Abstract
The ability to safely control transgene expression from viral vectors is a long-term goal in the gene therapy field. We have previously reported tight regulation of GFP expression in rat brain using a self-regulating tet-off rAAV vector. The immune responses against tet regulatory elements observed by other groups in nonhuman primates after intramuscular injection of tet-on encoding vectors raise concerns about the clinical value of tet-regulated vectors. However, previous studies have not examined immune responses following injection of AAV vectors into brain. Therefore, rat striatum was injected with tet-off rAAV harboring a therapeutic gene for Parkinson's disease, either hAADC or hGDNF. The expression of each gene was tightly controlled by the tet-off regulatory system. Using an ELISA developed with purified GST-tTA protein, no detectable immunogenicity against tTA was observed in sera of rats that received an intrastriatal injection of either vector. In contrast, sera from rats intradermally injected with an adenovirus containing either tTA or rtTA, as positive controls, had readily detectable antibodies. These observations suggest that tet-off rAAV vectors do not elicit an immune response when injected into rat brain and that these may offer safer vectors for Parkinson's disease than vectors with constitutive expression.
Keywords: Parkinson's disease, gene therapy, viral vectors, regulated gene expression, tTA, rtTA
Introduction
Parkinson's disease (PD) is a neurodegenerative disorder caused by loss of dopaminergic neurons in the substantia nigra pars compacta and a subsequent decrease in striatal dopamine (DA) levels with associated motor deficiencies. L-dopa is converted to DA by AADC (amino-acid decarboxylase) and has long been used to treat PD. However, as AADC levels decrease with progressive loss of DA neurons, L-dopa therapy becomes less effective and higher doses are needed to control symptoms, which may lead to debilitating dyskinesias. Currently, a clinical trial using rAAV (recombinant adeno-associated virus) as a gene therapy approach to deliver human AADC (hAADC) for PD patients is underway 1. This trial is based on behavioral improvement in rat and non-human primate models of PD 2,3. Another therapeutic gene therapy candidate for PD is GDNF (glial cell line-derived neurotrophic factor). GDNF gene delivery has been shown to protect DA neurons from neurodegeneration in rat and primate models of PD 4-8. On the other hand, undesirable side effects have been observed in trial of GDNF protein administration in PD patients 9 and continuous GDNF over-expression in rodents delivered by adenovirus (Ad) vectors 10. In addition, monkeys with over-expression of tyrosine hydroxylase (TH) and AADC in the context of a constitutively active AAV vector showed transient fever and motor hyperactivity, which is similar to symptoms induced by high doses of dopamine agonists 11. In order for gene therapy to be widely used for neurological disorders, it will be important to develop safer vectors that offer rescue approaches against unexpected, deleterious side effects.
A number of approaches have been considered to control transgene expression in brain by viral delivery. Tissue-specific promoters confine transgene expression to specific cell types, showing superior transduction efficiency and safety over the CMV (cytomegalovirus) promoter in rodent brain delivered by viral vectors, such as PDGF-β (platelet-derived growth factor-β), GFAP (glial fibrillary acidic protein), and NSE (neuron specific enolase) promoters 12-15. Alternatively, several regulated promoter systems have been developed to control transgene expression in vivo in the context of various viral backbones, including systems based on rapamycin 16,17, mifepristone 18, tetracycline 19 and ecdysone 18.
The tet system, which was originally developed by Gossen and Bujard 19, has proved to be efficient and reliable in controlling transgene expression in experimental models of neurological diseases 20-27. The tet regulatory systems consist of two components: the transactivator, tTA, or reverse transactivator, rtTA, and the tet-regulated element (TRE). The chimeric tTA fusion protein is comprised of the 23 kDa tet repressor (tetR) of Escherichia coli and a herpes simplex viral protein activation domain, VP16 (14 kDa). The TRE was created by fusing 7 repeats of the tet resistance operator (tetO) binding site with a minimal CMV promoter. In the tet-off system, tTA binds to TRE and induces transgene expression in the absence of tet or the tet analog doxycycline (dox). In this system, in the presence of dox, tTA binds to dox and detaches from TRE resulting in gene expression inhibition. In the tet-on system, gene expression is normally off unless dox is present. Dox stimulates binding of the reverse transactivator rtTA, which has 4 point mutations in the tetR domain, to the TRE 28.
Despite excellent regulation of gene expression with the tet systems in viral vectors, recent studies suggest that an immune response is elicited by rtTA after intramuscular delivery by plasmid, recombinant Ad or rAAV into non-human primates, resulting in the rapid loss of transgene expression 29-31. The two epitopes of rtTA that are involved in stimulating the cellular immune response, rtTA186 (FLEGLELII) and rtTA119 (FLCQQGFSL) 32, are also present in tTA. Most of the human population has been exposed to herpes simplex virus 33 and, thus, may have circulating antibodies against the VP16 portion of the tTA, which may block transgene expression and even lead to some side effects. However, the immune reaction in brain is substantially different from that in other organs, as it is an immune-privileged site 34. On the other hand, reports of immune responses against viral vectors have been reported following injection into the brain 35-37. Other studies have reported that no immune responses against tTA or rtTA were observed in rats and macaque injected with AAV vector containing tet regulatory elements into retina, another immune-privileged site 38-40. Therefore, tet-off AAV self-regulated vectors may be safe for clinical use in these tissues. The aims of this study were to directly test the humoral immune response against tTA following injection of a tet-regulated AAV regulated vector into rat brain and to evaluate the expression and regulation by dox of two therapeutic genes for Parkinson disease, hAADC and hGDNF.
Results
Tight regulation of hAADC or hGDNF expression in rats with intrastriatal injections of rAAVS3-hAADC or rAAVS3-hGDNF
To test whether the expression of hAADC or hGDNF could be tightly regulated by dox in vivo, rats received an intrastriatal injection of hAADC or hGDNF in the context of an AAV vector under the control of a tet-off self-regulated promoter, termed rAAVS3-hAADC or rAAVS3-hGDNF. Half of the rats were maintained on regular water to allow maximal expression of hAADC or hGDNF, while the other half was maintained on dox containing water (1 mg/ml) to suppress transgene expression. The striatae from both sides were removed to evaluate the expression of hAADC or hGDNF.
Expression of hAADC protein was evaluated by immunohistochemistry. Three weeks after injection, numerous cells expressing hAADC (10,107 ± 4016 cells, n=4) were observed in the “hAADC on” group, in which the rats were maintained on regular water (Figure 1A). The number of hAADC positive cells was decreased by ∼80% in the “hAADC off” group when AADC expression was turned off by dox ingestion (1,930 ± 214 cells, n=4, P<0.001, Figure 1B).
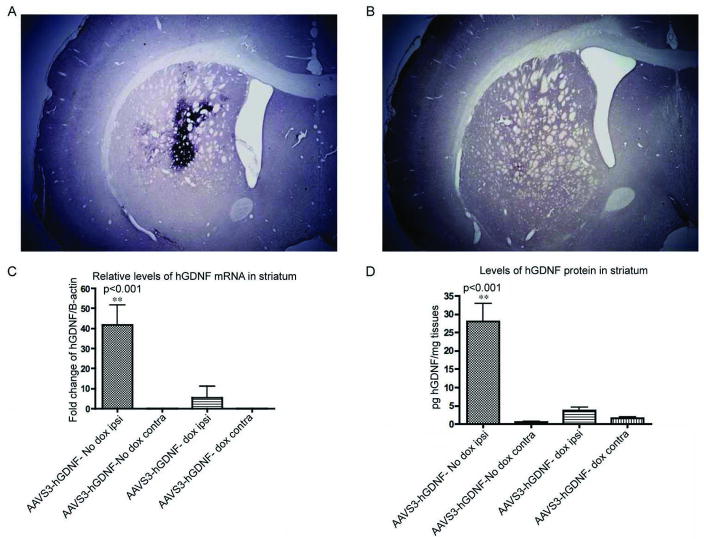
Figure 1. Expression of hAADC and hGDNF was tightly regulated by dox in rats that received an intrastriatal injection of either rAAV2S3hAADC or rAAV2S3hGDNF.
The representative immunohistochemical images show high hAADC expression in the striatum of rats maintained on the normal water (A) and low hAADC expression in rats maintained on the water containing dox (B). (C). Relative mRNA levels of hGDNF in striatum evaluated by qRT-PCR. The rats of rAAVS3-hGDNF in “on” state showed the highest expressional levels of hGDNF, which was significantly higher than the “hGDNF off” group (P<0.001). (D). hGDNF protein in the striatum measured by ELISA. The rats injected with rAAVS3-hGDNF maintained on normal water showed the highest levels of hGDNF protein on the ipsilateral side, which was significantly higher than that in the “hGDNF off” group (P<0.001).
The mRNA expression levels of hGDNF were assessed by qRT-PCR using total RNA isolated from the striatum on the viral vector injected and non-injected sides. Relative quantitation was performed using the mRNA levels of the endogenous control β-actin to normalize the target gene hGDNF RNA levels. hGDNF mRNA levels in the “hGDNF on” group (n=4) were 9.4 fold higher than those in the “off” group (n=5) at 5 weeks post injection (P<0.001). The signal for hGDNF mRNA in the non-injected side was below the detectable threshold (Figure 1C).
The amount of hGDNF protein in the striatum was evaluated by ELISA 5 weeks after injection. The highest level of hGDNF was observed in the hGDNF group not receiving dox, allowing hGDNF to be in the “on” state (28.06 ± 11.88 pg/mg tissue). In rats administered dox in the drinking water as well as in the striatae contralateral to the injection site, the amount of hGDNF was at or just above the level of detection (P<0.001, Figure 1D).
Purification of GST-tTA fusion protein from E.coli
Glutathione S-transferase (GST)-tTA fusion protein was purified to evaluate the immune response against the tet regulatory transactivator (tTA or rtTA). The coding region of tTA was obtained by PCR from the ptet-off plasmid. tTA fused with GST was expressed in E. coli BL21 (DE3) cells after cloning into pGEX-6P. A promoter inducible by isopropyl β-D-thiogalactoside (IPTG) controls the production of the fusion protein in the pGEX expression system. The induced GST-tTA was visualized by use of Coomassie blue staining on an SDS-PAGE gel (Figure 2B, lane 2). GST-tTA protein was purified by lysis of freeze-thawed bacterial pellets followed by incubation with the affinity column. Two bands were observed on the gel, with molecular masses of 63 and 50 kDa (Figure 2B, lane 5). Western blotting showed that both bands were recognized by the anti-tetR antibody (Figure 2B). As shown in Figure 2A, the molecular size of GST-tTA is 63 kDa, while the size of GST-tetR, which lacks the VP16 domain, is 49 kDa. This suggested that the lower molecular weight band was due to cleavage of the VP16 domain in the majority of purified protein. To address this possibility, every step in the purification was tracked (Figure 2B), which revealed that the GST-tTA fusion protein was cleaved when the frozen bacterial pellets were dissolved in PBS (phosphate buffered saline) buffer (Figure 2B, Lane 3 and 5). This cleavage was drastically reduced when fresh bacterial pellets were used (Figure 2A, Lane 4 and 6), and the full length purified protein (63 kDa) remained stable after many freeze-thaw cycles (data not shown). These purified proteins were used in the following experiments.
Figure 2. GST-tTA fusion protein purification.
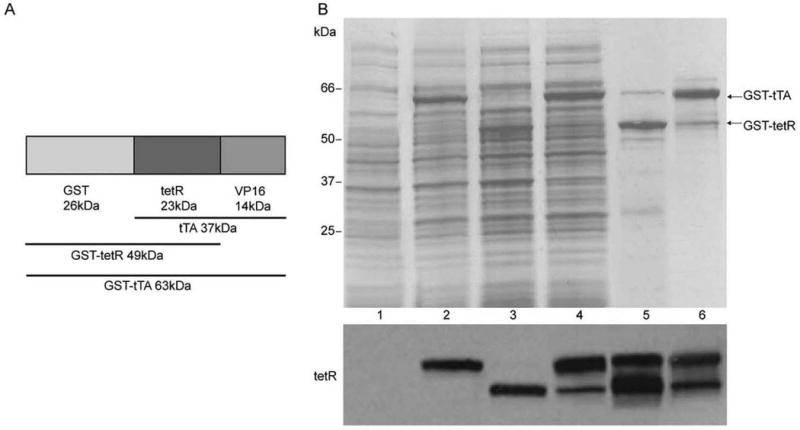
(A). Schematic map of the molecular mass of GST-tTA fusion protein. tTA is comprised of tet repressor protein (tetR) and a transactivator domain, VP16. (B). Upper panel: bacterial pellets transformed with pGEX-tTA were subjected to 4-15% SDS–PAGE followed by staining with Coomassie blue. Lane 1, culture lysate without IPTG induction. Lane 2, culture lysate with 1hr IPTG induction. Lane 3, cell lysate were frozen at -80°C overnight followed by thaw in PBS. Lane 4, fresh cell lysate dissolved in PBS. Lane 5, purified GST-tTA using freeze-thawed pellets. The top band is GST-tTA and the bottom band is GST-tetR, showing that the majority of the purified fusion proteins are broken down. However, the cleavage was significantly decreased using fresh bacterial pellets. The majority of the purified fusion proteins are GST-tTA (Lane 6). Bottom panel: western blot results detected by anti-tetR antibodies.
Establishment of an ELISA to evaluate the immunogenicity to tTA
To test the humoral immune response to tTA, an ELISA was developed. Wells were coated with 25 μg/ml of the purified GST-tTA protein, incubated with serial dilutions of anti-tetR antibody and HRP-conjugated secondary antibodies, and then detected using tetramethylbenzidine (TMB). GST alone at the same concentration was used as a negative control. The minimal and maximal detectable antibody concentrations against tetR were found to be 0.3 μg/ml and 15 μg/ml, respectively (data not shown). To test the ELISA, we first intradermally injected rAAV2S3-hrGFP (humanized renilla green fluorescent protein) into rats and then sera were collected 4 weeks after injection and analyzed by ELISA. No detectable antibodies were observed in the rAAVS3-hrGFP group (Figure 3). Next sera and plasma from rats intradermally injected with Ad containing the tet-off (Ad5BIE/SV40tTA) or tet-on (Ad-tet-on) system were collected. As shown in Figure 3, sera from 1 out of 3 rats that received Ad5BIE/SV40tTA showed a detectable level of anti-tTA (P<0.01), and plasma showed similar results (data not shown). The highest level of anti-tTA antibodies was observed in the sera from rats that were injected with Ad-tet-on (P<0.001, Figure 3).
Figure 3. Positive controls to verify the anti-tetR ELISA.
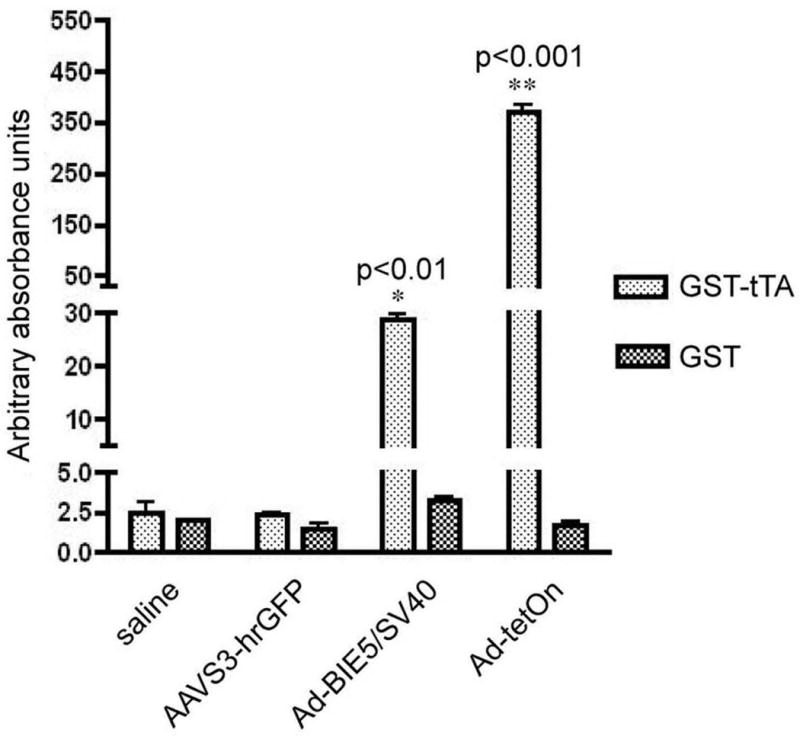
The positive controls included sera from rats that received intradermal injection of rAAV2S3hrGFP or an Ad containing either tet-off (AdBIE5/SV40) or tet-on (Ad-tet-on). The sera were collected at 4 weeks after injection. No detectable antibodies were observed in sera from any of the rats injected with rAAV2S3hrGFP. However, sera from 1 out of 3 rats that received AdBIE5/SV40 vector showed a detectable level of anti-tTA (P<0.01). The peak level was observed in the sera from rats that were injected intradermally with Ad-tet-on (P<0.001).
Lack of humoral immune response against tTA after intracranial injection of AAV vector haboring therapeutic genes and tet-off system
Humoral immune responses against tTA were assessed in rats that received intrastriatal injections of either rAAV2S3-hAADC or rAAV2S3-hGDNF using the ELISA developed above. The plasma was collected via cardiac puncture at 9 weeks post injection of rAAVS3-hAADC and 5 weeks after injection of rAAVS3-hGDNF.
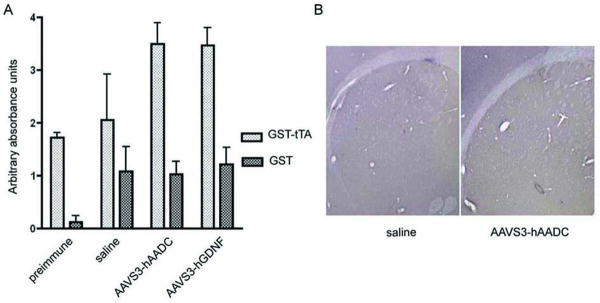
No detectable antibodies against tTA were observed in plasma from rats that were intrastriatally injected with either rAAV2S3-hAADC (n=21) or rAAV2S3-hGDNF (n=9) (Figure 4A). Pre-immune plasma and plasma from rats intrastriatally injected with saline (n=12) were used as negative controls (Figure 4A). In addition, 9 weeks post rAAV2S3-hAADC injection, inflammatory responses were evaluated by immunohistochemical staining for Iba1 (microglial marker). No microglial activation was observed in rats intrastriatally injected with either rAAV2S3-hAADC or saline (Figure 4B).
Figure 4. Lack of humoral immune response to tTA in rats that received an injection of rAAV harboring tTA into the striatum.
No detectable antibodies against tTA were observed in plasma from rats that were intrastriatally injected with either 5 μl of 1.6×10e13 vg/ml of rAAV2S3hAADC or 4 μl of 7.2×1013 vg/ml of rAAV2S3hGDNF (P>0.05). Plasma of rats injected with AAVhAADC was collected 9 weeks post injection (n=21), and the plasma of rats injected with AAVhGDNF was collected 5 weeks post injection (n=9). Pre-immune plasma and plasma from rats injected intrastriatally with saline were used as negative controls (n=12). (B) At 9 weeks post injection, microglial activity was assessed by immunochemical staining of Iba1 in rats injected with AAVS3-hAADC (n=3) and saline (n=3).
Discussion
This study shows that the expression of both hAADC and hGDNF is effectively regulated by dox in rat striatum delivered by rAAV tet-off regulatory vectors (rAAV2S3) using immunohistochemistry for hAADC protein, and ELISA and RT-PCR for hGDNF protein and mRNA, respectively. An ELISA was developed using purified GST-tTA fusion proteins to detect the neutralizing antibodies against tTA or rtTA. The stably detectable antibodies against tTA or rtTA were observed in the sera from rats intradermally injected with an Ad containing either tTA or rtTA, thus confirming the sensitivity of the ELISA developed. However, no antibodies against tTA were observed in rats intrastriatally injected with rAAV2S3hAADC or rAAV2S3hGDNF. No microglial activation in striatum was observed in rats intrastriatally injected with rAAV2S3hAADC. This could not be studied in the GDNF rats since they also received an injection of 6-OHDA into the striatum, which causes inflammation.
AADC and GDNF are two well studied candidates for gene therapy for Parkinson's disease. AAV-AADC gene therapy is already in clinical trial with no serious side effects observed to date 1. A clinical trial of AAV-GDNF gene therapy for Parkinson's disease will commence soon (personal communication). Ultimately, the chronic expression of a therapeutic transgene may lead to undesirable effects on brain function. This consideration is particularly important for the secreted neurotrophic factors, such as GDNF, whose receptors are widely expressed in the brain. Importantly, side effects due to continuous administration of GDNF protein have been reported previously 9,10. Even though lower levels of GDNF would be delivered through gene therapy, the effects of chronically increased levels of GDNF over years are unknown. We have found that the level of GDNF expressed off the tet-regulated promoter in the context of AAV is approximately 10-fold lower than off a CMV promoter (data not shown). However, these levels are still in the nM range and well above the Kd for GDNF signaling of 5pM. The lower levels of GDNF expressed off the tet-regulated promoter may actually offer another advantage over vectors conferring constitutively expressed GDNF which could conceivably cause more side effects. The current challenge for gene therapy is the development of an effective and safe transgene regulatory system. The viral tet-off regulatable system has been found to efficiently control gene expression in brain by our laboratory and other groups 20-22,24,41. We observed that eGFP expression could be efficiently turned off at the mRNA levels by over 99% in rat striatum using a tet-off AAV vector 41. This study extends these findings to two PD therapeutic gene candidates, hAADC and hGDNF, in a tet-off self-regulated AAV vector. Using immunohistochemistry, qRT-PCR and ELISA, we found that both hADDC and hGDNF expression were effectively down regulated by dox, but not to the same degree as eGFP in our previous study. This may be due to differences in turnover of mRNA or proteins. It remains to be determined whether the levels of residual transgene expression of hAADC or hGDNF would be sufficient to elicit biological effects.
Another important criterion for clinical translation of a regulated vector system for central nervous system diseases is to demonstrate that the regulatory elements are not immunogenic in the brain. Recent reports in the literature show that an immune response against rtTA was detected in non-human primates that received an intramuscular injection with rtTA-encoding plasmid, rAd or rAAV, resulting in dimished transgene expression 29-31. Other studies showed that lack of immune response against rtTA was associated with durable transgene regulation 30. tTA proteins possess two dominant epitopes of rtTA (FLEGLELII and FLCQQGFSL), and both have been shown to evoke cellular immune responses in mice 32. Favre and colleagues reported that the cellular immune responses correlated with the generation of anti-rtTA antibodies 30. To examine possible immunogenicity of AAV vectors harboring tet-off elements, GST-tTA fusion protein was purified and used to develop an ELISA that detects the neutralizing antibodies against tTA or rtTA. The ELISA can be applied to detect the antibodies against both tTA and rtTA, including the two domains, tetR/rtetR and VP16. To test the ELISA, sera from rats intradermally injected with rAAV2S3-hrGFP were first applied, and no detectable tTA antibodies were observed, which is in agreement with other studies. For example, no immune responses against tTA or rtTA were detected when rAAV containing tet-regulatory elements was intramuscularly injected into mice 32,42-44. To develop positive controls for the ELISA, rats received intradermal injection of Ad containing Ad5BIE/SV40tTA (Ad-tet-off) or Ad-tet-on. As expected, antibodies were detectable against tTA or rtTA in rats that received intradermal injection of adenovirus harboring tTA or rtTA, as peptides in the context of Ad are potent vaccines in rodents 45. Our results support the concept that tet-regulatory elements, when expressed from rAAV show weaker immunogenicity compared with rAd 46. However, immune responses to AAV capsid proteins have been reported to occur following intracranial injection of AAV viruses 35-37.
Humoral immune responses were assessed in rats injected intrastriatally with rAAV2S3hAADC or rAAV2S3hGDNF by ELISA. The levels of antibodies against tTA in AADC or GDNF group were not significantly different from negative controls, preimmune plasma or plasma from rats that received an intrastriatal injection of saline. Immunohistochemistry of Iba1 staining showed that no microglial activity was exhibited in striatum in rats injected with rAAV2S3hAADC. Both brain and retina are known to be immune privileged tissues in some respects 34. It has been reported that neither a humoral nor cellular response was observed when the tet-off or tet-on system was delivered into retina of rats or macaque monkeys by AAV, and the transgene showed sustained and long-term expression 38-40. In addition, transgene expression was stable following an intrastriatal injection of tet-on elements in the context of an Ad vector in mice with pre-existing systematic immunity against rtTA, which was elicited by intramuscular injection of tet-on encoding plasmids 26. We conclude that tTA is not immunogenic in rats following an intrastriatal injection of this self-regulated tet-off rAAV design.
In summary, the use of AAV tet-off vectors in the brain holds many advantages over vectors with constitutive expression, including relatively high transgene expression in the “on” state, depressed expression in the “off” state and non-immunogenicity of transactivator to the host. The combination of potent effects of AADC or GDNF and the rescue potential of the tet-off rAAV vector system offers a promising safer long-term gene therapy approach for PD than a vector with constitutive expression. To further assess the clinical usefulness of these self-regulating AAV tet-off vectors, studies need to focus on the evaluation the regulatory efficiency and immune responses against tTA in non-human primate brain.
Materials and Methods
Animals
Adult male Fisher 344 rats of 150-200g (Harlan, Indianapolis, IN) were housed in the Children's Memorial Research Center vivarium on a 12 hour light/dark cycle and provided with chow and water ad libitum. To generate positive control serum for the rtTA sequence, encoded within Ad vectors, we used male Lewis rats weighing 220-250g (Harlan, Indianapolis, IN), which were housed in the Gene Therapeutics Research Institute vivarium, UCLA. All animal procedures were conducted in accordance with institutional, USDA and NIH guidelines.
Vectors: pGEX-tTA, Ad5BIE/SV40tTA, rAAVS3-hrGFP, rAAVS3-hAADC, rAAVS3-hGDNF
The coding region of tTA was obtained by PCR from ptet-off (Clontech) using the forward primer, GAA TTC ATG TCT AGA TTA GAT AAA AGT AAA GTG (EcoRI site underlined) and the reverse primer, CTC GAG CTA CCC ACC GTA CTC GTC (XhoI site underlined). The PCR product was cloned into the EcoRI and XhoI sites of pBluescript SK (+) (Stratagene) and sequenced. The plasmid pSK-tTA was digested by EcoRI and XhoI followed by ligation of tTA into the expression vector pGEX-6p-1 (GE healthcare). The pGEX-tTA was grown in E. coli BL21 (DE3) for the production of recombinant proteins.
Ad5BIE/SV40tTA contains tTA driven by CMV promoter and the bidirectional tet-responsive promoter driving transgene and eGFP expression, as described previously 47. rAAVS3-hrGFP harbors hrGFP and tet regulatory elements placed between the internal terminal repeat sequences (ITR) in the serotype of AAV2 and were driven toward each other, as described previously 41. rAAVS3-hAADC or rAAVS3-hGDNF was made by replacing hrGFP in rAAVS3-hrGFP by hAADC or hGDNF and including a consensus Kozak sequence (5′-GCCGCCACCATG-3′).
Purification of GST-tTA fusion protein
To produce recombinant GST-tTA protein, bacterial cultures transformed with pGEX-tTA were grown in LB broth (Invitrogen, Carlsbad, CA) containing 50 μg/ml ampicillin with vigorous agitation at 37°C. IPTG was added to the medium at a final concentration of 1 mM after the OD600 reached 0.8 followed by additional 1 h induction. The bacterial cells were harvested by centrifugation at 6,000 g for 15 min at 4°C and then washed with cold 1× PBS (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.3) and re-suspended in lysis buffer (1×PBS containing 1% Triton-X100). The suspended cells were disrupted by sonication on ice and the cell solution was centrifuged at 12,000 g for 20 min. The supernatant was applied to the affinity matrix glutathione sepharose 4B column (GE healthcare, Uppsala, Sweden) with a bed volume of 2 ml, which had previously been washed and equilibrated with 1× PBS overnight. The column was washed three times with 20 ml 1× PBS containing 500 mM NaCl and then the fusion protein was eluted with elution buffer (50 mM Tris-HCl, pH 8.0, containing 2 mM reduced glutathione).
Western blot
SDS-PAGE and immunoblotting were performed in the mini-gel apparatus and submarine gel transfer systems (Bio-Rad), respectively. Samples separated on SDS-PAGE gels were transferred to a nitrocellulose membrane at a constant volt of 25v overnight at 4°C. The membrane was blocked by 5% nonfat milk in TBST (Tris-buffered saline/Tween 20, 20 mM Tris-HCl, pH 7.6, 140 mM NaCl, 0.05% Tween 20) for 2 h at room temperature, and then incubated with an antibody against the tet repressor (1:1000; MoBiTec, Goettingen, Germany) for 2 h. The membrane was washed with TBST three times followed by incubation with horseradish peroxidase (HRP) conjugated goat anti-rabbit secondary antibody (Santa-Cruz, CA; 1:5000) for 1 h at room temperature. Following five washes, the membrane was developed by supersignal west pico luminol/enhanced solution and west pico stable peroxide solution (Pierce, Appleton, WI) and exposed to film (Kodak BioMax Light Film, Fisher Scientific).
Recombinant AAV production
Recombinant AAV was prepared as previously described 41,48,49. Briefly, 2×107 HEK293T cells were plated into a 150 mm tissue culture plate one day before transfection. The transfection mixture contained 22.5 μg of the shuttle plasmid, 22.5 μg of pDG, and ddH2O at a final volume of 750 μl followed by addition of 750 μl CaCl2 and 1500 μl 2× HBS (280 mM NaCl, 1.48 mM Na2HPO4, 50 mM Hepes, pH 7.05). 72hrs after transfection, the cell pellets were harvested and dissolved in 15ml lysis buffer (50 mM Tris-HCl, pH 8.5, 150 mM NaCl) followed by 3 freeze-thaw cycles. The supernatant was obtained by centrifugation at 10,000 g for 10 min followed by incubation with 200 U benzonase for 10 min at 37°C and centrifugation at 5000 g for 10min. The viral lysate was applied to a 15-60% discontinuous iodoxanol gradient followed by centrifugation at 69,000 g for 15 hrs at 18°C. The virus was collected in the 40% layer, and was purified by FPLC on a heparin column (Amersham, NJ).
Stereotactic surgery
All surgical procedures were performed stereotaxically under aseptic conditions. Rats were anesthetized using 3% isoflorane during the surgery. The rats were lesioned by injection with 16 μg 6-OHDA (Sigma) dissolved in saline, into the left medial forebrain bundle (MFB) at a rate of 1.0 μl/min. The injection co-ordinates were -4.3 mm A/P, -1.2 mm M/L, -8.3 mm D/V. 3 weeks post injection, 5 μl of 1.6×1013 vector genomes (vg)/ml of rAAVS3-hAADC (n=21) or saline (n=5) was unilaterally injected into two striatal sites using a 10 μl Hamilton syringe and 26G needle (Hamilton, Reno, NV, USA) at a rate of 0.5 μl/min. The needle was slowly removed at a rate of 1 mm/min following holding in the injection site for 5 min to allow for the viral diffusion. The coordinate sites were +1 mm A/P, -2.5 mm M/L, -5 mm D/V and -1 mm A/P, -3.5 mm M/L, -5.5 mm D/V.
In the rAAVS3-hGDNF group, the rats were unilaterally injected with 4 μl of 7.2×1013vg/ml of rAAVS3-hGDNF (n=9) or saline (n=7) into striatum. The co-ordinate sites were +1 mm A/P, +2 mm M/L, -5 mm D/V and -1 mm A/P, +3 mm M/L, -5 mm D/V. At 7 days after injection, the rats were partially lesioned by injection of 16 μg 6-OHDA into striatum. The injection co-ordinates were 0 mm A/P, +2.5 mm M/L, -5.0 mm D/V. After surgery, the rats were returned to the cages and then to the vivarium after their respiratory rate had stabilized.
Intradermal injection and collection of sera and plasma
Rats were anesthetized by inhalation of 3% isoflorane using a Stoelting stereotaxic system and a nose cone following shaving their back fur. Rats were injected intradermally at a 30° angle using an allergy syringe with a 26G needle (BD, Franklin Lakes, NJ). The vectors injected were either Ad or AAV diluted in sterile saline and included 1×109 vg/ml of Ad5BIE/SV40tTA (n=3); or 1×109, 1×1010, or 1×1011 vg/ml of rAAVS3-hrGFP (n=12). All rats were injected with a total volume of 100 μl into three injection sites. Control rats received injections of saline (n=4). After injection, animals were returned to their cages and returned to the vivarium once their respiratory rate had stabilized. Rats were boosted with 100 μl of the same solution using the same procedure nineteen days after the first injection. Ten days after the second injection, rats were anesthestized by intraperitoneal injection of sodium pentobarbital (50 mg/kg in 0.4 ml saline) and sera and plasma were collected from heart in heparinized and Eppendorf tubes, respectively. Rats were then euthanized by thoractomy and checked for lack of respiration pain reflex.
Anti-rtTA serum of Lewis rats, which was elicited by intradermal injection with Ad-tet-on, was kindly provided by Maria G Castro (Xiong et al., 2006; 2008). Briefly, rats were injected intradermally with 1×109 pfu/100ul of Ad-TetON and were boosted with an identical dose of vector two weeks later. Serum was collected two weeks after the second boost (n=5), as described above.
ELISA for detecting tTA/rtTA antibodies
An ELISA protocol was optimized by combining different concentrations of GST-tTA and anti-tetR antibody and varying the incubation temperature and time. 96-well ELISA plates (Nunc, Rochester, NY) were coated with 100 μl of 25 μg/ml per well of either GST-tTA or GST dissolved in coating buffer (150 mM Na2CO3, 350mM NaHCO3, pH 9.6) for 7 hours at room temperature. The wells were rinsed three times with TBST and then blocked with 200 μl of 1% bovine serum albumin (BSA; Sigma, St. Louis, MO) overnight at 4°C. Then, 100 μl per well of a serial dilution of anti-tetR antibody (MoBiTec, Goettingen, German; 1:1000, 1:2000, 1:5000, 1:10,000, 1:20,000 and 1:50,000) in TBST containing 1% BSA was added and incubated for 1 h at 37°C. For the experimental group, 100μl of the sera or plasma from intradermally or intrastratially injected rats diluted 1:100 in TBST with 1% BSA were added into each well and incubated for 1 h at 37°C. The wells were rinsed three times and 100 μl of secondary antibody (goat anti rat or rabbit) conjugated to horseradish peroxidase (HRP) (Calbiochem, Darmstadt, Germany; 1:10,000) was added and incubated for 1 hour at 37°C. The wells were rinsed five times and bound antibody was detected by adding 100 μl of the HRP substrate, TMB (Calbiochem) at room temperature. The reaction was stopped by adding 100 μl of 1 N HCl and the absorbance was read at 450 nm. The arbitrary absorbance units were converted from the OD based on the standard curve established by rabbit anti-tetR antibodies.
Immunohistochemistry and collection of plasma
The data reported in this manuscript used plasma and brain tissue from some rats from large behavioral studies of the effects of regulated hAADC and hGDNF gene delivery in rat models of Parkinson's disease, which will be reported separately. At 9 weeks post-injection of rAAVS3-hAADC, 5 ml of blood was drawn from the heart of anesthetized rats for plasma and then the rats were perfused transcardially with 0.9% saline followed by 4% paraformaldehyde in PBS. Then, the brains were removed and fixed in 4% paraformaldehyde for 24 hrs at 4°C followed by incubation with cold 30% sucrose for an additional 24 hrs. Serial coronal sections at 40 μm were made through the striatum using a sliding microtome (Leica, McHenry, IL) and kept in sets of six. Immunohistochemistry of hAADC or Iba1 was performed by incubation of anti-human AADC primary antibody (Calbiochem; 1:2,500) or anti-Iba1 (Wako; 1:400) overnight at room temperature followed by incubation in biotinylated IgG secondary antibody for 2 hrs (Vector laboratories; 1:200) and visualization by diaminobenzidine (DAB, Sigma). For Iba1 staining, the 6-OHDA-lesion striatum from other projects was used as positive controls. Sections were mounted on slides and dehydrated and coverslipped. Results were evaluated using microscopy and morphometry using NeuroLucida™ software (MicroBrightField, Inc., Williston, VT). The raw cell number of AADC positive cells was obtained by counting and summing the total number of AADC immunoreactive cells in every sixth section through the whole striatum and multiplying by 6. This number was corrected to obtain the total number of cells expressing AADC using Abercrombie's formula (raw number*40μm/(40μm+13μm)), with 13μm being the measured average diameter of AADC positive cells.
ELISA to evaluate hGDNF expression and collection of plasma
Rats were sacrificed 5 weeks after virus injection by blood withdrawal from the heart followed by cardiac perfusion with ice-cold saline while under pentobarbital anesthesia. The striata from both hemispheres of the brain were quickly dissected under a dissecting microscope (Wild M7A, Switzerland) and collected in individual tubes. Then they were frozen on dry ice, and stored at - 80°C until protein and RNAs were isolated. The brain samples were homogenized on ice with 9 volumes by weight of homogenization buffer (0.1% Tween 20, 0.5% bovine serum albumin, 2 nM EDTA) containing protease inhibitor cocktail (aprotinin 2 μg/ml, leupeptins 2 μg/ml, benzethonium chloride 0.1 mM, PMSF 0.2 mM in PBS), followed by centrifugation at 17,200 rpm for 10 min at 4°C. The supernatant from each sample was collected in a fresh tube for ELISA. 1ml TriReagent (Molecular Research Center, Inc., Cincinnati, Ohio) was added to the pellet and RNA was isolated according to the manufacturer's directions.
Quantitative analysis of the levels of hGDNF was assessed using human GDNF ELISA development kit (R & D Systems, Inc., Minneapolis, MN) and following the protocol from the manufacturer. Briefly, a 96-well Evencoat microplate was coated with 100 μl of capture antibody for 1hr followed by washing with wash buffer. Then, 100 μl of samples or standards were added. After incubating for 1.5 hrs, the microplate was washed. Then, 100 μl of detection antibody was added and incubated for 1.5 hrs. The plate was washed again and incubated with streptavidin-HRP, followed by substrate solution. The OD of each well was measured after the stop solution was added using a microplate reader set at 450nm. The OD from cerebellum was assumed to be the threshold level for detection of hGDNF. OD in striatum was applied to a standard curve generated using recombinant hGDNF protein (R&D Systems, Inc., Minneapolis, MN) used to calculate the amount of hGDNF protein.
qRT-PCR
The samples in TriReagent for total RNA isolation were stored for 5 min at room temperature. 0.2 ml chloroform was added for phase separation, and 0.5 ml isopropanol was added to precipitate RNA. The total RNA samples were treated with TURBO DNA-free kit (Ambion, Inc., Austin, TX) to remove any contaminating genomic DNA, according to the manufacturer's instructions. The concentration of RNA was determined by reading the O.D. at 260 nm, and the samples were diluted to 5 ng/μl for qRT-PCR analysis. Quantitative analysis of the expression of hGDNF mRNAs from the brain samples was assessed using the Perkin Elmer 7500 System (Applied Biosystems, Inc., Foster City, CA) and the TaqMan one step PCR master mix reagent kit (Applied biosystems, Inc., Foster City, CA). In one qRT-PCR reaction, 25 ng of total RNA was added to a 96-well MicroAmp plate (Applied biosystems, Inc., Foster City, CA), which contained 12.5 μl 2× master mix, 0.625 μl 40× multiscribe and RNase inhibitor mix, 0.25 μl forward and reverse primers (10 mM), and 0.5 μl Taqman probe (5 μM). Nuclease-free water was added to bring the final volume of the reaction to 25 μl. The qRT-PCR reaction conditions were 30 min at 48°C, then 15 min at 95°C, followed by 40 cycles of denaturation at 95°C for 15 s and annealing/extension at 59°C for 1 min. Rat β-actin was used as an endogenous control to normalize the results of qRT-PCR for hGDNF mRNA in each sample. The relative quantitation of hGDNF mRNA is expressed as the fold-change of hGDNF mRNA over β-actin mRNA. The primers and probes for assaying hGDNF mRNA were designed specifically to recognize only human GDNF. The following primers/probes were used: human GDNF, forward 5′- CTG ACT TGG GTC TGG GCT ATG-3′, probe, 5′- TGC GAT GCA GCT GAG ACA ACG TAC G-3′, reverse, 5′- TTG TCA CTC ACC AGC CTT CTA-3′; rat β-actin, forward 5′- TCA CCC ACA CTG TGC CCA TCT ATG A-3′, probe, 5′- ACG CGC TCC CTC ATG CCA TCC TGC GT-3′, reverse 5′- CAT CGG AAC CGC TCA TTG CCG ATA G-3′.
Statistical analysis
Statistical analyses were performed by ANOVA using GraphPad Prism software, and the significance of intergroup differences was determined using Tukey's multiple comparison tests. Differences of P<0.05 were considered statistically significant.
Acknowledgments
We thank Dr. Christina Khodr, Northwestern University, for editorial assistance. The authors thank Ms. Jianping Xie and Mrs. Xue Song Wang for their excellent technical support. This work was supported by NIH grants NS31957 and U54NS045309, the Harry F. and Elaine M. Chaddick Foundation and the Medical Research Institute Council of Children's Memorial Hospital. The authors are also grateful for the support of the Chicago Biomedical Consortium to the viral vector core at the Children's Memorial Research Center.
Footnotes
Disclosure/Conflict of Interest: No author has a conflict of interest or competing financial interest in the work reported.
References
- 1.Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70:1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- 2.Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14:564–570. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Pernaute R, Harvey-White J, Cunningham J, Bankiewicz KS. Functional effect of adeno-associated virus mediated gene transfer of aromatic L-amino acid decarboxylase into the striatum of 6-OHDA-lesioned rats. Mol Ther. 2001;4:324–330. doi: 10.1006/mthe.2001.0466. [DOI] [PubMed] [Google Scholar]
- 4.Choi-Lundberg DL, Lin Q, Schallert T, Crippens D, Davidson BL, Chang YN, et al. Behavioral and cellular protection of rat dopaminergic neurons by an adenoviral vector encoding glial cell line-derived neurotrophic factor. Exp Neurol. 1998;154:261–275. doi: 10.1006/exnr.1998.6887. [DOI] [PubMed] [Google Scholar]
- 5.Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 6.Choi-Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, et al. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275:838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- 7.Bilang-Bleuel A, Revah F, Colin P, Locquet I, Robert JJ, Mallet J, et al. Intrastriatal injection of an adenoviral vector expressing glial-cell-line-derived neurotrophic factor prevents dopaminergic neuron degeneration and behavioral impairment in a rat model of Parkinson disease. Proc Natl Acad Sci U S A. 1997;94:8818–8823. doi: 10.1073/pnas.94.16.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnston LC, Eberling J, Pivirotto P, Hadaczek P, Federoff HJ, Forsayeth J. Clinically relevant effects of AAV2-GDNF on the dopaminergic nigrostriatal pathway in aged Rhesus monkeys. Hum Gene Ther. 2009 doi: 10.1089/hum.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kordower JH, Palfi S, Chen EY, Ma SY, Sendera T, Cochran EJ, et al. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson's disease. Ann Neurol. 1999;46:419–424. doi: 10.1002/1531-8249(199909)46:3<419::aid-ana21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Lapchak PA, Araujo DM, Hilt DC, Sheng J, Jiao S. Adenoviral vector-mediated GDNF gene therapy in a rodent lesion model of late stage Parkinson's disease. Brain Res. 1997;777:153–160. doi: 10.1016/s0006-8993(97)01100-1. [DOI] [PubMed] [Google Scholar]
- 11.During MJ, Samulski RJ, Elsworth JD, Kaplitt MG, Leone P, Xiao X, et al. In vivo expression of therapeutic human genes for dopamine production in the caudates of MPTP-treated monkeys using an AAV vector. Gene Ther. 1998;5:820–827. doi: 10.1038/sj.gt.3300650. [DOI] [PubMed] [Google Scholar]
- 12.Fu H, Samulski RJ, McCown TJ, Picornell YJ, Fletcher D, Muenzer J. Neurological correction of lysosomal storage in a mucopolysaccharidosis IIIB mouse model by adeno-associated virus-mediated gene delivery. Mol Ther. 2002;5:42–49. doi: 10.1006/mthe.2001.0514. [DOI] [PubMed] [Google Scholar]
- 13.Feng X, Eide FF, Jiang H, Reder AT. Adeno-associated viral vector-mediated ApoE expression in Alzheimer's disease mice: low CNS immune response, long-term expression, and astrocyte specificity. Front Biosci. 2004;9:1540–1546. doi: 10.2741/1323. [DOI] [PubMed] [Google Scholar]
- 14.Paterna JC, Moccetti T, Mura A, Feldon J, Bueler H. Influence of promoter and WHV post-transcriptional regulatory element on AAV-mediated transgene expression in the rat brain. Gene Ther. 2000;7:1304–1311. doi: 10.1038/sj.gt.3301221. [DOI] [PubMed] [Google Scholar]
- 15.Andersen JK, Frim DM, Isacson O, Breakefield XO. Herpesvirus-mediated gene delivery into the rat brain: specificity and efficiency of the neuron-specific enolase promoter. Cell Mol Neurobiol. 1993;13:503–515. doi: 10.1007/BF00711459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye X, Rivera VM, Zoltick P, Cerasoli F, Jr, Schnell MA, Gao G, et al. Regulated delivery of therapeutic proteins after in vivo somatic cell gene transfer. Science. 1999;283:88–91. doi: 10.1126/science.283.5398.88. [DOI] [PubMed] [Google Scholar]
- 17.Rivera VM, Clackson T, Natesan S, Pollock R, Amara JF, Keenan T, et al. A humanized system for pharmacologic control of gene expression. Nat Med. 1996;2:1028–1032. doi: 10.1038/nm0996-1028. [DOI] [PubMed] [Google Scholar]
- 18.Serguera C, Bohl D, Rolland E, Prevost P, Heard JM. Control of erythropoietin secretion by doxycycline or mifepristone in mice bearing polymer-encapsulated engineered cells. Hum Gene Ther. 1999;10:375–383. doi: 10.1089/10430349950018823. [DOI] [PubMed] [Google Scholar]
- 19.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ralph GS, Bienemann A, Harding TC, Hopton M, Henley J, Uney JB. Targeting of tetracycline-regulatable transgene expression specifically to neuronal and glial cell populations using adenoviral vectors. Neuroreport. 2000;11:2051–2055. doi: 10.1097/00001756-200006260-00048. [DOI] [PubMed] [Google Scholar]
- 21.Lee YB, Cosgrave AS, Glover CP, Bienemann A, Heywood D, Hobson RJ, et al. Increased utility in the CNS of a powerful neuron-specific tetracycline-regulatable adenoviral system developed using a post-transcriptional enhancer. J Gene Med. 2005;7:576–583. doi: 10.1002/jgm.694. [DOI] [PubMed] [Google Scholar]
- 22.Blesch A, Conner J, Pfeifer A, Gasmi M, Ramirez A, Britton W, et al. Regulated lentiviral NGF gene transfer controls rescue of medial septal cholinergic neurons. Mol Ther. 2005;11:916–925. doi: 10.1016/j.ymthe.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Nuber S, Petrasch-Parwez E, Winner B, Winkler J, von Horsten S, Schmidt T, et al. Neurodegeneration and motor dysfunction in a conditional model of Parkinson's disease. J Neurosci. 2008;28:2471–2484. doi: 10.1523/JNEUROSCI.3040-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu B, Wang S, Brenner M, Paton JF, Kasparov S. Enhancement of cell-specific transgene expression from a Tet-Off regulatory system using a transcriptional amplification strategy in the rat brain. J Gene Med. 2008;10:583–592. doi: 10.1002/jgm.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong W, Goverdhana S, Sciascia SA, Candolfi M, Zirger JM, Barcia C, et al. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J Virol. 2006;80:27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong W, Candolfi M, Kroeger KM, Puntel M, Mondkar S, Larocque D, et al. Immunization against the transgene but not the TetON switch reduces expression from gutless adenoviral vectors in the brain. Mol Ther. 2008;16:343–351. doi: 10.1038/sj.mt.6300375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curtin JF, Candolfi M, Puntel M, Xiong W, Muhammad AK, Kroeger K, et al. Regulated expression of adenoviral vectors-based gene therapies: therapeutic expression of toxins and immune-modulators. Methods Mol Biol. 2008;434:239–266. doi: 10.1007/978-1-60327-248-3_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 29.Chenuaud P, Larcher T, Rabinowitz JE, Provost N, Joussemet B, Bujard H, et al. Optimal design of a single recombinant adeno-associated virus derived from serotypes 1 and 2 to achieve more tightly regulated transgene expression from nonhuman primate muscle. Mol Ther. 2004;9:410–418. doi: 10.1016/j.ymthe.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Favre D, Blouin V, Provost N, Spisek R, Porrot F, Bohl D, et al. Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus. J Virol. 2002;76:11605–11611. doi: 10.1128/JVI.76.22.11605-11611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Latta-Mahieu M, Rolland M, Caillet C, Wang M, Kennel P, Mahfouz I, et al. Gene transfer of a chimeric trans-activator is immunogenic and results in short-lived transgene expression. Hum Gene Ther. 2002;13:1611–1620. doi: 10.1089/10430340260201707. [DOI] [PubMed] [Google Scholar]
- 32.Ginhoux F, Turbant S, Gross DA, Poupiot J, Marais T, Lone Y, et al. HLA-A*0201-restricted cytolytic responses to the rtTA transactivator dominant and cryptic epitopes compromise transgene expression induced by the tetracycline on system. Mol Ther. 2004;10:279–289. doi: 10.1016/j.ymthe.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Corey L, Reeves WC, Holmes KK. Cellular immune response in genital herpes simplex virus infection. N Engl J Med. 1978;299:986–991. doi: 10.1056/NEJM197811022991805. [DOI] [PubMed] [Google Scholar]
- 34.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 35.Peden CS, Manfredsson FP, Reimsnider SK, Poirier AE, Burger C, Muzyczka N, et al. Striatal readministration of rAAV vectors reveals an immune response against AAV2 capsids that can be circumvented. Mol Ther. 2009;17:524–537. doi: 10.1038/mt.2008.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunningham J, Pivirotto P, Bringas J, Suzuki B, Vijay S, Sanftner L, et al. Biodistribution of adeno-associated virus type-2 in nonhuman primates after convection-enhanced delivery to brain. Mol Ther. 2008;16:1267–1275. doi: 10.1038/mt.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadaczek P, Forsayeth J, Mirek H, Munson K, Bringas J, Pivirotto P, et al. Transduction of non-human primate brain with adeno-associated virus serotype 1: vector trafficking and immune response. Hum Gene Ther. 2008 doi: 10.1089/hum.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stieger K, Mendes-Madeira A, Meur GL, Weber M, Deschamps JY, Nivard D, et al. Oral administration of doxycycline allows tight control of transgene expression: a key step towards gene therapy of retinal diseases. Gene Ther. 2007;14:1668–1673. doi: 10.1038/sj.gt.3303034. [DOI] [PubMed] [Google Scholar]
- 39.Stieger K, Le Meur G, Lasne F, Weber M, Deschamps JY, Nivard D, et al. Long-term doxycycline-regulated transgene expression in the retina of nonhuman primates following subretinal injection of recombinant AAV vectors. Mol Ther. 2006;13:967–975. doi: 10.1016/j.ymthe.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Folliot S, Briot D, Conrath H, Provost N, Cherel Y, Moullier P, et al. Sustained tetracycline-regulated transgene expression in vivo in rat retinal ganglion cells using a single type 2 adeno-associated viral vector. J Gene Med. 2003;5:493–501. doi: 10.1002/jgm.367. [DOI] [PubMed] [Google Scholar]
- 41.Jiang L, Rampalli S, George D, Press C, Bremer EG, O'Gorman MR, et al. Tight regulation from a single tet-off rAAV vector as demonstrated by flow cytometry and quantitative, real-time PCR. Gene Ther. 2004;11:1057–1067. doi: 10.1038/sj.gt.3302245. [DOI] [PubMed] [Google Scholar]
- 42.Rendahl KG, Leff SE, Otten GR, Spratt SK, Bohl D, Van Roey M, et al. Regulation of gene expression in vivo following transduction by two separate rAAV vectors. Nat Biotechnol. 1998;16:757–761. doi: 10.1038/nbt0898-757. [DOI] [PubMed] [Google Scholar]
- 43.Bohl D, Salvetti A, Moullier P, Heard JM. Control of erythropoietin delivery by doxycycline in mice after intramuscular injection of adeno-associated vector. Blood. 1998;92:1512–1517. [PubMed] [Google Scholar]
- 44.Bohl D, Bosch A, Cardona A, Salvetti A, Heard JM. Improvement of erythropoiesis in beta-thalassemic mice by continuous erythropoietin delivery from muscle. Blood. 2000;95:2793–2798. [PubMed] [Google Scholar]
- 45.Xiang ZQ, Yang Y, Wilson JM, Ertl HC. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology. 1996;219:220–227. doi: 10.1006/viro.1996.0239. [DOI] [PubMed] [Google Scholar]
- 46.Jooss K, Chirmule N. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Ther. 2003;10:955–963. doi: 10.1038/sj.gt.3302037. [DOI] [PubMed] [Google Scholar]
- 47.Ebert AD, Chen F, He X, Cryns VL, Bohn MC. A tetracycline-regulated adenovirus encoding dominant-negative caspase-9 is regulated in rat brain and protects against neurotoxin-induced cell death in vitro, but not in vivo. Exp Neurol. 2005;191(1):S80–94. doi: 10.1016/j.expneurol.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 48.Grimm D, Kern A, Rittner K, Kleinschmidt JA. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- 49.Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]