Abstract
Cryoablation has emerged as a primary therapy to treat prostate cancer. While effective, the assumption that freezing serves as a ubiquitous lethal stress is challenged by clinical experience and experimental evidence demonstrating time-temperature related cell death dependence. The age-related transformation from an androgen-sensitive (AS) to an androgen-insensitive (AI) phenotype is a major challenge in the management of prostate cancer. AI cells exhibit morphological changes and treatment resistance to many therapies. Since this resistance has been linked with α6β4 integrin overexpression as a result of androgen receptor (AR) loss, we investigated whether α6β4 integrin expression, as a result AR loss, contributes to the reported increased freeze tolerance of AI prostate cancer. A series of studies using AS (LNCaP LP and PC-3 AR) and AI (LNCaP HP and PC-3) cell lines were designed to investigate the cellular mechanisms contributing to variations in freezing response. Investigation into α6β4 integrin expression revealed that AI cell lines overexpressed this protein, thereby altering morphological characteristics and increasing adhesion characteristics. Molecular investigations revealed a significant decrease in caspase 8, 9, and 3 levels AI cells following freezing. Inhibition of α6β4 integrin resulted in increased caspase activity following freezing (similar to AS cells) and enhanced cell death. These data demonstrate that AI cells show an increase in post-freeze susceptibility following inhibition of α6β4 integrin function. Further understanding the role of androgen-receptor related α6β4 integrin expression in prostate cancer cells responses to freezing might lead to novel options for neo-adjunctive treatments targeting the AR signaling pathway.
Keywords: androgen receptor, integrin, cryosurgery, apoptosis, caspase
Introduction
Prostate cancer continues to significantly impact many men each year. In 2006, more than 230,000 new cases were diagnosed with nearly 40,000 deaths reported in the United States 1,2. For the management of prostate cancer, cryosurgery has emerged as an effective tool for the treatment of localized disease 3–5. In 2008 the American Urological Society’s Best Practices Panel on Cryosurgery for Localized Prostate Cancer recommended prostate cryoablation as a primary and salvage therapy for all stages of localized disease 6. This recommendation followed a landmark ten-year retrospective analysis of 370 patients which demonstrated outcome levels at least equivalent to other therapeutic options (77% cancer-free based on sextant biopsy and 80% and 74% PSA negative outcomes for low and medium risk patients) 7. This minimally invasive procedure utilizes the multi-modal destructive forces of the freezing process to effectively ablate targeted tissue 8–10 in a manner that allows for decreased hospitalization time, reduced postoperative morbidity, rapid return to normal daily activities, and reduced overall treatment cost 11–17. Typically, prostate tumors are initially androgen sensitive (AS), and, as such, androgen ablation is a common first line in therapy. Prior to a cryoablative procedure, androgen ablation is often used to shrink the prostate to a more manageable volume (<50cc). Although androgen ablation is often initially effective, the therapy often fails within two years as the disease progresses to a hormone refractory state 18,19 due in part to selection pressure (i.e. selection of androgen independent cells). This advanced stage disease is often aggressive and metastatic, as well as resistant to many therapeutic options 20,21. A compelling link between androgen sensitivity and freeze response was reported in 2008 by Klossner et al. In this study, AS prostate cancer cells were found to be significantly more responsive to freezing damage than AI cells. This sensitivity difference was directly correlated with the presence or absence of an identifiable androgen receptor (AR). This study reported for the first time that the freeze sensitivity of prostate cancer could be negatively impacted by long term androgen deprivation. Given the high diagnostic incidence of prostate cancer and the high recurrence rate 12,13, strategies to develop and improve primary and salvage treatment options are warranted.
Integrins are extracellular transmembrane (ECM) obligate heterodimeric proteins with non-covalent α and β subunits that mediate external cell functions (such as cell motility, polarity, attachment, and shape) as well as control intracellular signaling pathways that regulate cell proliferation, survival, and apoptosis 22,23. For attachment-dependent prostatic cells, integrins form structures that mediate attachment of intracellular intermediate filaments and actin cytoskeleton with ECM proteins (i.e. collagen, fibronectin, and laminin) and basal cells 24,25. α6β4 integrins are a important component of the hemidesmosome expressed at the basal surface in most stratified epithelial cells. Regarding morphology, the α6β4 integrin links the cytoskeleton intermediate filaments (filopodia, lamellae, and retraction fibers) formed on cell borders to laminin-5 in ECM. In normal glandular prostate parenchyma, only the basal cells express integrins connecting them to the substratum. It has been reported that the basal epithelial cell lining is the first morphologic features to be lost in prostate cancer development.26 The action of the α6β4 integrin has been verified in integrin function blocking studies (specific to α6β4 integrin) which resulted in the slowing of cytoskeletal structure formation 27. Furthermore, knockout experiments directly confirm that elimination of integrin expression leads to decreases in each cell proliferation, adhesion, anchorage-dependent growth, and abnormal cell spreading 28,29. In fact, integrins regulate ECM survival cues to such a extent that experiments providing additional ECM (fibronectin) in vitro result in improved survival and anti-apoptotic cellular responses 30. Further, inhibition of integrin signaling function activates apoptotic cell death cascades 31 in many cell systems.
Previous studies have shown that the AR and the androgen signaling pathway are integral in prostate cancer progression and treatment resistance. Bonaccorsi 32–34 and Evangelou 35 have indicated that AR expression directly affects integrin expression. Loss of AR dramatically increases the expression of α6β4 integrins inducing morphological changes, increased cell invasion and proliferation 32,36–38. This AR-mediated upregulation in AI cells is important because integrin signaling thereby activates proliferation and survival cues, resulting in cancer progression 29,33,34,39–42. Integrin expression has also been shown to influence cell survival following cryopreservation 43. More specifically, the freezing process causes damage to microtubules and actin filament structural support fibers by extracellular ice, thermal contraction, alteration in intracellular solute levels, pH, etc. Together these mechanisms disrupt integrin mediated substrate attachment and intracellular signaling pathways which are essential for cell survival 44,45.
In this study we investigated the influence of integrin expression in prostate cancer cell response to freezing injury. Further, we explored the potential of integrin inhibition as an adjunctive approach to increasing cryosurgical efficacy in both AS and AI prostate cancer. While it is appreacated that temperatures of <−40°C typically results in complete cell destruction, studies have demonstrated that at temperatures associated with the margin or periphery of the cryogenic lesion (iceball) there maybe a degree of cell survival and as such the potential for cancer reoccurrence. As such, this study focused on temperatures associated with the iceball margin as a means of understating cellular response and improving cryoablation efficacy. Our data demonstrate that the inhibition of integrin activity during freezing leads to substantial increases in apoptotic cascade activity and cell death in prostate cancer cells. Further, we find differential responses to in AS vs AI prostate cancer cells to integrin inhibition.
Materials and Methods
Cell Culture
The androgen sensitive (AS) human prostate cancer cell line, LNCaP (LNCap LP), was obtained from the American Type Culture Collection (ATCC). The PC-3 AR cell line (AS) was transfected with the full-length AR and selected for stable expression, and the PC-3 cell line (androgen-independent, AI) was transfected with an empty vector. Both lines were obtained from Dr. Joachim B. Schnier (Department of Biochemistry and Molecular Medicine at the University of California at Davis 46). The LNCaP HP (high passage) cell line (AI) was created by repeated culture (over 60 passages) of the LNCaP cell line in low-hormone medium (RPMI-1640 supplemented with 10% charcoal stripped serum [Biomeda] and 1% penicillin/streptomycin [Mediatech, Inc.]). Cultures were maintained at 37°C, 5% CO2/95% air in RPMI-1640 growth medium (Caisson Laboratories) supplemented with 10% fetal calf serum (Atlanta Biologicals) and 1% penicillin/streptomycin (Mediatech, Inc.). PC-3 AR culture media was supplemented with 100 μg/ml hygromycin B (Calbiochem). Cultures were grown in Falcon 75 cm2 T-flasks with media exchange every 3 days. Experimental subcultures were prepared in Costar 96-well, removable strip plates at 18,000 cells/well.
Freezing Protocol
Costar strip wells (100μl medium/well) were placed into an aluminum block partially submerged in a cooling bath pre-set to the target temperature. The sample temperature was monitored with a type-T thermocouple with measurements taken at regular intervals. To prevent sample super-cooling, ice nucleation was initiated by contact with a cold metallic probe when sample temperature reached −2°C (± 1°C). Following nucleation, samples were held for an additional 12 min (15 min total) to allow for thermal equilibration. Samples were then allowed to thaw at room temperature and once completely thawed returned to normothermic culture conditions.
Cell Viability
Cell viability was assessed using the alamarBlue™ assay (Invitrogen) in HBSS (1:20 dilution) every other day following the freezing insult. Cell cultures were exposed to alamarBlue™ for 1 hour at 37°C and then analyzed using a Tecan SPECTRAFluorPlus plate reader (TECAN Austria GmbH) with an excitation of 530nm and emission of 590nm. Subsequently, cell culture media were replenished and returned to normal culture.
Integrin Function Blocking Assay
Blocking of α6β4 integrin function was accomplished by incubating cells in suspension (180,000 cells/ml) in the presence of 40μg/ml anti-α6β4 integrin anti- α6, and anti- β4 antibodies (Chemicon International) for 1 hour at 37°C. Subsequently, cells were plated into 96-well Costar strip plates at 18,000 cells/well, and cultured for 24 hours. Samples were then frozen after a 24 hour post exposure recovery period.
Fluorescent Imaging
To assess the mode of freeze-associated cell death, cells were cultured in Costar 96-well, strip plates and frozen to −15°C for 15 min. Samples were then assessed via triple labeling using the fluorescent probes (Molecular Probes) Hoechst (blue fluorescence, 0.06μg/μl), propidium iodide (red fluorescence, 0.007μg/μl), and YO-PRO-1 (green fluorescence, 0.8μM) to detect living cells, necrotic cells (freeze-ruptured), and apoptotic cells, respectively. Probes were added to each sample and incubated in the dark for 20 minutes. After incubation, samples were visualized via fluorescence microscopy using a Zeiss Axiovert 200M microscope at 240X magnification.
Western Blot
Samples were cultured in 100mm Petri dishes and frozen at −15°C for 15 minutes. Cell lysates (detached and adherent) were collected on ice at 1, 3, 6, 12, and 24 hours post-thaw using ice-cold RIPA (Radio-Immunoprecipitation Assay) cell lysis buffer with phosphatase inhibitor (sodium fluoride 1mM, sodium orthovanadate 1mM, sodium pyrophosphate 1mM), leupeptin (1ug/ml), PMSF (phenylmethylsulphonylfluoride 1mM), and 1x Halt Protease Cocktail Inhibitor (Pierce). Samples were homogenized by vortex mixing and centrifuged at 16,000 × g for 15 minutes at 4°C. Protein concentrations were determined using the bicinchonic acid protein assay (Pierce) and quantified using a Tecan SpectraFluorPlus spectrophotometer. Equal amounts of protein (25μg) were loaded for each sample and separated on a 10% SDS-PAGE gel (Bio-Rad). Proteins were transferred to PVDF membranes (Bio-Rad), blocked with 3% BSA solution containing 0.05% Tween 20, and incubated at 4°C overnight in the presence of each antibody (anti-human β-tubulin [BD Pharmingen], anti-human α6 integrin [Chemicon International], anti-human β4 integrin [Chemicon International], anti-human AKt [Cell Signaling], or anti-human pAKt [Cell Signaling]). Membranes were then washed three times with 0.05% Tween-20 in PBS and exposed with horseradish peroxidase conjugated secondary antibodies. Membranes were visualized using a Fujifilm LAS-3000 luminescent image analyzer for detection. All protein levels were compared to tubulin (protein loading control) and quantitative assessment was conducted via densometric analysis using the Fuji software.
Caspase Activity Assays
Protein samples for caspase-3, −8, −9 activity assays were collected from samples in 100mm Petri dishes frozen at −15oC for 15 minutes. Cell lysates were collected on ice at 1, 3, 6, 12, and 24 hours post-thaw using ice-cold RIPA cell lysis buffer without protease or phosphatase inhibitors. Protein concentrations were quantified and assessed. Equal amounts of protein (50μg) were assessed in duplicate for caspase activity using the BD ApoAlert™ Caspase Fluorescent Assay Kits for Caspase-3, −8, and −9. Sample fluorescence (caspase activity) was quantified using the TECAN spectrofluorometer and converted to fold change in activity based on non-frozen control values.
Data Analysis
Fluorescence units were converted to percent survival based on non-frozen experimental control (37°C). Viability experiments were repeated a minimum of 3 times with a intra experiment repeat of 8 replicates. Western Blot fluorescent imaging and protease activity assays were conducted on a minimum of 3 separate experiments. Calculations of standard error were performed and statistical significance was determined by single-factor analysis of variance (ANOVA).
Results
Cell Adhesion is Affected in AI Cells by Increased Expression of α6β4 Integrins
It is known that morphological changes occur during prostate cancer progression from AS to AI phenotypes, and ECM proteins have been reported to be involved with these changes 34,47. Previous studies have demonstrated this phenomena in both the LNCaP and PC3 cell lines utilized in this study 48. Specifically it has been shown that the LNCaP HP and PC3 cells lines are AI whereas the LNCaP LP and PC3-AR and AS cells. However, it is not known if changes in integrin expression are involved with the reported freeze difference in responses of AS and AI prostate cancer. Phase-contrast microscopy reveals that significant morphological changes occur with transition to AI (Figure 1). LNCaP LP (AS) exhibit large, slender, cytoplasmic projections that form prominent spaces between adjacent cells. Interestingly, LNCaP HP (AI) lack cell projections allowing cells to proliferate within close proximity. PC-3 AR (AS) exhibited broad cytoskeletal attachment allowing fewer cells to proliferate per unit area, whereas many more PC-3 cells (AI) were able to concentrate into a given area.
Figure 1. Phase contrast photomicrographs of a AS and AI cells in culture.
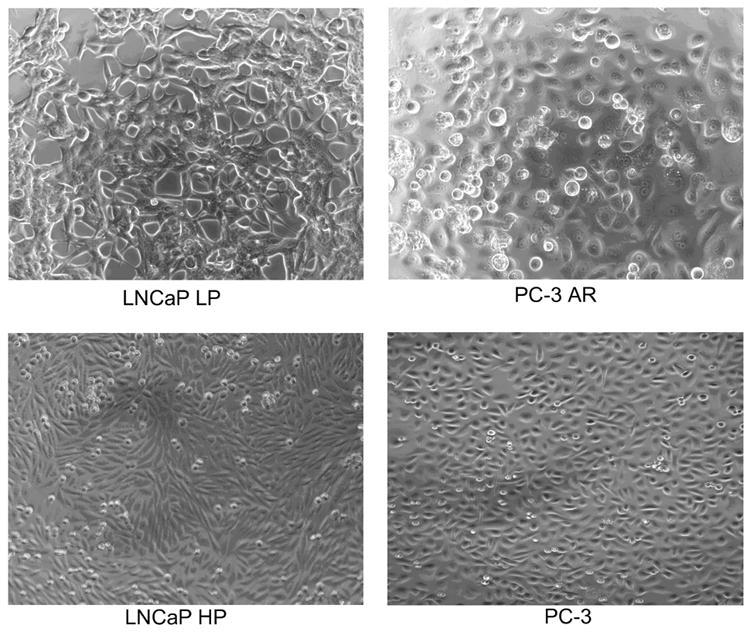
AS LNCaP LP exhibited prominent filopodia that allowed cells to remain separated in culture, while AI LNCaP HP and PC-3 demonstrated tight cell packing that increased cells per unit area.
With the observed morphological alterations, investigations into the molecular basis that may be responsible for attachment-related changes and the possible involvement of integrins were conducted. Further, the effect of freezing on integrin expression was also examined for its potential influence on cellular freeze response. Western blot analysis of integrin expression revealed significant alterations in the α6 and β4 integrin subunits following freezing (Figure 2). AS (LNCaP LP and PC-3 AR) cells exhibited significantly lower levels of both α6 and β4 integrin subunits overall. Additionally, the AS cells exhibited a temporal decrease in both α6 and β4 subunits post-freeze. AI cells (LNCaP HP and PC-3) exhibited greater basal levels of β4 and α6 integrin subunits. Furthermore, it was found that following a freeze insult, the AI cells demonstrated a temporal increase in integrin levels (Figure 2). Specifically, it was found that 12 hours following freezing, a peak in β4 levels occurred whereas a peak in α6 levels was found 24 hours post-freeze. These data demonstrate a unique response of AI cells following freezing where integrin levels increased significantly compared to the AS cells. With the direct connection between integrin expression and cell survival/proliferation pathways, and previous reports demonstrating the increase freeze resistance of AI prostate cancer cells, it seemed plausible that integrin expression and resistant signal transduction pathways may directly affect cell response to freezing.
Figure 2. Western blot analysis of integrin protein levels (α6 and β4 subunit) in AS and AI prostatic cancer cells following freezing.
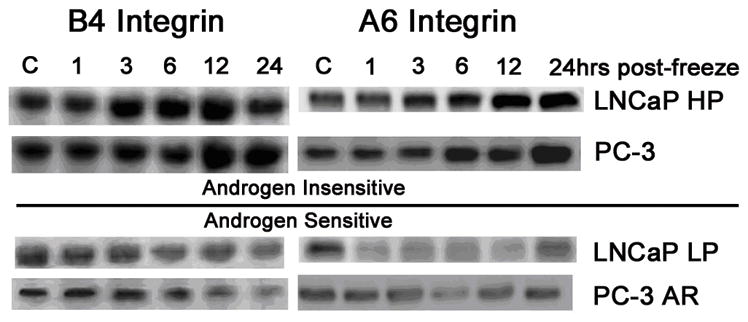
(LNCaP HP and PC-3) exhibited greater expression levels of each subunit,which increased in expression up to 12 hours (for the β4 subunit) and 24 hours (for the α6 subunit) following freezing. AS cells (LNCaP LP and PC3 AR) showed lower integrin expression levels overall and post-freeze analysis indicated a decline over a 24-hour period. Blots pictures are representative of 3 independent experimental repeats (N=3).
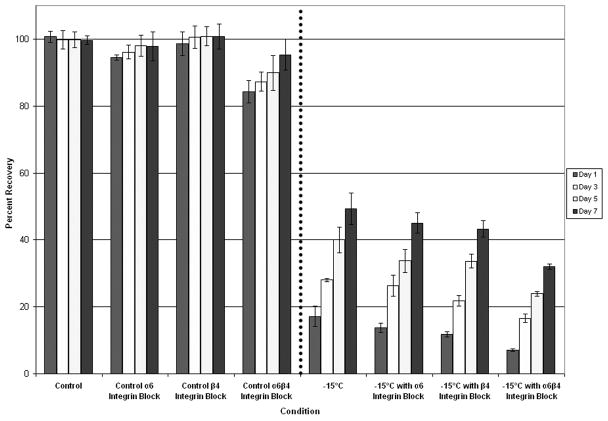
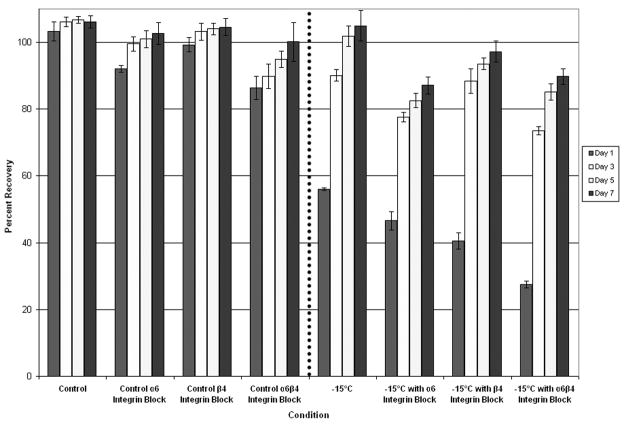
Inhibition of Integrin Function During Freezing Decreases AI Cell Viability More Significantly than AS Cell Viability
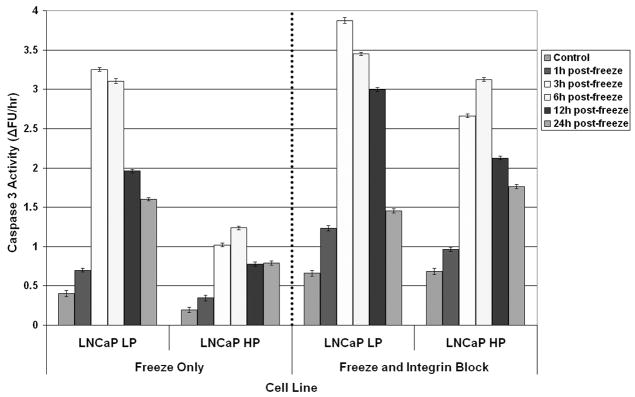
Studies with AI prostate cancer cells, which express greater levels of α6β4 integrin as a result of AR loss, have shown that increased α6β4 integrin ECM cell signaling correlates with greater freeze resistance 32,36–38. To further investigate α6β4 integrin influence on freeze response, competitive antibody inhibition studies using anti-α6 and anti-β4 integrin antibodies were conducted to block integrin signaling and function. Samples were frozen and post-thaw recovery (1 to 7 days) was assessed (Figure 3). Analysis of cell viability over the 7 day recovery period revealed that each of the surviving populations recovered and that the rate of recovery was proportional to the initial day 1 survival. Interestingly, different freeze responses were observed in AS and AI. AS cells exhibited a decrease in day 1 post-freeze cell viability after exposure to integrin function blocking compared to freeze alone (Figure 3A). While separate blocking of α6 and β4 integrin functions yielded decreases in post-freeze cell viability, blocking the α6β4 complement had the greatest effect on reducing post-freeze viability. With inhibition of α6β4 integrin function, LNCaP LP exhibited an overall decrease in post-freeze viability of 10.1% ± 0.5% from freeze alone (7% vs. 17 % respectively, P<0.01). Similar results were observed from the AS PC-3 AR cells where blocking of the α6β4 complex resulted in an 18% reduction in overall cell survival compared to cells frozen to −15°C without blocking (30% vs. 12% respectively P<0.01) (Data not shown). Interestingly, compared with AS cells, AI cells exhibited a much greater reduction in post-freeze cell survival following the integrin function-blocking assay (p < 0.05), indicating greater dependence on that signaling mechanism (Figure 3B). Similarly, inhibition of separate subunits yielded lesser reductions in post-freeze cell viability vs. the inhibition of both subunits. For α6β4 integrin function inhibition, LNCaP HP exhibited a decrease in post-freeze cell viability of 28.7% ± 0.7% overall (27% vs. 56% respectively P<0.01). The greater sensitivity of AI cell lines to disruption of integrin signaling pathways indicated a putative influence of integrin signaling on cell survival following a freezing insult. Further, through the integrin α6β4 complex blocking, AI cell susceptibility to freezing injury was similar to that of the native AS cell populations which contained lower basal integrin expression levels. These data reveal that integrins play a substantial role in cell response to freezing.
Figure 3. Post-freeze viability of prostate cancer cells following integrin function blocking.
The effects of α6, β4, and α6β4 integrin function blocking (40μg/ml) upon freeze response were analyzed using the metabolic indicator, alamarBlue. The AS cell line LNCaP LP (A) exhibited small decreases in cell viability following exposure to integrin function blocking, while α6β4 blocking showed the most significant effect compared to separate subunits. AI LNCaP HP (B) showed a greater decrease in post-freeze viability, while α6β4 integrin function blocking exhibited the greatest effect. These data indicated a differential response between AS and AI cell lines demonstrating that α6β4 integrin function blocking had greater effects on AI cell lines. Data presented represents a minimum of 3 experimental repeats with a intra experimental replication of 8 (N≥3, N≥24)
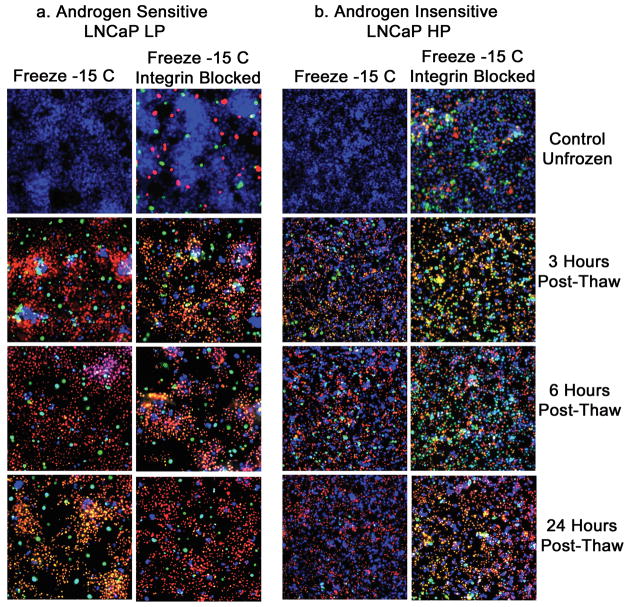
Integrin Function-Blocked AI Prostate Cell Lines Exhibited Differentially Increased Freezing-Induced Apoptosis and Necrosis
The significant reduction in post-freeze viability observed in the AI cells as a result of α6β4 integrin function inhibition prompted the investigation into what modes of cell death were affected. Thus, in order to evaluate total levels and temperature characteristics in terms of apoptotic and necrotic cell death following freezing, α6β4 integrin blocking assays were conducted. Samples were frozen to −15°C and assessed by a triple-fluorescent probe (Hoechst [blue]) to assess viable cells, propidium iodide [red] to assess necrotic cells, and YO-PRO-1 [green] to assess apoptotic cell (Figure 4). Fluorescent microscopy analysis of the LNCaP LP cells (AS) revealed low levels of both apoptosis and necrosis in non-freeze controls (Figure 4A). Samples subjected to freezing to −15°C resulted in a time dependent increase in the level of apoptosis peaking at 3 hours post-freeze and remaining elevated to 24 hours. Compared with non-treated, the α6β4 integrin-blocked AS LNCaP LP cells exhibited slight increases in the levels of apoptosis and necrosis for control (unfrozen) samples. This slight increase was also observed in the post-freeze samples. The fluorescent micrographic data did not reveal any substantial alteration in either population which correlated with the viability data for the AS cell populations. Similar results were also noted in the AS PC3 AR cell population (Data not shown). Quantitative analysis for freeze alone at 3 hours post-freeze revealed, the following: LNCaP LP = 22.1% ± 1.2% viability, 58.2% ± 0.8% necrosis, and 19.7 ± 1.5% apoptosis, while the 3 hour post-freeze sample treated with α6β4 integrin function blocking assay exhibited 20.5% ± 0.4% viability, 55.6% ± 1.6% necrosis, and 23.9% ± 1.1% apoptosis. Similar results were again noted with the AS PC-3 AR cells (Data not shown). These data indicated that α6β4 integrin blocking during freezing did not significantly (p >0.05) alter the apoptotic and necrotic cell death cascades in the AS cell populations. Compared to AS cell lines, the AI cells, when subjected to freezing, exhibited lower levels of overall apoptosis and necrosis. Apoptosis and necrosis were minimal in control (unfrozen) samples. Following freeze exposure, apoptotic and necrotic levels increased with a shift in peak apoptotic activity at 6 hours post-freeze with a rapid decline by 24 hours. Further, the overall levels of both apoptosis and necrosis were significantly lower in the AI vs. AS cell populations. Comparison of the freeze only AI samples with the α6β4 integrin blocking samples revealed that the functional blocking resulted in a substantial increase in the level of apoptotic cell death post-thaw. Post-freeze time points revealed increased levels of apoptosis and necrosis with peak levels occurring after 3 hours post-freeze, which represented a more rapid apoptotic induction compared with freeze alone. Quantitative analysis for freeze alone at 3 hours post-freeze revealed the following: LNCaP HP = 63.7% ± 1.4% viability, 23.4% ± 0.6% necrosis, and 12.9% ± 1.1% apoptosis, while the 3 hour post-freeze sample treated with α6β4 integrin blocking assays exhibited 43.3% ± 1.5% viability, 25.1% ± 0.9% necrosis, and 31.6 ± 0.4% apoptosis. This represented a greater than 2 fold increase in apoptosis (19% overall) as a result of integrin blocking. Similar results were noted with the AI PC-3 cell population (Data not shown). These data indicate that α6β4 integrin function blocking during freezing significantly (p < 0.05) increases apoptotic cell-death cascades. Interestingly, the AI cell lines exhibited much greater increases in apoptosis compared with AS cell lines. This correlates with metabolic viability data indicating that AI cell lines are more greatly affected by integrin inhibition. The data suggest that the increased integrin level in the AI cell population is responsible for the increased resistance to freezing-injury.
Figure 4. Fluorescent micrographs of AS and AI prostate cancer cells following freeze exposure with and without integrin inhibition.
Total levels of necrotic and apoptotic cell death were evaluated in LNCaP LP (A) and LNCaP HP (B) cells treated with 40μg/ml anti-α6β4 integrin antibody. Samples were frozen at −15°C and triple-probe fluorescent micrographs were taken after 3h, 6h, and 24h using Hoechst (blue) to assess viable cells, propidium iodide (red) to assess necrotic cells, and YO-PRO-1 (green) to assess apoptotic cells. A) Compared to freeze alone, AS cell line LNCaP LP with function-blocked integrins showed only slight increases in necrotic and apoptotic cells. B) Function blocked LNCaP HP exhibited significant increases (p < 0.05) in necrotic and apoptotic cell death at every tested time point, indicating that AI cell lines more greatly depended on integrin signaling pathways for increased freezing resistance.
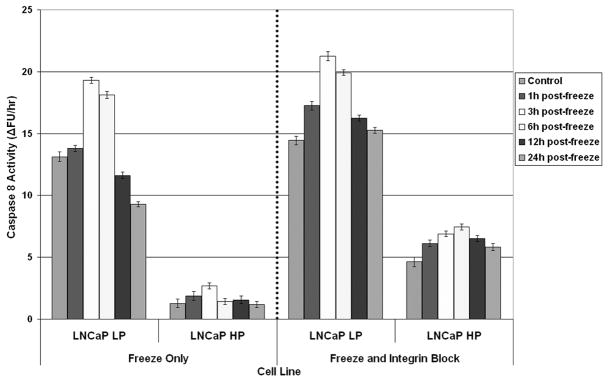
Integrin Function Blocked LNCaP HP and PC-3 Exhibited Greater Post-freeze Caspase Activity than Freeze Alone
Fluorescent micrographic and viability data support the notion of increased integrin levels playing a critical role in AI prostate cancer cell survival. To further investigate these phenomena, apoptotic cell death signal transduction pathway proteins (caspases) were analyzed to determine both the effect of freezing as well as integrin blocking. Representative caspases were selected based on their association with either membrane-linked (caspase 8) or mitochondrial-linked (caspase 9) apoptotic induction. Additionally, analysis of the down stream executioner caspase 3 was also conducted to verify signal transduction leading to cell death (Figure 5A, B, C). Analysis of caspase 8 following freezing revealed a high degree of activity peaking 3 hours post-freeze in the AS LNCaP population (Figure 5A). This level of activity was consistent in both the freeze only and integrin blocked samples. Analysis of the AI LNCaP HP samples revealed a substantial reduction in caspase 8 activity following freezing. Interestingly, when integrins were blocked, the AI cells showed a marked increase in caspase 8 activity compared to freeze only samples. This increase in activity was observed to be greater than 2.8 fold (2.6 RFU vs. 7.5 RFU respectively). These data demonstrate a marked alteration in caspase 8 (membrane-linked apoptosis) levels following freezing and integrin blocking. Investigation of the effect of freezing on caspase 9 activity (mitochondrial-linked apoptosis) revealed that following freezing there was a marked increase in caspase 9 activity. This increase was similar to that observed with caspase 8 activity (Figure 5B). In contrast to caspase 8, integrin blocking was much less effective on caspase 9 activity overall in both the AS and AI populations. Peak caspase 9 activities increased from 10RFU to 17RFU in the AI LNCaP HP samples as a result in integrin blocking which represented less than a 0.7 fold change. This was in stark contrast to the greater than 2.8 fold increase in caspase 8 activity. Analysis of down stream transduction of apoptotic signals revealed that following freezing, caspase 3 was also activated in both the AS and AI populations (Figure 5C). It was further noted that there was little alteration in caspase 3 activity between the freeze only and integrin blocked AS cell populations. In the AI cells, integrin blocking resulted in a significant increase in overall caspase 3 activity. These data (~2.5 fold increase) suggest that the activation of caspase 8 by integrin blocking translated into an increase in cell death. Similar results for caspase 8, 9, and 3 activity following freezing and integrin blocking were also observed in the AS PC-3 AR and AI PC-3 cell populations.
Figure 5. Caspase activity analysis in AI and AS prostate cancer cells following freezing and integrin blocking.
Caspase-8 (A), Caspase-9 (B), and Caspase-3 (C) activities were determined for LNCaP LP, LNCaP HP, PC-3 AR, and PC-3 cell lines treated with an α6β4 integrin function blocking assay. Cells were frozen and cell lysates were collected at regular intervals up to 24-hours post-freeze. Caspase activity was based on the ability to convert non-fluorescent substrate to its cleaved, fluorescent form. Compared to freeze alone, integrin function blocked AS cell lines LNCaP LP and PC-3 AR exhibited slightly increased levels of caspase-activity that peaked after 3-hours post-freeze. Interestingly, integrin function blocked AI cell lines LNCaP HP and PC-3 exhibited significantly (p < 0.05) greater levels of caspase activity (compared to freeze alone) that peaked after 6-hours post-freeze. These data indicated that integrin function blocking induced differentially greater cell death responses in AI cell lines. Data presented represents 3 experimental repeats (N=3).
Discussion
Cryosurgery is an option for treating either early or advanced stage (localized) prostate cancer and its efficacy has been well documented 12,49. For patients with localized (early or advanced stage) prostate cancer, cryosurgery demonstrates superior efficacy and improved long-term disease-free control. Despite successes in disease treatment, cryosurgery still experiences low, yet significant, recurrence rates which are greater for advanced stage carcinomas 12,13. Furthermore, the progression of prostate cancer to an AI, treatment resistant form (reviewed in 50) remains a therapeutic challenge, especially since increased integrin expression has been implicated with advanced stage disease 24,28,51. Therefore, we examined whether or not changes in AR expression (AS to AI) affected α6β4 integrin expression resulting in changes of prostate cancer tolerance to freezing. We demonstrated that AI cells, which lack AR expression, exhibited increased levels of α6β4 integrin expression which correlated significantly with increased post-freeze survival by mediating reduced caspase activity.
Similarly, Pawar et al.52 reported on α6 integrin cleavage as a feasible sensitizer for human prostate cancer to ionizing radiation. In this regards, the ECM environment changes dramatically following radiation therapy, which like cryoablation, alters the soluble and insoluble components of ECM. Importantly, the ability of the tumor cell to survive radiation therapy or cryoablation appears to be dependent on the level of integrin expression on the cell surface.
In this study we investigated the changes that occurred in integrin expression between AS and AI cells and their influence on freezing response. It has been recognized for many years that morphological changes in prostate cancer occur as tumors progress to advanced disease. In fact this morphological progression is the basis underlying the Gleason scale of prostate cancer differentiation. The initial identification of α6β4 integrin as a significant molecular difference between AS and AI cells resulted from recent studies showing that AR-mediated α6β4 integrin expression is responsible for morphological changes in filopodia and lamellae formation as observed in our model prostate cancer cell lines 32–34,53 (Figure 1).
Investigation into the involvement of α6β4 integrin in freeze response stemmed from a reports by Klossner et al. 48,54 demonstrating the freeze tolerance differential between AS and AI prostate cancer cells. Other studies showed that the cytoskeleton is a major target in freeze induced cell death 43. Western blot analysis of α6β4 integrin expression post-freeze further indicated that ECM protein expression might affect freezing survival through a reduction of apoptosis and increase in cell attachment and survival signaling. The increase in integrin signaling could provide for survival signaling to intercellular pathways via intermediate filaments, actin filaments, and kinase pathways such as the PI3K/AKt pathway 44,45. The results of the current study support this hypothesis and demonstrate that increased integrin attachment and signaling resulted in increased cell survival following freezing. Further, the importance of α6β4 integrin expression in freeze response was verified using a function-blocking assay, which demonstrated that AS cells exhibited a statistically smaller decrease in post-freeze viability compared with AI cells. These studies revealed that the increased freeze survival of AI cells was reduced by α6β4 integrin inhibition to levels comparable with AS cells treated with freeze alone.
The significant increase in cell death in AI cells with inhibited α6β4 integrin prompted the investigation into which alterations in cell death pathways might have occurred. Studies have shown that integrins regulate the apoptotic processes 30,31, but their role in the freeze response of AS and AI cells remained unknown. Fluorescent micrographic analysis provided a qualitative assessment showing that α6β4 integrin function-blocked AI cells exhibited a significant increase in caspase activity peaking at 3 hours post-freeze (versus of 6 hours post-freeze for freeze alone). Caspase activity assays provided qualitative analysis, corroborating an increase in the levels of apoptotic activity in AI cells following integrin blocking. Caspase-3 activity analysis revealed that, compared to freeze alone at −15°C, α6β4 integrin function-blocked AS cells showed slight increases in caspase-3 activity whereas, in the AI cells a substantial increase in caspase activity was observed by integrin blocking. Furthermore, AI cell lines exhibited increased caspase-3 activation (up to 24 hours post-freeze), which contrasted with freeze alone that showed declining caspase-3 activity over the same interval. This finding indicates that α6β4 integrin inhibition resulted in greater relative apoptotic activity in AI cells leading to a reduction in post-freeze viability. Following integrin blocking, AI cells showed caspase-3 activity levels that were comparable with those observed for AS cells. Werner et al.55 noted that increases in integrin-related caspase-3 activity result from disrupted hemidesmosome assembly, which may also occur in the freezing processes. A similar differential increase in caspase-8 activity was observed for AI cells compared with AS cells. This alteration in caspase 8 activity indicates that α6β4 integrin inhibition during freezing resulted in an activation of the membrane mediated apoptotic pathway. These findings are in accord with research showing that integrin signaling and caspase-8 activity are linked 56. Taken together, these findings indicate that inhibition of integrin signaling pathways might lead to “cross-talk” with transmembrane receptors to increase membrane-mediated apoptotic cell death when cells are unable to receive survival signaling from cell attachment proteins. Finally, caspase-9 showed similar differential increases for AI cells. However, compared to caspase-8 activity increases, the increase in caspase-9 activity suggests that integrin-related activation of both the membrane and mitochondrial-linked signaling pathways may be involved in the overall AI cell response to freezing. Additionally, caspase-9 exhibited peak activity at 3 hours, which indicates a shift to earlier apoptotic activation (compared with 6 hours for freeze alone).
In conclusion, the data support the hypothesis that AR-mediated changes in integrin expression differentially affects the freeze responses of AS and AI prostate cancer cells. Increased α6β4 integrin expression as a result of AR loss resulted in reduced caspase activity which could be transiently reversed through integrin binding inhibition. Studies have shown that cell adhesion and the accompanying cytoskeletal elements are important factors in treatment resistance of advanced malignant disease 57, and the identification of α6β4 integrin as a mediator of freeze response provides an exciting opportunity to increase the efficacy of cryosurgery through neo-adjuvant treatment options. Reports by our group 58–60 and Le Pivert 61 have detailed the advantages of using neo-adjunctive, low-dose chemotherapeutic agents to weaken prostate tumors prior to cryosurgery, which has shown to be effective beyond that of either treatment alone. Thus, it may be possible to adjust extant integrin targeting treatments 62 to inhibit α6β4 integrin, which may be overexpressed in prostate cancer, and combine that therapy with cryotherapy to achieve reduced prostate cancer disease recurrence and increase patient quality of life.
Acknowledgments
This study was funded in part by Galil Medical USA, Inc. (Plymouth Meeting, PA), Cell Preservation Services, Inc. (Owego, NY), and The National Institutes of Health.
Reference List
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006 Mar-Apr;56(2):106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Kendirci M, Bejma J, Hellstrom WJ. Update on erectile dysfunction in prostate cancer patients. Curr Opin Urol. 2006 May;16(3):186–95. doi: 10.1097/01.mou.0000193407.05285.d8. [DOI] [PubMed] [Google Scholar]
- 3.Baust JG, Gage AA, Clarke D, Baust JM, Van Buskirk R. Cryosurgery--a putative approach to molecular-based optimization. Cryobiology. 2004 Apr;48(2):190–204. doi: 10.1016/j.cryobiol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Grubb RL, 3rd, Vardi IY, Bhayani SB, Kibel AS. Minimally invasive approaches to localized prostate carcinoma. Hematol Oncol Clin North Am. 2006 Aug;20(4):879–95. doi: 10.1016/j.hoc.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Mouraviev V, Polascik TJ. Update on cryotherapy for prostate cancer in 2006. Curr Opin Urol. 2006 May;16(3):152–6. doi: 10.1097/01.mou.0000193393.54598.9f. [DOI] [PubMed] [Google Scholar]
- 6.Babaian RJ, Donnelly B, Bahn D, Baust JG, Dineen M, Ellis D, et al. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol. 2008 Nov;180(5):1993–2004. doi: 10.1016/j.juro.2008.07.108. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JK, Miller RJ, Jr, Ahmed S, Lotz MJ, Baust J. Ten-year biochemical disease control for patients with prostate cancer treated with cryosurgery as primary therapy. Urology. 2008 Mar;71(3):515–8. doi: 10.1016/j.urology.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 8.Baust JG, Gage AA. Progress toward optimization of cryosurgery. Technol Cancer Res Treat. 2004 Apr;3(2):95–101. doi: 10.1177/153303460400300202. [DOI] [PubMed] [Google Scholar]
- 9.Gage AA, Baust JG. Cryosurgery for tumors - a clinical overview. Technol Cancer Res Treat. 2004 Apr;3(2):187–99. doi: 10.1177/153303460400300212. [DOI] [PubMed] [Google Scholar]
- 10.Onik GM, Cohen JK, Reyes GD, Rubinsky B, Chang Z, Baust J. Transrectal ultrasound-guided percutaneous radical cryosurgical ablation of the prostate. Cancer. 1993 Aug;72(4):1291–9. doi: 10.1002/1097-0142(19930815)72:4<1291::aid-cncr2820720423>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed S, Lindsey B, Davies J. Salvage cryosurgery for locally recurrent prostate cancer following radiotherapy. Prostate Cancer Prostatic Dis. 2005;8(1):31–5. doi: 10.1038/sj.pcan.4500774. [DOI] [PubMed] [Google Scholar]
- 12.Bahn DK, Lee F, Badalament R, Kumar A, Greski J, Chernick M. Targeted cryoablation of the prostate: 7-year outcomes in the primary treatment of prostate cancer. Urology. 2002 Aug;60(2 Suppl 1):3–11. doi: 10.1016/s0090-4295(02)01678-3. [DOI] [PubMed] [Google Scholar]
- 13.Bahn DK, Lee F, Silverman P, Bahn E, Badalament R, Kumar A, et al. Salvage cryosurgery for recurrent prostate cancer after radiation therapy: a seven-year follow-up. Clin Prostate Cancer. 2003 Sep;2(2):111–4. doi: 10.3816/cgc.2003.n.018. [DOI] [PubMed] [Google Scholar]
- 14.Chin JL, Touma N. Current status of salvage cryoablation for prostate cancer following radiation failure. Technol Cancer Res Treat. 2005 Apr;4(2):211–6. doi: 10.1177/153303460500400210. [DOI] [PubMed] [Google Scholar]
- 15.Katz AE, Rukstalis DB. Introduction. Recent scientific and technological advances have challenged the traditional treatment options for patients with localized prostate cancer. Urology. 2002 Aug;60(2 Suppl 1):1–2. doi: 10.1016/s0090-4295(02)01851-4. [DOI] [PubMed] [Google Scholar]
- 16.Long JP, Bahn D, Lee F, Shinohara K, Chinn DO, Macaluso JN., Jr Five-year retrospective, multi-institutional pooled analysis of cancer-related outcomes after cryosurgical ablation of the prostate. Urology. 2001 Mar;57(3):518–23. doi: 10.1016/s0090-4295(00)01060-8. [DOI] [PubMed] [Google Scholar]
- 17.Wong WS, Chinn DO, Chinn M, Chinn J, Tom WL, Tom WL. Cryosurgery as a treatment for prostate carcinoma: results and complications. Cancer. 1997 Mar;79(5):963–74. [PubMed] [Google Scholar]
- 18.Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin. 2002 May-Jun;52(3):154–79. doi: 10.3322/canjclin.52.3.154. [DOI] [PubMed] [Google Scholar]
- 19.Kish JA, Bukkapatnam R, Palazzo F. The treatment challenge of hormone-refractory prostate cancer. Cancer Control. 2001 Nov-Dec;8(6):487–95. doi: 10.1177/107327480100800603. [DOI] [PubMed] [Google Scholar]
- 20.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004 Jan;10(1):33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 21.Powell SM, Brooke GN, Whitaker HC, Reebye V, Gamble SC, Chotai D, et al. Mechanisms of androgen receptor repression in prostate cancer. Biochem Soc Trans. 2006 Dec;34(Pt 6):1124–7. doi: 10.1042/BST0341124. [DOI] [PubMed] [Google Scholar]
- 22.Brakebusch C, Fassler R. The integrin-actin connection, an eternal love affair. EMBO J. 2003 May;22(10):2324–33. doi: 10.1093/emboj/cdg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998 Apr;10(2):220–31. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 24.Calaluce R, Bearss DJ, Barrera J, Zhao Y, Han H, Beck SK, et al. Laminin-5 beta3A expression in LNCaP human prostate carcinoma cells increases cell migration and tumorigenicity. Neoplasia. 2004 Sep-Oct;6(5):468–79. doi: 10.1593/neo.03499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu C, Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol. 2001 Nov;155(4):505–10. doi: 10.1083/jcb.200108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mol AJ, Geldof AA, Meijer GA, van der Poel HG, van Moorselaar RJ. New experimental markers for early detection of high-risk prostate cancer: role of cell-cell adhesion and cell migration. J Cancer Res Clin Oncol. 2007 Oct;133(10):687–95. doi: 10.1007/s00432-007-0235-8. [DOI] [PubMed] [Google Scholar]
- 27.Rabinovitz I, Mercurio AM. The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J Cell Biol. 1997 Dec;139(7):1873–84. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carloni V, Romanelli RG, Mercurio AM, Pinzani M, Laffi G, Cotrozzi G, et al. Knockout of alpha6 beta1-integrin expression reverses the transformed phenotype of hepatocarcinoma cells. Gastroenterology. 1998 Aug;115(2):433–42. doi: 10.1016/s0016-5085(98)70210-0. [DOI] [PubMed] [Google Scholar]
- 29.Grashoff C, Thievessen I, Lorenz K, Ussar S, Fassler R. Integrin-linked kinase: integrin’s mysterious partner. Curr Opin Cell Biol. 2004 Oct;16(5):565–71. doi: 10.1016/j.ceb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Morgan M, Saba S, Gower W. Fibronectin influences cellular proliferation and apoptosis similarly in LNCaP and PC-3 prostate cancer cell lines. Urol Oncol. 2000 Jul;5(4):155–9. doi: 10.1016/s1078-1439(99)00058-7. [DOI] [PubMed] [Google Scholar]
- 31.Chatterjee S, Brite KH, Matsumura A. Induction of apoptosis of integrin-expressing human prostate cancer cells by cyclic Arg-Gly-Asp peptides. Clin Cancer Res. 2001 Oct;7(10):3006–11. [PubMed] [Google Scholar]
- 32.Bonaccorsi L, Carloni V, Muratori M, Salvadori A, Giannini A, Carini M, et al. Androgen receptor expression in prostate carcinoma cells suppresses alpha6beta4 integrin-mediated invasive phenotype. Endocrinology. 2000 Sep;141(9):3172–82. doi: 10.1210/endo.141.9.7640. [DOI] [PubMed] [Google Scholar]
- 33.Bonaccorsi L, Carloni V, Muratori M, Formigli L, Zecchi S, Forti G, et al. EGF receptor (EGFR) signaling promoting invasion is disrupted in androgen-sensitive prostate cancer cells by an interaction between EGFR and androgen receptor (AR) Int J Cancer. 2004 Oct;112(1):78–86. doi: 10.1002/ijc.20362. [DOI] [PubMed] [Google Scholar]
- 34.Bonaccorsi L, Muratori M, Marchiani S, Forti G, Baldi E. The androgen receptor and prostate cancer invasion. Mol Cell Endocrinol. 2006 Feb;246(1–2):157–62. doi: 10.1016/j.mce.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Evangelou A, Letarte M, Marks A, Brown TJ. Androgen modulation of adhesion and antiadhesion molecules in PC-3 prostate cancer cells expressing androgen receptor. Endocrinology. 2002 Oct;143(10):3897–904. doi: 10.1210/en.2002-220156. [DOI] [PubMed] [Google Scholar]
- 36.Davis TL, Rabinovitz I, Futscher BW, Schnolzer M, Burger F, Liu Y, et al. Identification of a novel structural variant of the alpha 6 integrin. J Biol Chem. 2001 Jul;276(28):26099–106. doi: 10.1074/jbc.M102811200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jasavala R, Martinez H, Thumar J, Andaya A, Gingras AC, Eng JK, et al. Identification of putative androgen receptor interaction protein modules: cytoskeleton and endosomes modulate androgen receptor signaling in prostate cancer cells. Mol Cell Proteomics. 2007 Feb;6(2):252–71. doi: 10.1074/mcp.M600169-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Nagakawa O, Akashi T, Hayakawa Y, Junicho A, Koizumi K, Fujiuchi Y, et al. Differential expression of integrin subunits in DU-145/AR prostate cancer cells. Oncol Rep. 2004 Oct;12(4):837–41. [PubMed] [Google Scholar]
- 39.Edlund M, Miyamoto T, Sikes RA, Ogle R, Laurie GW, Farach-Carson MC, et al. Integrin expression and usage by prostate cancer cell lines on laminin substrata. Cell Growth Differ. 2001 Feb;12(2):99–107. [PubMed] [Google Scholar]
- 40.Persad S, Attwell S, Gray V, Delcommenne M, Troussard A, Sanghera J, et al. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci U S A. 2000 Mar;97(7):3207–12. doi: 10.1073/pnas.060579697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu C. ILK interactions. J Cell Sci. 2001 Jul;114(Pt 14):2549–50. doi: 10.1242/jcs.114.14.2549. [DOI] [PubMed] [Google Scholar]
- 42.Wu D, Thakore CU, Wescott GG, McCubrey JA, Terrian DM. Integrin signaling links protein kinase Cepsilon to the protein kinase B/Akt survival pathway in recurrent prostate cancer cells. Oncogene. 2004 Nov;23(53):8659–72. doi: 10.1038/sj.onc.1207900. [DOI] [PubMed] [Google Scholar]
- 43.Koenigsmann MP, Koenigsmann M, Notter M, Neuloh M, Mucke C, Thiel E, et al. Adhesion molecules on peripheral blood-derived CD34+ cells: effects of cryopreservation and short-term ex vivo incubation with serum and cytokines. Bone Marrow Transplant. 1998 Dec;22(11):1077–85. doi: 10.1038/sj.bmt.1701484. [DOI] [PubMed] [Google Scholar]
- 44.Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, et al. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003 Apr;17(7):926–40. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu BL, McGrath J. Response of cytoskeleton of murine osteoblast cultures to two-step freezing. Acta Biochim Biophys Sin (Shanghai) 2005 Dec;37(12):814–8. doi: 10.1111/j.1745-7270.2005.00121.x. [DOI] [PubMed] [Google Scholar]
- 46.Schnier JB, Nishi K, Gumerlock PH, Gorin FA, Bradbury EM. Glycogen synthesis correlates with androgen-dependent growth arrest in prostate cancer. BMC Urol. 2005;5:6. doi: 10.1186/1471-2490-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foty RA, Steinberg MS. Cadherin-mediated cell-cell adhesion and tissue segregation in relation to malignancy. Int J Dev Biol. 2004;48(5–6):397–409. doi: 10.1387/ijdb.041810rf. [DOI] [PubMed] [Google Scholar]
- 48.Klossner DP, Baust JM, VanBuskirk RG, Gage AA, Baust JG. Cryoablative response of prostate cancer cells is influenced by androgen receptor expression. BJU Int. 2008 May;101(10):1310–6. doi: 10.1111/j.1464-410X.2008.07499.x. [DOI] [PubMed] [Google Scholar]
- 49.Onik G. Image-guided prostate cryosurgery: state of the art. Cancer Control. 2001 Nov-Dec;8(6):522–31. doi: 10.1177/107327480100800607. [DOI] [PubMed] [Google Scholar]
- 50.Bhandari MS, Petrylak DP, Hussain M. Clinical trials in metastatic prostate cancer--has there been real progress in the past decade? Eur J Cancer. 2005 Apr;41(6):941–53. doi: 10.1016/j.ejca.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Davies G, Jiang WG, Mason MD. Cell-cell adhesion molecules and signaling intermediates and their role in the invasive potential of prostate cancer cells. J Urol. 2000 Mar;163(3):985–92. [PubMed] [Google Scholar]
- 52.Pawar SC, Dougherty S, Pennington ME, Demetriou MC, Stea BD, Dorr RT, et al. alpha6 integrin cleavage: sensitizing human prostate cancer to ionizing radiation. Int J Radiat Biol. 2007 Nov-Dec;83(11–12):761–7. doi: 10.1080/09553000701633135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jennbacken K, Gustavsson H, Welen K, Vallbo C, Damber JE. Prostate cancer progression into androgen independency is associated with alterations in cell adhesion and invasivity. Prostate. 2006 Nov;66(15):1631–40. doi: 10.1002/pros.20469. [DOI] [PubMed] [Google Scholar]
- 54.Klossner DP, Robilotto AT, Clarke DM, VanBuskirk RG, Baust JM, Gage AA, et al. Cryosurgical technique: assessment of the fundamental variables using human prostate cancer model systems. Cryobiology. 2007 Dec;55(3):189–99. doi: 10.1016/j.cryobiol.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Werner ME, Chen F, Moyano JV, Yehiely F, Jones JC, Cryns VL. Caspase proteolysis of the integrin beta4 subunit disrupts hemidesmosome assembly, promotes apoptosis, and inhibits cell migration. J Biol Chem. 2007 Feb;282(8):5560–9. doi: 10.1074/jbc.M603669200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol. 2001 Oct;155(3):459–70. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeRoock IB, Pennington ME, Sroka TC, Lam KS, Bowden GT, Bair EL, et al. Synthetic peptides inhibit adhesion of human tumor cells to extracellular matrix proteins. Cancer Res. 2001 Apr;61(8):3308–13. [PubMed] [Google Scholar]
- 58.Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Chemo-cryo combination therapy: an adjunctive model for the treatment of prostate cancer. Cryobiology. 2001 Jun;42(4):274–85. doi: 10.1006/cryo.2001.2333. [DOI] [PubMed] [Google Scholar]
- 59.Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Addition of anticancer agents enhances freezing-induced prostate cancer cell death: implications of mitochondrial involvement. Cryobiology. 2004 Aug;49(1):45–61. doi: 10.1016/j.cryobiol.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Clarke DM, Robilotto AT, VanBuskirk RG, Baust JG, Gage AA, Baust JM. Targeted induction of apoptosis via TRAIL and cryoablation: a novel strategy for the treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2007;10(2):175–84. doi: 10.1038/sj.pcan.4500920. [DOI] [PubMed] [Google Scholar]
- 61.Le Pivert P, Haddad RS, Aller A, Titus K, Doulat J, Renard M, et al. Ultrasound guided combined cryoablation and microencapsulated 5-Fluorouracil inhibits growth of human prostate tumors in xenogenic mouse model assessed by luminescence imaging. Technol Cancer Res Treat. 2004 Apr;3(2):135–42. doi: 10.1177/153303460400300206. [DOI] [PubMed] [Google Scholar]
- 62.Martinez-Montiel MP, Munoz-Yague MT. Biologic therapies for chronic inflammatory bowel disease. Rev Esp Enferm Dig. 2006 Apr;98(4):265–91. doi: 10.4321/s1130-01082006000400006. [DOI] [PubMed] [Google Scholar]