Abstract
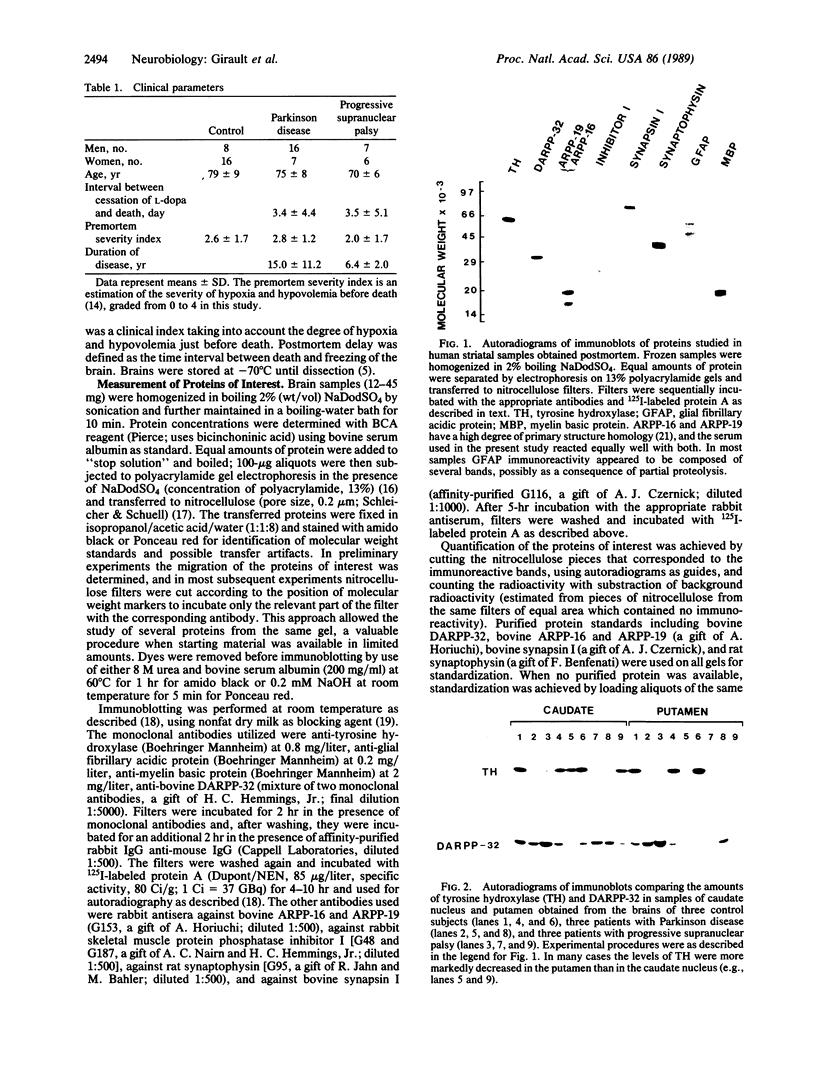
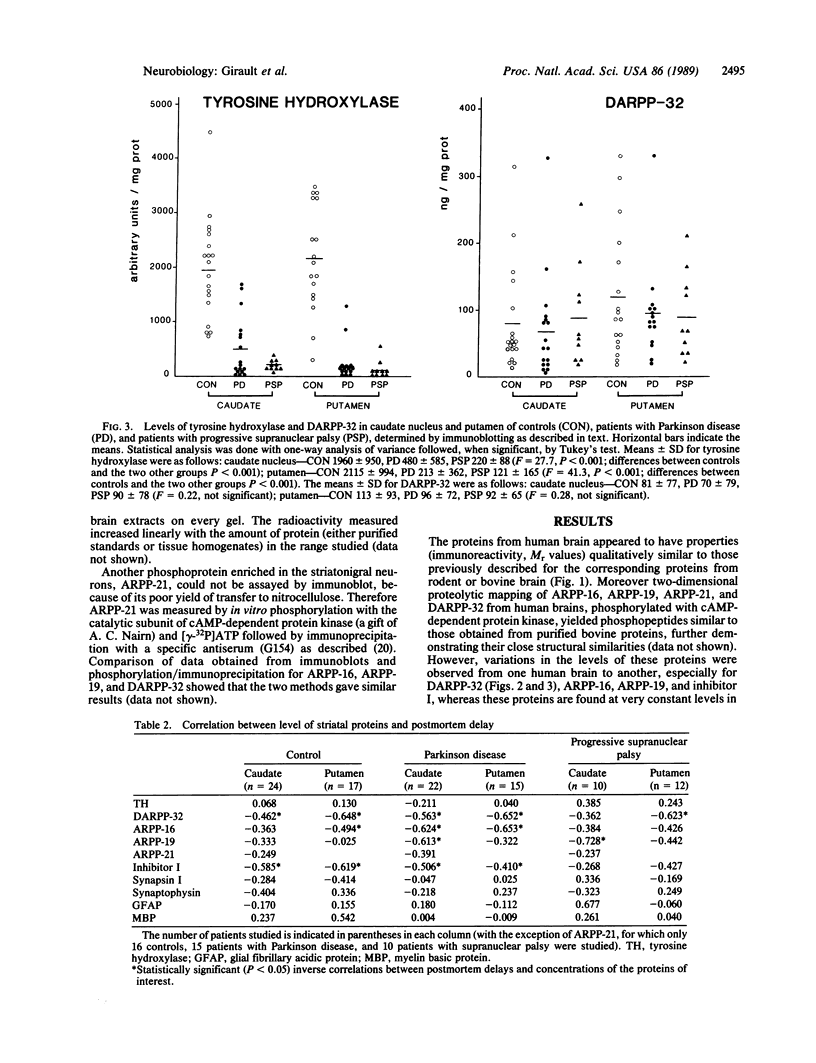
This study was undertaken to evaluate the levels of cAMP-regulated phosphoproteins in the striatum of patients with neurodegenerative diseases of the dopaminergic system. Postmortem samples of caudate nucleus and putamen from 24 control subjects, 23 patients with Parkinson disease, and 13 patients with progressive supranuclear palsy were studied with immunoblotting techniques. The levels of tyrosine hydroxylase were reduced in patients with Parkinson disease (levels were 24% and 10% of controls in caudate nucleus and putamen, respectively) and with progressive supranuclear palsy (levels were 11% and 6% of controls in caudate nucleus and putamen, respectively). Five phosphoproteins, which are present in striatal neurons and are likely to play a role in the postsynaptic actions of dopamine, were measured. These included ARPP-16, ARPP-19, ARPP-21 (cAMP-regulated phosphoproteins of Mr 16,000, 19,000, and 21,000, respectively), DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of Mr 32,000), and phosphatase inhibitor I. The levels of these phosphoproteins were inversely correlated with postmortem delay. In brains of patients with Parkinson disease or progressive supranuclear palsy with postmortem delays comparable to those of controls, the levels of these proteins as well as those of synaptic (synapsin I and synaptophysin) and glial (glial fibrillary acidic protein and myelin basic protein) markers were not significantly modified. We conclude that the levels of several phosphoproteins involved in signal transduction in striatal neurons are not altered in Parkinson disease and progressive supranuclear palsy. This observation supports the view that the striatal output neurons are intact in both diseases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cash R., Raisman R., Ploska A., Agid Y. Dopamine D-1 receptor and cyclic AMP-dependent phosphorylation in Parkinson's disease. J Neurochem. 1987 Oct;49(4):1075–1083. doi: 10.1111/j.1471-4159.1987.tb09996.x. [DOI] [PubMed] [Google Scholar]
- Doucet G., Descarries L., Garcia S. Quantification of the dopamine innervation in adult rat neostriatum. Neuroscience. 1986 Oct;19(2):427–445. doi: 10.1016/0306-4522(86)90272-1. [DOI] [PubMed] [Google Scholar]
- Girault J. A., Shalaby I. A., Rosen N. L., Greengard P. Regulation by cAMP and vasoactive intestinal peptide of phosphorylation of specific proteins in striatal cells in culture. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7790–7794. doi: 10.1073/pnas.85.20.7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein: regional, tissue, and phylogenetic distribution. J Neurosci. 1986 May;6(5):1469–1481. doi: 10.1523/JNEUROSCI.06-05-01469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Monfort J. C., Javoy-Agid F., Hauw J. J., Dubois B., Agid Y. Brain glutamate decarboxylase in Parkinson's disease with particular reference to a premortem severity index. Brain. 1985 Jun;108(Pt 2):301–313. doi: 10.1093/brain/108.2.301. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Hemmings H. C., Jr, Walaas S. I., Greengard P. DARPP-32 and phosphatase inhibitor-1, two structurally related inhibitors of protein phosphatase-1, are both present in striatonigral neurons. J Neurochem. 1988 Jan;50(1):257–262. doi: 10.1111/j.1471-4159.1988.tb13258.x. [DOI] [PubMed] [Google Scholar]
- Navone F., Jahn R., Di Gioia G., Stukenbrok H., Greengard P., De Camilli P. Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. J Cell Biol. 1986 Dec;103(6 Pt 1):2511–2527. doi: 10.1083/jcb.103.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet C. C., Miller P. E., Hemmings H. C., Jr, Walaas S. I., Greengard P. DARPP-32, a dopamine- and adenosine 3':5'-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III. Immunocytochemical localization. J Neurosci. 1984 Jan;4(1):111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyanagi K., Takahashi H., Wakabayashi K., Ikuta F. Selective decrease of large neurons in the neostriatum in progressive supranuclear palsy. Brain Res. 1988 Aug 23;458(2):218–223. doi: 10.1016/0006-8993(88)90464-7. [DOI] [PubMed] [Google Scholar]
- Pierot L., Desnos C., Blin J., Raisman R., Scherman D., Javoy-Agid F., Ruberg M., Agid Y. D1 and D2-type dopamine receptors in patients with Parkinson's disease and progressive supranuclear palsy. J Neurol Sci. 1988 Sep;86(2-3):291–306. doi: 10.1016/0022-510x(88)90106-2. [DOI] [PubMed] [Google Scholar]
- Ruberg M., Javoy-Agid F., Hirsch E., Scatton B., LHeureux R., Hauw J. J., Duyckaerts C., Gray F., Morel-Maroger A., Rascol A. Dopaminergic and cholinergic lesions in progressive supranuclear palsy. Ann Neurol. 1985 Nov;18(5):523–529. doi: 10.1002/ana.410180503. [DOI] [PubMed] [Google Scholar]
- STEELE J. C., RICHARDSON J. C., OLSZEWSKI J. PROGRESSIVE SUPRANUCLEAR PALSY. A HETEROGENEOUS DEGENERATION INVOLVING THE BRAIN STEM, BASAL GANGLIA AND CEREBELLUM WITH VERTICAL GAZE AND PSEUDOBULBAR PALSY, NUCHAL DYSTONIA AND DEMENTIA. Arch Neurol. 1964 Apr;10:333–359. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]





