot plus end tracks, similarly to other EB1 dependent plus end tracking proteins in the interior of the cell. (Bottom) Once Shot's CH domains bind actin, Shot's dynamic shifts binding along the lattice of microtubules and functionally cross-linking actin to microtubules. This mode of Shot's dynamics is EB1 independent.
Why does Shot possess these dual mechanisms (tip vs. lattice) for microtubule interaction? This remains unknown, but we can speculate based on our understanding of other microtubule-associated proteins. Microtubule dynamic instability is used as a mechanism to search intracellular space for docking and stabilization during kinetochore microtubule capture and polarization of migrating cells. Perhaps association with the microtubule plus end allows spectraplakins to search for activating inputs at the cell cortex or in actin-rich subregions of the cell. Different modes of microtubule association may also allow spectraplakins to regulate microtubule dynamics in different ways. A common theme emerging from studies of other +TIPs is that many of them function is as regulators of microtubule dynamic instability. Because the majority of subunit addition and loss occurs at the plus end, +TIPs are perfectly positioned to influence the conformation of tubulin at this site. We present evidence that Shot also can influence microtubule dynamics through a different mechanism, by cross-linking microtubules to the actin network to prevent lateral microtubule displacement by motor proteins.
Previous studies of the role of mouse ACF7 have clearly demonstrated a role for this protein as a “guide” to direct microtubule growth along actin stress fibers to assist them in targeting to focal adhesions (Wu et al., 2008). Our data show that, in S2 cells, Shot-mediated cross-linking performs an additional function by cross-linking microtubules to actin in order to resist whip-like lateral displacements by motor proteins. Many Drosophila cell types exist without a dominant interphase microtubule organizing center, instead organizing acentriolar networks to organize their cytoplasm (Rogers et al., 2008). We speculate that, in the absence of a structural anchor at the minus end, acentriolar cells rely upon actin-microtubule cross-linking to maintain their organization and to resist forces produced by motor proteins. These forces may be generated during organelle transport, especially if the motors associated with the surface of one organelle engage multiple microtubules. Shot is, therefore, likely to play an important role in maintaining microtubule organization for the efficient motor-mediated delivery of organelles and other cargo to specific destinations within the cell. Furthermore, this activity is consistent with the observation that the microtubule network in mushroom body neurons in Shot mutants exhibits gross disorganization and an overall loss of microtubule polarity (Reuter et al., 2002).
Based on our data we have developed a speculative model in which “inactive” Shot associates with a growing microtubule plus end by its interaction with EB1. Dynamic instability allows microtubules to probe the cytoplasm for an activating signal in a “search-and-capture” mechanism. On receiving the activating stimulus, Shot changes its conformation, releases from EB1, and engages actin with is dual CH domains and the microtubule lattice with its GAS2 domain. In this conformation, it is able to cross-link microtubules to actin to influence their growth trajectory or to stabilize the network against forces produced by motors. Testing these ideas will require careful observation of Shot dynamics in the developing embryo where cells are responding to extracellular cues, as well as genetic analyses to determine how the EB1–Shot interaction contributes to development.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mark Peifer, Bob Duronio, Greg Matera, and members of the Rogers, Slep, and Peifer laboratories for helpful input during the design of this project and critical reading of the manuscript. This work was supported by a grant from the American Cancer Society PF-08-152-01-CSM (to D.A.A.), by National Institutes of Health grant T32 HD046369-05 (to K.D.G.), and by National Institutes of Health grant R01 GM-081645 and the American Heart Association and March of Dimes (to S.L.R.).
Abbreviations used:
- +TIPs
microtubule plus end-interacting proteins
- 3′-UTR
3′-untranslated region of mRNA.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-01-0011) on March 24, 2010.
REFERENCES
- Bernier G., Mathieu M., De Repentigny Y., Vidal S. M., Kothary R. Cloning and characterization of mouse ACF7, a novel member of the dystonin subfamily of actin binding proteins. Genomics. 1996;38:19–29. doi: 10.1006/geno.1996.0587. [DOI] [PubMed] [Google Scholar]
- Drabek K., et al. Role of CLASP2 in microtubule stabilization and the regulation of persistent motility. Curr. Biol. 2006;16:2259–2264. doi: 10.1016/j.cub.2006.09.065. [DOI] [PubMed] [Google Scholar]
- Fukata M., Watanabe T., Noritake J., Nakagawa M., Yamaga M., Kuroda S., Matsuura Y., Iwamatsu A., Perez F., Kaibuchi K. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–885. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Gong T. W., Besirli C. G., Lomax M. I. MACF1 gene structure: a hybrid of plectin and dystrophin. Mamm. Genome. 2001;12:852–861. doi: 10.1007/s00335-001-3037-3. [DOI] [PubMed] [Google Scholar]
- Goode B. L., Wong J. J., Butty A. C., Peter M., McCormack A. L., Yates J. R., Drubin D. G., Barnes G. Coronin promotes the rapid assembly and cross-linking of actin filaments and may link the actin and microtubule cytoskeletons in yeast. J. Cell Biol. 1999;144:83–98. doi: 10.1083/jcb.144.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriounov D., Leung C. L., Liem R. K. Protein products of human Gas2-related genes on chromosomes 17 and 22 (hGAR17 and hGAR22) associate with both microfilaments and microtubules. J. Cell Sci. 2003;116:1045–1058. doi: 10.1242/jcs.00272. [DOI] [PubMed] [Google Scholar]
- Gregory S. L., Brown N. H. kakapo, a gene required for adhesion between and within cell layers in Drosophila, encodes a large cytoskeletal linker protein related to plectin and dystrophin. J. Cell Biol. 1998;143:1271–1282. doi: 10.1083/jcb.143.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honnappa S., et al. An EB1-binding motif acts as a microtubule tip localization signal. Cell. 2009;138:366–376. doi: 10.1016/j.cell.2009.04.065. [DOI] [PubMed] [Google Scholar]
- Hwang E., Kusch J., Barral Y., Huffaker T. C. Spindle orientation in Saccharomyces cerevisiae depends on the transport of microtubule ends along polarized actin cables. J. Cell Biol. 2003;161:483–488. doi: 10.1083/jcb.200302030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa J. H., Mullins R. D. Spatial and temporal relationships between actin-filament nucleation, capping, and disassembly. Curr. Biol. 2007;17:395–406. doi: 10.1016/j.cub.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakesisoglou I., Yang Y., Fuchs E. An epidermal plakin that integrates actin and microtubule networks at cellular junctions. J. Cell Biol. 2000;149:195–208. doi: 10.1083/jcb.149.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama A., Karakesisoglou I., Wong E., Vaezi A., Fuchs E. ACF 7, an essential integrator of microtubule dynamics. Cell. 2003;115:343–354. doi: 10.1016/s0092-8674(03)00813-4. [DOI] [PubMed] [Google Scholar]
- Kulic I. M., Brown A. E., Kim H., Kural C., Blehm B., Selvin P. R., Nelson P. C., Gelfand V. I. The role of microtubule movement in bidirectional organelle transport. Proc. Natl. Acad. Sci. USA. 2008;105:10011–10016. doi: 10.1073/pnas.0800031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R., Gustus C., Terada Y., Uetake Y., Matuliene J. CHO1, a mammalian kinesin-like protein, interacts with F-actin and is involved in the terminal phase of cytokinesis. J. Cell Biol. 2002;156:783–790. doi: 10.1083/jcb.200109090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz V. A., Miller K. G. A class VI unconventional myosin is associated with a homologue of a microtubule-binding protein, cytoplasmic linker protein-170, in neurons and at the posterior pole of Drosophila embryos. J. Cell Biol. 1998;140:897–910. doi: 10.1083/jcb.140.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Harris K. L., Whitington P. M., Kolodziej P. A. short stop is allelic to kakapo, and encodes rod-like cytoskeletal-associated proteins required for axon extension. J. Neurosci. 2000;20:1096–1108. doi: 10.1523/JNEUROSCI.20-03-01096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Kolodziej P. A. Short Stop provides an essential link between F-actin and microtubules during axon extension. Development. 2002a;129:1195–1204. doi: 10.1242/dev.129.5.1195. [DOI] [PubMed] [Google Scholar]
- Lee S., Kolodziej P. A. The plakin Short Stop and the RhoA GTPase are required for E-cadherin-dependent apical surface remodeling during tracheal tube fusion. Development. 2002b;129:1509–1520. doi: 10.1242/dev.129.6.1509. [DOI] [PubMed] [Google Scholar]
- Lee S., et al. The F-actin-microtubule crosslinker Shot is a platform for Krasavietz-mediated translational regulation of midline axon repulsion. Development. 2007;134:1767–1777. doi: 10.1242/dev.02842. [DOI] [PubMed] [Google Scholar]
- Leung C. L., Sun D., Zheng M., Knowles D. R., Liem R. K. Microtubule actin cross-linking factor (MACF): a hybrid of dystonin and dystrophin that can interact with the actin and microtubule cytoskeletons. J. Cell Biol. 1999;147:1275–1286. doi: 10.1083/jcb.147.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandato C. A., Bement W. M. Actomyosin transports microtubules and microtubules control actomyosin recruitment during Xenopus oocyte wound healing. Curr. Biol. 2003;13:1096–1105. doi: 10.1016/s0960-9822(03)00420-2. [DOI] [PubMed] [Google Scholar]
- McCartney B. M., McEwen D. G., Grevengoed E., Maddox P., Bejsovec A., Peifer M. Drosophila APC2 and Armadillo participate in tethering mitotic spindles to cortical actin. Nat. Cell Biol. 2001;3:933–938. doi: 10.1038/ncb1001-933. [DOI] [PubMed] [Google Scholar]
- Prokop A., Uhler J., Roote J., Bate M. The kakapo mutation affects terminal arborization and central dendritic sprouting of Drosophila motorneurons. J. Cell Biol. 1998;143:1283–1294. doi: 10.1083/jcb.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter J. E., Nardine T. M., Penton A., Billuart P., Scott E. K., Usui T., Uemura T., Luo L. A mosaic genetic screen for genes necessary for Drosophila mushroom body neuronal morphogenesis. Development. 2002;130:1203–1213. doi: 10.1242/dev.00319. [DOI] [PubMed] [Google Scholar]
- Rodriguez O. C., Schaefer A. W., Mandato C. A., Forscher P., Bement W. M., Waterman-Storer C. M. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat. Cell Biol. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- Rogers G. C., Rusan N. M., Peifer M., Rogers S. L. A multicomponent assembly pathway contributes to the formation of acentrosomal microtubule arrays in interphase Drosophila cells. Mol. Biol. Cell. 2008;19:3163–3178. doi: 10.1091/mbc.E07-10-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. L., Rogers G. C., Sharp D. J., Vale R. D. Drosophila EB1 is important for proper assembly, dynamics, and positioning of the mitotic spindle. J. Cell Biol. 2002;158:873–884. doi: 10.1083/jcb.200202032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. L., Wiedemann U., Stuurman N., Vale R. D. Molecular requirements for actin-based lamella formation in Drosophila S2 cells. J. Cell Biol. 2003;162:1079–1088. doi: 10.1083/jcb.200303023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röper K., Brown N. H. Maintaining epithelial integrity: a function for gigantic spectraplakin isoforms in adherens junctions. J. Cell Biol. 2003;162:1305–1315. doi: 10.1083/jcb.200307089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röper K., Gregory S. L., Brown N. H. The ‘spectraplakins’: cytoskeletal giants with characteristics of both spectrin and plakin families. J. Cell Sci. 2002;115:4215–4225. doi: 10.1242/jcs.00157. [DOI] [PubMed] [Google Scholar]
- Rothenberg M. E., Rogers S. L., Vale R. D., Jan L. Y., Jan Y. N. Drosophila pod-1 crosslinks both actin and microtubules and controls the targeting of axons. Neuron. 2003;39:779–791. doi: 10.1016/s0896-6273(03)00508-7. [DOI] [PubMed] [Google Scholar]
- Salmon W. C., Adams M. C., Waterman-Storer C. M. Dual-wavelength fluorescent speckle microscopy reveals coupling of microtubule and actin movements in migrating cells. J. Cell Biol. 2002;158:31–37. doi: 10.1083/jcb.200203022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Soriano N., Travis M., Dajas-Bailador F., Gonçalves-Pimentel C., Whitmarsh A. J., Prokop A. Mouse ACF7 and Drosophila short stop modulate filopodia formation and microtubule organisation during neuronal growth. J. Cell Sci. 2009;122:2534–2542. doi: 10.1242/jcs.046268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A. W., Kabir N., Forscher P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J. Cell Biol. 2002;158:139–152. doi: 10.1083/jcb.200203038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slep K. C., Rogers S. L., Elliott S. L., Ohkura H., Kolodziej P. A., Vale R. D. Structural determinants for EB1-mediated recruitment of APC and spectraplakins to the microtubule plus end. J. Cell Biol. 2005;168:587–598. doi: 10.1083/jcb.200410114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., Liem R. K. Plakins in development and disease. Exp. Cell Res. 2007;313:2189–2203. doi: 10.1016/j.yexcr.2007.03.039. [DOI] [PubMed] [Google Scholar]
- Strumpf D., Volk T. Kakapo, a novel cytoskeletal-associated protein is essential for the restricted localization of the neuregulin-like factor, vein, at the muscle-tendon junction site. J. Cell Biol. 1998;143:1259–1270. doi: 10.1083/jcb.143.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A., Prokop A., Yamamoto M., Sugimura K., Uemura T., Betschinger J., Knoblich J. A., Volk T. Shortstop recruits EB1/APC1 and promotes microtubule assembly at the muscle-tendon junction. Curr. Biol. 2003;13:1086–1095. doi: 10.1016/s0960-9822(03)00416-0. [DOI] [PubMed] [Google Scholar]
- Sun D., Leung C. L., Liem R. K. Characterization of the microtubule binding domain of microtubule actin crosslinking factor (MACF): identification of a novel group of microtubule associated proteins. J. Cell Sci. 2001;114:161–172. doi: 10.1242/jcs.114.1.161. [DOI] [PubMed] [Google Scholar]
- Tamai K., Sawamura D., Choi Do H. Y., Li K., Uitto J. Molecular biology of the 230-kD bullous pemphigoid antigen. Cloning of the BPAG1 gene and its tissue-specific expression. Dermatology. 1994;189(suppl 1):27–33. doi: 10.1159/000246924. [DOI] [PubMed] [Google Scholar]
- Vorobjev I. A., Rodionov V. I., Maly I. V., Borisy G. G. Contribution of plus and minus end pathways to microtubule turnover. J. Cell Sci. 1999;112:2277–2289. doi: 10.1242/jcs.112.14.2277. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer C., Duey D. Y., Weber K. L., Keech J., Cheney R. E., Salmon E. D., Bement W. M. Microtubules remodel actomyosin networks in Xenopus egg extracts via two mechanisms of F-actin transport. J. Cell Biol. 2000;150:361–376. doi: 10.1083/jcb.150.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer C. M., Salmon E. D. Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J. Cell Biol. 1997;139:417–434. doi: 10.1083/jcb.139.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Kodama A., Fuchs E. ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell. 2008;135:137–148. doi: 10.1016/j.cell.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.