Abstract
Background
Chemoprophylaxis is recommended for medical patients at moderate to high risk of venous thromboembolism (VTE) and is now a requirement of the Joint Commission on Accreditation of Healthcare Organizations. To see who receives prophylaxis and how far hospitals will need to go to meet this requirement, we examined VTE prophylaxis patterns at US hospitals.
Methods
We conducted a retrospective cohort study of adult patients with seven medical diagnoses considered to carry moderate to high risk of VTE at 376 acute care facilities in 2004–2005. We excluded patients on warfarin or with hospital stays of <2 days. VTE prophylaxis was assessed by billing codes for any heparin or compression device. We classified patient risk using a VTE risk prediction model.
Results
Of 351,535 patients included, 36% received prophylaxis by hospital day 2. Prophylaxis rates were highest among patients with certain VTE risk factors, including mechanical ventilation (67%), restraints (57%), central lines (55%), obesity (46%), and prior VTE (44%). The median hospital rate was 31% (IQR 19% to 42%); only 3% of hospitals had rates >70%. Compared to patients at low risk of VTE (<0.05%), patients at high risk (>1.0%) were more likely to receive prophylaxis (52% vs. 34%, p < 0.001). Hospitals with high rates of prescribing for high-risk patients also had high rates for low-risk patients.
Conclusions
VTE prophylaxis rates at US hospitals are substantially below Joint Commission targets, even for patients at highest risk of VTE.
Key words: venous thromboembolism (VTE), hospital mortality, chemoprophylaxis
INTRODUCTION
Despite decades of research on measures to prevent venous thromboembolism (VTE), it remains a major source of morbidity and mortality for hospitalized patients, with as many as 16% of high-risk medical patients developing VTE during their hospital stay.1,2 Pharmacologic prophylaxis with subcutaneous heparin reduces the risk of VTE by approximately 50%,3 and is therefore recommended for specific groups of patients at high risk of developing VTE. Guidelines produced by the American College of Chest Physicians (ACCP) recommend thromboprophylaxis for patients hospitalized for stroke, acute myocardial infarction, heart failure, respiratory disease, sepsis, and cancer.2
Studies conducted more than a decade ago have shown that physicians often fail to use this important therapy in a variety of high-risk settings, including the perioperative period, during critical illness, and among other high-risk medical patients.4–6 More recently, several large, multinational observational studies have demonstrated that approximately half of all medical patients met ACCP criteria for high risk of VTE, and of these, approximately 60% of US patients received some sort of VTE prophylaxis.7,8 However, these studies enrolled fewer than 10,000 US patients at a limited number of hospitals and did not characterize the variability in prescribing practices among US hospitals or physicians.
Since then, the Joint Commission on Accreditation of Healthcare Organizations has recommended that all hospitalized medical patients receive prophylaxis against VTE within 2 days of admission or have documentation as to why no VTE prophylaxis was given.9 To better understand who receives VTE prophylaxis, and to see how far hospitals will need to go to meet the new Joint Commission requirements, we examined patterns of use of VTE prophylaxis among a large and representative sample of hospitalized medical patients in the US.
METHODS
Setting and Patients
We identified a sample of patients discharged between January 1, 2004 and June 30, 2005 from 376 acute care facilities in the US that participated in Premier’s Perspective, a database used to measure quality and resource utilization, which has been described previously.10 Participating hospitals represent all regions of the US and are similar in composition to US hospitals; however, in comparison to information contained in the American Hospital Association annual survey, Perspective hospitals are more likely to be located in the South and in urban areas. Available data elements for each patient include sociodemographic information, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, as well as a date-stamped log of all items and billed services, including diagnostic tests, medications, and other treatments. Hospitals are characterized by size, region, setting, and teaching status. Permission to conduct the study was obtained from the Institutional Review Board at Baystate Medical Center.
We included all general medical patients aged ≥18 years whose diagnosis placed them at moderate to high risk of VTE according to the ACCP recommendations.2 This included patients with pneumonia, septicemia, or respiratory failure with pneumonia, heart failure, chronic obstructive pulmonary disease (COPD), stroke, and urinary tract infection. Diagnoses were assessed using ICD-9-CM codes. We did not include patients with acute coronary syndromes because of the frequent use of anticoagulation for the primary diagnosis. We also excluded patients who received warfarin on hospital day 1 or 2, because they would not be considered at risk of VTE, and patients whose length of stay was ≤ 2 days, because such cases would not be included in the Joint Commission measure and because the value of VTE prophylaxis in such cases is unknown.
Risk Factors
For each patient, we extracted age, gender, race/ethnicity, and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD-9-CM secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser.11 We used a combination of ICD-9 codes and charges for medications and supplies to identify the presence of risk factors that have been previously linked to VTE, including paralysis, cancer (metastatic, solid tumor, and lymphoma), chemotherapy/radiation, prior VTE, use of estrogens and estrogen modulators, inflammatory bowel disease, nephrotic syndrome, myeloproliferative disorders, obesity, smoking, central venous catheter, inherited or acquired thrombophilia, mechanical ventilation, urinary catheter, decubitus ulcer, HMGco-A reductase inhibitors, restraints, diabetes, and varicose veins.
Definitions of Prophylaxis
We considered VTE prophylaxis to have been provided if the patient received at least a single dose of low molecular weight heparin (LMWH), unfractionated heparin (UH), a pneumatic compression device, or compression stockings during the first 2 days of hospitalization. We considered the patient to have received standard prophylaxis if either LMWH or UFH was initiated at the recommended dosage (40 mg daily or 5,000 units twice or thrice daily, respectively) by hospital day 2 and continued for the duration of the hospitalization, or until diagnosis and treatment for VTE occurred. Because we did not have access to medication administration records, it was not possible to discern whether a missing charge for prophylaxis represented the failure to properly order or administer the medication or represented instead a failure to bill completely.
Statistical Analysis
Univariate predictors of any VTE prophylaxis were assessed using chi-square tests. We also assessed the degree to which the use of measures to prevent VTE varied according to patient risk [low (<0.5%), intermediate (0.5% to 1.0%) and high (>1.0%)] using a risk prediction instrument developed by the authors using this dataset. The instrument included the following factors: age, sex, primary diagnosis, inflammatory bowel disease, thrombophilia, obesity, cancer, central venous catheter, mechanical ventilation, urinary catheter, active chemotherapy, and steroids. In order to distinguish the relative contributions of physician and hospital practices from patient risk, we stratified the physicians and hospitals into quartiles of prescribing and then examined the proportion of patients in each risk group who received prophylaxis. All analyses were performed using the Statistical Analysis System (version 9.1, SAS Institute, Inc., Cary, NC).
RESULTS
Our sample included 351,535 patients from 376 hospitals (Table 1). Most patients were over age 65, 58% were female, and 66% were white. All patients had a primary diagnosis that made them potential candidates for VTE prophylaxis, the most common being pneumonia (31%) and congestive heart failure (24%). In addition, many patients had additional risk factors for thromboembolism, such as cancer (14%), paralysis (7%), or diabetes (32%). Others had procedures such as mechanical ventilation (5%), central lines (3%), or urinary catheters (14%) that result in immobilization. Most patients were cared for by internists (54%) or family practitioners (20%). The majority of patients were cared for at hospitals in the South (55%).
Table 1.
Patient and Hospital Characteristics Associated with Early VTE Prophylaxis
| Total | Early prophylaxis (by Day 2) | ||||
|---|---|---|---|---|---|
| Predictors | N | Column % | N | Row % | p-value |
| 351,535 | 100.0 | 125,433 | 35.7 | ||
| Demographics | |||||
| Age | <0.001 | ||||
| 18–49 | 38,773 | (11.0) | 12,457 | (32.1) | |
| 50–64 | 69,311 | (19.7) | 26,582 | (38.4) | |
| 65–79 | 123,387 | (35.1) | 46,069 | (37.3) | |
| 80+ | 120,064 | (34.2) | 40,325 | (33.6) | |
| Gender | <0.001 | ||||
| Male | 147,910 | (42.1) | 54,211 | (36.7) | |
| Female | 203,616 | (57.9) | 71,218 | (35.0) | |
| Race | <0.001 | ||||
| White | 231,184 | (65.8) | 81,739 | (35.4) | |
| Black | 55,712 | (15.8) | 22,517 | (40.4) | |
| Hispanic | 13,745 | (3.9) | 4,742 | (34.5) | |
| Other | 50,894 | (14.5) | 16,435 | (32.3) | |
| Primary diagnosis | <0.001 | ||||
| Community-acquired pneumonia | 108,639 | (30.9) | 33,098 | (30.5) | |
| Septicemia | 11,576 | (3.3) | 4,494 | (38.8) | |
| Chronic obstructive pulmonary disease | 45,888 | (13.1) | 15,152 | (33.0) | |
| Respiratory failure | 13,015 | (3.7) | 8,007 | (61.5) | |
| Congestive heart failure | 85,222 | (24.2) | 36,359 | (42.7) | |
| Stroke | 49,557 | (14.1) | 20,656 | (41.7) | |
| Urinary tract infection | 37,638 | (10.7) | 7,667 | (20.4) | |
| Attending specialty | <0.001 | ||||
| Internist | 190,284 | (54.1) | 70,459 | (37.0) | |
| GP/family medicine | 71,765 | (20.4) | 19,118 | (26.6) | |
| Cardiologist | 25,508 | (7.3) | 10,467 | (41.0) | |
| Pulmonologist | 21,009 | (6.0) | 8,955 | (42.6) | |
| Nephrology | 8,522 | (2.4) | 2,521 | (29.6) | |
| Other | 34,447 | (9.8) | 13,913 | (40.4) | |
| Insurance payor | <0.001 | ||||
| Medicare traditional | 235,033 | (66.9) | 82,534 | (35.1) | |
| Medicare-managed care | 16,347 | (4.7) | 6,477 | (39.6) | |
| Medicaid | 23,120 | (6.6) | 8,511 | (36.8) | |
| Private | 61,505 | (17.5) | 22,388 | (36.4) | |
| Self-pay/uninsured/other | 15,530 | (4.4) | 5,523 | (35.6) | |
| Risk factors for VTE | |||||
| Admitted from skilled nursing facility | 4,460 | (1.3) | 1,523 | (34.1) | 0.03 |
| Paralysis | 25,311 | (7.2) | 10,770 | (42.6) | <0.001 |
| Restraints | 4,759 | (1.4) | 2,690 | (56.5) | <0.001 |
| Decubitus ulcer | 10,591 | (3.0) | 4,143 | (39.1) | <0.001 |
| Cancer | 48,180 | (13.7) | 16,527 | (34.3) | <0.001 |
| Chemotherapy | 1,033 | (0.3) | 358 | (34.7) | 0.49 |
| Prior venous thromboembolism | 5,553 | (1.6) | 2,445 | (44.0) | <0.001 |
| Pregnancy | 27 | (0.0) | 7 | (25.9) | 0.29 |
| Estrogens | 5,368 | (1.5) | 1,594 | (29.7) | <0.001 |
| Estrogen modulators | 2,648 | (0.8) | 818 | (30.9) | <0.001 |
| Secondary diagnosis of atrial fibrillation | 57,192 | (16.3) | 25,134 | (43.9) | <0.001 |
| Prosthetic heart valve | 3,250 | (0.9) | 1,145 | (35.2) | 0.59 |
| Congestive heart failure | 35,116 | (10.0) | 15,673 | (44.6) | <0.001 |
| Respiratory failure-secondary diagnosis | 24,760 | (7.0) | 13,144 | (53.1) | <0.001 |
| Inflammatory bowel disease | 1,149 | (0.3) | 382 | (33.2) | 0.08 |
| Nephrotic syndrome | 794 | (0.2) | 319 | (40.2) | 0.008 |
| Myeloproliferative disorder | 3,118 | (0.9) | 1,063 | (34.1) | 0.06 |
| Paroxysmal nocturnal hemoglobinuria | 9 | (0.0) | 3 | (33.3) | 0.88 |
| Obesity | 26,705 | (7.6) | 12,169 | (45.6) | <0.001 |
| Smoking | 47,139 | (13.4) | 18,150 | (38.5) | <0.001 |
| Varicose veins | 309 | (0.1) | 98 | (31.7) | 0.15 |
| Central line | 10,662 | (3.0) | 5,872 | (55.1) | <0.001 |
| Inherited or acquired thrombophilia | 295 | (0.1) | 127 | (43.1) | 0.008 |
| Diabetes mellitus | 110,717 | (31.5) | 42,407 | (38.3) | <0.001 |
| Mechanical ventilation | 18,808 | (5.4) | 12,585 | (66.9) | <0.001 |
| Computed risk of VTE | <0.001 | ||||
| <0.5% | 278,807 | (79.3) | 94,195 | (33.8) | |
| 0.5% to 1.0% | 49,587 | (14.1) | 19,221 | (38.8) | |
| >1.0% | 23,132 | (6.6) | 12,013 | (51.9) | |
| Hospital characteristics | |||||
| Region | <0.001 | ||||
| South | 193,848 | (55.1) | 68,258 | (35.2) | |
| Midwest | 66,364 | (18.9) | 25,744 | (38.8) | |
| West | 38,175 | (10.9) | 10,435 | (27.3) | |
| Northeast | 53,148 | (15.1) | 20,996 | (39.5) | |
| Number of beds | <0.001 | ||||
| 0–200 | 61,287 | (17.4) | 15,931 | (26.0) | |
| 201–300 | 57,205 | (16.3) | 17,491 | (30.6) | |
| 301–500 | 126,695 | (36.0) | 46,454 | (36.7) | |
| >500 | 106,348 | (30.3) | 45,557 | (42.8) | |
| Teaching hospital | 117,949 | (33.6) | 50,336 | (42.7) | <0.001 |
| Rural hospital | 75,058 | (21.4) | 21,174 | (28.2) | <0.001 |
VTE Prophylaxis Rates
Thirty-six percent of patients received some form of prophylaxis by the second hospital day, and 11% received chemoprophylaxis at the usual dosage throughout their entire stay (standard prophylaxis). Of patients who did receive prophylaxis, 34% received unfractionated heparin, 60% low molecular weight heparin, and 13% some sort of compression. An additional 9% of patients received prophylaxis that began after hospital day 2. Rates of prophylaxis varied according to primary diagnosis, from 62% of patients with respiratory failure to only 20% of patients with UTI. Having certain additional VTE risk factors beyond primary diagnosis was associated with receipt of prophylaxis. The highest rates were seen among patients who appeared to be immobilized, as indicated by mechanical ventilation (67%), restraints (57%), central lines (55%), urinary catheters (43%), paralysis (43%), or decubitus ulcers (39%). Patients with obesity, nephrotic syndrome, inherited thrombophilias, or a history of VTE also had high rates of prophylaxis. Cancer patients actually had a slightly lower rate of prophylaxis than non-cancer patients (34% vs. 36%, p < 0.001). Use of prophylaxis varied by region and was highest in the Northeast and lowest in the West. Rates were also highest at large hospitals (43% at hospitals with >500 beds vs. 26% at hospitals with <200 beds, p < 0.001) and teaching institutions (43% vs. 32% at non-teaching institutions). Patients cared for by pulmonologists were most likely (43%) and those cared for by family practitioners least likely (27%) to receive prophylaxis.
Variation in Rates and Choice of Prophylaxis Across Hospitals and Physicians
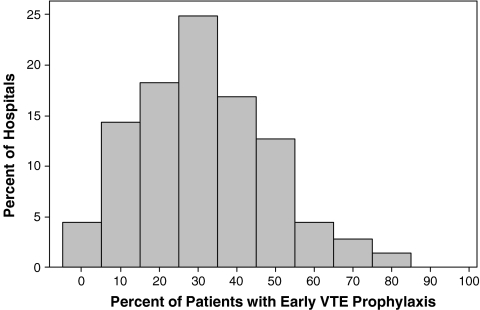
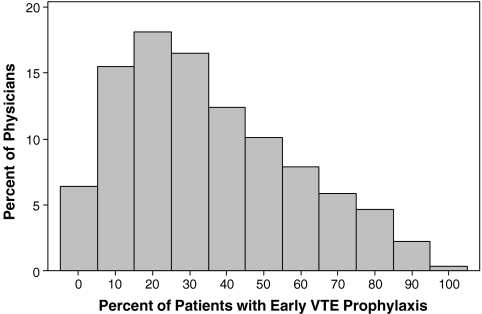
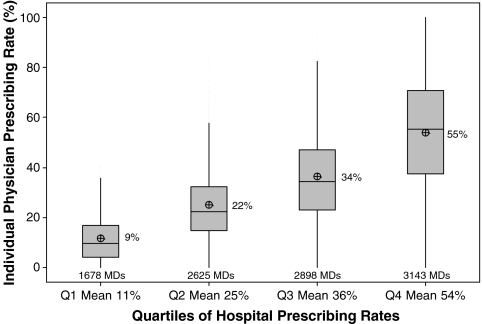
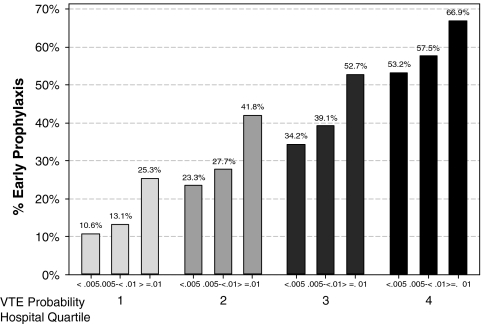
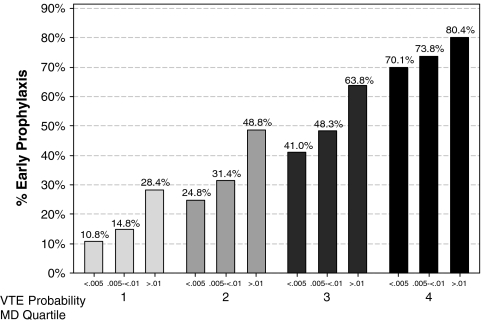
There was substantial variation in prescribing rates across hospitals (Fig. 1) and physicians (Fig. 2). Among hospitals contributing at least 100 patients, the median rate was 31% and the interquartile range was 19% to 42%. Physician prescribing rates tended to cluster around the hospital rates, with the wide variation in all four quartiles (Fig. 3). We found that receipt of VTE prophylaxis varied with a patient’s predicted risk of VTE—52% of patients with a VTE risk of > 1.0% received prophylaxis compared to 34% of patients with a VTE risk of <0.5%. When hospitals (Fig. 4) and physicians (Fig. 5) were stratified into quartiles based on their use of prophylaxis, the effect of patient risk was still apparent. However, with few exceptions, the probability of a patient receiving prophylaxis was more dependent on the hospital in which they were treated and the attending physician than the patient’s individual predicted risk, so that a low-risk patient at a hospital with a high prescribing rate was more likely to receive prophylaxis than a high-risk patient at a hospital with a lower prescribing rate.
Figure 1.
Prescribing rates of VTE prophylaxis across 362 US hospitals.
Figure 2.
Prescribing rates among 10,353 physicians.
Figure 3.
Variation in individual physician prescribing rates for early prophylaxis within quartiles of hospital prescribing rate. For each hospital quartile, the mean hospital rate is noted.
Figure 4.
Quartiles of hospital prophylaxis rates stratified by patient risk for VTE.
Figure 5.
Quartiles of physician prophylaxis rates stratified by patient risk for VTE.
The choice of which chemoprophylaxis was used appeared to depend more on the physician than the hospital in which care was delivered. Approximately 25% hospitals and 23% of physicians used low molecular weight heparin exclusively, while 13% of hospitals and 29% of physicians used unfractionated heparin exclusively.
DISCUSSION
Despite decades of attention, well-publicized guidelines, and ongoing quality improvement efforts, rates of VTE prophylaxis among general medical patients remain stubbornly low. In this cross-sectional study of patients from 376 US hospitals, representing approximately 10% of all patients hospitalized in the US, we found that less than 40% of patients who appeared to be candidates for VTE prophylaxis according to the ACCP guidelines actually received any. As has been observed with other treatments, we found that prophylaxis rates vary varied widely, not only across hospitals, but also among attending physicians within hospitals, and that physician and hospital practice outweighed the patients’ own risk of VTE in determining whether or not a patient would receive prophylaxis.
The rates of prophylaxis observed in the hospitals included in our study were lower than those reported in other contemporary studies. For example, both the prospective ENDORSE study8 and the IMPROVE registry7 found that prophylaxis rates among US medical patients meeting ACCP criteria exceeded 60%. There may be several explanations for this discrepancy. First, we measured only prophylaxis begun by hospital day 2, whereas the other studies included prophylaxis at any time. Using that measure, however, only increased our rates slightly, to 45%. Second, we had no direct information on either patient mobility or whether patients had been admitted for comfort care only. Some of the patients in our sample may not have been candidates if they were mobile or comfort measures only. It is likely that excluding both types of patients would have improved our overall rates. Finally, these other studies included limited numbers of US patients and hospitals. Reports of prophylaxis rates at individual institutions, especially after quality improvement initiatives, may be substantially higher. We also found that in 3% of our study hospitals, more than 70% of patients received prophylaxis, but we also found a number of hospitals where prophylaxis was seldom used. Small, rural, non-teaching hospitals tended to have the lowest rates of prophylaxis, and these may not have been included in other studies. The rate we observed in large teaching hospitals is closer to what others have observed.
Because pharmacological prophylaxis involves painful injections between one and three times daily, carries a risk of thrombocytopenia and bleeding, and can be expensive, it should be reserved for patients at moderate to high risk for VTE. The estimation of risk, however, is not straightforward, and the Joint Commission does not endorse a specific risk prediction tool.9 While prior studies have focused on prophylaxis rates among patients meeting ACCP criteria for prophylaxis, they did not evaluate the effects of additional risk factors and were thus unable to assess how prescribing patterns were influenced by the overall risk of VTE. Although our sample consisted entirely of patients considered to be in need of prophylaxis according to current guidelines, we found that physicians appear to prescribe prophylaxis more selectively, taking into account individual risk factors. As evidence of this risk-based decision making, we noted that patients at highest risk were 50% more likely to get prophylaxis than those at low risk.
Finally, we found that which form of chemoprophylaxis a patient receives is largely determined by the preferences of the physicians and hospitals where they received care. In contrast to ENDORSE and IMPROVE, we found that low molecular weight heparin was prescribed more often than unfractionated heparin. For patients receiving recommended prophylaxis, just over 1/3 of hospitals used one method of chemoprophylaxis almost exclusively, perhaps reflecting formulary decisions or prescribing guidelines, and 49% of physicians prescribed just one form of chemoprophylaxis, with more choosing unfractionated heparin. This pattern is not surprising, given that neither form of heparin has been definitively shown to be more effective, safer, or overall less expensive.
Our study has a number of limitations. First, it is a retrospective study based on hospital claims data. Nevertheless, the highly detailed nature of our dataset allowed us to identify risk factors (e.g., urinary catheter) and treatments that cannot be obtained from commonly used sources of data. Second, we used an inclusive definition of prophylaxis, giving credit for even limited use of heparin or pneumatic compression. While a far smaller percentage of patients in our study received standard prophylaxis, this may reflect a combination of inaccurate billing and physician error. Third, venous thromboembolism is an area that has received substantial attention from quality improvement organizations and professional societies, and rates today may be higher than those seen even a few years ago.
Despite decades of advocacy for greater use of VTE prophylaxis, VTE remains a common complication of hospitalization, and medical patients continue to comprise the majority of patients with hospital-acquired VTE. The new Joint Commission reporting measures may help to redress this problem. Our results suggest that most institutions still have a long way to go to achieve the Joint Commission goals. Our findings also have important implications for the implementation of Joint Commission reporting. Our study confirmed that almost all doctors prescribe VTE prophylaxis12 and that they are more likely to prescribe it for higher risk patients than lower risk ones. Still, the differences in rates between hospitals are much greater than the differences among patient risk groups within hospitals. The true determinant of hospital rates remains unknown, but they may reflect either local beliefs about the value of prophylaxis or quality improvement initiatives (e.g., checklists or other reminder systems). Although higher rates may be achievable by encouraging physicians to prescribe VTE prophylaxis for almost all medical patients,13 it is not clear that such an unbalanced strategy will result in better patient care. Our results suggest that within hospitals, patient risk had only a modest influence on physician decision making. Consequently, as prophylaxis rates rise for moderate and high-risk patients (appropriate prescribing), they also increase for low-risk patients (overprescribing). The Joint Commission measures, however, require justification only for failure to prescribe; they do not recognize or discourage overprescribing. Given that symptomatic VTE may occur in less than 1% of medical inpatients14 and that VTE prophylaxis has not been shown to decrease mortality,3 concerns about bleeding risks and the costs associated with prescribing for low-risk patients should be taken seriously.15,16 Further improvements in VTE prophylaxis for medical patients might best be served by the development of better risk stratification tools and reporting metrics that balance underprescribing with overprescribing.
Acknowledgements
This study was supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation. The funding source had no role in the study design, analysis or interpretation of the data.
Conflict of Interest None disclosed.
References
- 1.Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341:793–800. doi: 10.1056/NEJM199909093411103. [DOI] [PubMed] [Google Scholar]
- 2.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American college of chest physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 3.Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: A meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1476–1486. doi: 10.1001/archinte.167.14.1476. [DOI] [PubMed] [Google Scholar]
- 4.Stratton MA, Anderson FA, Bussey HI, et al. Prevention of venous thromboembolism: Adherence to the 1995 American college of chest physicians consensus guidelines for surgical patients. Arch Intern Med. 2000;160:334–340. doi: 10.1001/archinte.160.3.334. [DOI] [PubMed] [Google Scholar]
- 5.Keane MG, Ingenito EP, Goldhaber SZ. Utilization of venous thromboembolism prophylaxis in the medical intensive care unit. Chest. 1994;106:13–4. doi: 10.1378/chest.106.1.13. [DOI] [PubMed] [Google Scholar]
- 6.Goldhaber SZ, Dunn K, MacDougall RC. New onset of venous thromboembolism among hospitalized patients at Brigham and Women’s Hospital is caused more often by prophylaxis failure than by withholding treatment. Chest. 2000;118:1680–4. doi: 10.1378/chest.118.6.1680. [DOI] [PubMed] [Google Scholar]
- 7.Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: Findings from the international medical prevention registry on venous thromboembolism. Chest. 2007;132:936–45. doi: 10.1378/chest.06-2993. [DOI] [PubMed] [Google Scholar]
- 8.Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): A multinational cross-sectional study. Lancet. 2008;371:387–94. doi: 10.1016/S0140-6736(08)60202-0. [DOI] [PubMed] [Google Scholar]
- 9.The Joint Commission on the Accreditation of Healthcare Organizations. Venous Thromboembolism (VTE) Core Measure Set. 2009.
- 10.Rothberg MB, Pekow PS, Liu F, et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3:91–102. doi: 10.1002/jhm.290. [DOI] [PubMed] [Google Scholar]
- 11.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Cook D, Tkaczyk A, Lutz K, McMullin J, Haynes RB, Douketis J. Thromboprophylaxis for hospitalized medical patients: A multicenter qualitative study. J Hosp Med. 2009;4:269–75. doi: 10.1002/jhm.461. [DOI] [PubMed] [Google Scholar]
- 13.Maynard G, Stein J. Preventing Hospital-Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [Google Scholar]
- 14.Dunn AS, Brenner A, Halm EA. The magnitude of an iatrogenic disorder: A systematic review of the incidence of venous thromboembolism for general medical inpatients. Thromb Haemost. 2006;95:758–62. [PubMed] [Google Scholar]
- 15.Schuurman B, Heijer M, Nijs AM. Thrombosis prophylaxis in hospitalised medical patients: Does prophylaxis in all patients make sense? Neth J Med. 2000;56:171–6. doi: 10.1016/S0300-2977(00)00011-5. [DOI] [PubMed] [Google Scholar]
- 16.Bergmann JF, Segrestaa JM, Caulin C. Prophylaxis against venous thromboembolism. Bmj. 1992;305:1156. doi: 10.1136/bmj.305.6862.1156-a. [DOI] [PMC free article] [PubMed] [Google Scholar]