Summary
It is now well established that post-learning sleep is beneficial for human memory performance [1–5]. Meanwhile, human and animal studies demonstrate that learning-related neural activity is re-expressed during post-training non-rapid eye movement sleep (NREM) [6–9]. NREM sleep processes appear to be particularly beneficial for hippocampus-dependent forms of memory [1–3, 10]. These observations suggest that learning triggers the reactivation and reorganization of memory traces during sleep, a systems-level process that in turn enhances behavioral performance. Here, we hypothesized that dreaming about a learning experience during NREM sleep would be associated with improved performance on a hippocampus-dependent spatial memory task. Subjects (n=99) were trained on a virtual navigation task, and then retested on the same task 5 hours after initial training. Improved performance at retest was strongly associated with task-related dream imagery during an intervening afternoon nap. Task-related thoughts during wakefulness, in contrast, did not predict improved performance. These observations suggest that sleep-dependent memory consolidation in humans is facilitated by the offline reactivation of recently formed memories, and furthermore, that dream experiences reflect this memory processing. That similar effects were not seen during wakefulness suggests that these mnemonic processes are specific to the sleep state.
Results and Discussion
Subjects were trained on a virtual navigation task, and then retested on the same task 5hrs after initial training (see Experimental Procedures and Figure 1). As previously seen using the same task [11], subjects who napped following training improved significantly more at retest than those who remained awake during the retention interval (Wake improvement=25.6±56.9 sec (S.E.), n=49; Sleep improvement=187.9±41.8 sec, n=50; main effect of condition: F1,95=5.34, p=.02, see Experimental Procedures for analysis details).
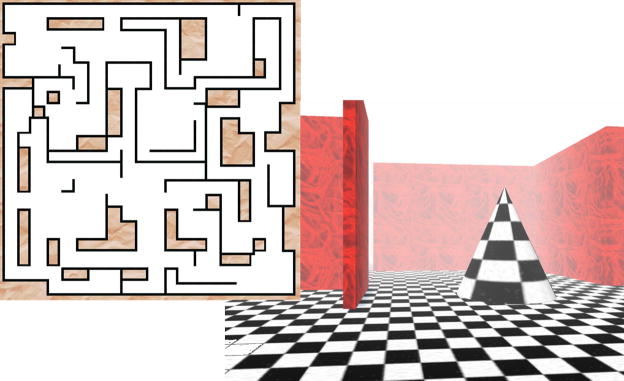
Figure 1. The virtual maze task.
In this spatial memory task, subjects first learned the layout of a complex maze (Left, level 3 shown). Route memory was then probed across a series of trials, as subjects repeatedly navigated to a specified goal point, beginning from pseudorandomized starting locations. An example view of the maze environment is pictured (Right). For summary of task-related mentation, see Supplemental Table S1.
Dreaming of the Task Predicts Improved Performance at Retest
Task-related mentation was strongly associated with enhanced performance at retest, whether measured by questionnaire or by open-ended verbal report (see Experimental Procedures). In the forced-choice questionnaire protocol (Fig 3, bottom), participants in the Sleep group who reported maze-related mentation following task training (n=12; 55%) improved significantly more at retest than Sleep participants without maze-related mentation (n=10, t20=4.02, p<.001; Supplemental Figure S1, left). In contrast, although a number of Wake participants also reported maze-related mentation on the questionnaire (n=11; 40%), this waking mentation was not associated with improved performance (p>.8, Supplemental Figure S1 right). Thus, as hypothesized, task-related mentation was associated with enhanced performance only when it occurred in subjects who slept, and not in those who remained awake (2 (condition: Wake/Sleep) × 2 (mentation: related/unrelated) ANOVA: interaction: p=.006; main effect of mentation: p=.01; Supplemental Figure S1).
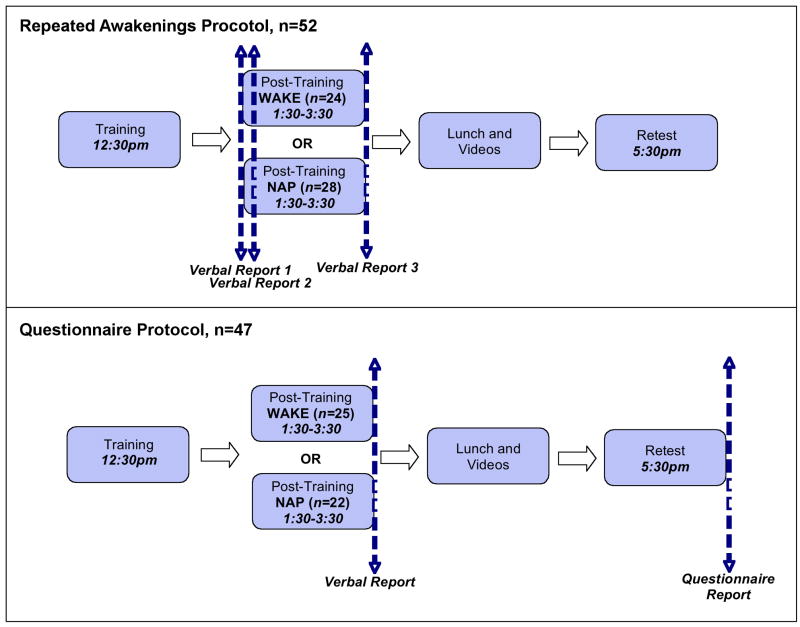
Figure 3. Protocol for collection of subjective reports.
“Repeated Awakenings” participants (n=52) were interrupted for reporting during the sleep onset period, and provided a total of three verbal reports during the post-training Sleep/Wake period. “Questionnaire Protocol” participants (n=47) were not interrupted during sleep onset, and instead provided only one verbal report at the end of the nap, in addition to completing a questionnaire regarding task-related mentation at the end of the study. Total sleep time (TST) did not differ between these participant subsets (p>.3).
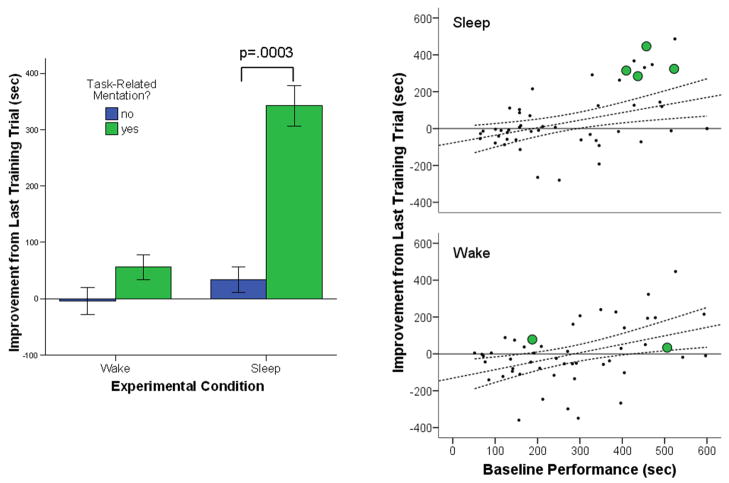
The same pattern was evident when examining open-ended verbal reports (Figure 2, left; see also Figure 3). The four participants in the sleep group (8.0%) who made reference to the maze in their verbal dream reports ranked among those with the largest post-sleep performance improvements in the sample (Mann-Whitney rank order test: U=14.0, p=.005; Figure 2, Left), improving tenfold more than Sleep participants (n=46) without task-related reports (t48=3.88, p=.0003; Welch’s test for unequal variance: t1,5.96=52.8, p=.0004; Figure 2, Left). Three of these subjects reported task-related mentation at sleep onset (following at least one minute of continuous sleep), while the fourth reported a maze-related dream after awakening from Stage 2 sleep at the end of the nap period. Again, maze-related verbal reports from subjects in the Wake group were not associated with improvement in completion time, with the two Wake group subjects who reported thoughts of the maze task performing similarly to the Wake group as a whole (Figure 2, Left; p>.6 for effect of mentation within Wake group; 2 (condition: Wake/Sleep) × 2 (mentation: related/unrelated) ANOVA: main effect of mentation: F1,95=6.94, p=.009; condition × mentation interaction: F1,95=3.11, p=.08).
Figure 2. Participants with maze-related verbal reports improved more than other subjects at retest.
Left: Sleep subjects with verbal reports related to the maze improved tenfold more at retest than did participants without task-related mentation. In contrast, thoughts about the task during Wake did not provide a similar benefit. Error bars = SEM. Right: Baseline performance was a strong predictor of later improvement (regression lines and 95% CI lines for all subjects). Sleep participants reporting maze-related dreams (n=4, large circles) were amongst those with the poorest baseline performance, but improved significantly more at retest than other poor performers. In contrast, subjects who reported thoughts of the maze task during Wake (n=2, large circles) did not differ from others in terms of baseline performance, and improved similarly to those with comparable baseline performance. See also Supplemental Figure S1.
Task-Related Reports are not Veridical Reiterations of the Learning Experience
Maze-related elements of the verbal reports are listed in Supplemental Table S1. Surprisingly, none of these six reports consisted of exact, veridical “replays” of the learning experience. The reports do not, for example, describe the specific objects, locations, or routes of which the task is comprised. Instead, while participants’ reports were unquestionably related to the maze, they consisted of remote associates and memories thematically related to the task, or of isolated fragments and thoughts about the maze-navigation experience. Two subjects reported hearing the music associated with the task, one subject reported thinking of the upcoming retest, and three reports described other “maze-like” environments (see Supplemental Table S1). This lack of exact replay mirrors that observed in animal studies of neuronal memory reactivation, in that patterns of neural activity in rodent sleep statistically resemble patterns seen in the same networks during prior waking experience, but are never strictly identical to those activity patterns [6–8]. Similarly, subjective reports in the present study suggest that memory consolidation in sleep is not necessarily associated with the veridical reiteration of an experience, at least within conscious awareness.
Baseline Performance Predicts Task-Related Dreaming
Although subjects reporting task-related mentation on the questionnaire measure performed similarly at baseline to those who did not (p>.6), those with maze-related verbal reports were among those who performed most poorly at training, completing the maze significantly more slowly at baseline than other subjects (t97=2.14, p=.03; Figure 2, Right). Critically, however, these subjects with maze-related verbal reports continued to show enhanced improvement even when baseline performance was included as a covariate (F1,47=9.00, p=.004; Figure 2, Right), indicating that poor performance at baseline alone cannot explain the memory enhancement associated with task-related dreaming. In summary, it appears that those subjects who reported dreaming about the task also experienced greater difficulty in learning the maze and, having continued to process this information during subsequent sleep, improved more than other subjects at retest.
Sleep Architecture and Performance
Subjects obtained an average of 45.0 (± 16.2 S.D.) minutes of NREM sleep during the post-learning nap (see Supplemental Table S2). However, amount of sleep obtained was not correlated with improvements in performance at retest, nor did amount or % time in any individual sleep stage predict performance. In light of previous studies using hippocampus-dependent tasks, however, this lack of correlation between performance and sleep architecture is not particularly surprising. Total sleep time does not appear to be an important predictor of sleep-dependent consolidation for hippocampus-dependent memory, with even brief periods of sleep leading to performance benefits similar to those following a full night [1, 12, 13]. Meanwhile, although a substantial body of literature suggests a causal connection between NREM sleep and hippocampus-dependent memory [1–3, 9, 10, 14–19], reports of significant correlations between amounts of NREM sleep and performance have been the exception, rather than the rule. It may be that “sleep stage” categorization is unable to capture the more specific electrophysiological and neurochemical features on which neural plasticity in the sleeping brain depends.
Memory Reactivation and Cognition in NREM Sleep
These cognitive data link post-sleep performance improvements to the reactivation of specific task-related memories in the sleeping brain, as displayed in dream content. As such, the present data argue against non-specific accounts of the mnemonic benefits of sleep, such as the “interference hypothesis” holding that sleep is beneficial primarily due to an absence of sensory input that might otherwise interfere with task memory [e.g. 20], or the synaptic homeostasis model, which suggests that all synapses are equivalently activated and modified during sleep [21]. To the contrary, an association of post-sleep performance with specific task-related mnemonic content suggests, that the performance benefit of sleep is indeed a result of the reactivation of specific memories in the sleeping brain.
Contrary to popular notions of dreaming, reports of mental activity are quite common in NREM sleep [22, 23]. Here, we focused on NREM because of its apparent close relationship to hippocampus-dependent memory consolidation in humans [1–3, 24], and its association with robust memory trace reactivation in rodents [6, 7, 25]. That three of four Sleep group verbal reports mentioning the maze were collected from very early in the sleep period (following as little as one minute of sleep) is not surprising, as the animal literature indicates that memory reactivation during NREM is strongest immediately following learning [7, 8, 26]. Human studies also suggest that effects of learning on the sleep EEG are most visible shortly after sleep onset [e.g. 27, 28] and our own previous work has detected effects of learning on sleep-onset mentation, but not on dreams recalled the following morning [29, 30].
Critically important is the finding that memory enhancement in the present study was selectively predicted by related mentation during sleep, while thinking of the maze during wakefulness was unrelated to later performance. That this waking “rehearsal” of the maze did not benefit subsequent performance indicates that these mnemonic processes active in sleep are in fact, specific to the sleep state, and suggests that general levels of motivation and engagement cannot easily account for the observed results; more motivated subjects do not simply think about the task more and try harder to improve their performance at retest. Further, this observation points to the importance of the neurophysiological state within which memory reprocessing occurs. NREM sleep is characterized by dramatic neurochemical and electrophysiological changes thought to facilitate memory consolidation [2, 9, 24, 31–34], including a sharp drop in acetylcholine levels [34, 35] and the emergence of 12–15 Hz sleep spindles in the cortical EEG that are temporally correlated with high-frequency EEG “ripples” in the hippocampus [15, 36–38].
As a final note, it is important to distinguish this sleep-dependent improvement from the mental rehearsal during waking that has previously been shown to improve spatial performance [39, 40]. These rehearsal effects are typically seen after repeated, intentional mental reiterations of learned material. In contrast, our data reflect improvement correlated with naturally occurring thought and imagery related to, but distinctly different from the learned material, and do not reflect intentional “rehearsal” of learned information. Future research might fruitfully compare the effects of intentional rehearsal during wakefulness to spontaneous task-related mentation during sleep.
Conclusions
We observed that dreaming of a spatial learning task predicted enhanced post-nap memory performance. However, it is not our contention that dream experiences cause memory consolidation during sleep. Instead, we propose that task-related dream experience and the subsequent behavioral enhancement of memory performance both result from an underlying process of memory reactivation and consolidation in sleep. Thus, dreaming may be a reflection of the brain processes supporting sleep-dependent memory processing. In combination with prior brain imaging and cellular-level analyses, our data also provide evidence at the phenomenological level that sleep-dependent memory processing is not necessarily associated with offline reiteration of recent experiences in their original form. Instead, memories may be enhanced, in part, through a more nuanced process that slowly integrates critical elements of recent experience into established remote and semantic memory networks, facilitating the gradual evolution of more flexible, and ultimately more useful, memory representations.
Experimental Procedures
Participants
Participants (n=99) included 55 female and 44 male college students, ages 18–30. By self report, all subjects were free of psychiatric and sleep disorders, and of medications known to interfere with sleep. Subjects were instructed to abstain from alcohol, caffeine, and drug use in the 24hrs prior to the study, and to maintain a regular sleep schedule prior to participation, and they filled out a 3-day sleep log documenting their pre-study sleep schedule. The protocol was approved by the institutional review board of Beth Israel Deaconess Medical Center.
Procedures
Subjects were trained on a virtual navigation task at 12:30pm and were retested at 5:30pm, 5 hours after initial training. Participants arrived at the laboratory at 11:00am, where they gave written consent and were wired for polysomnographic (PSG) recording. Wake subjects were also wired for PSG, but not recorded. At 12:30pm, participants filled out the Stanford Sleepiness Scale (SSS) [41] and began training on the maze task (see below). Training lasted approximately 45 minutes. Following training, subjects were informed of group (Wake or Sleep) assignment. During the subsequent retention interval, Sleep participants (n=50) lay down for a 90-min sleep opportunity, while Wake subjects (n=49) remained awake in the laboratory. Wake subjects began the retention interval with a 20min period of “quiet wakefulness”, during which they sat silently, not engaged in any particular activity, after which they were allowed to watch videos until retesting. Sleep subjects slept in a darkened room while EEG, EOG, and EMG were monitored and recorded. Because of our theoretical focus on NREM processes, restricting subjects’ sleep to NREM stages was critical to the present design, allowing analysis of the effects of NREM sleep in the absence of the confounding influence of REM. Thus, participants were awoken at the first signs of REM. This procedure follows from prior work addressing the effects of NREM sleep on memory [1, 42], and allows the isolation of NREM-specific physiology. See Supplemental Table S2 for sleep architecture data.
Collection of mentation reports
Prior to the post-training Sleep or Wake period, subjects were instructed that when prompted, they should provide a detailed verbal report of “everything that was going through your mind”. During the sleep onset period, dream recall is exceptionally high [43–46], and experimental awakenings can yield dream reports related to pre-sleep learning as much as 45% of the time[29]. However, in the present study there was a concern that interruptions at sleep onset would disrupt the nap, reduce total sleep time (TST), and interfere with memory consolidation. Thus, two complementary methods were used to assess whether task-related thought and imagery were present (see Figure 3). One group of subjects (“Repeated Awakenings Protocol”, n=52) was prompted to provide three open-ended verbal reports of their current subjective experience during the post-training period. For Sleep subjects, these reports were elicited a) just prior to the initiation of sleep, b) after 1min of continuous sleep (“sleep onset” reports), and c) at the termination of the nap period. As a control for any sleep disruption introduced by awakening subjects during the initial minutes of the nap, a second group of subjects (“Questionnaire Protocol”, n=47) was not interrupted for reports during sleep onset, providing only one open-ended verbal report at the termination of the Sleep period. The two awakening protocol groups did not differ significantly in TST, indicating that sleep onset interruptions in the Repeated Awakenings protocol did not overtly interfere with their ability to sleep (t48=0.87, p>.3). Thus, our primary analyses treat these subsets as a single subject group. Participants in the Questionnaire protocol further answered a forced-choice question at the end of the study, reporting whether they had experienced any task-related mentation during the period between training and retesting (see Figure 3). Specifically, they were asked, “During the time in between when you first saw the maze, and when you were tested on it again, were you thinking about, dreaming about, or imagining the maze game? (Yes/No)”.
Verbal reports were classified as related to the maze-learning task only in cases where the maze task was explicitly and unambiguously mentioned in the report (see Supplemental Table S1). Overall, while 100% of Wake subjects queried were able to provide at least one mentation report, 22% of Sleep subjects were unable to recall any mental experience in response to the prompt(s) delivered.
Following a 30min lunch break, all subjects watched PG-rated videos in the laboratory until retest (Figure 3). Study staff monitored participants in order to ensure napping did not occur. At 5:30pm, subjects again filled out the SSS and were then retested on the maze task. No between group differences were seen in SSS scores, gender distribution, age, trait dream recall frequencies, or pre-study sleep schedule (Table 1).
Table 1.
Participant Characteristics
| Baseline Performance (Last Training Trial) | SSS at Training | SSS at Retest | Age | % Female | Trait Dream Recall | Mean Bed Time from Pre-Study Log | Mean Wake Time from Pre-Study Log | |
|---|---|---|---|---|---|---|---|---|
| Wake Group (n=49) | ||||||||
| Related Mentation (n=2) | 231.5 ±207.7 sec | 2.0 ±0.0 | 2.5 ±0.7 | 20.5 ±2.12 | 100% | 3.00 ±0.00 | 12:48 ±1.50 | 8:45 ±0:21 |
| No Related Mentation (n=47) | 255.2 ±122.3 sec | 2.9 ±1.0 | 2.8 ±1.0 | 20.9 ±2.40 | 50% | 2.90 ±0.53 | 12:20 ±1:08 | 8:24 ±0:46 |
| Sleep Group (n=50) | ||||||||
| Related Mentation (n=4) | 538.5 ±123.0 sec | 2.8±1.3 | 2.3 ±0.5 | 21.0 ±1.41 | 50% | 3.00 ±0.00 | 12:03 ±0:31 | 8:17 ±0:40 |
| No Related Mentation (n=46) | 255.1 ±201.2 sec | 2.8 ±1.0 | 2.0 ±0.8 | 21.5 ±2.98 | 50% | 2.86 ±0.69 | 12:33 ±1:09 | 8:35 ±1:02 |
Notes. There were no significant differences between groups in sleepiness ratings, age, gender distribution, trait dream recall, or sleep schedule prior to the study. Means±SD. SSS=Stanford Sleepiness Scale. Longer completion times indicate poorer baseline performance. Trait dream recall is assessed as # of dreams recalled per week, as reported on the pre-study sleep log. For Nap sleep architecture data, see Supplemental Table S2.
Virtual Maze-Learning Task
The task was a simple 3D graphical environment designed using the Abashera Maze Editor (Magnus Norman Software, Figure 1), and implemented on a PC computer. The maze did not include unique landmarks, and participants began each trial from a different starting point, thus encouraging the use of a hippocampus-dependent spatial strategy, rather than the memorization of a particular path. The 12:30pm training session lasted for approximately 45 minutes, and consisted of three distinct phases: a skill-level pretest, an exploration phase, and a series of test trials. The development of the “skill-level pretest” was based on pilot data indicating substantial inter-individual differences in baseline maze navigation skills. During this 20-minute pretest, participants were asked to repeatedly navigate to particular goal points, within mazes of increasing difficulty. In order to equate subjective maze difficulty across participants, subjects were assigned to one of 4 maze “difficulty levels”, which differed in size and complexity, based on their performance during pretest evaluation. Next, during the exploration phase, subjects spent 5 minutes exploring a novel maze at their assigned difficulty level, during which time they were instructed to view as much of the maze as possible, and to remember it as well as possible. In particular, subjects were instructed to remember the location of a particular object in the maze (a “tree”, from which all exploration began). Following this exploration period, subjects continued to navigate through the maze environment during a series of three test trials, in which they were instructed to reach the goal point (the “tree”) as quickly as possible. Test trials began from three different starting points, equidistant from the goal. The order of starting points was counterbalanced across subjects, and all subjects received the same counterbalanced order at training and at retest. In order to avoid feelings of frustration, and to cap training time at a maximum of 1hr, a cutoff time of 10min was established for each trial. If a participant failed to reach the goal within 10min, the trial was terminated, and the next trial begun. As in several prior studies employing virtual environment learning tasks [e.g. 47–49], performance on each trial was defined as time required to reach the goal, here with a maximum of 10min. Improvement across the course of the study was calculated as the change in performance from the last (third) training trial, to the mean performance on three retest trials (trials 4–6) performed at 5:30pm.
Data Analysis Considerations
All comparisons described here were based on specific pre hoc hypotheses. Primary analyses employed a 2(Sleep v Wake) × 2(related mentation v no related mentation) ANOVA, followed by planned paired comparisons. Importantly, caution had to be used in applying inferential tests of statistical significance to small and unequal samples, as normality and variance of the population distribution is difficult to estimate based on small subject groups. Although tests for heterogeneity of variance were non-significant, we took a conservative approach and applied multiple methods to confirm the robustness of small-sample effects. In addition to the Student’s t-test, we applied nonparametric Mann-Whitney rank order tests to all comparisons involving small subsamples. Critical contrasts were also conducted using Welch’s unequal variance t-tests, which compare sample distributions in a manner similar to the t-test, but does not assume homogeneity of variance. As reported above, these analyses uniformly confirmed our primary results, demonstrating the statistical robustness of the observed effects.
Highlights
Improved spatial memory performance is predicted by task-related dream experience.
Task-related thoughts during waking are unrelated to memory performance.
Findings are discussed in light of models of sleep-dependent memory consolidation.
Supplementary Material
Acknowledgments
We thank Mariette Asare and Erin Miles for assistance with data collection, and to Jon Chamberlain for his contributions to development of the maze navigation task. We also thank John Antrobus, Daniel Schacter, and Daniel Gilbert for their comments on this manuscript. This research was supported by grants R01-MH48832, R01-MH65292, and T32-HL07901 from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006;86:241–247. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006 doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 3.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 4.Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Curr Biol. 2006;16:1290–1294. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Walker MP. A refined model of sleep and the time course of memory formation. Behav Brain Sci. 2005;28:51–64. doi: 10.1017/s0140525x05000026. discussion 64–104. [DOI] [PubMed] [Google Scholar]
- 6.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 7.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 8.Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci. 1999;19:4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Phillips C, Degueldre C, Del Fiore G, Aerts J, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt C, Peigneux P, Muto V, Schenkel M, Knoblauch V, Munch M, de Quervain DJ, Wirz-Justice A, Cajochen C. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci. 2006;26:8976–8982. doi: 10.1523/JNEUROSCI.2464-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wamsley EJ, Stickgold R. Virtual maze learning is enhanced by a short daytime nap containing only NREM sleep. Sleep. 2008;31:A386. [Google Scholar]
- 12.Lahl O, Wispel C, Willigens B, Pietrowsky R. An ultra short episode of sleep is sufficient to promote declarative memory performance. J Sleep Res. 2008;17:3–10. doi: 10.1111/j.1365-2869.2008.00622.x. [DOI] [PubMed] [Google Scholar]
- 13.Tucker MA, Fishbein W. The impact of sleep duration and subject intelligence on declarative and motor memory performance: how much is enough? J Sleep Res. 2009;18:304–312. doi: 10.1111/j.1365-2869.2009.00740.x. [DOI] [PubMed] [Google Scholar]
- 14.Ramadan W, Eschenko O, Sara SJ. Hippocampal sharp wave/ripples during sleep for consolidation of associative memory. PLoS One. 2009;4:e6697. doi: 10.1371/journal.pone.0006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molle M, Eschenko O, Gais S, Sara SJ, Born J. The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur J Neurosci. 2009;29:1071–1081. doi: 10.1111/j.1460-9568.2009.06654.x. [DOI] [PubMed] [Google Scholar]
- 16.Rasch B, Buchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–1429. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 17.Orban P, Rauchs G, Balteau E, Degueldre C, Luxen A, Maquet P, Peigneux P. Sleep after spatial learning promotes covert reorganization of brain activity. Proc Natl Acad Sci U S A. 2006;103:7124–7129. doi: 10.1073/pnas.0510198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clemens Z, Fabo D, Halasz P. Twenty-four hours retention of visuospatial memory correlates with the number of parietal sleep spindles. Neurosci Lett. 2006;403:52–56. doi: 10.1016/j.neulet.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 19.Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132:529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Wixted JT. The psychology and neuroscience of forgetting. Annu Rev Psychol. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]
- 21.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Wamsley EJ, Hirota Y, Tucker MA, Smith MR, Antrobus JS. Circadian and ultradian influences on dreaming: A dual rhythm model. Brain Res Bull. 2007;71:347–354. doi: 10.1016/j.brainresbull.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen TA. A review of mentation in REM and NREM sleep: “covert” REM sleep as a possible reconciliation of two opposing models. Behav Brain Sci. 2000;23:851–866. doi: 10.1017/s0140525x0000399x. discussion 904–1121. [DOI] [PubMed] [Google Scholar]
- 24.Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci. 2007;11:442–450. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 26.Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 28.Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- 29.Wamsley EJ, Perry K, Djonlagic I, Reaven LB, Stickgold R. Cognitive replay of visuomotor learning at sleep onset: temporal dynamics and relationship to task performance. Sleep. 2010;33:59–68. doi: 10.1093/sleep/33.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stickgold R, Malia A, Maguire D, Roddenberry D, O’Connor M. Replaying the game: hypnagogic images in normals and amnesics. Science. 2000;290:350–353. doi: 10.1126/science.290.5490.350. [DOI] [PubMed] [Google Scholar]
- 31.Schabus M, Gruber G, Parapatics S, Sauter C, Klosch G, Anderer P, Klimesch W, Saletu B, Zeitlhofer J. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27:1479–1485. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 32.Walker MP. The role of slow wave sleep in memory processing. J Clin Sleep Med. 2009;5:S20–26. [PMC free article] [PubMed] [Google Scholar]
- 33.Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008 doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- 34.Gais S, Born J. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc Natl Acad Sci U S A. 2004;101:2140–2144. doi: 10.1073/pnas.0305404101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn Sci. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- 36.Clemens Z, Molle M, Eross L, Barsi P, Halasz P, Born J. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain. 2007 doi: 10.1093/brain/awm146. [DOI] [PubMed] [Google Scholar]
- 37.Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 39.Avanzino L, Giannini A, Tacchino A, Pelosin E, Ruggeri P, Bove M. Motor imagery influences the execution of repetitive finger opposition movements. Neurosci Lett. 2009;466:11–15. doi: 10.1016/j.neulet.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 40.Vieilledent S, Kosslyn SM, Berthoz A, Giraudo MD. Does mental simulation of following a path improve navigation performance without vision? Brain Res Cogn Brain Res. 2003;16:238–249. doi: 10.1016/s0926-6410(02)00279-3. [DOI] [PubMed] [Google Scholar]
- 41.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 42.Tucker MA, Fishbein W. Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Sleep. 2008;31:1–7. doi: 10.1093/sleep/31.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogel G. Sleep onset mentation. In: Antrobus JS, Ellman S, editors. The Mind in Sleep. 2. New York: Wiley; 1991. [Google Scholar]
- 44.Foulkes D, Schmidt M. Temporal sequence and unit composition in dream reports from different stages of sleep. Sleep. 1983;6:265–280. doi: 10.1093/sleep/6.3.265. [DOI] [PubMed] [Google Scholar]
- 45.Foulkes D, Spear PS, Symonds JD. Individual differences in mental activity at sleep onset. J Abnorm Psychol. 1966;71:280–286. doi: 10.1037/h0023581. [DOI] [PubMed] [Google Scholar]
- 46.Palagini L, Gemignani A, Feinberg I, Guazzelli M, Campbell IG. Mental activity after early afternoon nap awakenings in healthy subjects. Brain Res Bull. 2004;63:361–368. doi: 10.1016/j.brainresbull.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Walker BN, Lindsay J. Navigation performance with a virtual auditory display: effects of beacon sound, capture radius, and practice. Human factors. 2006;48:265–278. doi: 10.1518/001872006777724507. [DOI] [PubMed] [Google Scholar]
- 48.Sakthivel M, Patterson PE, Cruz-Neira C. Gender differences in navigating virtual worlds. Biomed Sci Instrum. 1999;35:353–359. [PubMed] [Google Scholar]
- 49.Fortenbaugh FC, Hicks JC, Hao L, Turano KA. High-speed navigators: Using more than what meets the eye. J Vis. 2006;6:565–579. doi: 10.1167/6.5.3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.