Abstract
Background
Little information is available about the association of depression with long-term control of glycemia, blood pressure, or lipid levels in patients with diabetes.
Objective
To determine whether minor and major depression at study enrollment compared with no depression are associated with higher average HbA1c, systolic blood pressure (SBP) and LDL cholesterol over the long term in patients with an indication for or receiving drug treatment.
Design
Cohort study.
Patients
A total of 3,762 patients with type 2 diabetes mellitus enrolled in the Pathways Epidemiologic Study in 2001–2002 and followed for 5 years.
Main Measures
Depression was assessed at study enrollment using the Patient Health Questionnaire-9 (PHQ-9). SBP and information on cardiovascular co-morbidity were abstracted from medical records, and LDL cholesterol and HbA1c measured during clinical care were obtained from computerized laboratory data during a median of 4.8 years’ follow-up.
Key Results
Among those with an indication for or receiving drug treatment, after adjustment for demographic and clinical characteristics, average long-term HbA1c, SBP, and LDL cholesterol did not differ in patients with comorbid diabetes and minor or major depression compared with those with diabetes alone.
Conclusions
The adverse effect of depression on outcomes in patients with diabetes may not be mediated in large part by poorer glycemic, blood pressure, or lipid control. Further study is needed of the biologic effects of depression on patients with diabetes and their relation to adverse outcomes.
KEY WORDS: depression, diabetes, glycemia, hypertension, hyperlipidemia
INTRODUCTION
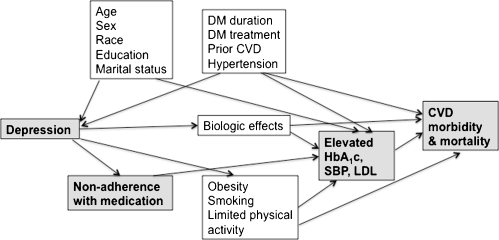
In patients with diabetes, comorbid depression is associated with higher mortality and higher risk of major complications.1–5 As shown in Fig. 1, this association may be due to biologic effects of depression on the hypothalamic-pituitary axis, platelet adhesion, inflammation, or the autonomic nervous system, resulting in less favorable cardiovascular outcomes. Alternatively, the association may be due to non-adherence with medication leading to worse control of glycemia, systolic blood pressure (SBP), and/or LDL cholesterol, resulting in higher cardiovascular disease rates (shaded boxes in Fig. 1).
Figure 1.
Causal diagram for the association of depression with poor risk factor control and cardiovascular disease morbidity and mortality in patients with diabetes. DM = diabetes mellitus, CVD = cardiovascular disease.
The purpose of this analysis was to examine whether there is an association of depression with worse long-term control of glycemia, SBP, and LDL cholesterol in patients with diabetes. Because depression is associated with poorer medication adherence in patients with diabetes,6 we hypothesized that inadequate adherence with medication would be an important mediator in the proposed relationship between depression and control of glycemia, SBP, and LDL cholesterol. We thus restricted our analyses to patients with indications for or receiving drug treatment for these risk factors.
We used data from a prospective study of patients with type 2 diabetes with assessment of depression at study enrollment and 5-year follow-up of health outcomes. We tested the hypothesis that during follow-up, major or minor depression at enrollment compared with no depression would be associated with higher average HbA1c, higher average SBP among patients with hypertension, and higher average LDL cholesterol among those ever treated with lipid-lowering agents.
METHODS
During 2001–2002, the Pathways Epidemiologic Study enrolled 4,839 patients with diabetes at Group Health.7 An aim of this study was to develop a risk-prediction model for poor clinical risk factor control, including glycemic, blood pressure, and lipid control. The study was approved by the Group Health Human Subjects Review Committee. At study enrollment, participants completed a self-administered questionnaire that assessed demographic characteristics, diabetes duration and treatment, and health behaviors. History of cardiovascular disease (myocardial infarction or coronary revascularization), cerebrovascular disease (stroke or carotid endarterectomy), and peripheral vascular disease (peripheral vascular disease procedure or amputation) before enrollment were assessed by review of the outpatient medical record. A physician diagnosis of hypertension before enrollment and a new diagnosis of hypertension during follow-up were also ascertained by medical record review. In 50 duplicate medical record reviews, the observed agreement for the occurrence of a myocardial infarction or stroke was 93% (95% CI 88–96%), and the kappa statistic was 0.82 (95% CI 0.72–0.91), indicating excellent agreement.
Measurement of Depression
The Patient Health Questionnaire-9 (PHQ-9) was used to screen for depression at study enrollment. A recent meta-analysis of 14 studies reported a sensitivity of 0.80 (95% CI 0.71 to 0.87) and specificity of 0.92 (95% CI 0.88 to 0.95) for a diagnosis of probable major depression by PHQ-9, compared with a structured psychiatric interview.8 A diagnosis of probable major depression on the PHQ-9 requires a report of five or more symptoms for more than half the days in the preceding 2 weeks, including either depressed mood or anhedonia.9,10 A diagnosis of probable minor depression requires a report of two to four symptoms for more than half the days in the preceding 2 weeks, including either depressed mood or anhedonia.
Measurement of Risk Factor Control at Study Enrollment and During Follow-up
SBP was abstracted from the medical record from ambulatory visit notes, not including emergency room visits. Abstractors recorded the SBP taken closest in time to study enrollment and to the first through fifth anniversaries of the study enrollment date (a maximum of six values). The average (standard deviation, SD) number of SBP values available per person was 3.9 (1.3). All values for LDL cholesterol and HbA1c measured during clinical care were obtained from computerized laboratory data during the period from study enrollment through a maximum of 5 years’ follow-up. The average (SD) number of values available during follow-up was 5.1 (2.9) for LDL cholesterol and 9.7 (4.7) for HbA1c. Data availability was similar across depression status groups at study enrollment.
Selection of Patients for Analysis
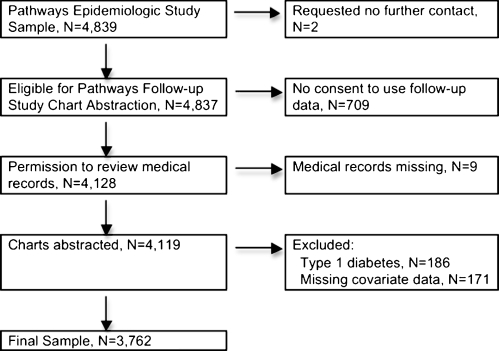
In the final sample for this analysis, there were 3,762 patients (Fig. 2) who consented to medical record review or for whom we had a waiver of consent (those who had died, were too ill to be contacted, or were unreachable by phone). Patients with type 1 diabetes (n = 186) were excluded. Each analysis included only those patients whose clinical outcomes could plausibly be affected by adherence with prescribed medications, that is, patients who had an indication for or were receiving drug treatment for each of the risk factors. For the analysis of glycemic control, all patients with at least one HbA1c value were included (n = 3,762). Patients entered the SBP analysis at study enrollment if they had a physician diagnosis of hypertension before enrollment or entered the analysis during follow-up (n = 355) at the time of a new diagnosis of hypertension during follow-up (total n = 2,731 in the SBP analysis). Patients entered the LDL cholesterol analysis at enrollment if they had ever been treated with a lipid-lowering drug before enrollment or entered the analysis during follow-up (n = 1,543) when newly started on lipid-lowering medication (total n = 2,980 in the LDL analysis).
Figure 2.
Flow diagram for inclusion in the analysis.
Statistical Analysis
Patient characteristics were compared across the three depression groups using the chi-square and ANOVA tests. We compared adjusted mean HbA1c, SBP, and LDL cholesterol over follow-up time across the three depression groups: minor depression, major depression, and no depression (the reference group). First, we created figures using cubic splines to display unadjusted smoothed mean levels of HbA1c, SBP, and LDL cholesterol over time and to explore the functional form of these three risk factor control measures over time. These exploratory analyses demonstrated linear changes in risk factors over time. Next, we estimated depression group-level averages using marginal regression models, estimated using generalized estimating equations, to adjust for correlation due to repeated measurement of each individual. Initial regression models included interactions between depression at study enrollment and time to allow different mean rates of change (slope) across the three depression groups. We found no evidence of differences in the rate of change in risk factor control measures by the Wald test and did not include this interaction in the models we present.
Regression models were also used to adjust for potential confounders. We fit two models to each risk factor control measure, with the second model including additional covariates. Model 1 was adjusted only for demographic characteristics (age, gender, race, education, and marital status), follow-up time, and the interaction of age with follow-up time. The interaction term for age by follow-up time was included in the model to allow change in risk factor levels to depend upon age at study enrollment, more thoroughly controlling for confounding by age. This interaction term was significant with p < 0.001 and thus was retained in the model. Model 2 was additionally adjusted for clinical characteristics, including diabetes duration, diabetes treatment at study enrollment (diet vs. oral hypoglycemic vs. insulin with or without oral agents), hypertension diagnosis, and clinically evident cardiovascular disease (coronary disease, cerebrovascular disease, or peripheral vascular disease) before study enrollment.
RESULTS
A total of 3,762 patients were available for analysis. Compared with those not included in the analysis, those included were slightly older, and fewer were women. They had a longer average duration of diabetes, slightly higher HbA1c and LDL cholesterol at enrollment, and a larger proportion had hypertension, but the proportion with major and minor depression at enrollment was similar. Among the patients included in the analysis, compared with the no depression group, those with major depression at study enrollment were younger, heavier, and more sedentary, and included more women, current smokers, and patients with a history of cardiovascular disease (Table 1). Characteristics of those with minor depression were generally intermediate between those with no depression and those with major depression.
Table 1.
Characteristics of the Study Cohort by Depression Status at Study Enrollment
| Characteristic | Overall | No depression | Minor depression | Major depression | p-value* |
|---|---|---|---|---|---|
| N = 3762 | N = 2995 | N = 319 | N = 448 | ||
| Age, mean (SD), years | 64.2 (12.6) | 64.8 (12.3) | 64.6 (13.3) | 60.0 (13.1) | <0.001 |
| Women, N (%) | 1800 (47.9) | 1384 (46.2) | 153 (48.0) | 263 (58.7) | <0.001 |
| Non-white, N (%) | 737 (19.6) | 564 (18.8) | 82 (25.7) | 91 (20.3) | 0.01 |
| Some college or more, N (%) | 2833 (75.3) | 2293 (76.6) | 217 (68.0) | 323 (72.1) | <0.001 |
| Single marital status, N (%) | 1261 (33.5) | 953 (31.8) | 112 (35.1) | 196 (43.8) | <0.001 |
| Duration of diabetes, mean (SD), years | 8.8 (8.4) | 8.7 (8.4) | 10.2 (9.1) | 8.9 (7.4) | 0.01 |
| Diabetes treatment, N (%) | |||||
| None or diet | 988 (26.3) | 832 (27.8) | 73 (22.9) | 83 (18.5) | <0.001 |
| Oral hypoglycemic | 1742 (46.3) | 1416 (47.3) | 145 (45.5) | 181 (40.4) | |
| Any insulin | 1032 (27.4) | 747 (24.9) | 101 (31.7) | 184 (41.1) | |
| Hypertension, N (%) | 2411 (64.1) | 1902 (63.5) | 219 (68.7) | 290 (64.7) | 0.18 |
| Cardiovascular disease, N (%) | 910 (24.2) | 686 (22.9) | 94 (29.5) | 130 (29.0) | 0.001 |
| Cerebrovascular disease, N (%) | 256 (6.8) | 188 (6.3) | 28 (8.8) | 40 (8.9) | 0.04 |
| Peripheral vascular disease, N (%) | 96 (2.6) | 78 (2.6) | 10 (3.1) | 8 (1.8) | 0.32 |
| Body mass index, mean (SD), kg/m2 | 31.8 (7.2) | 31.2 (6.8) | 32.2 (7.0) | 35.2 (9.3) | <0.001 |
| Current smoking, N (%) | 313 (8.3) | 215 (7.2) | 34 (10.7) | 64 (14.3) | <0.001 |
| Physical activity ≤1 day/week, N (%) | 1236 (32.9) | 877 (29.3) | 144 (45.1) | 215 (48.0) | <0.001 |
| HbA1c at enrollment, mean (SD), % | 7.8 (1.6) | 7.7 (1.5) | 7.9 (1.6) | 8.1 (1.7) | <0.001 |
| SBP at enrollment, mmHg | 136.0 (20.2) | 136.1 (19.7) | 136.8 (22.9) | 134.3 (20.9) | 0.20 |
| LDL cholesterol at enrollment, mean (SD), mg/dl | 111.5 (34.7) | 111.6 (34.5) | 108.5 (32.3) | 112.8 (37.0) | 0.33 |
*p-value for test of differences between depression groups
Cardiovascular disease = myocardial infarction or coronary revascularization before study enrollment
Cerebrovascular disease = stroke or carotid endarterectomy before study enrollment
Peripheral vascular disease = peripheral vascular procedure or amputation before study enrollment
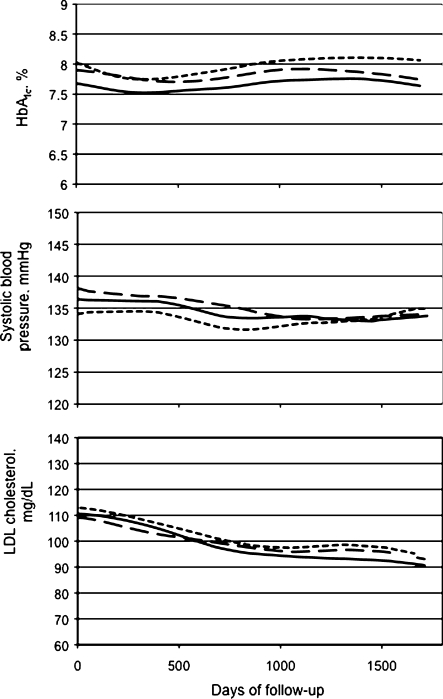
Figure 3 shows smoothed unadjusted mean levels of HbA1c, SBP, and LDL cholesterol over a median of 4.8 years’ follow-up by depression status at study enrollment. Among all participants, in analyses adjusted only for depression, there was a slight increase in mean HbA1c levels over time (+0.03% per year, p < 0.001). Conversely, both SBP and LDL cholesterol level declined on average over time (−0.7 mmHg per year, p < 0.001, and −4.3 mg/dl per year, p < 0.001, respectively).
Figure 3.
Smoothed unadjusted mean HbA1c, systolic blood pressure (SBP), and LDL cholesterol over follow-up according to depression status at study enrollment. Solid line is for patients without depression, long dashed line for those with minor depression, and short dashed line for those with major depression.
Table 2 shows the adjusted mean difference and 95% CI for HbA1c, SBP, and LDL cholesterol during follow-up for the minor depression and major depression groups compared with the no depression group. Differences are sequentially adjusted for demographic characteristics and for clinical characteristics. After adjustment for demographic variables, patients with minor and major depression had slightly higher average HbA1c over follow-up than those with no depression (+0.19%, 95% CI 0.06–0.31, and +0.22%, 95% CI 0.08–0.35, respectively). These differences became non-significant after further adjustment for clinical characteristics. For SBP and LDL cholesterol levels, there were no differences in mean level during follow-up, either before or after adjustment for patient characteristics.
Table 2.
Mean Difference (95% CI) in HbA1c, Systolic Blood Pressure (SBP), and LDL Cholesterol Over 5-year Follow-up in the Minor Depression and Major Depression Groups vs. the no Depression Groupa
| Minor depression (n = 319) | Major depression (n = 448) | |
| HbA1c, % | ||
| Adjusted for demographic variablesb | 0.19 (0.06, 0.31) | 0.22 (0.08, 0.35) |
| Adjusted for demographic and clinical characteristicsc | 0.12 (0.00, 0.24) | 0.10 (-0.03, 0.24) |
| Minor depression (n = 246) | Major depression (n = 333) | |
| SBP, mmHg | ||
| Adjusted for demographic variablesb | 0.7 (−1.2, 2.6) | −0.5 (−2.1, 1.2) |
| Adjusted for demographic and clinical characteristicsc | 0.4 (−1.4, 2.3) | −0.5 (−2.1, 1.1) |
| Minor depression (n = 246) | Major depression (n = 326) | |
| LDL cholesterol, mg/dl | ||
| Adjusted for demographic variablesb | 0.2 (−3.5, 4.0) | −0.5 (−4.3, 3.2) |
| Adjusted for demographic and clinical characteristicsc | 0.5 (−3.2, 4.2) | 0.7 (−3.0, 4.4) |
aThe HbA1c analysis included all 3,762 patients (2,995 with no depression); the SBP analysis included 2,731 patients with a physician diagnosis of hypertension (2,152 patients with no depression); the LDL cholesterol analysis included 2,980 patients who received lipid-lowering treatment (2,408 patients with no depression)
bDemographic characteristics: age, gender, race, education, and marital status; all models also adjusted for follow-up time and interaction of age with follow-up time
cClinical characteristics: diabetes duration, diabetes treatment (diet vs. oral hypoglycemic vs. insulin with or without oral agents), clinical cardiovascular disease, and hypertension diagnosis
DISCUSSION
In this population-based study of patients with type 2 diabetes, we found that compared with no depression, major depression and minor depression were associated with slightly higher average HbA1c levels during follow-up after adjustment for demographic characteristics. However, these associations were attenuated and became non-significant after adjustment for clinical characteristics including diabetes duration and treatment and cardiovascular disease. There was no difference by depression status in average SBP or LDL cholesterol level during follow-up.
The strengths of our study include the population-based design, the careful assessment of depression status at study enrollment, the availability of detailed information on patient characteristics that may be related both to depression and to risk factor control measures, and access to automated data on all measurements of HbA1c and LDL cholesterol conducted at Group Health during follow-up. We did not find strong evidence of differential risk factor measurement across the depression groups, possibly because all individuals under study were members of the same health plan. Regarding study power, the width of the confidence intervals in Table 2 provides an indication of the differences in risk factor levels detectable with this sample size. Considering the estimates adjusted for demographic and clinical characteristics, the study was able to exclude a difference between those with major depression vs. no depression greater than 0.24% for HbA1c, greater than 1.1 mmHg for SBP, and greater than 4.4 mg/dl for LDL cholesterol. Thus, this study had adequate power to detect clinically important differences in risk factor control.
Limitations of this study include the assessment of depression at only one point in time, the possible misclassification of depression status by the PHQ-9, the possibility of unknown or unmeasured confounding factors, and the study of only three risk factors: glycemia, blood pressure, and lipid levels. Further, the study included only patients enrolled at Group Health and only those who gave permission to use medical information during follow-up. These patients may have received medical care that differs from care available in other settings, and those who gave permission may have differed from those who declined permission in unmeasured ways. Finally, the association between diabetes and depression is thought to be bidirectional,11 with diabetes increasing risks of depressive illness as well as depression increasing the risk of diabetes onset. Therefore, it is possible that declines in health prior to study enrollment led to differences in depression status at enrollment, so that the observed differences represent a continuation of a process that began prior to our study of these individuals.
Few studies have examined the association of depression with long-term risk factor control in individuals with diabetes. A small study of 110 African-American patients with diabetes reported no association of depression with glycemic, blood pressure, or lipid control over a 3-year period.12 A recent study in US veterans with type 2 diabetes reported a slightly higher average adjusted HbA1c (+0.13%, 95% CI 0.03–0.22) in patients with depression compared with those without depression during an average 4.1 years’ follow-up.13 In that study, diabetes and depression diagnoses and cardiovascular co-morbidity were all identified using International Classification of Diseases, 9th revision (ICD-9) codes only. The prevalence of depression in the VA study was only 6%, compared with 12% with major depression and 20% with either major or minor depression in our study. This difference in depression prevalence is consistent with the finding that physicians make an accurate diagnosis in only 40% to 50% of patients with comorbid major depression and diabetes.7 In the VA study, adjustment for clinical characteristics had relatively little effect on the strength of the association between depression and glycemic control, while in our study, which included medical record review to assess cardiovascular co-morbidity, adjustment for these characteristics attenuated the association. These findings suggest that careful measurement of confounding factors is critical in studies of patients with co-morbid diabetes and depression, and that after thorough adjustment for confounding, an association of depression with long-term glycemic control is weak or absent.
Depression in individuals with diabetes is associated with higher mortality,1–4 higher risk of major complications,1,5 and less favorable functional outcomes.14 Prior publications suggest that compared with patients without depression, patients with comorbid diabetes and depression have poorer self-reported diabetes self-management15 and specifically report a lower frequency of checking blood glucose levels, less physical activity, a less healthy diet, and lower adherence to oral hypoglycemic, antihypertensive, and lipid-lowering medications.6 However, no or few differences have been found between depressed and nondepressed patients with diabetes on physician-ordered tests such as number of HbA1c or lipid panels drawn per year or the percent of patients receiving an annual retinal exam.6 Because depressed patients with diabetes have a significantly higher number of primary care visits per year,16 physicians may have more opportunity to order tests and specialist examinations and to increase treatment intensity. Such increased attention could potentially improve risk factor control.
The findings of no association of depression with long-term risk factor control in the present study, and the relatively weak association of depression with HbA1c in the study by Richardson et al., are surprising. These results suggest that an adverse effect of depression on outcomes in patients with diabetes may not be mediated in large part by poorer control of these risk factors and that biologic factors need further study. In studies of both community respondents and those with cardiovascular disease, depression has been associated with adverse biologic effects,17 including dysregulation of the hypothalamic-pituitary axis, increases in platelet adhesiveness18 and other pro-inflammatory markers19,20 such as interleukin-6 and C-reactive protein, and abnormalities in the autonomic nervous system such as decreased heart rate variability.21 Further study is needed in patients with diabetes of the biologic sequelae of depression and their relationship with adverse outcomes.
Acknowledgments
Supported by grant MH073686 from the National Institute of Mental Health, Bethesda, MD.
Conflict of interest
Dr. Ciechanowski is CEO and founder of Samepage, Inc., a consulting company providing services for improving patient-provider relationships; Dr. Lin has received honoraria from Health Star Communications and Prescott Medical; Dr. Katon has received honoraria for lectures from Wyeth, Lilly, Forest, and Pfizer, and is on advisory boards for Lilly and Wyeth; Dr. Von Korff has one grant to Group Health Research Institute funded by Johnson & Johnson and another grant pending.
Footnotes
Financial support: Supported by grant MH073686 from the National Institute of Mental Health, Bethesda, MD.
References
- 1.Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care. 2003;26(10):2822–8. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- 2.Katon WJ, Rutter C, Simon G, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care. 2005;28(11):2668–72. doi: 10.2337/diacare.28.11.2668. [DOI] [PubMed] [Google Scholar]
- 3.Katon W, Fan MY, Unutzer J, Taylor J, Pincus H, Schoenbaum M. Depression and diabetes: a potentially lethal combination. J Gen Intern Med. 2008;23(10):1571–5. doi: 10.1007/s11606-008-0731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin EH, Heckbert SR, Rutter CM, et al. Depression and increased mortality in diabetes: unexpected causes of death. Ann Fam Med. 2009;7(5):414–21. doi: 10.1370/afm.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63(4):619–30. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Lin EH, Katon W, Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27(9):2154–60. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 7.Katon WJ, Simon G, Russo J, et al. Quality of depression care in a population-based sample of patients with diabetes and major depression. Med Care. 2004;42(12):1222–9. doi: 10.1097/00005650-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007;22(11):1596–1602. doi: 10.1007/s11606-007-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 11.Golden SH, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751–9. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gary TL, Baptiste-Roberts K, Crum RM, Cooper LA, Ford DE, Brancati FL. Changes in depressive symptoms and metabolic control over 3 years among African Americans with type 2 diabetes. Int J Psychiatry Med. 2005;35(4):377–82. doi: 10.2190/BQ22-4HU0-P6EK-L7YX. [DOI] [PubMed] [Google Scholar]
- 13.Richardson LK, Egede LE, Mueller M, Echols CL, Gebregziabher M. Longitudinal effects of depression on glycemic control in veterans with type 2 diabetes. Gen Hosp Psychiatry. 2008;30(6):509–14. doi: 10.1016/j.genhosppsych.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Korff M, Katon W, Lin EH, et al. Work disability among individuals with diabetes. Diabetes Care. 2005;28(6):1326–32. doi: 10.2337/diacare.28.6.1326. [DOI] [PubMed] [Google Scholar]
- 15.Egede LE, Ellis C. The effects of depression on diabetes knowledge, diabetes self-management, and perceived control in indigent patients with type 2 diabetes. Diabetes Technol Ther. 2008;10(3):213–9. doi: 10.1089/dia.2007.0278. [DOI] [PubMed] [Google Scholar]
- 16.Simon GE, Katon WJ, Lin EH, et al. Diabetes complications and depression as predictors of health service costs. Gen Hosp Psychiatry. 2005;27(5):344–51. doi: 10.1016/j.genhosppsych.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Joynt KE, Whellan DJ, O'Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry. 2003;54(3):248–61. doi: 10.1016/S0006-3223(03)00568-7. [DOI] [PubMed] [Google Scholar]
- 18.Serebruany VL, Glassman AH, Malinin AI, et al. Enhanced platelet/endothelial activation in depressed patients with acute coronary syndromes: evidence from recent clinical trials. Blood Coagul Fibrinolysis. 2003;14(6):563–7. doi: 10.1097/00001721-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Vaccarino V, Johnson BD, Sheps DS, et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50(21):2044–50. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 20.Everson-Rose SA, Lewis TT, Karavolos K, Dugan SA, Wesley D, Powell LH. Depressive symptoms and increased visceral fat in middle-aged women. Psychosom Med. 2009;71(4):410–16. doi: 10.1097/PSY.0b013e3181a20c9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res. 2002;53(4):897–902. doi: 10.1016/S0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]