Abstract
INTRODUCTION
System level barriers have been associated with inadequate follow-up of abnormal cervical cytology.
OBJECTIVE
The aim of this study was to develop and evaluate an electronic tracking system to improve follow-up of abnormal Pap tests.
PROGRAM DESCRIPTION
We implemented an electronic medical record (EMR)-based Pap test tracking system at two clinical practices at an inner-city academic health center. The system generated a provider-specific monthly report of all abnormal Pap results, and provided a patient-specific Pap tracking table embedded in the EMR for each subject.
EVALUATION
We compared abnormal Pap test follow-up rates for the 24 months pre-intervention with rates 12 months following its implementation (post-intervention). The evaluation followed all subjects for 12 months from the date of their abnormal Pap test, looking for diagnostic resolution.
RESULTS
Subjects were young women (mean age = 30.5) of primarily white (42%) and African American (37%) descent, who spoke English (88%). Forty-eight percent were insured through publicly subsidized insurance. Controlling for type of abnormality and practice location, the adjusted mean time to resolution decreased significantly from 108 days (confidence interval, CI 105–112 days) in the pre-intervention period to 86 days (CI 81–91 days).
CONCLUSION
Our study cannot demonstrate that with follow up, we directly avoided cases of invasive cervical cancer. However, we show that in an at-risk urban population, an automated, EMR-based tracking system reduced the time to resolution, and increased the number of women who achieved diagnostic resolution.
KEY WORDS: pap smear, cervical cancer, papillomavirus
INTRODUCTION
Screening for cervical cancer with a Pap test is only as successful as the follow-up rate for an abnormal result. If a patient has a Pap test, yet does not receive appropriate follow-up for an abnormal result, then the opportunity to prevent or treat pre cancerous lesions or cervical cancer is missed and the Pap test is ineffective. This is specifically an issue in lower income and minority populations who experience a higher risk of cervical cancer1,2 and a higher rate of inadequate abnormal Pap test follow-up3. Multiple studies have documented the problem of inadequate follow-up of abnormal Pap tests3–11.
AIM
With the advent of electronic medical record (EMR) systems, there is great potential to address inadequate follow-up from a systems point of view. We developed a tracking system for our internal EMR, and evaluated this tracking system as an intervention to improve adequate follow-up of abnormal Pap tests.
PROGRAM DESCRIPTION
We developed a tracking system that has two components:
A tracking report of abnormal Pap tests generated for the providers each month
A Pap test tracking table embedded in the EMR
The first component, the tracking report, identified potential cases by searching for Pap test orders placed by a provider in the EMR. An interface between the EMR and the pathology reports was developed and from these text fields created a document in the EMR. It was then possible to extract and track the cytology report corresponding to the day the order was placed. Pathology reports are provided in relatively standard text-only format which is scanned for specific phrases. Initially, work was done in coordination with the pathology department to determine standardized Pap test result language. In addition, all possible combinations of added spaces, added hyphens, and lower-case versus capitalized letters were accounted for so that abnormal test result would not be excluded. This process involved multiple iterations of comparing tracking reports directly from pathology, and adjusting the text parse filters to ensure capture of any missed results. Additionally, a hierarchy of abnormality severity was developed so that if a Pap result mentioned both ASCUS (atypical squamous cells of undetermined significance) and HGSIL (high grade squamous intraepithelial lesion), for example, then it would be labeled with the more severe abnormality (HGSIL).
Documented phone and letter contacts from standardized templates in the EMR were included with dates. Appointment data for this tracking report were extracted from another system that manages the outpatient clinic scheduling. Colposcopy appointment dates, location and status appeared on the report, including cancelled and no-show appointments. When a gynecologist performs a colposcopy, they use a standard colposcopy procedure template, which can therefore be tracked. A completed colposcopy was considered resolution of the screening Pap abnormality, and at this point the subject fell off the tracking report. The tracking reports were generated in spreadsheet format with the relevant provider and subject identifiers and results (Table 1). The report was cumulative, meaning that unresolved abnormal Pap test results remained on the report until resolved.
Table 1.
Field Provided in Monthly Provider Pap Test Tracking Reports
| Demographic information: |
|---|
| • Practice location |
| • Provider name |
| • Subject medical record number (MRN) |
| • Subject name |
| Pap test information: |
| • Date of Pap test |
| • Result of Pap test |
| • HPV status (positive or negative, if tested) |
| • Result of LAST abnormal Pap test (if any) |
| • Date of LAST abnormal Pap test (if any) |
| Follow-up information: |
| • Date subject contacted of an abnormal result |
| • Method used to contact subject (eg. letter, phone call) |
| • Date of GYN follow-up appointment |
| • Status of GYN follow-up appointment (eg. future, arrived) |
| • Number of cancelled GYN appointments |
| • Practice location of GYN follow-up appointment |
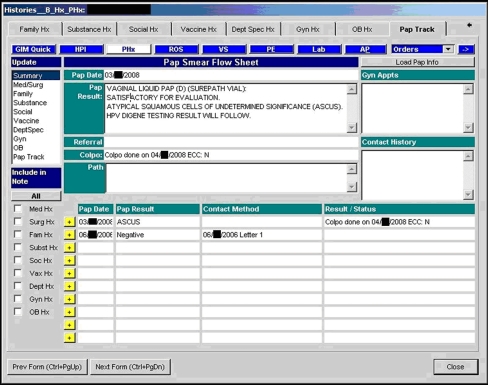
The second component of the tracking system was an individual Pap test tracking table within any individual subject record (see Fig. 1). This table showed the details of all past Pap test results, linked patient contacts, appointments and gynecology pathology results. The EMR tracking table gave providers efficient access to current Pap test status when seeing a patient in the office, and was another point of intervention during this visit.
Figure 1.
Example of an EMR Pap tracking table.
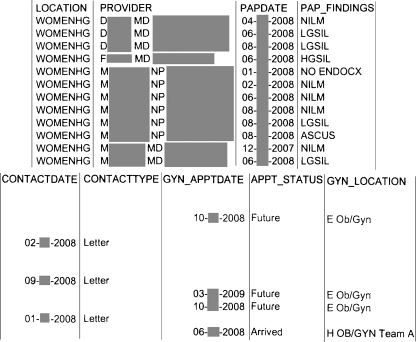
The tracking reports were distributed to each provider monthly and included all of their patients who had had an unresolved Pap test abnormality (Fig. 2). We purposefully delayed the reporting of the abnormal Pap test results by one to two months to allow time for the subject to be contacted and for the colposcopy to be scheduled, and for the list to reflect patients with true delays. The standard manner for informing providers about their abnormal Pap tests did not change, where the Pap test result arrived directly in the ordering provider’s EMR inbox for their review and action. Therefore, the paper tracking report serves as a second notice to providers of any abnormal Pap results, and highlighted patients who either did not keep or did not schedule a gynecology follow-up appointment, or who may never have been notified of their abnormal test results. Once the providers received copies of their individual Pap tracking reports there was no specific protocol about how they managed the information on these reports. No additional resources were given to providers to manage the information on the tracking reports.
Figure 2.
Example of a monthly abnormal Pap report.
EVALUATION
Study Design
We used a pre-test/post-test study design to evaluate whether implementation of a Pap tracking system 1) reduced the number of subjects with inadequate follow-up of abnormal Pap tests, and 2) reduced the time to follow-up. We compared inadequate Pap test follow-up rates prior to availability of the tracking system (pre-intervention) with inadequate follow-up rates following its implementation (post-intervention) at two clinical practices at an inner-city academic health center. The pre-intervention time period was December 2004 to December 2006. We allowed a 3-month implementation period for the tracking system, during which providers were trained and supported in its use. The tracking system was formally implemented on February 1, 2007 at one site, and April 1, 2007 at the second site. The post-intervention time period was therefore March 2007 to April 2008 for the first site, and May 2007 to June 2008 for the second site. All test results were followed for 12 months to determine if a diagnostic evaluation had been completed.
Study Subjects
Eligible subjects were 18 years of age or older, and had one of the following abnormal Pap test results:
Atypical squamous cells of undetermined significance with positive high-risk HPV serotype (ASCUS/HPV+)
Low-grade squamous intraepithelial lesion (LGSIL)
Atypical glandular cells of undetermined significance (AGC/AGUS)
Atypical squamous cells: cannot exclude high-grade squamous intraepithelial lesion (ASC-H)
High-grade squamous intraepithelial lesion (HGSIL)
Carcinoma in situ, or invasive cancer
Data Collection
We obtained an independent list from the pathology department of all abnormal Pap tests during the study time periods for each provider in the two clinical practices. Data were collected through retrospective electronic chart abstraction of all women with abnormal Pap tests, reviewed for 12 months following the abnormal test.
Independent Variables
Race/ethnicity was documented in the EMR as a single set of seven mutually exclusive responses, which we collapsed into “white,” “black/African American” and “other.” Primary language (nine categories) was collapsed into “English” and “non-English.” Health insurance coverage was grouped into private, public and no insurance. The type of cervical abnormality was collapsed into ASCUS/HPV+, AGC/AGUS, LGSIL, HGSIL, and all others. We also included a dichotomous variable to indicate in which clinical practice the subject was seen.
Dependent Variables
The primary outcome for the study was timeliness of diagnostic resolution of the abnormal Pap test. Diagnostic resolution was defined as a definitive tissue diagnosis (biopsy with pathology), or a clinical evaluation determining that no further evaluation was necessary12. We evaluated the outcome both as a dichotomous and continuous variable. For the dichotomous variable, we categorized subjects as to whether they had received diagnostic resolution by 365 days. For the continuous variable, we defined follow-up as the number of days to diagnostic resolution, top coding those who did not resolve to 366 days.
Data Analysis
Our primary research question was whether there was a difference between the pre-intervention and post-intervention groups. We calculated and tested for the differences in median time-to-resolution using the one-way Wilcoxon test, differences in mean time-to-resolution using the t-test and differences in percentages using the chi-squared test. We used the Cox-proportional hazard method for univariate and multivariate hazard analyses of likelihood of resolving within 365 days. From the model we then calculated an adjusted mean time-to resolution and confidence intervals. Those predictors that were significant at the p < 0.10 level in the bivariate analyses were included in the final multivariate models. All analyses were conducted using SAS v9.1 (SAS Institute, Cary NC).
RESULTS
Subject Demographics
Table 2 shows demographic and Pap abnormality characteristics for subjects in the pre-intervention period (n = 137) and post-intervention period (n = 69); 2.2% of the total 9164 Pap tests performed during the study time periods were abnormal. There were no statistically significant differences between the pre- and post-intervention groups in age, race/ethnicity, primary language, insurance status, or type of Pap abnormality (all p > 0.10). Overall, subject characteristics reflected the low income and minority populations cared for at our institution, with 60% of subjects publicly insured or uninsured, and more than 50% from a racial or ethnic minority group.
Table 2.
Subject Characteristics Before and After the Pap Tracking Intervention
| Pre-Intervention N = 137 n (%) | Post-Intervention N = 69 n (%) | P-value | |
|---|---|---|---|
| Age | 0.58 | ||
| 18–21 | 11 (8.0) | 6 (8.7) | |
| 22–26 | 33 (24) | 22 (32) | |
| 27–35 | 44 (32) | 17 (25) | |
| 36+ | 49 (36) | 24(35) | |
| Race | 0.85 | ||
| White | 57 (42) | 30 (43) | |
| Black | 53 (39) | 24 (35) | |
| Other | 27 (20) | 15 (22) | |
| Language | 0.66 | ||
| English | 122 (89) | 60 (87) | |
| Non-English | 15 (11) | 9 (13) | |
| Insurance | 0.16 | ||
| Private | 55 (40) | 28 (41) | |
| Public | 12 (8.8) | 12 (17) | |
| Uninsured | 70 (51) | 29 (42) | |
| Abnormality | 0.33 | ||
| ASCUS/HPV+a | 36 (26) | 24 (35) | |
| AGC/AGUSb | 5 (3.7) | 4 (5.8) | |
| LGSILc | 85 (62) | 39 (57) | |
| HGSILd | 6 (4.4) | 2 (2.9) | |
| ASC-He | 5 (3.7) | 0 (0) |
aASCUS = atypical squamous cells of undetermined significance; HPV = human papillomavirus
bAGC = atypical glandular cells; AGUS = atypical glandular cells of undetermined significance
c LGSIL = low-grade squamous intraepithelial lesion
dHGSIL = high-grade squamous intraepithelial lesion
eASC-H = atypical squamous cells: cannot exclude high-grade squamous intraepithelial lesion
Outcomes
Table 3 shows the pre- and post-intervention results for the two primary measures of diagnostic resolution. The bivariate comparisons did not show a statistically significant difference between the pre- and post-intervention period, although the direction of difference favored our hypothesis. In bivariate hazard analyses we found that the severity of cytologic abnormality and practice location were associated at the p < 0.10 level and therefore included in the multivariable analysis. Age, race/ethnicity, language, and insurance status were not associated with the outcome. After multivariate adjustment for type of Pap abnormality and practice location, subjects in the post-intervention period were significantly more likely to ever achieve diagnostic resolution (OR, 15.4; CI, 3.7–62) and more likely to achieve diagnostic resolution in a shorter period of time (HR, 1.40; CI, 1.03–1.9), relative to subjects in the pre-intervention period. The adjusted mean time to diagnostic resolution decreased from 108 days (CI 105–112 days) to 86 days (CI 81 to 91 days). Colposcopy results were similar between the two groups, (p = 0.63) with 14% of women with CIN 2 or more severe lesions.
Table 3.
Outcomes of Abnormal Pap Tests Before and After Pap Tracking Intervention
| Pre-Intervention N = 137 | Post-Intervention N = 69 | P-value | |
|---|---|---|---|
| Achieved resolution n (%) | 127 (93%) | 67 (97%) | 0.20 |
| MEDIAN days to resolution Median (IQRa) | 72 (47–112) | 58 (36–102) | 0.04 |
| Unadjusted MEAN days to resolution Mean (CI) | 108 (92–125) | 86 (68–105) | 0.11 |
| Adjustedb MEAN days to resolution Mean (CI) | 108 (105–112) | 86(81–91) | 0.0002 |
| Adjusted odds ratiob of EVER achieving resolution OR (95% CI) | ref (1.00) | 15.4 (3.7–62) | 0.0002 |
| Adjusted odds ratiob of resolving in a SHORTER period of time OR (95% CI) | ref (1.00) | 1.40 (1.03–1.9) | 0.03 |
| Colposcopy result | |||
| Non-neoplastic | 60 (48%) | 38 (57%) | 0.63 |
| CIN 1c | 47 (38%) | 19 (28%) | |
| CIN 2 | 11 (9%) | 6 (9%) | |
| CIN 3 | 6 (5%) | 3 (5%) | |
| Invasive cervical cancer | 0 (0%) | 1 (1%) | |
| Other | 1 (<1%) | 0 (0%) | |
a IQR = interquartile range
b Adjusted for type of Pap abnormality and practice location
c CIN = cervical intraepithelial neoplasia
DISCUSSION
We developed and evaluated an EMR based tracking system for abnormal Pap tests, in order to assist providers in ensuring all abnormalities reached diagnostic resolution. We found that this tracking system significantly improved follow-up of abnormal Pap tests. Although many practices have developed electronic or paper tracking methods requiring manual entry, we report here on a novel method of incorporating the tracking within an electronic medical record that when initially developed had no intrinsic design features allowing this to happen.
Multiple studies have documented the problem of inadequate follow-up of abnormal Pap tests3–11. Leyden and colleagues looked at women diagnosed with cervical cancer from January 1995 to December 2000 in seven comprehensive health plans, and found that 13% of all cancers were attributed to inadequate follow-up of an abnormal Pap test4. Other studies have documented that 30–49% of women had either no follow up or delays beyond 3 to 7 months in abnormal Pap test follow-up in minority, uninsured or Medicaid-insured, or low income populations3–11. Systems specifically in safety net institutions that care for these communities are critical to improve follow-up rates and so improve effectiveness of cervical cancer screening. Our results are focused on resolution of abnormal Pap tests, and not in actual prevention of cervical cancer. Larger studies would be needed to demonstrate that such systems directly result in fewer women progressing to invasive cancer.
It is likely a combination of both systems and patient barriers that impede adequate abnormal Pap test follow-up. Patient barriers include difficulty in keeping follow-up appointments, limited understanding of the significance of the abnormality, and other life-issues taking priority. Systems barriers include failure of the provider to be aware of an abnormal result, and limited capability to systematically track patients who do not keep follow-up appointments. Our program addressed the systems barriers by giving providers tools to allow them to more easily track subjects after an abnormal Pap test. Our higher baseline follow up rates may already reflect some of the benefits of an EMR system; however, delays persisted without a tracking system.
Most of the EMRs used in outpatient medicine were developed with a focus on billing and require significant information technology development in data collection, synthesis and distribution to develop a tracking function. Our system required synthesis from multiple data sources, including scheduling, ordering, registration, and pathology. Data collection challenges included text-only fields in pathology reports; reports with standard result syntax or field based reports would avoid these pitfalls. Distribution of the reports was not automated, requiring that personnel adjust the programming and run the reports each month. Even during the intervention, changes to one part of the system resulted in difficulties and changes needed to access other parts of the system. Therefore, the system required some finite but constant resources to maintain, generate, and distribute the tracking reports. Despite these challenges, the system is in active use, and providers have reported satisfaction with the systems’ ability to catch those cases that fall between the cracks. Most providers receive a monthly list of fewer than five patients. Of note, our work was supported through the medical center’s risk management department, given the quality improvement benefits of the system. Given that many health care systems have adopted self insurance for malpractice, risk management funds might be a source to support other primary care initiatives for tracking and case management which improve quality of care and reduces risk13.
One component of a medical home14 is the ability to conduct population-based management of care, including tracking of abnormal screening test results. Our system serves as an example of the successful development and utilization of such a tracking system toward management of an entire practice. Our tracking system did not provide any additional assistance or personnel to the provider after they are given the monthly tracking report. Due to the relatively small number of abnormal Pap tests, providers were able to utilize existing staff personnel and their own efforts to ensure follow-up was achieved. This might be more of an issue if this tracking model was applied to a larger-volume abnormality, such as abnormal mammograms, cholesterol results, or glycosylated hemoglobin results in patients with diabetes. Additional resources, in terms of case management15 or patient navigation16, along with electronic tracking systems, have been employed to provide the additional follow through necessary when more frequent rates of an abnormality are expected, including phone or mail contact with patients, and rescheduling of missed appointments.
Acknowledgements
Financial support for this study was provided by a grant from the Risk Management Fund at Boston Medical Center. This study was supported by Boston University School of Medicine, Boston, MA. Preliminary data were presented at the 2009 Annual Meeting of the Society of General Internal Medicine in Miami as a poster presentation.
Conflict of Interest None disclosed.
References
- 1.The American Cancer Society. What Are the Key Statistics About Cervical Cancer? http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_statistics_for_cervical_cancer_8.asp. Accessed January 28, 2010.
- 2.Schiffman MH, Brinton LA, Devesa SS. Cervical cancer. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. 2. New York: Oxford University Press; 1996. pp. 1090–116. [Google Scholar]
- 3.Peterson NB, Han J, Freund KM. Inadequate follow-up for abnormal Pap smears in an urban population. J Natl Med Assoc. 2003;95(9):825–32. [PMC free article] [PubMed] [Google Scholar]
- 4.Leyden WA, Manos MM, Geiger AM, et al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst. 2005;97(9):675–83. doi: 10.1093/jnci/dji115. [DOI] [PubMed] [Google Scholar]
- 5.Marcus AC, Crane LA, Kaplan CP, et al. Improving adherence to screening follow-up among women with abnormal Pap smears: results from a large clinic-based trial of three intervention strategies. Med Care. 1992;30(3):216–30. doi: 10.1097/00005650-199203000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Cartwight PS, Reed G. No-show behavior in a county hospital clinic. Am J Gynecol Health. 1990;6:15–21. [Google Scholar]
- 7.Laedtke TW, Dignan M. Compliance with therapy for cervical dysplasia among women of low socioeconomic status. South Med J. 1992;85(1):5–8. doi: 10.1097/00007611-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Lane DS. Compliance with referrals from a cancer-screening project. J Fam Pract. 1983;17(5):811–7. [PubMed] [Google Scholar]
- 9.Carey P, Gjerdingen DK. Follow-up of abnormal Papanicolaou smears among women of different races. J Fam Pract. 1993;37(6):583–7. [PubMed] [Google Scholar]
- 10.Nathoo V. Investigation of non-responders at a cervical cancer screening clinic in Manchester. Br Med J (Clin Res Ed) 1988;296(6628):1041–2. doi: 10.1136/bmj.296.6628.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paskett ED, White E, Carter WB, Chu J. Improving follow-up after an abnormal Pap smear: a randomized controlled trial. Prev Med. 1990;19(6):630–41. doi: 10.1016/0091-7435(90)90060-W. [DOI] [PubMed] [Google Scholar]
- 12.Freund KM, Battaglia TA, Calhoun E, et al. National Cancer Institute Patient Navigation Research Program: methods, protocol, and measures. Cancer. 2008;113(12):3391–9. doi: 10.1002/cncr.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Townsend RW. Formation and operation of a captive insurance company for malpractice coverage. Coll Rev. 1988;5:47–61. [PubMed] [Google Scholar]
- 14.National Committee for Quality Assurance (NCQA). Standards and Guidelines for Physician Practice Connections - Patient-Centered Medical Home (PPC-PCMH): 2008.
- 15.Norris SL, Nichols PJ, Caspersen CJ, et al. The effectiveness of disease and case management for people with diabetes. A systematic review. Am J Prev Med. 2002;22(4 Suppl):15–38. doi: 10.1016/S0749-3797(02)00423-3. [DOI] [PubMed] [Google Scholar]
- 16.Battaglia TA, Roloff K, Posner MA, Freund KM. Improving follow-up to abnormal breast cancer screening in an urban population. A patient navigation intervention. Cancer. 2007;109(2 Suppl):359–67. doi: 10.1002/cncr.22354. [DOI] [PubMed] [Google Scholar]