We explore the role of components that act both upstream and downstream of Slt2p in the Ptc1p-dependent regulation of ER inheritance and mitochondrial inheritance. Our findings are that Ptc1p is needed to inactivate the pool of Slt2p associated with the bud tip to promote the cortical distribution of the ER in daughter cells.
Abstract
Inheritance of the endoplasmic reticulum (ER) requires Ptc1p, a type 2C protein phosphatase of Saccharomyces cerevisiae. Genetic analysis indicates that Ptc1p is needed to inactivate the cell wall integrity (CWI) MAP kinase, Slt2p. Here we show that under normal growth conditions, Ptc1p inactivates Slt2p just as ER tubules begin to spread from the bud tip along the cortex. In ptc1Δ cells, the propagation of cortical ER from the bud tip to the periphery of the bud is delayed by hyperactivation of Slt2p. The pool of Slt2p that controls ER inheritance requires the CWI pathway scaffold, Spa2p, for its retention at the bud tip, and a mutation within Slt2p that prevents its association with the bud tip blocks its role in ER inheritance. These results imply that Slt2p inhibits a late step in ER inheritance by phosphorylating a target at the tip of daughter cells. The PI4P5-kinase, Mss4p, is an upstream activator of this pool of Slt2p. Ptc1p-dependant inactivation of Slt2p is also needed for mitochondrial inheritance; however, in this case, the relevant pool of Slt2p is not at the bud tip.
INTRODUCTION
In eukaryotic cells, the endoplasmic reticulum (ER) typically forms a continuous polygonal network of sheets and tubules that extends throughout the entire cytoplasmic space (Voeltz et al., 2002; Du et al., 2004). This distinctive morphology may have evolved to ensure accessibility of the ER and its unique functions, such as protein translocation, Ca2+ signaling, and lipid biosynthesis to all parts of the cell. In the budding yeast Saccharomyces cerevisiae, a meshwork of ER lines the cell cortex, closely apposed to the plasma membrane with several tubular connections through the cytoplasm to the nuclear envelope (Voeltz et al., 2002; Du et al., 2004). During cell division, the daughter cell acquires its own complement of cortical ER by an active, multistage inheritance mechanism. Early in the cell cycle, as a nascent bud is forming, a tubule of ER appears to emanate from the nuclear envelope, becomes oriented along the mother-bud axis, and then extends into the growing bud (Fehrenbacher et al., 2002; Estrada et al., 2003). Formation and vectorial extension of this segregation structure utilizes the type V myosin, Myo4p, and an associated protein She3p (Estrada et al., 2003). Stable retention of this tubule at the bud tip requires components of the exocyst vesicle-tethering complex (Wiederkehr et al., 2003; Reinke et al., 2004). Soon after this tubule becomes anchored at the bud tip, an ER network spreads from the bud tip along the cortex of the bud until it achieves a uniform distribution, similar in morphology to that seen in the mother cell. This final stage of ER inheritance requires the activity of a type 2C protein phosphatase, Ptc1p (Du et al., 2006).
In addition to its role in the regulation of ER inheritance, Ptc1p is also needed for the inheritance of mitochondria (Roeder et al., 1998), vacuoles (Du et al., 2006), and possibly peroxisomes (Jin et al., 2009). Interestingly, although Ptc1p acts only at a late stage of ER inheritance, the propagation of cortical ER from the bud tip, it is needed at an early stage of mitochondrial and vacuole inheritance (Roeder et al., 1998; Du et al., 2006). In the case of vacuole inheritance, the loss of Ptc1p may uncouple the linkage of the vacuole to the type V myosin, Myo2p, that directs vacuole movement into the bud (Jin et al., 2009).
At a biochemical level, the Ptc1p phosphatase is known to down-regulate two different mitogen-activated protein kinase (MAPK) cascades; the high osmolarity glycerol (HOG) pathway that responds to hyperosmotic and heat stress conditions (Martin et al., 2005) and the cell wall integrity (CWI) pathway that responds to disruptions of the cell surface (Huang and Symington, 1995). The ER inheritance defect of ptc1Δ mutant cells is suppressed by disruption of SLT2, the gene encoding the final kinase of the CWI MAP kinase cascade (Du et al., 2006), indicating that it is the overactivation of the CWI pathway that leads to the ER inheritance defect in ptc1Δ cells.
The Slt2 kinase can be activated through several different pathways (for review see Levin, 2005). In response to disruption of the cell wall or other sources of cell surface stress, the cell surface sensors Wsc1p and Mid2p activate the Rho1p GTPase by stimulating the Rho1 exchange factor, Rom2p. Active Rho1p, in turn, stimulates the yeast protein kinase C, Pkc1p, (Kamada et al., 1996), which then activates the CWI MAP kinase cascade. Production of PI4,5P2 at the cell surface also activates this pathway through the recruitment of Rom2p to the plasma membrane (Audhya and Emr, 2002). Even in the absence of an extracellular signal, Slt2p, the final member of the CWI cascade, undergoes cyclical activation and inactivation through the cell cycle, peaking at times of polarized growth (Zarzov et al., 1996). This temporal pattern may correlate with periods of cell surface stress due to cell wall remodeling. Many of the known downstream effects of Slt2p activation are mediated by the nuclear transcription factors Rlm1p and Swi4p (Watanabe et al., 1995; Baetz et al., 2001), and a major fraction of Slt2p can be found in the nucleus. Nonetheless, a pool of Slt2p is also found at sites of polarized growth (van Drogen and Peter, 2002). The targets of this cortical pool of Slt2p are unknown. Here, we explore the role of components that act both upstream and downstream of Slt2p in the Ptc1p-dependent regulation of ER inheritance. We also assess the role that these components play in mitochondrial inheritance. Our findings lead to the conclusion that Ptc1p is needed to inactivate the pool of Slt2p associated with the bud tip to promote the cortical distribution of the ER in daughter cells.
MATERIALS AND METHODS
Strain Construction
Yeast strains used in this study are listed in Table 1. To construct spa2Δ (SFNY1886 and SFNY1887) and ptc1Δ (SFNY1891 and SFNY1892) strains, DNA fragments containing the KanMX4 module were amplified by PCR using genomic DNA, from the spa2Δ and ptc1Δ strains from the yeast genome-wide gene-deletion collection, as templates. These DNA fragments contain 250–300 base pairs of the 5′ and 3′ noncoding sequences of SPA2 or PTC1 and replace the entire coding sequences of the SPA2 or PTC1 genes with the KanMX4 module. The slt2, rlm1, swi4, wsc1, and mid2 gene disruptions were constructed by the same strategy. SFNY1893 and SFNY1890 were made by integrating StuI-digested pRH475 into the ura3 locus of AAY202 and SEY6210. The mss4-102 and wild-type strains were generously provided by Dr. Scott Emr's lab, and NY2921 and NY2922 were made from a cross between SFNY1925 and NY1210.
Table 1.
Yeast strains used in this work
| Strain | Genotype | Source |
|---|---|---|
| NY1210 | MATa his3-200 leu2-3,112 ura3-52 | Novick lab collection |
| NY2920 | MATa his3-200 leu2-3,112 ura3-52 ptc1::his5 | Novick lab collection |
| SFNY 1255 | MATα his3-200 ura3-52 leu2-3,112::(LEU2, HMG1-GFP) | Ferro-Novick lab collection |
| SFNY 1610 | MATα his3-200 ura3-52 leu2-3,112::(LEU2, HMG1-GFP) ptc1::his5+ | Ferro-Novick lab collection |
| SFNY 1683 | MATa his3-200 leu2-3,112 ura3-52::(URA3, HMG1-GFP) | Ferro-Novick lab collection |
| SFNY 1684 | MATa his3-200 leu2-3,112 ura3-52::(URA3, HMG1-GFP) ptc1::his5+ | Ferro-Novick lab collection |
| SFNY 1886 | MATα his3-200 ura3-52 leu2-3,112::(LEU2, HMG1-GFP) ptc1::his5+spa2::KanMX4 | This study |
| SFNY 1887 | MATα his3-200 ura3-52 leu2-3,112::(LEU2, HMG1-GFP) spa2::KanMX4 | This study |
| SFNY 1890 | MATα his3-200 trp1-Δ901 lys2-801 suc2-Δ9 leu2-3,112 ura3-52::(URA3, HMG1-GFP) | This study |
| SFNY 1891 | MATα his3-200 trp1-Δ901 lys2-801 suc2-Δ9 leu2-3,112 ura3-52::(URA3, HMG1-GFP)ptc1::KanMX4 | This study |
| SFNY 1892 | MATα his3-200 trp1-Δ901 lys2-801 suc2-Δ9 leu2-3,112 ura3-52::(URA3, HMG1-GFP)mss4Δ::HIS3 ptc1::KanMX4 [YCplac-mss4-102] | This study |
| SFNY 1893 | MATα his3-200 trp1-Δ901 lys2-801 suc2-Δ9 leu2-3,112 ura3-52::(URA3, HMG1-GFP)mss4Δ::HIS3 [YCplac-mss4-102] | This study |
| SFNY 1921 | MATα his3-200 ura3-52 leu2-3,112::(LEU2, HMG1-GFP) ptc1::his5+rlm1::KanMX4 | This study |
| SFNY 1922 | MATα his3-200 ura3-52 leu2-3,112::(LEU2, HMG1-GFP) ptc1::his5+mid2::KanMX4 | This study |
| SFNY 1923 | MATα his3-200 ura3-52 leu2-3,112::(LEU2, HMG1-GFP) ptc1::his5+wsc1::KanMX4 | This study |
| SFNY 1924 | MATα his3-200 ura3-52 leu2-3,112::(LEU2, HMG1-GFP) ptc1::his5+swi4::KanMX4 | This study |
| SFNY 1925 | MATα his3-200 ura3-52 leu2-3,112::(LEU2, HMG1-GFP) ptc1::his5+slt2::KanMX4 | This study |
| NY 2921 | MATa his3-200 ura3-52 leu2-3,112::(LEU2, HMG1-GFP) slt2::KanMX4 | This study |
| NY 2922 | MATα his3-200 ura3-52 leu2-3,112 ptc1::his5+slt2::KanMX4 | This study |
| SEY6210 | MATα his3-200 trp1-Δ901 lys2-801 suc2-Δ9 leu2-3,112 ura3-52 | Scott Emr lab collection |
| AAY202 | MATα his3-200 trp1-Δ901 lys2-801 suc2-Δ9 leu2-3,112 ura3-52 mss4Δ::HIS3[YCplac-mss4-102] | Scott Emr lab collection |
Microscopy
The inheritance of ER, mitochondria was analyzed as previously described (Du et al., 2006).
Determination of the Level of Activated Slt2p during the Cell Cycle
One hundred fifty OD600 units of SFNY1683 (wild type) and SFNY1684 (ptc1Δ) grown in YPD were washed twice with 50 ml YPD (pH 3.9), resuspended in 300 ml YPD (pH 3.9) containing 6 μg/ml α-factor, and incubated at 30°C for 3 h. Fifteen milliliters of each culture was collected to prepare the t = 0 samples. The rest of the cells were washed twice with 50 ml SC-URA, resuspended in 300 ml SC-URA, and incubated at 30°C. During the incubation, 15 ml of each strain was collected at 15-min intervals to prepare samples. Five milliliters of each sample was fixed with 3% formaldehyde for 30 min to examine bud growth and ER distribution. Cells from the remaining 10 ml of each sample were collected, immediately washed with ice-cold water, and frozen in liquid nitrogen. After all time points were collected, cells were thawed on ice, lysates were made in ice-cold lysis buffer (20 mM PIPES, pH 6.8, 150 mM NaCl, 1 mM EDTA, 10 mM β-mercaptoethanol), and the level of phosphorylated Slt2p in each lysate was determined as previously described (Du et al., 2006).
RESULTS
Inactivation of Slt2p during the Cell Cycle Occurs Concomitantly with the Release of ER Tubules from the Bud Tip
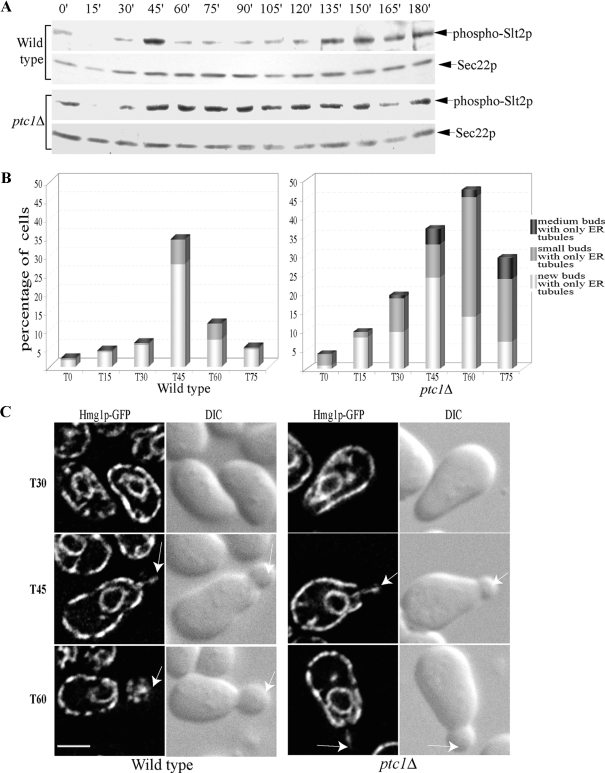
Genetic analysis suggests that, in ptc1 mutants, the failure to inactivate Slt2p by dephosphorylation during the cell cycle underlies the delay in the propagation of ER segregation tubules from their docking site at the bud tip to the cortex of the bud (Du et al., 2006). This proposal predicts that the timing of Slt2p inactivation in wild-type cells should closely correlate with the onset of delivery of ER tubules to the bud cortex. To assess this correlation, we followed both Slt2p phosphorylation and ER distribution over time in synchronized wild-type cells (Figure 1). Cells were arrested in the G1 phase with α-factor and then allowed to reenter the cell cycle by removal of the pheromone. Our finding that the level of activated Slt2p oscillated during the cell cycle was consistent with a previous study (Zarzov et al., 1996). After α-factor release, the level of phospho-Slt2p diminished within 15 min, reappeared within 30 min, peaked sharply at 45 min, and quickly dropped by 60 min (Figure 1A). Quantification of the number of budded cells revealed that ∼10% of the cells were budded at the 15-min time point, whereas ∼20% were budded at 30 min. The budded cells had only ER tubules along the axis and no cortical ER in the bud (Figure 1B). By the 45-min time point, 55% of the cells had developed a bud, and these were predominantly newly formed buds. Most new and small buds did not have cortical ER and ∼35% contained only ER segregation tubules. By the 60-min time point, the population of cells with new buds dramatically dropped to ∼10%, and the population with medium sized buds increased. The population of budded cells containing only axial ER tubules was reduced to ∼10% as the ER spread along the cortex of the growing buds (Figure 1, B and C). Additional cells entered mitosis at later time points. Our data show that, in wild-type cells, the level of activated Slt2p peaks during the period of the cell cycle in which buds emerge and ER segregation tubules are transported into the new buds. Inactivation of Slt2p occurs concomitantly with bud expansion and the propagation of cortical ER from the bud tip along the bud cortex.
Figure 1.
Ptc1p-dependent dephosphorylation of Slt2p normally occurs during the period of bud growth in which ER segregation tubules are delivered to the bud periphery from the bud tip. Both wild-type and ptc1Δ cells were arrested in the G1 phase of the cell cycle by α-factor treatment and then synchronously released from the block as described in Materials and Methods. (A) The level of phosphorylated Slt2p at each time point was determined by Western blot analysis. Sec22p was used as a loading control. (B) The percentage of budded cells that contained only ER segregation tubules along the axis within the bud. (C) Hmg1p-GFP-labeled ER images of representative cells and corresponding DIC images 30, 45, and 60 min after the removal of α-factor. Arrows point to the buds. Bars, 2 μm.
We next examined phosphorylation of Slt2p during bud development in synchronized ptc1Δ cells. The population of unbudded cells, newly budded cells, cells with small buds, cells with medium buds, and mitotic cells of the ptc1Δ mutant were comparable to that of the wild-type population at each time point (unpublished data). Thus Ptc1p is not required for bud growth. Slt2p in ptc1Δ cells was activated during bud initiation in a pattern similar to that in the wild-type strain (Figure 1A). However, the level of activated Slt2p in the ptc1Δ mutant continuously increased and was sustained during bud expansion in late S/G2 phases. During this time period a significant number of new small- and medium-budded cells were found to have only axial ER tubules and no cortical ER in the buds (Figure 1, B and C). Together with our previous finding, that disruption of SLT2 suppresses the ER inheritance defect of ptc1Δ cells (Du et al., 2006), these data support the hypothesis that Ptc1p regulates the inactivation of Slt2p during bud expansion, and inactivation of Slt2p is required for the timely propagation of ER from the docking site at the bud tip along the cortex of the growing bud.
Deletion of SPA2 Suppresses the ER Inheritance Defect in ptc1Δ Mutant Cells
Although the majority of Slt2p localizes to the nucleus, a small pool of this MAPK localizes to sites of polarized growth, including the tip of newly initiated buds (van Drogen and Peter, 2002). The known targets of the nuclear pool of Slt2p are transcription factors that control the expression of proteins implicated in cell wall biogenesis and cell cycle regulation (Madden et al., 1997; Jung and Levin, 1999; Baetz et al., 2001). The role of Slt2p at the bud tip has not yet been defined. Because it is the overactivation of Slt2p that is responsible for the delay in the release of ER segregation tubules from the bud tip in the ptc1Δ mutant, an intriguing possibility is that the Slt2p at the bud tip regulates the timing of ER inheritance progression by phosphorylating targets at this site. To begin to address this possibility, we examined ER inheritance in a spa2Δ ptc1Δ double mutant. Spa2p, a component of the polarisome polarity complex, interacts with kinases of the Slt2p MAPK cascade and is required for the recruitment of Slt2p to the bud tip (Sheu et al., 1998; van Drogen and Peter, 2002).
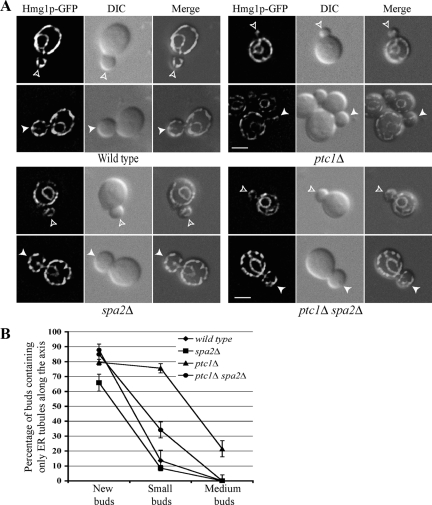
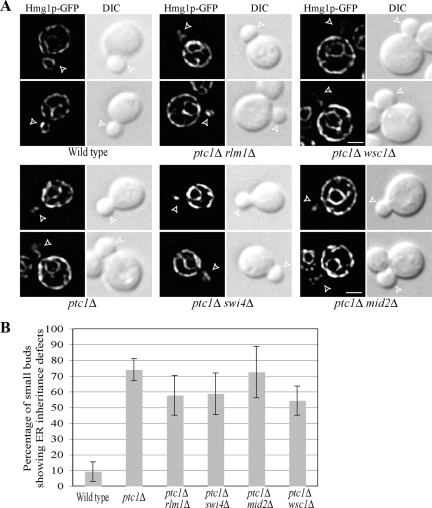
In wild-type cells, an ER tubule moves into newly formed buds, forms a stable contact at the bud tip and then quickly spreads along the bud cortex, whereas in ptc1Δ cells, the propagation of ER along the cortex is delayed (Du et al., 2006). As shown in Figure 2, deletion of SPA2 strongly suppressed the delay in ER inheritance resulting from the loss of Ptc1p. Approximately 30% of spa2Δ ptc1Δ small buds had tubules along the mother-bud axis as the sole inherited ER elements, much less than the 75% of ptc1Δ small buds with this phenotype. Nonetheless, the restoration of ER inheritance in the spa2Δ ptc1Δ double mutant was not complete. The percentage of spa2Δ ptc1Δ small buds that contain only ER tubules along the axis was still ∼20% above that of wild-type small buds. The remaining, cytosolic pool of activated Slt2p in the spa2Δ ptc1Δ double mutant may be able to phosphorylate targets at the bud tip, albeit with reduced efficiency, and thereby confer a residual delay in ER tubule delivery to the bud cortex. As in the case of wild-type cells with medium-sized buds, all the spa2Δ ptc1Δ medium-sized buds had ER tubules spread evenly around their cortexes. Loss of Spa2p had no detectable effect on the total level of activated Slt2p in either wild-type or ptc1Δ cells (Supplemental Figure S1). These data suggest that it is specifically the pool of Slt2p at the bud tip that serves as a critical regulator for the timing of cortical ER inheritance progression during the cell cycle. In support of this proposal, loss of either of the known nuclear targets of Slt2p, specifically Rlm1p and Swi4p, had no significant effect on the ER distribution in wild-type or ptc1Δ cells (Figure 3).
Figure 2.
Deletion of the SPA2 gene significantly suppresses the ER inheritance defect in ptc1Δ mutant cells. (A) Representative fluorescence, DIC, and fluorescence-DIC merged images of wild-type, spa2Δ, ptc1Δ, and ptc1Δ spa2Δ cells grown to early log phase in SC medium at 25°C. Open arrowheads and arrowheads point to small and medium buds, respectively. Bars, 2 μm. (B) The percentage of buds that contained only an ER tubule along the axis is plotted for new small and medium buds. Error bars, SEM from three independent experiments.
Figure 3.
Loss of the known nuclear targets of Slt2p, Rlm1p, and Swi4p or of the cell wall stress sensors, Wsc1p and Mid2p, has no significant effect on the ER distribution in ptc1Δ cells. (A) Representative fluorescence of the ER marker Hmg1-GFP and DIC images of wild-type, ptc1Δ, ptc1Δ rlm1Δ, ptc1Δ swi4Δ, ptc1Δ wsc1Δ, and ptc1Δ mid2Δ cells grown to early log phase in SC medium at 25°C. Open arrowheads point to small buds. Bars, 2 μm. (B) The ER distribution in small buds (0.3–0.5 diameter of mother cell) of all six strains shown in A was quantified. Error bars, SEM from three independent experiments.
An slt2 Mutation That Blocks Bud Tip Localization Suppresses the ER Inheritance Defect of ptc1Δ Cells
Suppression of the ER inheritance defect of ptc1Δ cells by deletion of spa2 could, in principle, be mediated by the mislocalization of some component other than Slt2p. To definitively establish Slt2p as the relevant component that is mislocalized in response to the loss of Spa2p, we sought mutations within Slt2p that would block its localization to the bud tip. We searched for a possible nuclear export sequence (NES) with the expectation that blocking nuclear export by altering this sequence would prevent bud tip localization of Slt2p and thereby allow a test of our hypothesis. A short sequence around residues L70 and L75 fit the loose NES consensus sequence of L-x(2,3)-[LIVFM]-x(2,3)-L-x-[LI] (La Cour et al., 2003; see Supplemental Figure S2A). We mutated leucines 70, 73, and 75 to alanine within the putative NES site and expressed the wild-type and mutant alleles as green fluorescent protein (GFP) fusions under control of the endogenous promoter. We refer to this mutation as the slt-LA allele. The wild-type Slt2-GFP fusion was concentrated in the nucleus, yet was also present in the cytosol (Figure 4A and Supplemental Figure S2C). As previously reported, a concentration of Slt2-GFP was seen above the cytosolic background at the tip of small-budded cells and at the neck in large-budded cells. In contrast, the Slt2-LA-GFP mutant protein was predominantly cytosolic, with a modest increased concentration in the nucleus and no apparent concentration at the bud tip or neck (Figure 4A and Supplemental Figure S2C). Some cells displayed a small bright spot that was not associated with the nucleus, bud tip, or neck. Although the mutant protein was not exclusively nuclear, as we had anticipated, we had nonetheless achieved our goal of blocking the bud tip localization of Slt2p.
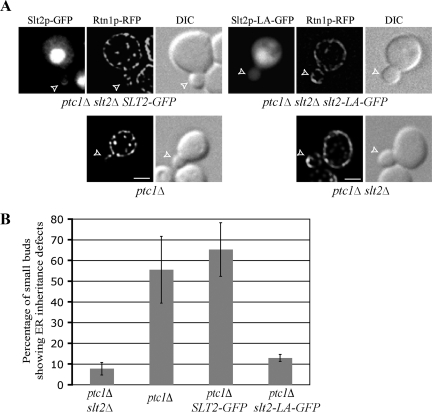
Figure 4.
The Slt2-LA mutation within the putative NES site blocks bud tip localization and suppresses the ER inheritance defects of ptc1Δ cells. (A) Slt2-GFP– or Slt2p-LA-GFP–, Rtn1p-RFP–labeled ER and DIC images of representative small-budded cells grown to early log phase in SC medium at 25°C. The strains examined are ptc1Δ slt2Δ, ptc1Δ, or ptc1Δ slt2Δ containing a plasmid expressing GFP-fusion Slt2p or Slt2p-LA from the endogenous promoter. Open arrowheads point to small buds. Bars, 2 μm. Analysis of 100 small-budded cells established that 87% of cells expressing Slt2-GFP exhibited bud tip localization, whereas 95% of Slt2-LA-GFP–expressing cells did not. (B) The ER distribution within small buds of the strains indicated above was quantified. Error bars, SEM from three independent experiments.
We next tested the effect of expression of Slt2-GFP and Slt2-LA-GFP on ER inheritance in ptc1Δ slt2Δ cells. As shown in Figure 4, ∼56% of small buds in ptc1Δ cells have an aberrant distribution of ER, whereas in ptc1Δ slt2Δ cells the value is reduced to 8%. Expression of Slt2-GFP complements the loss of Slt2p in ptc1Δ slt2Δ cells as indicated by the increase in the percentage of small buds with aberrant ER distribution to 65%. In contrast, expression of Slt2-LA-GFP in ptc1Δ slt2Δ cells did not alter the distribution of ER in small buds. Thus, with regards to its role in ER inheritance, our data are consistent with the proposal that Slt2p must be localized to the bud tip. One possible concern with this experiment is that the slt2-LA mutation may be inactivating the protein rather than just mislocalizing a pool. We have shown that the total phosphorylation level of slt2-LA mutation is comparable to that of wild-type Slt2 (Supplemental Figure S2B). We also present evidence in a later section that the Slt2-LA protein is still active with respect to mitochondrial inheritance, ruling out this possibility.
Mss4p Is Essential for the Activation of Slt2p in ptc1Δ Cells
Extensive studies have identified a number of upstream molecules that transmit signals to launch protective responses through the activation of Slt2p. Known activators of the Slt2p MAPK cascade include two putative cell surface sensors Wsc1p and Mid2p, the small GTPase Rho1p, and protein kinase C, Pkc1p (reviewed in Levin, 2005). In addition, increased production of the phospholipid, phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2), by the PI(4)P 5-kinase, Mss4p, at the plasma membrane is a key messenger activating the Slt2p MAPK cascade in response to heat treatment (Yoshida et al., 1994; Audhya and Emr, 2002). PI(4,5)P2 may act by recruiting Rom2p, a guanine nucleotide exchange factor for Rho1p, to the plasma membrane (Audhya and Emr, 2002).
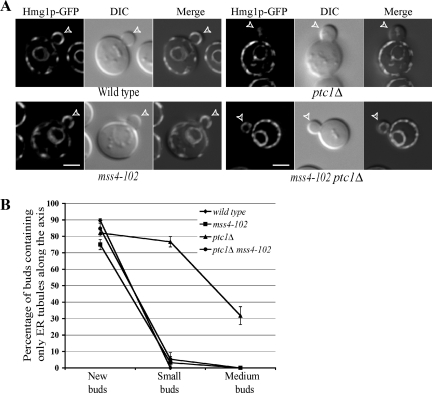
A variety of extracellular stimuli can induce an increase in the activity of Slt2p. However, even under normal growth conditions this kinase is transiently activated and localized to the bud tip at early stages of bud formation (Figure 1A; Zarzov et al., 1996; van Drogen and Peter, 2002). Several lines of evidence suggest that different upstream activators evoke distinct signaling responses. For example, heat shock–induced activation of Slt2p and salvage response are severely impaired in cells lacking either Wsc1p or Mid2p (Rajavel et al., 1999; Martin et al., 2000), whereas neither of these transmembrane proteins are needed for G2 arrest and Slt2p activation in response to actin depolarization (Harrison et al., 2001). Because Ptc1p controls the timing of Slt2p inactivation at later stages of bud growth, identification of essential upstream activators of Ptc1p-dependent regulation of Slt2p may provide clues to the mechanism of Slt2p regulation during the cell cycle. To this end, we tested the ability of mutations affecting the function of Wsc1p, Mid2p, or Mss4p to restore ER inheritance in a ptc1Δ mutant. Deleting the entire coding sequences of either the WSC1 gene or the MID2 gene had no significant effect on ER inheritance in ptc1Δ mutant cells (Figure 3). In contrast, the mss4-102 mutation completely suppressed the delay in the cortical propagation of ER tubules in the ptc1Δ mutant (Figure 5). Although mss4-102 cells are temperature-sensitive for growth, our observation that this mutation restored ER inheritance at 25°C is consistent with the previous observation that the mss4-102 gene product is largely defective in PI(4)P5-kinase activity, even at permissive growth temperatures (Audhya and Emr, 2002).
Figure 5.
Loss of Mss4p function restores ER inheritance in the ptc1Δ mutant cells. (A) Hmg1p-GFP–labeled ER, DIC, and GFP-DIC merged images of a representative small-budded cell grown in SC medium at 25°C. Bars, 2 μm. (B) The ER distribution in wild-type, mss4-102, ptc1Δ, and ptc1Δ mss4-102 budded cells was quantified. Error bars, SEM from three independent experiments.
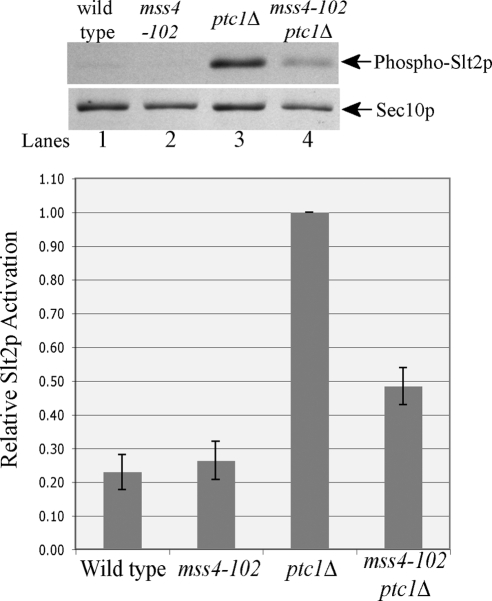
Based on the known function of Mss4p as a key element in the heat shock-induced activation of Slt2p, the suppression of the ptc1Δ ER inheritance defect by mss4-102 could reflect a decrease in the basal activity of Slt2p. To determine if, even in the absence of heat shock, Mss4p plays an essential role in Slt2p activation in the ptc1Δ mutant background, we examined Slt2p phosphorylation in ptc1Δ, mss4-102, and ptc1Δ mss4-102 cells. Under normal growth conditions at 25°C, Slt2p remained predominantly unphosphorylated in wild-type and mss4-102 cells (Figure 6, lanes 1 and 2). Consistent with our previous findings (Du et al., 2006), ptc1Δ cells showed a prominent accumulation of phosphorylated Slt2p, even in the absence of extracellular stress (Figure 6, lane 3). Loss of Mss4p function in ptc1Δ cells resulted in dramatically reduced phosphorylation of Slt2p (Figure 6, lane 4). We still detected about a twofold higher level of phospho-Slt2p in ptc1Δ mss4-102 cells compared with wild-type cells, even though the suppression of the ER inheritance defect by the mss4-102 mutation was complete (Figure 5B). Either there is a threshold for the amount of activated Slt2p needed to block the release of ER segregation tubules from their docking sites at the bud tip and the mss4-102 mutation reduces the level below the threshold, or the pool of Slt2p at the bud tip is particularly sensitive to Mss4p function.
Figure 6.
The function of Mss4p is essential for Slt2p activation in ptc1Δ mutant cells. Yeast lysates were extracted separately from strains SFNY1890 (lane 1), SFNY1891 (lane 3), SFNY1892 (lane 2), or SFNY1893 (lane 4). Sec10p was used as a loading control. To quantify the relative Slt2p activation, the density of the phospho-Slt2p band was divided by the density of Sec10p, and the normalized phospho-Slt2p value of each strain was divided by that of the ptc1Δ mutant. Error bars, SEM from three independent experiments.
Rom2p acts upstream of the CWI pathway and is concentrated at the tips of small buds (Manning et al., 1997). To explore the possibility that Ptc1p acts upstream of Rom2p to regulate the CWI pathway, we examined the localization of Rom2p in ptc1 cells but observed no difference from wild type (data not shown). This observation is consistent with a model in which Ptc1p acts well downstream of Rom2p in the regulation of the CWI pathway by directly dephosphorylating Slt2p.
Ptc1p Antagonizes Slt2p in Mitochondrial Inheritance, But the Active Pool Is Not at the Bud Tip and Does Not Require Mss4p
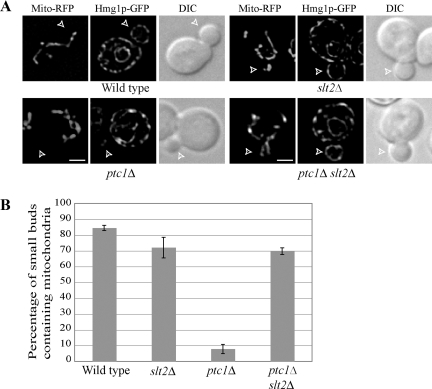
Ptc1p has also been implicated in mitochondrial inheritance (Roeder et al., 1998), although, it has not yet been determined if its role in this process involves the down-regulation of Slt2p. We found that the mitochondrial inheritance defect of ptc1Δ mutant cells was substantially suppressed in the ptc1Δ slt2Δ double mutant (Figure 7, A and B). Although only ∼10% of buds in ptc1Δ cells have mitochondria, ∼70% of buds in ptc1Δ slt2Δ cells contain mitochondria. Thus, as in the case of ER inheritance, Ptc1p is needed to down-regulate Slt2p function to allow mitochondrial inheritance.
Figure 7.
Deletion of SLT2 restores both ER and mitochondrial inheritance in the ptc1Δ mutant. (A) A plasmid expressing the F0ATP synthase mitochondrial targeting sequence fused to RFP (Mito-RFP) was transformed into the indicated strains. RFP fluorescence–labeled mitochondria, Hmg1p-GFP–labeled ER and DIC images in representative small-budded cells are shown. Open arrowheads point to small buds. Bars, 2 μm. (B) The percentage of small buds containing a mitochondrial segregation structure is presented. Error bars, SEM of three experiments.
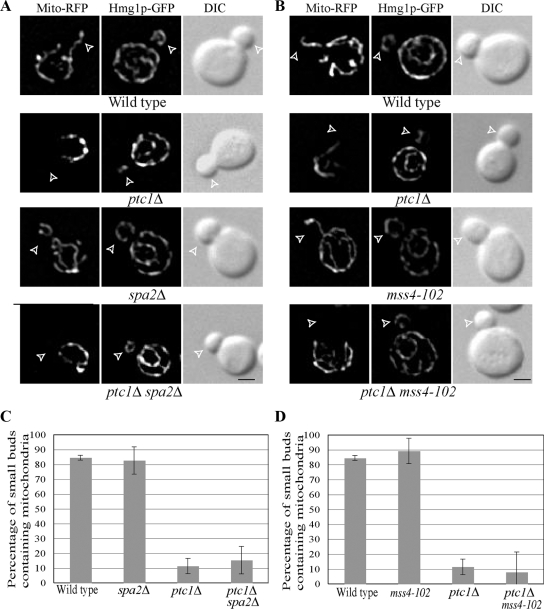
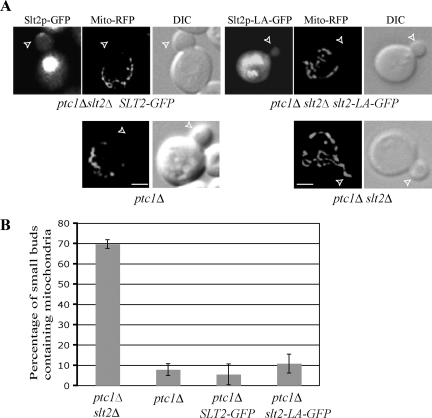
We next explored the effects of loss of Spa2p function on mitochondrial inheritance in ptc1Δ cells. In contrast to ER inheritance, mitochondrial inheritance was not restored in ptc1Δ cells by deletion of SPA2 (Figure 8, A and C), and deletion of SPA2 in a wild-type background had no effect on mitochondrial inheritance. These observations suggest that the pool of Slt2p retained at the bud tip by Spa2p is not relevant to mitochondrial inheritance. To confirm this point, we tested the effect of the slt2-LA mutation on mitochondrial inheritance in ptc1Δ slt2Δ cells (Figure 9). Expression of either Slt2-GFP or Slt2-LA-GFP caused a dramatic drop in the percentage of small buds containing mitochondria, indicating that, with respect to mitochondrial inheritance, Slt2-LA is functional. This confirms that the bud tip–associated pool of Slt2p is not required for the regulation of mitochondrial inheritance.
Figure 8.
The loss of either Spa2p (A) or Mss4p (B) function has no effect on mitochondrial inheritance in ptc1Δ mutant and wild-type cells. RFP fluorescence labeled mitochondria (Mito-RFP), Hmg1p-GFP–labeled ER and DIC images in representative small-budded cells are shown. Open arrowheads point to small buds. Bars, 2 μm. (C and D) The percentage of small buds containing a mitochondrial segregation structure is presented. Error bars, SEM of three experiments.
Figure 9.
The Slt2-LA mutation does not affect its function in mitochondrial inheritance. (A) Slt2-GFP or Slt2p-LA-GFP, RFP fluorescence-labeled mitochondria (Mito-RFP) and DIC images of representative small-budded cells grown to early log phase in SC medium at 25°C. The strains examined are ptc1Δ slt2Δ, ptc1Δ, or ptc1Δ slt2Δ containing a plasmid expressing Slt2-GFP or Slt2p-LA-GFP from the endogenous promoter. Open arrowheads point to small buds. Analysis of at least 100 small budded cells established that 85% of cells expressing Slt2-GFP exhibited bud tip localization, whereas 91% of Slt2-LA-GFP expressing cells did not. Bars, 2 μm. (B) The percentage of small buds containing mitochondrial segregation structures from the indicated strains was quantified. Error bars, SEM from three independent experiments.
In contrast to its effect on ER inheritance, the mss4-102 mutation failed to suppress the mitochondrial inheritance defect of ptc1Δ cells. This mutation also had no effect on mitochondrial inheritance in a wild-type background (Figure 8, B and D). These results suggest that the pool of Slt2p active in blocking mitochondrial inheritance is not dependent on Mss4p. Mss4p is found at the plasma membrane and therefore may selectively activate the cortical pool of Slt2p associated with Spa2p at sites of polarized growth.
DISCUSSION
The protein phosphatase, Ptc1p, controls the inheritance of several different organelles including the ER (Du et al., 2006), mitochondria (Roeder et al., 1998), vacuoles (Du et al., 2006; Jin et al., 2009), and peroxisomes (Jin et al., 2009). Here we have extended our analysis of its role in ER inheritance. We have provided additional evidence indicating that Ptc1p is needed to inactivate the MAP kinase, Slt2p, and have defined components that act upstream of Slt2p to regulate the location and activity of the pool controlling ER distribution.
Under normal growth conditions, Ptc1p acts only at a very late stage of ER inheritance; the propagation of ER from the bud tip along the bud cortex (Du et al., 2006). Early stages of ER inheritance, including the formation of a segregation tubule, Myo4p-dependent movement of the tubule into the bud and capture of the tubule at the bud tip, all appear largely unaffected by the loss of Ptc1p. Ptc1p is needed to down-regulate Slt2p as shown by the suppression of the ER inheritance defect in ptc1Δ slt2Δ double mutants (Du et al., 2006). Ptc1p inactivates Slt2p during its normal, cell cycle–dependant oscillation, just as the ER starts to propagate from its anchorage site at the bud tip along the bud cortex. In the absence of Ptc1p, Slt2p activity remains high throughout the cell cycle and the ER fails to spread from its anchorage site at the bud tip until later in the cycle.
The regulation of ER inheritance is specifically dependent upon the pool of Slt2p that is associated with Spa2p at the bud tip. This finding is fully consistent with the observed defect in the propagation of ER from the bud tip in ptc1Δ mutant cells. The simplest model is that the phosphorylation of some component at the tip of the bud by Slt2p can delay the normal spreading of ER from this site in ptc1Δ cells. The relevant target of the Slt2p kinase at the bud tip is not known; however, these observations limit the possible candidates. This mechanism is very reminiscent of a checkpoint control in that the activation of a kinase can at least temporarily block the progression of a cellular process, although the kinase is not actually necessary for the process per se. Further studies will be needed to identify the trigger(s) that initiate this putative checkpoint in organelle inheritance.
Mitochondrial inheritance, like ER inheritance, requires Ptc1p and, as in the case of ER inheritance, Ptc1p acts in opposition to Slt2p. We have, however, noted some important distinctions: Ptc1p controls mitochondrial inheritance by down-regulating a pool of Slt2p that is not retained at the bud tip by Spa2p. Furthermore, the slt2-LA mutation that prevents its association with the bud tip does not affect its function with regards to mitochondrial inheritance. These distinctions make sense in light of the different stages at which inheritance of ER and mitochondria are blocked in ptc1Δ cells. ER inheritance is blocked at a late stage with a tubule anchored at the tip of the bud but is unable to spread along its cortex, whereas mitochondrial inheritance is blocked at a very early stage, before the formation of a segregation structure in the mother cell. In addition, loss of Mss4p function did not suppress the mitochondrial inheritance defect of ptc1Δ cells, whereas the ER inheritance defect was fully suppressed by loss of Mss4p. Mss4p is an important upstream activator of the CWI pathway that is associated with the plasma membrane and may therefore specifically activate the cortical pool of Slt2p that regulates ER distribution. Mitochondrial inheritance is regulated by a pool of Slt2p that is apparently independent of Mss4p function. Further studies will be needed to identify the target of Slt2p at the bud tip that controls the propagation of ER along the cortex of the bud and the mechanism by which phosphorylation inhibits its activity.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Scott Emr (Cornell University) for providing the mss4-102 strain. This study was supported by National Institutes of Health Grant GM073892 to P.N. and S.F.N. Salary support for Y. D., S. S., and S.F.N. was provided by the Howard Hughes Medical Institute.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-06-0532) on March 31, 2010.
REFERENCES
- Audhya A., Emr S. D. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell. 2002;2:593–605. doi: 10.1016/s1534-5807(02)00168-5. [DOI] [PubMed] [Google Scholar]
- Baetz K., Moffat J., Haynes J., Chang M., Andrews B. Transcriptional coregulation by the cell integrity mitogen-activated protein kinase Slt2 and the cell cycle regulator Swi4. Mol. Cell. Biol. 2001;21:6515–6528. doi: 10.1128/MCB.21.19.6515-6528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Ferro-Novick S., Novick P. Dynamics and inheritance of the endoplasmic reticulum. J. Cell Sci. 2004;117:2871–2878. doi: 10.1242/jcs.01286. [DOI] [PubMed] [Google Scholar]
- Du Y., Walker L., Novick P., Ferro-Novick S. Ptc1p regulates cortical ER inheritance via Slt2p. EMBO J. 2006;25:4413–4422. doi: 10.1038/sj.emboj.7601319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada P., Kim J., Coleman J., Walker L., Dunn B., Takizawa P., Novick P., Ferro-Novick S. Myo4p and She3p are required for cortical ER inheritance in Saccharomyces cerevisiae. J. Cell Biol. 2003;163:1255–1266. doi: 10.1083/jcb.200304030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbacher K. L., Davis D., Wu M., Boldogh I., Pon L. A. Endoplasmic reticulum dynamics, inheritance, and cytoskeletal interactions in budding yeast. Mol. Biol. Cell. 2002;13:854–865. doi: 10.1091/mbc.01-04-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. C., Bardes E. S., Ohya Y., Lew D. J. A role for the Pkc1p/Mpk1p kinase cascade in the morphogenesis checkpoint. Nat. Cell Biol. 2001;3:417–420. doi: 10.1038/35070104. [DOI] [PubMed] [Google Scholar]
- Huang K. N., Symington L. S. Suppressors of a Saccharomyces cerevisiae pkc1 mutation identify alleles of the phosphatase gene PTC1 and of a novel gene encoding a putative basic leucine zipper protein. Genetics. 1995;141:1275–1285. doi: 10.1093/genetics/141.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Taylor Eves P., Tang F., Weisman L. S. PTC1 is required for vacuole inheritance and promotes the association of the myosin-V vacuole-specific receptor complex. Mol. Biol. Cell. 2009;20:1312–1323. doi: 10.1091/mbc.E08-09-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung U. S., Levin D. E. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 1999;34:1049–1057. doi: 10.1046/j.1365-2958.1999.01667.x. [DOI] [PubMed] [Google Scholar]
- Kamada Y., Qadota H., Python C. P., Anraku Y., Ohya Y., Levin D. E. Activation of yeast protein kinase C by Rho1 GTPase. J. Biol. Chem. 1996;271:9193–9196. doi: 10.1074/jbc.271.16.9193. [DOI] [PubMed] [Google Scholar]
- La Cour T., Gupta R., Rapacki K., Skriver K., Poulsen F. M., Brunak S. NESbase version 1.0, a database of nuclear export signals. Nuclear Acids Res. 2003;31:393–396. doi: 10.1093/nar/gkg101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden K., Sheu Y. J., Baetz K., Andrews B., Snyder M. SBF cell cycle regulator as a target of the yeast PKC-MAP kinase pathway. Science. 1997;275:1781–1784. doi: 10.1126/science.275.5307.1781. [DOI] [PubMed] [Google Scholar]
- Manning B. D., Padmanabha R., Snyder M. The Rho-GEF Rom2p localizes to sites of polarized cell growth and participates in cytoskeletal functions in Saccharomyces cerevisiae. Mol. Biol. Cell. 1997;8:1829–1844. doi: 10.1091/mbc.8.10.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H., Flandez M., Nombela C., Molina M. Protein phosphatases in MAPK signalling: we keep learning from yeast. Mol. Microbiol. 2005;58:6–16. doi: 10.1111/j.1365-2958.2005.04822.x. [DOI] [PubMed] [Google Scholar]
- Martin H., Rodriguez-Pachon J. M., Ruiz C., Nombela C., Molina M. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 2000;275:1511–1519. doi: 10.1074/jbc.275.2.1511. [DOI] [PubMed] [Google Scholar]
- Rajavel M., Philip B., Buehrer B. M., Errede B., Levin D. E. Mid2 is a putative sensor for cell integrity signaling in Saccharomyces cerevisiae. Mol. Cell. Biol. 1999;19:3969–3976. doi: 10.1128/mcb.19.6.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke C. A., Kozik P., Glick B. S. Golgi inheritance in small buds of Saccharomyces cerevisiae is linked to endoplasmic reticulum inheritance. Proc. Natl. Acad. Sci. USA. 2004;101:18018–18023. doi: 10.1073/pnas.0408256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder A. D., Hermann G. J., Keegan B. R., Thatcher S. A., Shaw J. M. Mitochondrial inheritance is delayed in Saccharomyces cerevisiae cells lacking the serine/threonine phosphatase PTC1. Mol. Biol. Cell. 1998;9:917–930. doi: 10.1091/mbc.9.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu Y. J., Santos B., Fortin N., Costigan C., Snyder M. Spa2p interacts with cell polarity proteins and signaling components involved in yeast cell morphogenesis. Mol. Cell. Biol. 1998;18:4053–4069. doi: 10.1128/mcb.18.7.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drogen F., Peter M. Spa2p functions as a scaffold-like protein to recruit the Mpk1p MAP kinase module to sites of polarized growth. Curr. Biol. 2002;12:1698–1703. doi: 10.1016/s0960-9822(02)01186-7. [DOI] [PubMed] [Google Scholar]
- Voeltz G. K., Rolls M. M., Rapoport T. A. Structural organization of the endoplasmic reticulum. EMBO Rep. 2002;3:944–950. doi: 10.1093/embo-reports/kvf202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Irie K., Matsumoto K. Yeast RLM1 encodes a serum response factor-like protein that may function downstream of the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 1995;15:5740–5749. doi: 10.1128/mcb.15.10.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr A., Du Y., Pypaert M., Ferro-Novick S., Novick P. Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol. Biol. Cell. 2003;14:4770–4782. doi: 10.1091/mbc.E03-04-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Ohya Y., Goebl M., Nakano A., Anraku Y. A novel gene, STT4, encodes a phosphatidylinositol 4-kinase in the PKC1 protein kinase pathway of Saccharomyces cerevisiae. J. Biol. Chem. 1994;269:1166–1172. [PubMed] [Google Scholar]
- Zarzov P., Mazzoni C., Mann C. The SLT2(MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO J. 1996;15:83–91. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.