Abstract
The absence of preexisting immunity against porcine adenovirus (Ad) serotype 3 (PAd3) and bovine Ad serotype 3 (BAd3) in humans makes them attractive alternatives to human Ad serotype 5 (HAd5) vectors. To determine whether there is significant cross-reactivity among HAd5, BAd3, and PAd3 at the level of cell-mediated immune responses, BALB/c mice were inoculated intraperitoneally with wild type (WT) or replication-defective (RD) HAd5, BAd3, or PAd3. Thirty-five days after the first inoculation, cross-reactive CD8+ cytotoxic T cells, as well as CD4+ Th1- and Th2-helper T cells, in the spleen were analyzed by ELISPOT, flow cytometry and cytotoxic T lymphocyte (CTL) assays. Virus neutralization assays were used to evaluate humoral cross-reactivity. CD8+ or CD4+ T cells primed with WT or RD HAd5, PAd3, or BAd3 demonstrated significant (P <0.005) reactivity with homologous Ad antigens, whereas, only minimal cross-reactivity was observed upon stimulation with heterologous Ad antigens. Ad-neutralizing antibodies were found to be homologous Ad-specific. Overall, these results suggest that there is no significant immunological cross-reactivity among HAd5, BAd3, and PAd3, thereby supporting the rationale for the use of BAd3 and PAd3 as alternative HAd vectors to circumvent anti-HAd immunity in humans.
Keywords: Adenoviral vectors, bovine adenovirus, cell-mediated immunity, cross-reactivity, gene therapy, nonhuman adenoviral vectors, porcine adenovirus, preexisting vector immunity
INTRODUCTION
Human adenoviruses (HAds) have been utilized as vehicles for gene transfer for therapeutic or prophylactic purposes. Numerous advantages offered by Ad vectors include the ease of large scale production, well-characterized biology, a broad host range, and their ability to transduce both dividing and non-dividing cells. Vectors based on HAd serotype 5 (HAd5) are currently the most widely studied.1 However, the clinical usefulness of these vectors is limited by preexisting immunity in the human population. HAds are ubiquitous human pathogens that generally cause subclinical to clinical infections and are often associated with activation of both humoral and cell-mediated immune responses. Furthermore, it has been clearly demonstrated that the early region 1 (E1)-deleted replication-deficient (RD) HAd vectors are capable of expressing viral early and late proteins at sufficient levels to stimulate virus-specific humoral and cellular immune responses.2,3 Humoral immunity neutralizes the viral vector and blocks transduction of susceptible cells, while cell-mediated immunity (CMI) is responsible for the elimination of Ad-transduced cells. Together, humoral and cellular immune responses against the Ad vector result in the transient expression of the transgene and necessitate repeat vector administration in most gene therapy protocols. Induction of immune responses following the first administration of the vector dampens the efficacy of subsequent administrations of the similar vector. Sequential administration of antigenically distinct Ad vectors has been proposed to circumvent these limitations.4,5,6
In order to expand the repertoire of Ad vectors, vectors derived from less prevalent HAd serotypes such as HAd3, HAd11, and HAd35 and nonhuman Ads such as bovine Ad (BAd), porcine Ad (PAd), ovine Ad, canine Ad, simian Ad, and fowl Ad, are being developed as alternatives or supplements to HAd5 vectors.7,8 In general, the humoral immune response among various HAd serotypes is serotype-specific9 though some cross-reactivity among Ad subgroups has been reported; however, the cross-reactive antibodies mostly are not virus cross-neutralizing.10 In contrast to humoral immune response, extensive cross-reactivity in HAd-specific cytotoxic T cells has been demonstrated,11,12,13 which is a potential concern for the use of HAd vectors in gene therapy applications. Both CD4+ and CD8+ T-cell responses against Ad in peripheral blood mononuclear cells (PBMCs) from healthy human adults have been demonstrated.11,14,15,16
To circumvent the problem of immunological cross-reactivity among HAd vectors, we explored the potential of vectors derived from nonhuman Ads such as BAd3 and PAd3. As these viruses are phylogenetically distant from HAds and have distinct host specificity, we hypothesized that there will be minimal or low immunological cross-reactivity among nonhuman and human Ads. Earlier, we demonstrated that no preexisting virus cross-neutralizing antibodies against PAd3 or BAd3 were detected in humans, and HAd5-neutralizing antibodies did not cross-neutralize PAd3 or BAd3.4,17 Furthermore, vectors based on PAd3 and BAd3 efficiently transduced several types of human and murine cells in culture17 and their internalization was independent of Coxsackievirus and Ad receptor (CAR), the primary HAd5 receptor. Moreover, HAd5, BAd3 and PAd3 appeared to utilize distinct receptors for cell internalization.18,19,20 Recently, we investigated the biodistribution and persistence of BAd3, PAd3, or HAd5 vectors in a mouse model and observed a distinct biodistribution pattern and prolonged persistence, especially with the BAd3-based vector.21 In this study, we evaluated cross-reactive humoral and cell-mediated immune responses among human, bovine and porcine Ads in a mouse model. Since most of gene therapy or vaccination studies were conducted with RD E1-deleted Ad vectors, we compared wild-type (WT) Ads (HAd5, PAd3, and BAd3) and their corresponding RD vectors (HAdΔE1E3, PAdΔE1E3, and BAdΔE1E3) to investigate the role of E1 deletion on CMI.
MATERIAL AND METHODS
Adenoviral vectors and antigen preparation
WT HAd5, PAd3, or BAd3 and RD HAdΔE1E3 (E1 & E3 deleted),22 PAdΔE1E3 (E1A & E3 deleted),23 or BAdΔE1E3 (E1A & E3 deleted)17 vectors were propagated in 293 (human embryonic kidney cells expressing Ad E1),24 FPRT HE1-5 (fetal porcine retina cells expressing Ad E1)23 and FBRT HE1 (fetal bovine retina cells expressing Ad E1),25 respectively, as described previously. Cells in monolayer cultures were grown in minimum essential medium (MEM) with 10% FetalClone III (Thermo Fisher Scientific, Rockford, IL) and 50 µg/ml gentamicin (Amresco Inc. Solon, OH). The virus purification was done by cesium chloride density gradient centrifugation.22 The infectivity of viruses was estimated by plaque assay on bovine-human hybrid (BHH2C),26 FPRT HE1-5, or FBRT HE1, and virus titers were expressed as plaque forming units (p.f.u.)/ml. For the preparation of Ad and control antigens, 293, FPRT HE1-5 or FBRT HE1 cells were infected with HAdΔE1E3, PAdΔE1E3, or BAdΔE1E3 at a multiplicity of infection (m.o.i.) of 10 p.f.u./cell and incubated in MEM with 2% FetalClone III. The cells were harvested when the infected cell monolayer displayed approximately 90% cytopathic effect (c.p.e.) and suspended in PBS. The harvested cells were subjected to three freeze-thaw cycles and supernatants were collected. Uninfected 293 cell lysates were prepared similarly and used as a control antigen. Ad and control antigens contained in 14 ml polystyrene round-bottom tubes (BD Biosciences, San Jose, CA) were placed on the transilluminator tray of Epi Chemi II Darkroom (UVP,LLC, Upland, CA) and irradiated with 365 nm UV light for 20 min. Protein concentration of each preparation was estimated with Coomassie Protein Assay Reagent (Thermo Fisher Scientific).
Animal inoculation
Eight-to-ten-week-old female BALB/c mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All animal inoculations were conducted in accordance with the guidelines and approval from Institutional Biosafety Committee and Purdue University Animal Care and Use Committee. Mice (5 animals per group) were inoculated intraperitoneally (on Day 0 and Day 21) with HAd5, PAd3, BAd3, HAdΔE1E3, PAdΔE1E3, or BAdΔE1E3 at a dose of 108 p.f.u. per mouse in a volume of 100 µl PBS++ (Phosphate buffer saline supplemented with 0.01 % MgCl2 and 0.01 % CaCl2). In order to increase the chances of identifying cross-reactive cell-mediated immune responses among HAd5, PAd3 and BAd3, it was important to elicit very high levels of immune responses against the homologous Ad. Therefore, two inoculations were done with each virus preparation. Mock-inoculated mice served as negative controls. At day 35, mice were euthanized, and the blood samples and spleens were collected. Spleens were pressed individually through a 70 µm cell strainer (BD Biosciences) using the rubber plunger of a 6 ml syringe. Erythrocytes were lysed with ACK lysing buffer (Lonza, Walkersville, MD) and splenocytes were washed and suspended in 5 ml splenocyte medium [Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 25 mM HEPES, 2mM Glutamine, 10 % FetalClone III, 1 × non-essential amino acids (Invitrogen), 1 × sodium pyruvate (Invitrogen), 50 ng/ml 2-mercaptoethanol (Invitrogen) and 50 µg/ml gentamicin].
CD4+ and CD8+ T cells preparation for ELISPOT and flow cytometry
Splenocytes were processed for purification of CD4+ and CD8+ T cells using immunomagnetic methods as per manufacturer’s protocol (Miltenyi Biotec, Auburn, CA). Briefly, the splenocytes were incubated with CD4 (L3T4) or CD8 (Ly-2) MicroBeads (Miltenyi Biotec) at the concentration of 10 µl per 107 splenocytes for 15 min at 4°C. The cells were then washed with PBS supplemented with 0.5% bovine serum albumin (BSA) fraction V (EMD Chemicals Inc, Gibbstown, NJ) and 2mM EDTA and passed through a LS column (Miltenyi Biotec) placed in a QuadroMACS separator (Miltenyi Biotec) and positively selected CD4+ or CD8+ T cells were eluted out. The purity of purified CD4+ or CD8+ splenocytes was found to be more than 90% as determined by flow cytometry.
Preparation of stimulator cells
As previously described, NIH3T3 cells (syngeneic to BALB/c) were used as stimulator cells.27,28 NIH3T3 cells were infected with HAdΔE1E3, PAdΔE1E3, or BAdΔE1E3 at the m.o.i. of 1,000 p.f.u./cell. Mock-infected NIH3T3 cells were taken as control. At 48 h post-infection, cells were harvested, centrifuged at 200 × g for 5 min, resuspended in 5 ml of MEM with 20 µg of mitomycin C (Sigma-Aldrich, St. Louis, MO) per ml and incubated for 45 min at 37°C. The cells were then thoroughly washed four times with MEM containing 5% heat-inactivated FetalClone III. The number of viable cells were determined by trypan blue dye exclusion method.
Preparation of effector cells
HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3- or mock-primed stimulator cells were seeded in 6-well plates at a density of 5 × 105 cells per well. The CD8+ spleen cells (2.5 × 105) from HAd5-, PAd3-, BAd3-, HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3-, or mock-inoculated mice were layered onto the each type of stimulator cells and incubated for 5 days in splenocyte medium supplemented with IL-2 (50 units/ml). Cells were collected and added to the freshly prepared stimulator cells in 12-well plates in duplicate (one well for flow cytometry and the other well for CTL assay) and cultured for an additional two days. Brefeldin A (eBioscience, San Diego, CA) at a concentration of 3 µg/ml was added into each well 12 h before harvesting the cells for flow cytometry.
CD4+ spleen cells from HAd5-, PAd3-, BAd3-, HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3- or mock-inoculated mice were aliquoted into 12-well plates in quadruplicates at a concentration of 2.5 × 105 cells per well and stimulated with Ad or mock antigen (20 µg/ml) for 20 h in splenocyte medium. Brefeldin A at a concentration of 3 µg/ml was added into each well 12 h before harvesting CD4+ cells for flow cytometry.
Preparation of target cells
HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3- or mock-infected target NIH3T3 cells were prepared using the same protocol as stimulator cell preparation, but the cells were not treated with mitomycin C.
Colorimetric CTL assays
CTL assays were done using a previously described procedure with some modifications.29 Briefly, the effector and target cells were mixed at 5:1 ratio (in triplicates) in 96-well plates and incubated at 37°C for 20 h. Target cells without the effector cells were cultured as a control. After incubation, cells were gently washed with PBS, and 200 µl of 0.036% neutral red solution in PBS was added to stain the unlysed cells for 30 min. Cells were washed twice with PBS and lysed with 250 µl of 0.05M acetic acid-0.05% sodium dodecyl sulfate solution. The amount of the dye released was estimated colorimetrically by measuring the optical density (OD) at 570 nm with Kinetic Microplate Reader (Molecular Devices, Sunnyvale, CA). The results were expressed as a percent lysis of target cells calculated by the formula: % lysis = [(OD of control well − OD of well with effector cells)/OD of control well] × 100.
Enzyme-linked-immunospot (ELISPOT) assays
Ad-specific CD4+ cellular immune responses were assessed by IFNγ (Th1 type) or IL-4 (Th2 type) ELISPOT assays using positively selected CD4+ T cells from HAd5-, PAd3-, BAd3-, HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3- or mock-inoculated mice. ELISPOT assays were done according to a previously described protocol.30 Briefly, 96-well plates (Millipore, Bedford, MA) were precoated overnight with 100 µl/well of 2 µg/ml of purified rat anti-mouse IFNγ or rat anti-mouse IL-4 (BD Biosciences) and were blocked with 1% BSA in PBS. The plates were washed thoroughly with 0.5% tween-20 in PBS (PBS-T) and incubated with CD4+ T cells (2.5 × 105 per well) in triplicates in 100 µl splenocyte medium containing Ad or control antigen at a concentration of 20 µg/ml. After 20 h of incubation, plates were washed and incubated overnight at 4°C with 100 µl of biotinylated rat anti-mouse IFNγ or biotinylated rat anti-mouse IL-4 (BD Biosciences). The plates were washed and incubated for 30 min with 1:5,000 dilution of ExtrAvidin-alkaline phosphatase (Sigma-Aldrich). After 8 washes with PBS-T, the plates were developed with nitro-blue-tetrazolium/5-bromo-4-chloro-3indolyl-phophate chromogen (Sigma-Aldrich). The reaction was stopped using tap water, and plates were air-dried. The numbers of spots were quantified using an ELISPOT Bioreader 5000 (ImmunoBioSystem, The Colony, TX).
IFNγ-ELISPOT assays for CD8+ splenocytes from HAd5-, PAd3-, BAd3-, HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3- or mock-inoculated mice were performed as described above, using 2.5 × 105 CD8+ splenocytes and 5 × 104 HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3- or mock infected target NIH3T3 cells in place of the Ad antigen.
Virus cross-neutralization assays
Serum samples from mice inoculated with HAd5-, PAd3-, BAd3-, HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3-, or mock-inoculated mice were incubated at 56°C for 30 min to inactivate the complement. Neutralizing antibodies against WT HAd5, PAd3 or BAd3 in each serum sample were evaluated as described earlier.23 Briefly, two-fold serial dilutions of each serum sample in 96-well plates were reacted with 100 p.f.u. of HAd5, PAd3 or BAd3 for 1 h at 37°C followed by the addition of 5,000 appropriate cells in each well. HAd5, PAd3, or BAd3 neutralization assay was done in 293, FPRT HE1, or MDBK cells, respectively. The plates were incubated at 37°C for 5–7 days for the development of c.p.e. The virus neutralizing antibody titer was the reciprocal of the highest serum dilution that completely prevented the development of c.p.e.
Flow cytometry
Stimulated CD4+ or CD8+ T cells from HAd5-, PAd3-, BAd3-, HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3- or mock-inoculated mice were harvested, centrifuged and washed with FACS buffer (PBS supplemented with 1% BSA). Cells were preincubated with anti-CD16/CD32 (0.5 µg per 106 cells) (eBioscience) for 10 min on ice to block Fc-receptors. CD4 or CD8 cell surface antigens were stained with fluorescein isothiocyanate (FITC) anti-mouse CD4 (L3T4) or FITC anti-mouse CD8a (Ly-2) (eBioscience), and cells were fixed for 20 min in darkness in a fixation buffer containing 4% paraformaldehyde. For intracellular cytokine staining, cells were washed twice with permeabilization buffer (eBioscience), and then simultaneously stained with phycoerythrin (PE) anti-mouse IL-4 and allophycocyanin (APC) anti-mouse IFNγ (eBioscience). Spleen cells were incubated for 20 min at room temperature in darkness, washed once in permeabilization buffer and finally resuspended in flow cytometry staining buffer (eBioscience). Unstained and single fluorochrome stained cells were included as controls. Cells were gated using forward and side scatter parameters for dead cell exclusion in a BD FACSCanto II flow cytometer (BD biosciences). In each sample, 50,000 events were measured and data analyzed using BD FACSDiva software (BD Biosciences) to determine the percent cytokine producing CD4+ or CD8+ T cells.
Statistical analysis
A two way ANOVA model was used to test the statistical significance between or within the groups. All statistical analyses were applied in PROC GLM with CONTRAST option in (SAS Institute Inc., Cary, NC, USA). For all tests, P < 0.005 was considered significant.
RESULTS
Virus cross-neutralizing antibodies among WT or RD HAd5, BAd3, and PAd3
Before evaluating the cross-reactive CMI among HAd5, PAd3, and BAd3, we reconfirmed our earlier observation that there was an absence of virus-cross-neutralizing antibody responses among these viruses.4,31 Mice were inoculated intraperitoneally on Day 0 and boosted on Day 21 with HAd5, PAd3, BAd3, HAdΔE1E3, PAdΔE1E3, BAdΔE1E3, or phosphate-buffered saline (PBS), and blood samples were collected on Day 35 post-inoculation. The serum samples were tested for their ability to neutralize WT HAd5, PAd3, or BAd3. Highest levels of virus neutralizing antibodies (> 5,000) were detected with homologous virus and antiserum combinations (HAd5 with anti-HAd5 or anti-HAdΔE1E3 serum, PAd3 with anti-PAd3 or anti-PAdΔE1E3 serum, and BAd3 with anti-BAd3 or anti-BAdΔE1E3 serum) (Table 1). Very low levels of virus neutralizing antibody titers (< 30), comparable to the background (PBS control), were observed with heterologous virus and antiserum combinations. Virus-neutralizing antibody titers in the homologous system were slightly higher (though not statistically significant) with WT compared to RD Ads.
Table 1.
Cross-neutralizing serum antibody titers from HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3-, HAd-WT-, PAd-WT-, or BAd-WT-inoculated mice. Serum samples were evaluated for HAd-WT, PAd-WT, or BAd-WT cross-neutralization titers by virus-neutralization assays as mentioned in materials and methods. Values are reported as the average ± standard deviation from five animals per group.
| Ad- inoculated mice groups (N=5) |
Anti-Ad cross-neutralizing antibody titer | ||
|---|---|---|---|
| HAd-WT | PAd-WT | BAd-WT | |
| HAdΔE1E3 | 5120.00 ± 2560.00 | 18.57 ± 10.69 | 17.14 ± 11.13 |
| HAd-WT | 6217.14 ± 2902.77 | 17.14 ± 11.13 | 18.57 ± 10.69 |
| PAdΔE1E3 | 28.57 ± 24.78 | 4022.86 ± 1368.38 | 25.71 ± 13.97 |
| PAd-WT | 27.14 ± 12.54 | 4754.29 ± 2736.76 | 21.43 ± 13.45 |
| BAdΔE1E3 | 25.71 ± 13.97 | 22.86 ± 12.54 | 5120.00 ± 2560.00 |
| BAd-WT | 24.29 ± 11.34 | 22.86 ± 12.54 | 5485.71 ± 2303.32 |
Cross-reactivity of CD4+ T cells among WT or RD HAd5, BAd3, and PAd3
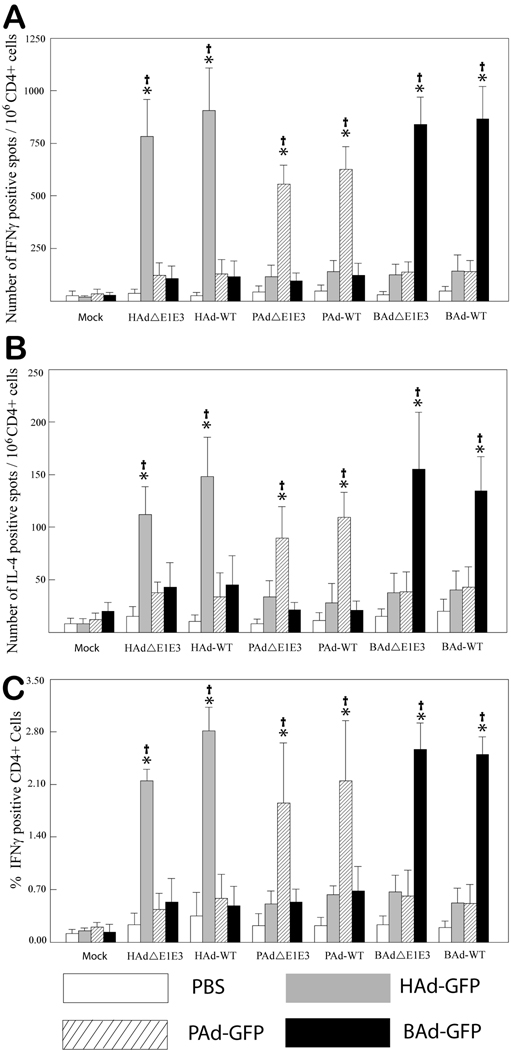
Since CMI is responsible for the elimination of virus-transduced cells, the usefulness of PAd3 or BAd3 vectors for human gene delivery is dependent upon the successful evasion of HAd5-sepcific cellular immunity, in addition to virus-neutralizing antibodies. CD4+ T cells exert their effect via the secretion of cytokines and are classified into Th1 or Th2 subsets. In general, the Th1 type CD4+ T cells secrete cytokines such as IFNγ, IL-2 and TNFα and are responsible for generating strong cellular immune response against virus-transduced cells, while the Th2 type CD4+ T cells produce cytokines such as IL-4, IL-5, IL-6 and IL-10 that promote the proliferation and differentiation of B cells and are associated with humoral immune responses. The positively selected CD4+ T cells from HAd5-, PAd3-, BAd3-, HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3-, or PBS-inoculated mice were stimulated with the UV-inactivated HAd-, PAd-, BAd-, or PBS-infected cell lysate. The Ad-infected cell lysate provided both early regulatory proteins and late structural proteins. Activation of CD4+ T cells (both Th1 and Th2 type) was detected by observing their ability to secrete IFNγ or IL-4 upon stimulation with an antigen lysate. The frequency of antigen-specific CD4+ T cells secreting IFNγ or IL-4 was determined by ELISPOT assays and flow cytometry. In ELISPOT assay, the highest numbers of IFNγ-specific spots were observed following the stimulation of CD4+ T cells with homologous virus antigen (Fig. 1A); these numbers were significantly higher (P < 0.005) compared to those with mock or heterologous antigen stimulation. There was no or minimal stimulation with heterologous antigen compared to mock. Similarly, significantly (P < 0.005) higher numbers of IL-4-specific spots were detected on homologous stimulation compared to those observed with mock or heterologous stimulation (Fig. 1B). Again, there was no or minimal stimulation with heterologous antigen compared to mock. Interestingly, the numbers of IFNγ positive spots with homologous stimulation were approximately 4–6 folds higher as compared to the numbers of IL-4 positive spots following similar antigen stimulation, suggesting the predominance of the Th1 type of immune response.
Figure 1. Cross-reactivity of positively selected CD4+ splenocytes from HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3-, HAd-WT-, PAd-WT-, BAd-WT-, or mock-inoculated mice.
Splenocytes were positively selected with anti-CD4 monoclonal antibody-coated magnetic beads and were stimulated with HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3-, or mock-infected cell lysate for 20 h. The number of cells expressing IFNγ (A) or IL-4 (B) was measured by enzyme-linked immunospot (ELISPOT) assay. (C) The percentage of CD4+ cells expressing IFNγ was measured by flow cytometry assay. Values are reported as the average ± standard deviation for five animals per group. *P < 0.005 versus values at mock stimulation within each treatment group. †P < 0.005 for homologous stimulation versus heterologous stimulation within each treatment group.
The results obtained with ELISPOT assays were further corroborated by flow cytometry. The CD4+ T cells, stimulated in a similar fashion as that for ELISPOT assay, were stained for surface CD4 and intracellular IFNγ or IL-4. Similar to the results obtained with ELISPOT assays, highest frequencies of IFNγ-positive CD4+ T cells were observed following homologous stimulation, which were significantly (P < 0.005) higher compared to those with mock or heterologous stimulation (Fig. 1C). No significant (P > 0.005) differences in the frequencies of IFNγ-positive CD4+ T cells were observed on heterologous stimulation compared to mock stimulation, suggesting no or minimum cross-reactivity in Th1 type CD4+ cells among all three Ads. Both ELISPOT and flow cytometry assays did not show significant (P > 0.005) differences in the frequencies of IFNγ or IL-4 positive CD4+ T cells either from WT or RD HAd5, PAd3, or BAd3 on homologous stimulation. In contrast to the ELISPOT assays, we were unable to detect any IL-4 positive CD4+ T cells by flow cytometry.
Cross-reactivity of CD8+ T cells among WT or RD HAd5, BAd3, and PAd3
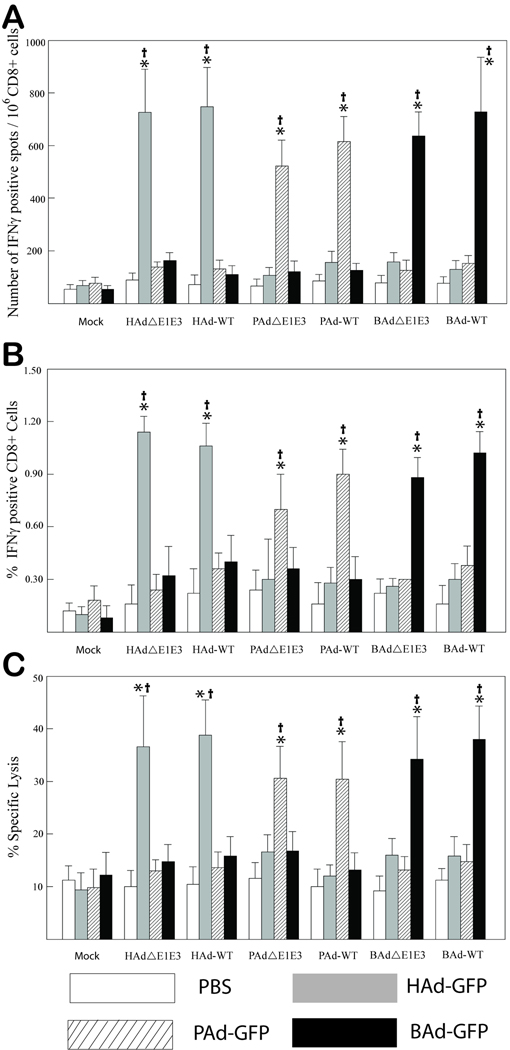
CD8+ T cells are the primary effector cells responsible for the identification and elimination of virus transduced cells.32 In order to evaluate the cross-reactivity among human and non-human Ads, the positively selected CD8+ T cells from HAd5-, PAd3-, BAd3-, HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3-, or PBS-inoculated mice were stimulated with HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3-, or PBS-infected syngeneic NIH3T3 cells, which provided both early and late viral proteins presented via the major histocompatibility complex class 1 (MHCI) molecules. The specific activation of CD8+ T cells was assessed by determining their ability to secrete IFNγ following stimulation with Ad-infected cells and their cytolytic activity against the Ad-infected target cells. The frequency of activated CD8+ T cells was determined by IFNγ ELISPOT assay and flow cytometry, and the functional cytotoxicity was evaluated by their ability to lyse HAdΔE1E3-, PAdΔE1E3-, or BAdΔE1E3-transduced NIH3T3 target cells. In ELISPOT assays, the highest numbers of IFNγ-specific spots were observed upon stimulation of CD8+ T cells with NIH3T3 cells-pulsed with homologous virus; these numbers were significantly (P < 0.005) higher compared to those with mock or heterologous stimulation (Fig. 2A). No significant (P > 0.005) differences in the number of spots obtained on heterologous stimulation compared to mock stimulation were observed, indicating absence of or minimal cross-reactivity of CD8+ T cells among all three Ads.
Figure 2. Cross-reactivity of positively selected CD8+ splenocytes from HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3-, HAd-WT-, PAd-WT-, BAd-WT- , or mock-inoculated mice.
(A) Splenocytes were positively selected with anti-CD8 monoclonal antibody-coated magnetic beads and were stimulated with HAdΔE1E3-, PAd3ΔE1E3-, BAd3ΔE1E3-, or mock-infected and chemically inactivated syngeneic NIH3T3 stimulator cells. The number of cells expressing IFNγ was measured by enzyme-linked immunospot (ELISPOT) assay. (B) The percentage of CD8+ cells expressing IFNγ was measured by flow cytometry. (C) Neutral red uptake cytotoxic T lymphocyte (CTL) assay. Positively selected CD8+ cells from HAdΔE1E3-, PAdΔE1E3-, BAdΔE1E3-, HAd-WT-, PAd-WT-, BAd-WT-, or mock-inoculated mice were stimulated with HAdΔE1E3-, PAd3ΔE1E3-, BAd3ΔE1E3-, or mock-infected and chemically inactivated syngeneic NIH3T3 stimulator cells for 7 days. The target cells were HAdΔE1E3-, PAd3ΔE1E3-, BAd3ΔE1E3-, or mock-infected NIH3T3 cells. The effector to target cell ratio was 5:1. Values are reported as the average ± standard deviation for five animals per group. *P < 0.005 versus values at mock stimulation within each treatment group. †P <0.005 for homologous stimulation versus heterologous stimulation within each treatment group.
Flow cytometry data were consistent with the ELISPOT results. Highest frequencies of IFNγ-positive CD8+ T cells were detected upon stimulation with homologous viral antigen (Fig. 2B). The frequency of activated CD8+ T cells upon homologous stimulation was significantly (P < 0.005) higher compared to that with mock or heterologous stimulation. No significant (P >0.005) differences in the frequency of IFNγ-positive CD8+ T cells were observed on heterologous stimulation compared to mock stimulation.
To determine whether CTLs induced by WT or RD HAd5, PAd3, or BAd3 were cross-reactive, CTLs were tested for their ability to lyse target NIH3T3 cells transduced with HAdΔE1E3, PAdΔE1E3, or BAdΔE1E3. Significant (P < 0.005) lysis of the virus-infected NIH3T3 target cells was observed on incubation with homologous CTLs, whereas, incubation with heterologous CTLs resulted in the absence of or minimal lysis of target cells compared to mock controls (Fig. 2C). ELISPOT, flow cytometry and CTL assays did not detect significant (P > 0.005) differences in the activation of IFNγ-positive CD8+ T cells either from WT or RD HAd5, PAd3, or BAd3 upon homologous stimulation.
DISCUSSION
Clinical usefulness of HAd-based vectors is somewhat hampered due to the high prevalence of anti-HAd5 immunity in the human population. To circumvent this limitation, vectors derived from rare HAd serotypes or Ads from nonhuman species are being investigated as alternate vectors. While most of the Ad-specific antisera do not cross-neutralize Ads from different subgroups, extensive cross-reactivity of cellular immunity among heterologous Ad serotypes has been reported which may hinder the “sero-switch” strategy.11,12,13 In this study, we evaluated the degree of cross-reactivity of immune responses among HAd5, PAd3, and BAd3. We demonstrated that WT or RD HAd5, PAd3, and BAd3 elicit potent humoral as well as cellular immune responses which are mostly homologous Ad-specific.
Though hyperimmune HAd5-, PAd3- or BAd3-specific serum raised in rabbits or mice has been shown to have significant cross-reacting ELISA antibodies among HAd5, PAd3, or BAd3, these antisera could neutralize only the homologous Ad.4 Consistent with previous observations, sera from mice inoculated with WT or RD HAd5, PAd3, or BAd3 showed exceptionally high neutralizing antibody titers only in homologous virus-antiserum combinations, suggesting a significant variation in virus neutralizing epitopes among these Ads. Although the absence of cross-neutralizing antibodies among HAd5, PAd3 and BAd3 is encouraging, the presence of cross-reactive, non-neutralizing antibodies could impair the in-vivo efficacy of Ad vectors.33,34 Systemic administration of Ad vectors in individuals with cross-reactive humoral immunity may result in the formation of immune complexes and activation of complement pathways. Furthermore, opsonizing antibodies may mediate antibody-dependent cellular cytotoxicity and phagocytosis via natural killer cells or macrophages. Additional in vivo assays are needed to determine if these observations are applicable to PAd3 or BAd3.
The presence of T cell responses against epitopes primarily present in the hexon as well as in the fiber, penton or E1A proteins of Ad has been documented in humans or mice.35,36,37,38 Some of the epitopes are highly conserved among different Ad serotypes and are responsible for immunological cross-reactivity.11,12,13,14,39 Structural and phylogenetic analyses of the hexon protein show evolutionary relationships of human and nonhuman Ads.40 Minimal levels of cellular cross-reactivity observed in the current study may be an indication of some cross-reactive epitopes among HAd5, PAd3, and BAd3.
In contrast to high frequencies of IFNγ-secreting CD4+ T cells on a homologous stimulation, fewer IL-4 secreting CD4+ T cells were detected by ELISPOT assay. There was no significant increase in IL-4-positive CD4+ T cells above background levels by flow cytometry. These results, consistent with earlier reports, support the predominance of a Th1 type of cell response following Ad inoculation.14,41,42
In this study, the induction of neutralizing antibodies and the activation of T-cell responses by either WT or RD human or nonhuman Ad vectors were comparable. This suggested that the development of immune responses was mainly against the antigens expressed by both WT and RD Ads. Most of the Ad vectors used in gene therapy are RD because of deletions in the E1 region, which is essential for viral replication and transcriptional activation of other viral genes. Leaky expression of viral early and late genes by the first generation (E1-deleted) Ad vectors was most likely responsible for the activation of CMI.2,3 The HAd5, PAd3 and BAd3 can efficiently transduce many cell lines of murine origin,17 however, these viruses do not replicate in murine cell lines or in mice.43 (data not shown).
Cellular immunity targeting the transgene-encoded epitopes has also been reported to be responsible for both the elimination of transduced cells and for limiting the duration of transgene expression.35,44,45,46 Such transgene-specific immune responses will depend on the nature and antigenicity of the transgene-encoded protein. In the current study, RD HAdΔE1E3, PAdΔE1E3, or BAdΔE1E3 vectors with or without a transgene were evaluated.
Re-administration of the same vector is limited in efficacy because of the generation of vector-specific immune responses. Since HAd5-, PAd3- and BAd3-specific antibodies were not cross-neutralizing and the cellular immunity did not show significant cross-reactivity, it is hypothesized that sequential administration of these vectors will be effective. Consistent with this hypothesis, our recent study showed that a BAd3 vector-based vaccine in BALB/c mice can overcome exceptionally high levels of preexisting anti-HAd5 immunity to generate similar levels of humoral and cell-mediated immune responses against the transgene.31 This also suggests that the cross-reactive but non-neutralizing antibodies have minimal role in affecting the in vivo efficacy of BAd3-based vectors.
The absence of significant cross-reactivity in virus-neutralizing antibodies and/or CMI among HAd5, PAd3, and BAd3 in mouse models may not necessarily reflect the situation in humans. Cellular immune responses to Ad vectors in genetically heterogeneous human populations may be influenced by the presence of diverse MHC alleles capable of presenting different viral antigens. While future clinical trials in humans are needed to determine whether these observations are applicable to humans, the presence of low levels of immunological cross-reactivity among HAd5, PAd3, or BAd3 indicates the potential usefulness of nonhuman Ad-derived vectors for evasion of preexisting anti-HAd5 immunity.
ACKNOWLEDGEMENTS
This work was supported by Public Health Service grant CA110176 from the National Cancer Institute. We are thankful to Jane Kovach for her excellent secretarial assistance and Ching-Yun Chang for help with statistical analyses.
REFERENCES
- 1.Edelstein ML, Abedi MR, Wixon J, Edelstein RM. Gene therapy clinical trials worldwide 1989– 2004-an overview. J Gene Med. 2004;6:597–602. doi: 10.1002/jgm.619. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moffatt S, Hays J, HogenEsch H, Mittal SK. Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: implications in gene therapy. Virology. 2000;272:159–167. doi: 10.1006/viro.2000.0350. [DOI] [PubMed] [Google Scholar]
- 5.Morral N, O'Neal W, Rice K, Leland M, Kaplan J, Piedra PA, et al. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci USA. 1999;96:12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mastrangeli A, Harvey BG, Yao J, Wolff G, Kovesdi I, Crystal RG, et al. "Sero-switch" adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum Gene Ther. 1996;7:79–87. doi: 10.1089/hum.1996.7.1-79. [DOI] [PubMed] [Google Scholar]
- 7.Bangari DS, Mittal SK. Development of nonhuman adenoviruses as vaccine vectors. Vaccine. 2006;24:849–862. doi: 10.1016/j.vaccine.2005.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone D, Lieber A. New serotypes of adenoviral vectors. Curr Opin Mol Ther. 2006;8:423–431. [PubMed] [Google Scholar]
- 9.Wold WS, Horwitz MS. Adenoviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Walters Kluwer Health-Lippincott Williams & Wilkins; 2007. pp. 2395–2436. [Google Scholar]
- 10.Bauer U, Flunker G, Bruss K, Kallwellis K, Liebermann H, Luettich T, et al. Detection of antibodies against adenovirus protein IX, fiber, and hexon in human sera by immunoblot assay. J Clin Microbiol. 2005;43:4426–4433. doi: 10.1128/JCM.43.9.4426-4433.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakay M, Szalay K, Beladi I, Balint E, Lengyel A, Adam E, et al. Cross-reactivity between human adenoviruses in delayed-type hypersensitivity. APMIS. 2005;113:197–202. doi: 10.1111/j.1600-0463.2005.apm1130307.x. [DOI] [PubMed] [Google Scholar]
- 12.Heemskerk B, Veltrop-Duits LA, van VT, ten Dam MM, Heidt S, Toes RE, et al. Extensive cross-reactivity of CD4+ adenovirus-specific T cells: implications for immunotherapy and gene therapy. J Virol. 2003;77:6562–6566. doi: 10.1128/JVI.77.11.6562-6566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith CA, Woodruff LS, Rooney C, Kitchingman GR. Extensive cross-reactivity of adenovirus-specific cytotoxic T cells. Hum Gene Ther. 1998;9:1419–1427. doi: 10.1089/hum.1998.9.10-1419. [DOI] [PubMed] [Google Scholar]
- 14.Olive M, Eisenlohr LC, Flomenberg P. Quantitative analysis of adenovirus-specific CD4+ T-cell responses from healthy adults. Viral Immunol. 2001;14:403–413. doi: 10.1089/08828240152716646. [DOI] [PubMed] [Google Scholar]
- 15.Flomenberg P, Piaskowski V, Truitt RL, Casper JT. Characterization of human proliferative T cell responses to adenovirus. J Infect Dis. 1995;171:1090–1096. doi: 10.1093/infdis/171.5.1090. [DOI] [PubMed] [Google Scholar]
- 16.Flomenberg P, Piaskowski V, Truitt RL, Casper JT. Human adenovirus-specific CD8+ T-cell responses are not inhibited by E3-19K in the presence of gamma interferon. J Virol. 1996;70:6314–6322. doi: 10.1128/jvi.70.9.6314-6322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bangari DS, Shukla S, Mittal SK. Comparative transduction efficiencies of human and nonhuman adenoviral vectors in human, murine, bovine, and porcine cells in culture. Biochem Biophys Res Commun. 2005;327:960–966. doi: 10.1016/j.bbrc.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Bangari DS, Sharma A, Mittal SK. Bovine adenovirus serotype 3 utilizes sialic acid as a cellular receptor for virus entry. Virology. 2009;392:162–168. doi: 10.1016/j.virol.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bangari DS, Mittal SK. Porcine adenovirus serotype 3 internalization is independent of CAR and alpha(v)beta(3) or alpha(v)beta(5) integrin. Virology. 2005;332:157–166. doi: 10.1016/j.virol.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Bangari DS, Sharma A, Mittal SK. Bovine adenovirus type 3 internalization is independent of primary receptors of human adenovirus type 5 and porcine adenovirus type 3. Biochemical and Biophysical Research Communications. 2005;331:1478–1484. doi: 10.1016/j.bbrc.2005.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma A, Bangari DS, Tandon M, Pandey A, HogenEsch H, Mittal SK. Comparative analysis of vector biodistribution, persistence and gene expression following intravenous delivery of bovine, porcine and human adenoviral vectors in a mouse model. Virology. 2009;386:44–54. doi: 10.1016/j.virol.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noblitt LW, Bangari DS, Shukla S, Knapp DW, Mohammed S, Kinch MS, et al. Decreased tumorigenic potential of EphA2-overexpressing breast cancer cells following treatment with adenoviral vectors that express EphrinA1. Cancer Gene Therapy 11(11):757–766, 2004 Nov. 2004:757–766. doi: 10.1038/sj.cgt.7700761. [DOI] [PubMed] [Google Scholar]
- 23.Bangari DS, Mittal SK. Porcine adenoviral vectors evade preexisting humoral immunity to adenoviruses and efficiently infect both human and murine cells in culture. Virus Res. 2004;105:127–136. doi: 10.1016/j.virusres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 25.van Olphen AL, Tikoo SK, Mittal SK. Characterization of bovine adenovirus type 3 E1 proteins and isolation of E1-expressing cell lines. Virology. 2002;295:108–118. doi: 10.1006/viro.2002.1389. [DOI] [PubMed] [Google Scholar]
- 26.van Olphen AL, Mittal SK. Development and characterization of bovine x human hybrid cell lines that efficiently support the replication of both wild-type bovine and human adenoviruses and those with E1 deleted. J Virol. 2002;76:5882–5892. doi: 10.1128/JVI.76.12.5882-5892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 28.Vogels R, Zuijdgeest D, van RR, Hartkoorn E, Damen I, de Bethune MP, et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol. 2003;77:8263–8271. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parish CR, Mullbacher A. Automated colorimetric assay for T cell cytotoxicity. J Immunol Methods. 1983;58:225–237. doi: 10.1016/0022-1759(83)90277-6. [DOI] [PubMed] [Google Scholar]
- 30.Sambhara S, Switzer I, Kurichh A, Miranda R, Urbanczyk L, James O, et al. Enhanced antibody and cytokine responses to influenza viral antigens in perforin-deficient mice. Cell Immunol. 1998;187:13–18. doi: 10.1006/cimm.1998.1314. [DOI] [PubMed] [Google Scholar]
- 31.Singh N, Pandey A, Jayashankar L, Mittal SK. Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of pre-existing immunity against Human Adenovirus. Mol Ther. 2008;16:965–971. doi: 10.1038/mt.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doherty PC, Turner SJ. Memories of virus-specific CD8+ T cells. Immunol Cell Biol. 2004;82:136–140. doi: 10.1046/j.0818-9641.2004.01241.x. [DOI] [PubMed] [Google Scholar]
- 33.Pichla-Gollon SL, Lin SW, Hensley SE, Lasaro MO, Herkenhoff-Haut L, Drinker M, et al. Effect of preexisting immunity on an adenovirus vaccine vector: in vitro neutralization assays fail to predict inhibition by antiviral antibody in vivo. J Virol. 2009;83:5567–5573. doi: 10.1128/JVI.00405-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perreau M, Kremer EJ. The conundrum between immunological memory to adenovirus and their use as vectors in clinical gene therapy. Mol Biotechnol. 2006;34:247–256. doi: 10.1385/MB:34:2:247. [DOI] [PubMed] [Google Scholar]
- 35.Jooss K, Ertl HC, Wilson JM. Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. J Virol. 1998;72:2945–2954. doi: 10.1128/jvi.72.4.2945-2954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molinier-Frenkel V, Lengagne R, Gaden F, Hong SS, Choppin J, Gahery-Segard H, et al. Adenovirus hexon protein is a potent adjuvant for activation of a cellular immune response. J Virol. 2002;76:127–135. doi: 10.1128/JVI.76.1.127-135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rawle FC, Knowles BB, Ricciardi RP, Brahmacheri V, Duerksen-Hughes P, Wold WS, et al. Specificity of the mouse cytotoxic T lymphocyte response to adenovirus 5. E1A is immunodominant in H-2b, but not in H-2d or H-2k mice. J Immunol. 1991;146:3977–3984. [PubMed] [Google Scholar]
- 38.McKelvey T, Tang A, Bett AJ, Casimiro DR, Chastain M. T-cell response to adenovirus hexon and DNA-binding protein in mice. Gene Ther. 2004;11:791–796. doi: 10.1038/sj.gt.3302232. [DOI] [PubMed] [Google Scholar]
- 39.Olive M, Eisenlohr L, Flomenberg N, Hsu S, Flomenberg P. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum Gene Ther. 2002;13:1167–1178. doi: 10.1089/104303402320138952. [DOI] [PubMed] [Google Scholar]
- 40.Rux JJ, Kuser PR, Burnett RM. Structural and phylogenetic analysis of adenovirus hexons by use of high-resolution x-ray crystallographic, molecular modeling, and sequence-based methods. J Virol. 2003;77:9553–9566. doi: 10.1128/JVI.77.17.9553-9566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 42.Perreau M, Kremer EJ. Frequency, proliferation, and activation of human memory T cells induced by a nonhuman adenovirus. J Virol. 2005;79:14595–14605. doi: 10.1128/JVI.79.23.14595-14605.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duncan SJ, Gordon FC, Gregory DW, McPhie JL, Postlethwaite R, White R, et al. Infection of mouse liver by human adenovirus type 5. J Gen Virol. 1978;40:45–61. doi: 10.1099/0022-1317-40-1-45. [DOI] [PubMed] [Google Scholar]
- 44.Tripathy SK, Black HB, Goldwasser E, Leiden JM. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Haecker SE, Su Q, Wilson JM. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum Mol Genet. 1996;5:1703–1712. doi: 10.1093/hmg/5.11.1703. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Jooss KU, Su Q, Ertl HC, Wilson JM. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3:137–144. [PubMed] [Google Scholar]