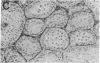
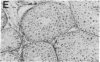
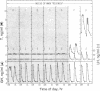
Abstract
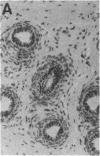
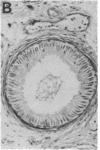
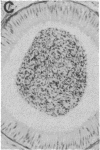
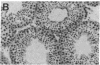
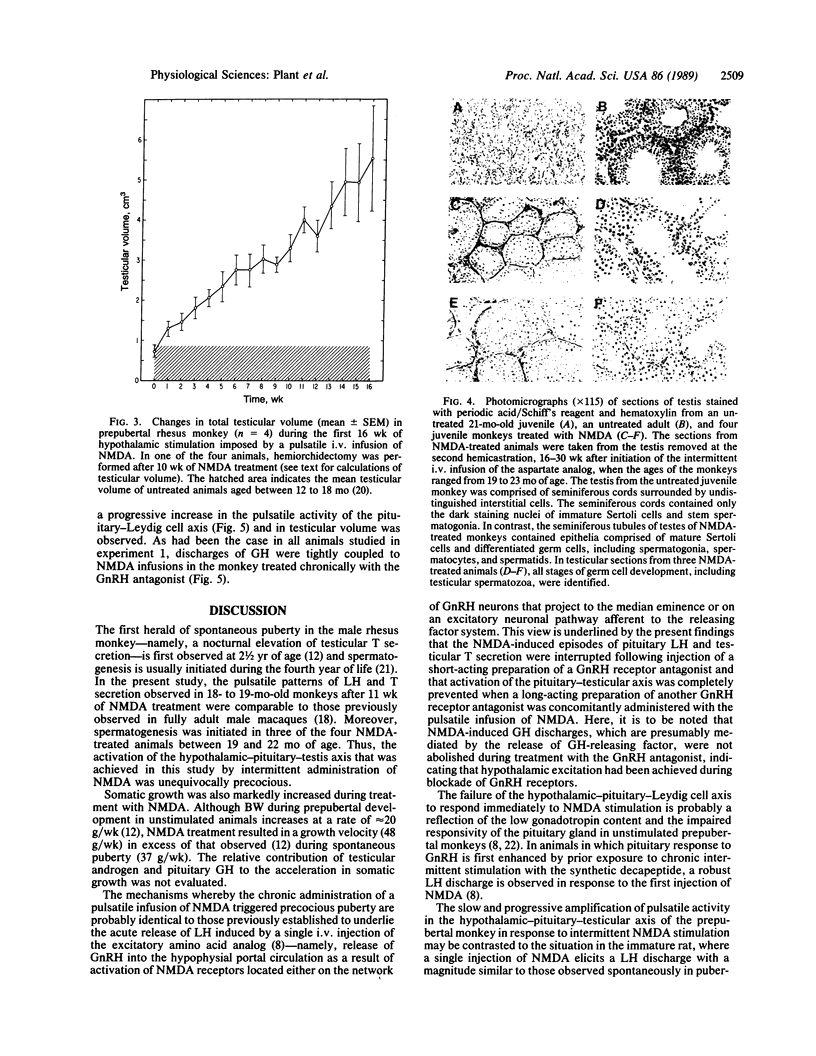
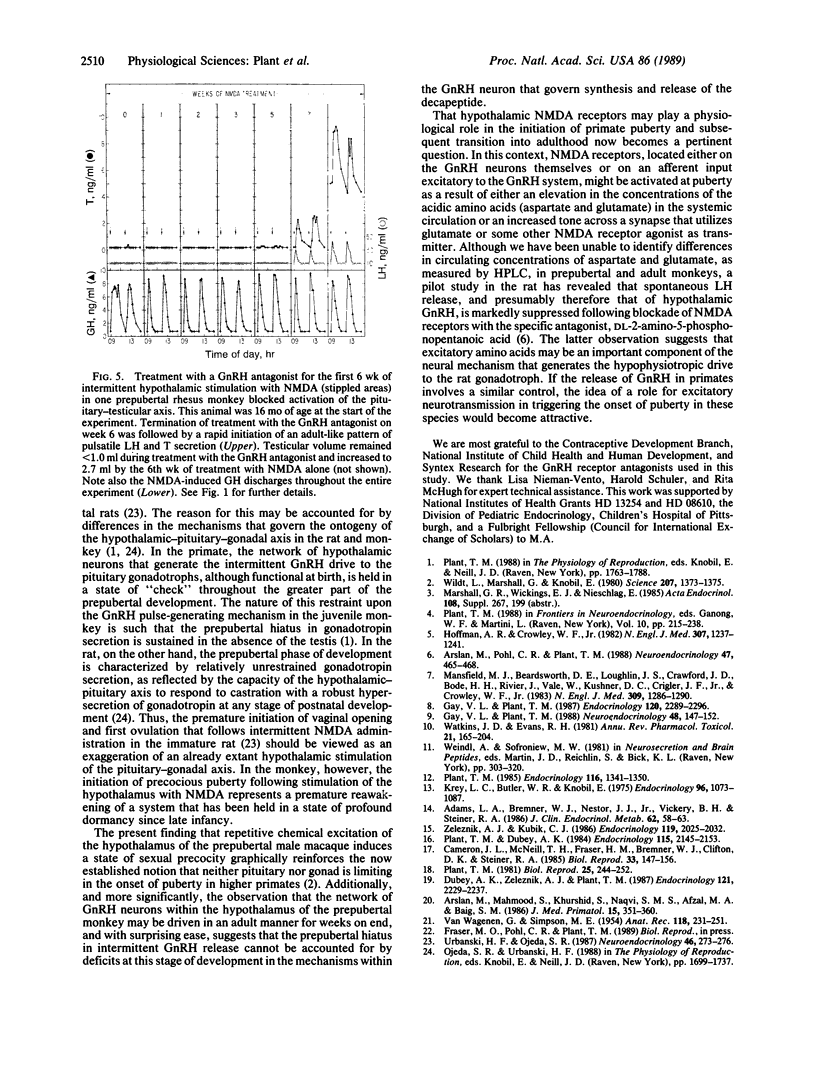
Gonadal quiescence prior to puberty in primates results from a diminished secretion of the pituitary gonadotropic hormones, follicle-stimulating hormone and luteinizing hormone, which, in turn, is occasioned by an interruption of pulsatile release of gonadotropin-releasing hormone (GnRH) from the hypothalamus during this phase of development. A discharge of GnRH may be provoked from the hypothalamus of prepubertal monkeys, however, by an i.v. injection of N-methyl-D-aspartate (NMDA), an analog of the putative excitatory neurotransmitter, aspartate. Since this action of NMDA is blocked by the specific NMDA receptor antagonist, DL-2-amino-5-phosphonopentanoic acid, the release of GnRH is likely mediated by NMDA receptors located either on the GnRH neurons themselves or on afferents to the GnRH cells. We report here that prolonged intermittent NMDA stimulation of GnRH neurons within the hypothalamus of the juvenile monkey for 16-30 wk results, with surprising ease, in the onset of precocious puberty with full activation of the hypothalamic-pituitary-Leydig cell axis and initiation of spermatogenesis. These findings demonstrate that, in primates, the network of hypothalamic GnRH neurons, which in adulthood provides the drive to the gonadotropin-secreting cells of the anterior pituitary gland, must now be viewed together with the pituitary and gonads as a nonlimiting component of the control system that governs the onset of puberty in these species.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. A., Bremner W. J., Nestor J. J., Jr, Vickery B. H., Steiner R. A. Suppression of plasma gonadotropins and testosterone in adult male monkeys (Macaca fascicularis) by a potent inhibitory analog of gonadotropin-releasing hormone. J Clin Endocrinol Metab. 1986 Jan;62(1):58–63. doi: 10.1210/jcem-62-1-58. [DOI] [PubMed] [Google Scholar]
- Arslan M., Mahmood S., Khurshid S., Naqvi S. M., Afzal M. A., Baig S. M. Changes in circulating levels of immunoreactive follicle stimulating hormone, luteinizing hormone, and testosterone during sexual development in the rhesus monkey, Macaca mulatta. J Med Primatol. 1986;15(5):351–359. [PubMed] [Google Scholar]
- Arslan M., Pohl C. R., Plant T. M. DL-2-amino-5-phosphonopentanoic acid, a specific N-methyl-D-aspartic acid receptor antagonist, suppresses pulsatile LH release in the rat. Neuroendocrinology. 1988 May;47(5):465–468. doi: 10.1159/000124951. [DOI] [PubMed] [Google Scholar]
- Cameron J. L., McNeill T. H., Fraser H. M., Bremner W. J., Clifton D. K., Steiner R. A. The role of endogenous gonadotropin-releasing hormone in the control of luteinizing hormone and testosterone secretion in the juvenile male monkey, Macaca fascicularis. Biol Reprod. 1985 Aug;33(1):147–156. doi: 10.1095/biolreprod33.1.147. [DOI] [PubMed] [Google Scholar]
- Dubey A. K., Zeleznik A. J., Plant T. M. In the rhesus monkey (Macaca mulatta), the negative feedback regulation of follicle-stimulating hormone secretion by an action of testicular hormone directly at the level of the anterior pituitary gland cannot be accounted for by either testosterone or estradiol. Endocrinology. 1987 Dec;121(6):2229–2237. doi: 10.1210/endo-121-6-2229. [DOI] [PubMed] [Google Scholar]
- Gay V. L., Plant T. M. N-methyl-D,L-aspartate elicits hypothalamic gonadotropin-releasing hormone release in prepubertal male rhesus monkeys (Macaca mulatta). Endocrinology. 1987 Jun;120(6):2289–2296. doi: 10.1210/endo-120-6-2289. [DOI] [PubMed] [Google Scholar]
- Gay V. L., Plant T. M. Sustained intermittent release of gonadotropin-releasing hormone in the prepubertal male rhesus monkey induced by N-methyl-DL-aspartic acid. Neuroendocrinology. 1988 Aug;48(2):147–152. doi: 10.1159/000125002. [DOI] [PubMed] [Google Scholar]
- Hoffman A. R., Crowley W. F., Jr Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med. 1982 Nov 11;307(20):1237–1241. doi: 10.1056/NEJM198211113072003. [DOI] [PubMed] [Google Scholar]
- Krey L. C., Butler W. R., Knobil E. Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I. Gonadotropin secretion. Endocrinology. 1975 May;96(5):1073–1087. doi: 10.1210/endo-96-5-1073. [DOI] [PubMed] [Google Scholar]
- Mansfield M. J., Beardsworth D. E., Loughlin J. S., Crawford J. D., Bode H. H., Rivier J., Vale W., Kushner D. C., Crigler J. F., Jr, Crowley W. F., Jr Long-term treatment of central precocious puberty with a long-acting analogue of luteinizing hormone-releasing hormone. Effects on somatic growth and skeletal maturation. N Engl J Med. 1983 Nov 24;309(21):1286–1290. doi: 10.1056/NEJM198311243092104. [DOI] [PubMed] [Google Scholar]
- Plant T. M. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta). Endocrinology. 1985 Apr;116(4):1341–1350. doi: 10.1210/endo-116-4-1341. [DOI] [PubMed] [Google Scholar]
- Plant T. M., Dubey A. K. Evidence from the rhesus monkey (Macaca mulatta) for the view that negative feedback control of luteinizing hormone secretion by the testis is mediated by a deceleration of hypothalamic gonadotropin-releasing hormone pulse frequency. Endocrinology. 1984 Dec;115(6):2145–2153. doi: 10.1210/endo-115-6-2145. [DOI] [PubMed] [Google Scholar]
- Plant T. M. Time courses of concentrations of circulating gonadotropin, prolactin, testosterone, and cortisol in adult male rhesus monkeys (Macaca mulatta) throughout the 24 h light-dark cycle. Biol Reprod. 1981 Sep;25(2):244–252. doi: 10.1095/biolreprod25.2.244. [DOI] [PubMed] [Google Scholar]
- Urbanski H. F., Ojeda S. R. Activation of luteinizing hormone-releasing hormone release advances the onset of female puberty. Neuroendocrinology. 1987 Sep;46(3):273–276. doi: 10.1159/000124831. [DOI] [PubMed] [Google Scholar]
- VAN WAGENEN G., SIMPSON M. E. Testicular development in the rhesus monkey. Anat Rec. 1954 Feb;118(2):231–251. doi: 10.1002/ar.1091180209. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Wildt L., Marshall G., Knobil E. Experimental induction of puberty in the infantile female rhesus monkey. Science. 1980 Mar 21;207(4437):1373–1375. doi: 10.1126/science.6986658. [DOI] [PubMed] [Google Scholar]
- Zeleznik A. J., Kubik C. J. Ovarian responses in macaques to pulsatile infusion of follicle-stimulating hormone (FSH) and luteinizing hormone: increased sensitivity of the maturing follicle to FSH. Endocrinology. 1986 Nov;119(5):2025–2032. doi: 10.1210/endo-119-5-2025. [DOI] [PubMed] [Google Scholar]