Abstract
Aims
To develop a risk score to quantify bleeding risk in outpatients with or at risk of atherothrombosis.
Methods and results
We studied patients in the REACH Registry, a cohort of 68 236 patients with/at risk of atherothrombosis. The outcome of interest was serious bleeding (non-fatal haemorrhagic stroke or bleeding leading to hospitalization and transfusion) over 2 years. Risk factors for bleeding were assessed using modified regression analysis. Multiple potential scoring systems based on the least complex models were constructed. Competing scores were compared on their discriminative ability via logistic regression. The score was validated externally using the CHARISMA population. From a final cohort of 56 616 patients, 804 (1.42%, 95% confidence interval 1.32–1.52) experienced serious bleeding between baseline and 2 years. A nine-item bleeding risk score (0–23 points) was constructed (age, peripheral arterial disease, congestive heart failure, diabetes, hypertension, smoking, antiplatelets, oral anticoagulants, hypercholesterolaemia). Observed incidence of bleeding at 2 years was: 0.46% (score ≤6); 0.95% (7–8); 1.25% (9–10); 2.76% (≥11). The score's discriminative performance was consistent in CHARISMA and REACH (c-statistics 0.64 and 0.68, respectively); calibration in the CHARISMA population was very good (modified Hosmer-Lemeshow c2 = 4.74; P = 0.69).
Conclusion
Bleeding risk increased substantially with a score >10. This score can assist clinicians in predicting the risk of serious bleeding and making decisions on antithrombotic therapy in outpatients.
Keywords: Bleeding risk, Atherothrombosis, Antithrombotic therapy
Introduction
Antithrombotics are commonly used for the treatment and prevention of ischaemic events in patients with established atherothrombosis or with risk factors.1 Although effective, they increase the risk of bleeding, which may negate their benefits. Indeed, bleeding events are associated with worse short- and long-term outcomes.2,3
Prolonged or even lifetime antithrombotic therapy is becoming commonplace. Coronary artery disease (CAD) patients are treated with aspirin for life and, whenever an acute coronary syndrome occurs or a coronary stent is placed, undergo protracted treatment with a thienopyridine in addition to aspirin. Before embarking on such treatment, it is important to assess both the benefits and the risks of these therapies. For example, guidelines recommend continuing double antiplatelet therapy for over 12 months after implantation of a drug-eluting stent (DES) in patients at low risk of bleeding.4 While multivariable modelling of bleeding risk has been performed in outpatients with atherothrombosis in whom anticoagulant or antiplatelet therapy is considered,5 there is, however, no tool to assess the long-term bleeding risk in clinical practice. A score to quantify risk of bleeding would be helpful for clinical decision-making, particularly if it allowed comparison of the risks associated with use of antithrombotic therapy. This could help clinicians select the proper treatment modality [coronary artery bypass graft (CABG) vs. percutaneous coronary intervention vs. medical therapy] and assist in the selection of stent type (drug eluting vs. bare metal).
Methods
We studied patients enrolled in the Reduction of Atherothrombosis for Continued Health (REACH) Registry,6 a prospective registry of patients aged at least 45 years, with established cerebrovascular disease (CVD), CAD, peripheral arterial disease (PAD), or with at least three atherosclerosis risk factors. The design of the REACH Registry has been described.6 In brief, the REACH Registry is an observational registry designed to provide clinical follow-up of 68 236 outpatients from 44 countries in North America, Latin America, Western Europe, Eastern Europe, Middle East, Asia, and Australia. Patients were enrolled over an initial 7 month recruitment period (December 2003 to June 2004) on a worldwide basis. Data regarding medical history, risk factors, characteristics, and management were collected at baseline. Follow-up assessments tracked specified clinical events at 12 ± 3 and 21 ± 3 months in all countries (yielding a maximum follow-up of 24 months). In the USA, additional follow-up took place at 6 and 18 months.6
This protocol was submitted to institutional review boards according to local requirements and signed informed consent was required for all patients.
Measures
The outcome of interest was an episode of serious bleeding, defined as non-fatal haemorrhagic stroke or bleeding leading to both hospitalization and transfusion. Events were recorded at each of the four follow-up assessments. We created a binary outcome based on an observation of at least one bleeding event, or no recorded bleeding event as of the last available assessment. This outcome was designed to provide a conservative estimate of bleeding events over the 2 year period. Bleeding episodes of lesser severity may have significant impact for patients and their physicians, but are difficult to capture reliably.
Risk factors
The 65 variables collected in the baseline case report form were analysed as potential predictors of bleeding. Demographic data included age, sex, ethnicity, whether the patient lived alone, education, and employment status. Age was categorized into 10 year intervals up to 75 years. Atherothrombotic risk factors were: advanced age (≥65 years in men, ≥70 in women), smoking, diabetes, diabetic nephropathy, hypertension, hypercholesterolaemia, ankle brachial index (ABI) <0.9, carotid stenosis, and carotid plaques.
History of ischaemic disease was divided into CVD (documented transient ischaemic attack or ischaemic stroke); CAD (stable/unstable angina, myocardial infarction, coronary angioplasty/stenting, and CABG); and PAD (historic or current intermittent claudication associated with one of: ABI <0.9, lower-limb arterial surgery, or lower-limb amputation). For each, we also considered summary variables of CVD, CAD, and PAD defined as including one of the variables described earlier.
Physical measurements included weight, height, waist circumference, blood pressure, and ABI. Body mass index (BMI) was classified into five 1 point intervals between 25 and 35 kg/m2; a separate binary variable classifying patients as obese (BMI at least 30 kg/m2) was created. Weight was categorized into 20 kg increments between 60 and 100 kg. Waist circumference was used to create a binary measure of obesity using the National Cholesterol Education Program Adult Treatment Panel III cut-off (at least 88 cm for women; at least 102 for men).7 Biochemical measurements included serum creatinine; fasting blood glucose, total cholesterol, and triglycerides; and microalbuminuria. Current or past medical conditions included carotid angioplasty/stenting, carotid surgery, congestive heart failure (CHF), atrial fibrillation/flutter, aortic valve stenosis, abdominal aortic aneurysm, diabetes, hypertension, and smoking status.
Current chronic therapies were categorized into three classes: cardiovascular agents (calcium-channel antagonists, beta-blockers, nitrates/other anti-anginal agents, diuretics, angiotensin-converting-enzyme inhibitors, angiotensin II receptor blockers, other antihypertensives, peripheral arterial claudication medications); antidiabetic agents (insulin, biguanides, sulfonylureas, thiazolidinediones, other); non-steroidal anti-inflammatory drugs; lipid-lowering drugs (statins or other); and other medications with an antithrombotic or anticoagulant effect, such as antiplatelets (aspirin or other) or oral anticoagulants. A four-category summary variable was created for the two antiplatelet agents, coding patients as using aspirin alone, another antiplatelet agent, both, or neither.
From this initial pool of variables, we eliminated variables for which data were missing in >5% of patients. There was no imputation for these variables, which were diabetic nephropathy; ABI <0.9; carotid stenosis (asymptomatic); carotid plaques; intermittent claudication associated with ABI <0.9; ethnicity; education; waist-based obesity; aortic valve stenosis; abdominal aortic aneurysm; and the biochemical measurements.
Statistical analysis
The aim was to create a score for predicting risk of serious bleeding that would be both easy to use and easy to calculate from routinely available clinical data.
Using bleeding as the dichotomous outcome variable, we assessed the univariate relationship between each factor and outcome. All variables were assessed using logistic regression analysis, with a selection criterion of P < 0.05. The baseline category for qualitative variables was either the lowest category (in the case of ordinal variables) or the category containing the largest proportion of patients. To maximize the usable population size, the availability of data for >95% of patients was also retained as a criterion for variable entry. The resulting list of potential factors was then further restricted according to ease of assessment in a clinical setting and to their known association with bleeding. Given the large number of variables, the overlap interactions and correlations were not studied.
Multivariable analysis
Stepwise logistic regression produces highly variable results,8 even if split or cross-validation is employed.9 We therefore chose a modified regression technique employing multiple regressions on bootstrap resamples.10,11 In essence, we generated multiple bootstrap samples to which the same automatic selection techniques were applied. Selection of the final model was based on the resulting estimates of the distribution of the model selection process; in practice, the percentage of analyses in which the variables were selected.10 To generate parsimonious models, we used Akaike's Information Criterion for best-fit model selection.
Using the resulting ordering of factors, we compared models for the n-highest ranked factors. Discrimination was assessed by calculating receiver operating characteristic (ROC) curves. To maximize the accuracy of the parameter estimates, the maximum possible sample size was used for each logistic regression; in other words, all patients with data for the selected factors.
We built multiple potential scoring systems based on the least complex models, retaining similar discriminative capacity to that of the full model. The scores were based on nearest integer approximations of constant multiples of the regression coefficients. Competing scores were compared for discriminative ability via logistic regression.
External validation
External validation was carried out using the 15 603 patients enrolled in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial.12 The clinical characteristics of the CHARISMA trial and REACH Registry populations were very similar.
In CHARISMA, the bleeding endpoint was based on the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) definition.13 For the validation, we used a combination of severe and moderate bleeding. Severe bleeding was defined as fatal bleeding, intracranial haemorrhage, or bleeding causing haemodynamic compromise (requiring blood, fluid replacement, inotropic support, or surgical intervention). Moderate bleeding was defined as bleeding requiring blood transfusion but not resulting in haemodynamic compromise.
Discrimination was assessed using c-statistics. Calibration was assessed using quartile plots; mean predicted and observed risks; and modified Hosmer–Lemeshow c2 statistics. We also calculated the coefficient of contingency, which quantifies the lack of model fit adjusting for the sample size.
Results
For the global registry of 68 236, follow-up rates were good: 93.3% at 12 months and 75% at 21 months. A total of 65 441 (95.9%) patients attended at least one follow-up assessment. We excluded 852 of these patients due to incomplete outcome data, yielding a final sample size of 64 589 (94.7%). The mean age of the patient population was 68.6 years and 36.2% were female. The patients' other baseline characteristics are shown in Table 1. On those 64 589 patients, 903 serious bleeding events (1.4%) were recorded. The details of the composite outcome are shown in Table 2.
Table 1.
Baseline characteristics of the population and univariate analysis of potential risk factors
| Characteristic | n (%) | Haemorrhage, n (%) |
OR (95% CI) | P-valuea | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Demographics/physical characteristics | |||||
| Gender | |||||
| Female | 23 386 (36.2) | 318 (35.2) | 23 068 (36.2) | 1 | 0.52 |
| Male | 41 155 (63.8) | 585 (64.8) | 40 570 (63.8) | 1.05 (0.91–1.20) | |
| Age, years | |||||
| 45–54 | 7231 (11.2) | 45 (5.0) | 7186 (11.3) | 1 | <0.001 |
| 55–64 | 15 083 (23.4) | 150 (16.6) | 14 933 (23.5) | 1.60 (1.15–2.24) | |
| 65–74 | 23 703 (36.8) | 313 (34.7) | 23 390 (36.8) | 2.14 (1.56–2.92) | |
| 75+ | 18 366 (28.5) | 394 (43.7) | 17 972 (28.3) | 3.50 (2.57–4.77) | |
| Lives alone | |||||
| No | 50 947 (80.0) | 689 (77.0) | 50 258 (80.0) | 1 | 0.02 |
| Yes | 12 748 (20.0) | 206 (23.0) | 12 542 (20.0) | 1.20 (1.02–1.40) | |
| Employment status | |||||
| Full time | 10 718 (16.8) | 95 (10.6) | 10 623 (16.9) | 0.54 (0.44–0.67) | <0.001 |
| Part time | 3786 (5.9) | 49 (5.5) | 3737 (5.9) | 0.79 (0.59–1.06) | |
| Unemployed | 4267 (6.7) | 39 (4.4) | 4228 (6.7) | 0.56 (0.40–0.77) | |
| Retired | 39 862 (62.5) | 647 (72.5) | 39 215 (62) | 1 | |
| Incapacity | 3311 (5.2) | 51 (5.7) | 3260 (5.2) | 0.95 (0.71–1.26) | |
| Other | 1876 (2.9) | 12 (1.3) | 1864 (3.3) | 0.39 (0.22–0.69) | |
| Weight, kg | |||||
| <60 | 8284 (12.9) | 113 (12.6) | 8171 (12.9) | 1 | 0.98 |
| 60–80 | 27 440 (42.7) | 389 (43.3) | 27 051 (42.7) | 1.04 (0.84–1.28) | |
| >80–100 | 20 610 (32.1) | 288 (32.1) | 20 322 (32.1) | 1.02 (0.82–1.28) | |
| >100 | 7869 (12.3) | 108 (12.0) | 7761 (12.3) | 1.01 (0.77–1.31) | |
| Atherothrombotic risk factors | |||||
| Smoking status | |||||
| Never | 27 016 (43.1) | 329 (37.2) | 26 687 (43.2) | 1 | <0.001 |
| Former | 26 177 (41.7) | 424 (48.0) | 25 753 (41.7) | 1.34 (1.16–1.54) | |
| Current | 9510 (15.2) | 131 (14.8) | 9379 (15.2) | 1.13 (0.92–1.39) | |
| Diabetes | |||||
| No | 36 287 (56.2) | 465 (51.5) | 35 822 (56.3) | 1 | 0.004 |
| Yes | 28 298 (43.8) | 438 (48.5) | 27 860 (43.7) | 1.21 (1.06–1.38) | |
| High blood pressure | |||||
| No | 11 825 (18.3) | 107 (11.8) | 11 718 (18.4) | 1 | <0.001 |
| Yes | 52 759 (81.7) | 796 (88.2) | 51 963 (81.6) | 1.68 (1.37–2.05) | |
| Hypercholesterolaemia | |||||
| No | 18 006 (27.9) | 300 (33.3) | 17 706 (27.8) | 1 | <0.001 |
| Yes | 46 512 (72.1) | 602 (66.7) | 45 910 (72.2) | 0.77 (0.67–0.89) | |
| Ischaemic disease | |||||
| CVD | |||||
| No | 46 696 (72.3) | 594 (65.8) | 46 102 (72.4) | 1 | <0.001 |
| Yes | 17 893 (27.7) | 309 (34.2) | 17 584 (27.6) | 1.36 (1.19–1.57) | |
| CAD | |||||
| No | 26 259 (40.7) | 345 (38.2) | 25 914 (40.7) | 1 | 0.13 |
| Yes | 38 330 (59.3) | 558 (61.8) | 37 772 (59.3) | 1.11 (0.97–1.27) | |
| PAD | |||||
| No | 56 737 (87.8) | 741 (82.1) | 55 996 (87.9) | 1 | <0.001 |
| Yes | 7852 (12.2) | 162 (17.9) | 7690 (12.1) | 1.59 (1.34–1.89) | |
| Stable angina | |||||
| No | 44 506 (70.0) | 590 (66.7) | 43 916 (70.1) | 1 | 0.03 |
| Yes | 19 041 (30.0) | 295 (33.3) | 18 746 (29.9) | 1.17 (1.02–1.35) | |
| CABG | |||||
| No | 51 117 (79.6) | 668 (74.5) | 50 449 (79.7) | 1 | <0.001 |
| Yes | 13 118 (20.4) | 229 (25.5) | 12 889 (20.3) | 1.34 (1.15–1.56) | |
| Current or previous medical conditions | |||||
| Atrial fibrillation | |||||
| No | 56 471 (89.3) | 714 (80.1) | 55 757 (89.5) | 1 | <0.001 |
| Yes | 6743 (10.7) | 177 (19.9) | 6566 (10.5) | 2.11 (1.78–2.49) | |
| CHF | |||||
| No | 54 561 (86.1) | 665 (74.7) | 53 896 (86.3) | 1 | 0.001 |
| Yes | 8782 (13.9) | 225 (25.3) | 8557 (13.7) | 2.13 (1.83–2.48) | |
| Carotid surgery | |||||
| No | 60 992 (95.5) | 839 (93.7) | 60 153 (95.5) | 1 | 0.01 |
| Yes | 2868 (4.5) | 56 (6.3) | 2812 (4.5) | 1.43 (1.09–1.88) | |
| Current chronic therapy | |||||
| Anticoagulants | |||||
| No | 54 875 (87.7) | 669 (76.1) | 54 206 (87.9) | 1 | <0.001 |
| Yes | 7662 (12.3) | 210 (23.9) | 7452 (12.1) | 2.28 (1.95–2.67) | |
| Antiplatelet agents | |||||
| None | 13 725 (21.3) | 195 (21.6) | 13 530 (21.3) | 1 | <0.001 |
| Acetylsalicylic acid | 34 939 (54.2) | 425 (47.1) | 34 514 (54.3) | 0.85 (0.72–1.01) | |
| Other antiplatelet | 7401 (11.5) | 116 (12.9) | 7285 (11.5) | 1.10 (0.88–1.39) | |
| Both | 8412 (13.0) | 166 (18.4) | 8246 (13.0) | 1.40 (1.13–1.72) | |
| Diuretics | |||||
| No | 38 447 (59.8) | 447 (49.7) | 38 000 (59.9) | 1 | <0.001 |
| Yes | 25 876 (40.2) | 452 (50.3) | 25 424 (40.1) | 1.51 (1.32–1.72) | |
| Biochemistry | |||||
| Serum creatinine, mg/dL | 1.1 ± 0.7b | 1.3 ± 0.8b | 1.1 ± 0.7b | 1.13 (1.08–1.18)c | <0.001 |
CABG, coronary artery bypass graft; CAD, coronary arterial disease; CHF, congestive heart failure; CI, confidence interval; CVD, cardiovascular disease; OR, odds ratio; PAD, peripheral arterial disease; SD, standard deviation.
aGlobal P for each category; univariate analysis.
bMean ± SD.
cPer 1 SD.
Table 2.
Characteristics of the composite outcome
| Composite outcome | n (%) | Bleeding leading to both hospitalization and transfusion | Non-fatal haemorrhagic stroke | n (%) |
|---|---|---|---|---|
| Missing | 852 (1.3) | Missing | Missing | 852 (100) |
| No | 63 686 (98.6) | Missing | No | 216 (0.3) |
| No | Missing | 48 (0.1) | ||
| No | No | 63 422 (99.6) | ||
| Yes | 903 (1.4) | Missing | Yes | 2 (0.2) |
| No | Yes | 118 (13.1) | ||
| Yes | Missing | 1 (0.1) | ||
| Yes | No | 775 (85.8) | ||
| Yes | Yes | 7 (0.8) |
The population retained for the final multivariable analysis was a subset of the study population (n = 56 616; 87.7%) who had data available for each of the 17 factors selected in the multivariable analysis. In this final population, 804 serious bleeding were recorded (804/56 616: 1.42%; confidence interval 1.32, 1.52).
Univariate factors
Based on univariate analyses of each of the 49 factors and bleeding, we excluded the factors without relationship to the outcome of interest (P > 0.05), including smoking, unstable angina, myocardial infarction, coronary angioplasty/stenting, sex, formal education, the two BMI factors, weight, systolic blood pressure, carotid angioplasty/stenting, three cardiovascular drugs (calcium-channel antagonists, beta-blockers, ACE-inhibitors), statins, other lipid-lowering agents, at least one lipid-lowering agent, three antidiabetic agents (biguanides, sulfonylureas, others), non-steroidal anti-inflammatory drugs, and physician age.
The resulting potential factors were then further restricted according to ease of assessment in a clinical setting, and the plausibility of a causal association with bleeding (ethnic origin, height, other antihypertensive drugs, other antidiabetic agents, and, finally, physician specialty, practice type, and geographic location were eliminated). This provided a list of 18 factors: four risk factors (advanced age, type I or II diabetes, hypertension, hypercholesterolaemia); four indications of ischaemic disease (CVD, stable angina, CABG, PAD); three demographic factors (age, living alone or not, employment status); four medical conditions (carotid surgery, CHF, atrial fibrillation, smoking); and three medications (antiplatelets, oral anticoagulants, diuretics). Advanced age as a binary risk factor was not associated with the outcome when age classes were accounted for (P > 0.5), and was therefore not included separately in the following analyses. Estimates of the relationships between risk of bleeding and the 17 remaining factors are shown in Table 1.
Bootstrap regressions
The sample for the bootstrap regression was the subset of the study population (n = 56 616; 87.7%) with data available for all 17 of the selected factors. A total of 804 patients [1.42% (95% confidence interval 1.32–1.52) of the bootstrap population], and 99 patients (1.2%) of the excluded population, had experienced at least one bleeding event. The difference in bleeding rates between patients with and without missing values was not significant (P = 0.22).
One thousand bootstrap samples were generated and analysed according to backwards stepwise logistic regression, using the Akaike Information Criterion as the stopping criterion. Five variables (age, antiplatelet agents, anticoagulants, hypertension, smoking) were selected in >98% of the regression analyses. Four more variables (CHF, diabetes, hypercholesterolaemia, PAD) were selected in >85% of analyses. The variables CVD, employment type, and CABG were selected in >60% of cases. All other variables were selected in <60% of the regressions.
The discriminative power for each of the logistic regressions on ordered selections of the highest ranked n-factors, with n taking values from 1 to 17 (full model), was assessed. The maximum value of c-statistic (0.68) was obtained for the 15 factor model; however, models with as few as nine variables (c-statistic = 0.68) showed no substantial reduction in discrimination.
Score construction
Four scores were created, containing 8–11 variables. Logistic regression onto the score values produced very similar results in terms of discrimination to the derivative models, hence the nine-factor regression model was chosen (c-statistic = 0.68) as the least complex model that retained the majority of the predictive value. The Hosmer–Lemeshow test for goodness of fit was not statistically significant (P = 0.432), which is in accordance with an acceptable calibration.
The final bleeding risk score sheet (Table 3) contained one demographic factor (age, 2–6 points); two predictors related to medical history (PAD, 1 point; CHF, 2 points); four comorbidities or lifestyle characteristics (diabetes, 1; hypercholesterolaemia, 1; hypertension, 2; smoking 1–2 points); and two medication regimens (antiplatelets, 1–4; oral anticoagulants, 4 points). The maximum possible score is 23, although the maximum observed score was 21. This score very closely approximated the complete nine-factor regression model, with a correlation of 0.993 between the predicted probabilities from the regression and score.
Table 3.
Bleeding risk score sheet
| Factor | Points |
|||
|---|---|---|---|---|
| Age, years | 45–54 | 55–64 | 65–74 | 75 + |
| 0 | 2 | 4 | 6 | |
| Peripheral arterial disease | No | Yes | ||
| 0 | 1 | |||
| Congestive heart failure | No | Yes | ||
| 0 | 2 | |||
| Diabetes | No | Yes | ||
| 0 | 1 | |||
| Hypercholesterolaemia | No | Yes | ||
| 1 | 0 | |||
| Hypertension | No | Yes | ||
| 0 | 2 | |||
| Smoking | Never | Former | Current | |
| 0 | 1 | 2 | ||
| Antiplatelet agents | None | ASA | Other | Both |
| 0 | 1 | 2 | 4 | |
| Oral anticoagulants | No | Yes | ||
| 0 | 4 | |||
ASA, acetylsalicylic acid.
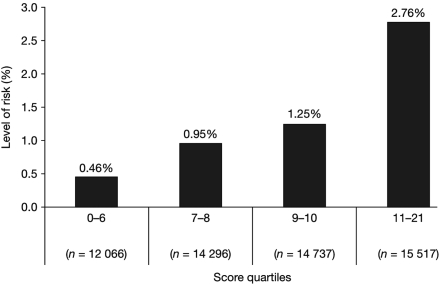
Risk stratification using our score was quite effective in classifying the risk level of patients, with a more than six-fold increase in risk between the highest and lowest quartiles: a 2 year incidence of 0.46% in patients with a score of ≤6 vs. 2.76% in patients with a score of ≥11 (Figure 1).
Figure 1.
Risk stratification using the score (percentages indicate 2 year risk of serious bleeding).
External validation
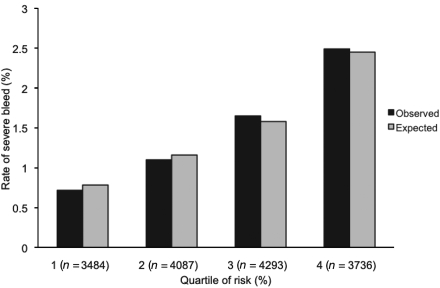
In the CHARISMA population, 487 (3.1%) severe bleeding events were recorded. The discrimination performance of the bleeding risk score was quite similar in the CHARISMA and REACH patient populations (c-statistics 0.64 and 0.68, respectively). The coefficient of contingency was 2.30. The modified Hosmer–Lemeshow c2 showed a goodness-of-fit (8.25; P = 0.31), indicating very good calibration, which is illustrated in Figure 2. The distribution between observed and expected rate was compared using a Hosmer–Lemeshow χ2 test. The P-value was 0.31, which means the observed and expected event rates can be considered equal.
Figure 2.
Calibration plots: observed events in the CHARISMA population vs. events expected using the score.
Clinical application
Using the bleeding risk score sheet, patient information for age, PAD, CHF, diabetes, hypercholesterolaemia, hypertension, smoking, antiplatelets, and oral anticoagulants is matched with the value for each variable, and the assigned number of points summed. The total is then matched with the estimated 2 year risk (Figure 1).
Discussion
We have developed and externally validated a risk score for serious bleeding in outpatients with atherothrombosis receiving a broad range of antithrombotic therapies. The score is easy to use and comprises clinical items that can be collected in a few minutes. This is, to our knowledge, the first score designed to predict bleeding in stable patients with atherothrombosis. There are other risk scores to evaluate bleeding risk, but these were designed for patients with atrial fibrillation on oral anticoagulants,14 for patients with acute coronary syndromes,15 or for patients undergoing percutaneous coronary intervention.16 In all of these, particularly those in acute hospitalized settings, the patient population characteristics and frequency of use of coronary interventions differ markedly from those experienced by stable outpatients with atherothrombosis. An additional value of the current score is that it was built using a large contemporary registry population representative of routine clinical practice across many geographic areas, and may therefore have better external validity than one derived from the highly selected patient populations participating in randomized clinical trials.17,18 We showed a dramatic increase in bleeding risk with a score >10. Therefore it seems important to weigh the role of antithrombotic strategies carefully in clinical situations when a patient has a risk score >10. As expected, antithrombotics were among the factors that most heavily impact the score (4 points for dual antiplatelet therapy and oral anticoagulation). Obviously, the risk of bleeding needs to be evaluated with a parallel assessment of the risk of thrombosis. Since bleeding and thrombosis share several risk factors, this clinical dilemma is not easily resolved, and the resulting choices may vary according to clinical setting. Hypercholesterolaemia had an apparent association with reduced bleeding. The exact pathophysiological mechanisms underlying this putative protection remain unclear.
Some risk factors for bleeding previously identified in studies of hospitalized patients were not included in this outpatient score. For example, creatinine clearance had been eliminated first for missing data (>5%). However, forcing this factor into the score did not improve the model's discriminative performance. Likewise, the score does not include anthropometric variables. Body mass index and weight as categories did not demonstrate independent association with bleeding and were therefore eliminated after univariate analysis. Some other potential risk factors were not collected in the REACH case report form (history of bleeding, peptic ulcer disease, cancer, baseline platelet count, haematocrit, etc.) and could not therefore be considered in the model.
Our score has potential uses in several clinical settings. Concerns have been raised about the prolonged risk of DES thrombosis,19 and continuation of dual antiplatelet therapy for up to 12 months with a DES is recommended20 vs. ≥1 month with a bare-metal stent. This score could be useful in assessing the benefit-to-risk ratio of implanting a bare-metal stent or a DES, by allowing physicians to compare the bleeding risk of dual vs. single prolonged antiplatelet therapy. It could also be important to assess bleeding risk when stents have to be placed in patients who already require chronic anticoagulants. The decision to continue or stop dual antiplatelet therapy 12 months after implanting a DES is a critical issue. The Food and Drug Administration (FDA) recommend that ‘clopidogrel treatment should continue for at least 12 months in patients with DESs who are at low risk for bleeding’.21 However, to our knowledge, there is no tool to assess the risk of bleeding in atherothrombotic patients. This score could prove very useful in this setting. Likewise, while an expert consensus recommended the use of proton pump inhibitors in patients at high risk of gastrointestinal bleeding,22 there are concerns that these agents may reduce the efficacy of clopidogrel.23 Even if this concern has been mitigated recently by the results of the COGENT randomized trial (D.L. Bhatt, TCT 2009, San Francisco, CA, late-breaking trial presentation), evaluating the risk of bleeding may assist in selecting the best candidates for gastroprotection.
There are several potential limitations to this analysis that deserve consideration. The definition of serious bleeding used for these analyses required either a haemorrhagic stroke or bleeding leading to both hospitalization and transfusion. However, the level of detail captured in the REACH case report form did not provide us with precise information about the nature of the bleeding events (location of bleeding, amount of blood loss and transfusion, clinical outcome). While this definition may underestimate the rate of major bleeding events, since it does not take into account events such as epidural haematoma or bleeding requiring hospitalization but no transfusion, the score underwent external validation using the CHARISMA database and the widely adopted and more sensitive GUSTO bleeding definition. The data regarding thienopyridines are limited to those available at the time the study was conducted and do not therefore include prasugrel. The exposure to antithrombotics is extrapolated from yearly data collection, but does not incorporate potential interim changes in medications between the annual visit. Likewise, the international normalized ratio achieved was not collected, yet impacts on the frequency and severity of bleeding.
We eliminated 16 variables for missing data; however, none of these appeared clinically relevant except for serum creatinine. This variable was therefore forced into the multivariable analysis (as well as weight) but did not increase the model's performance.
The c-statistic in the REACH population (0.68) might be considered low. However, the performance of a score is evaluated by its discrimination but also by its calibration,24 which was very good in the external validation. Finally, our c-statistic (0.68) was very similar to that of adopted bleeding risk scores (0.67 for the HEMORR2HAGES score;14 0.71 for the CRUSADE bleeding risk score15) and superior even to that obtained for the Thrombolysis in Myocardial Infarction (TIMI) risk score (0.65), which has been widely adopted into clinical practice.25
In conclusion, we have developed a simple bleeding risk score for use in outpatients. Prospective use of this score may help identify patients at increased risk of bleeding complications, and assist in the selection of antithrombotic treatments and in developing strategies to mitigate that risk.
Funding
This work was supported by sanofi-aventis, Bristol-Myers Squibb, and the Waksman Foundation (Tokyo, Japan). Funding to pay the Open Access publication charges for this article was provided by sanofi-aventis and Bristol Myers Squibb.
Conflict of interest: G.D. has no conflicts of interest. J.S.W. has received research grants from sanofi-aventis. G.B. has received research grants from sanofi-aventis. P.R. has received research grants from sanofi-aventis. M.J.A. has received research grants from AGA Medical, AstraZeneca, BMS, Boehringer Ingelheim, Novo Nordisk, Photo Thera, sanofi-aventis, and Schering-Plough; is a consultant for AGA Medical, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Eli Lilly & Co, Genentech, KOS, The Medicines Company, Merck, Novo Nordisk, PDL BioPharma, Inc., Pfizer, and sanofi-aventis; is on the Speaker's bureau for AstraZeneca, BMS, Boehringer Ingelheim, diaDexus, Genentech, The Medicines Company, Medscape, Novo Nordisk, PDL BioPharma Inc., and sanofi-aventis; is an advisory board member for AGA Medical, AstraZeneca, BMS, Boehringer Ingelheim, Eli Lilly & Co, Genentech, KOS, The Medicines Company, Merck, Novo Nordisk, Pfizer, and sanofi-aventis; and receives honoraria from AGA Medical, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, diaDexus, Eli Lilly & Co, Genentech, KOS, The Medicines Company, Medscape, Merck, Novo Nordisk, PDL BioPharma, Inc., Pfizer, sanofi-aventis, TAP Pharmaceuticals-DSMB, and Schering-Plough. P.W.F.W. has received grant support from sanofi-aventis. E.M.O. receives research grants from AstraZeneca, Bristol-Myers Squibb, CV Therapeutics, Inc., Daiichi Sankyo, Datascope, Eli Lilly & Company, Sanofi-Aventis, Schering-Plough Corporation, The Medicines Company, provides consulting or other services for Abiomed, CV Therapeutics, Inc., Datascope, Inovise, Liposcience, Northpoint Domain, Pozen, Inc., Response Biomedical, sanofi-aventis, The Medicines Company, and WebMD (theheart.org), and has equity in Inovise. D.M.B. has no disclosures. R.B.D'A. has received research grants from the NIH/NHLBI and a Framingham contract; honoraria from sanofi-aventis (REACH Registry); and is a consultant/advisory board member for sanofi-aventis (REACH Registry). D.L.B. discloses the following relationships: Research Grants—Bristol Myers Squibb, Eisai, Ethicon, Heartscape, sanofi-aventis, The Medicines Company; Consultant/Advisory Board—Arena, Astellas, Astra Zeneca, Bayer, Bristol Myers Squibb, Cardax, Centocor, Cogentus, Daiichi-Sankyo, Eisai, Eli Lilly, Glaxo Smith Kline, Johnson & Johnson, McNeil, Medtronic, Millennium, Molecular Insights, Otsuka, Paringenix, PDL, Philips, Portola, sanofi-aventis, Schering Plough, Scios, Takeda, The Medicines Company, Vertex. Ph.G.S. discloses the following relationships: Research Grant: sanofi-aventis (significant); Speakers bureau (all modest): Boehringer-Ingelheim, BMS, GSK, Medtronic, Nycomed, sanofi-aventis, Servier, The Medicines Company; Consulting/advisory board (all modest): Astellas, AstraZeneca, Bayer, Boehringer-Ingelheim, BMS, Endotis, GSK, Medtronic, MSD, Nycomed, sanofi-aventis, Servier, The Medicines Company. Stockholding: none.
Acknowledgements
The REACH Registry is endorsed by the World Heart Federation. A complete list of REACH investigators is accessible online at www.reachregistry.org. The REACH Registry enforces a no ghost-writing policy. This manuscript was written and edited by the authors, who take full responsibility for its content. The first draft was written by Ph.G.S. and G.D. We thank Sophie Rushton-Smith for her assistance with coordinating revisions and providing editorial help in preparing this manuscript, including editing, checking content and language, formatting, referencing, and preparing tables and figures. All manuscripts in the REACH Registry are prepared by independent authors who are not governed by the funding sponsors and are reviewed by an academic publications committee before submission. The funding sponsors have the opportunity to review manuscript submissions but do not have authority to change any aspect of a manuscript.
Appendix
Reach registry executive committee
D.L.B., MD, MPH, VA Boston Healthcare System and Brigham and Women's Hospital, Boston, MA, USA (chair); Ph.G.S., MD, Hôpital Bichat-Claude Bernard, Paris, France (chair); E.M.O., MD, Duke University Medical Center, Durham, NC, USA; Joachim Röther, MD, Klinikum Minden, Minden, Germany; P.W.F.W., MD, Emory University School of Medicine, Atlanta, GA, USA.
Reach registry global publication committee
M.J.A., MD, Northwestern University Medical School, Chicago, IL, USA; D.L.B., MD, MPH, Boston Healthcare System and Brigham and Women's Hospital, Boston, MA, USA (chair); R.D'A., PhD, Boston University, Boston, MA, USA; Kim Eagle, MD, University of Michigan, Ann Arbor, MI, USA; Shinya Goto, MD, PhD Tokai University School of Medicine, Isehara, Kanagawa, Japan; Alan T. Hirsch, MD, University of Minnesota School of Public Health and Minneapolis Heart Institute Foundation, Minneapolis, MN, USA; Chiau-Suong Liau, MD, PhD, Taiwan University Hospital and College of Medicine, Taipei, Taiwan; Jean-Louis Mas, MD, Centre Raymond Garcin, Paris, France; E.M.O., MD, Duke University Medical Center, Durham, NC, USA; Joachim Röther, MD, Klinikum Minden, Minden, Germany; Sidney C. Smith, MD, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA; Ph.G.S., MD, Hôpital Bichat-Claude Bernard, Paris, France (chair); P.W.F.W., MD, Emory University School of Medicine, Atlanta, GA, USA.
References
- 1.Hennekens CH. Update on aspirin in the treatment and prevention of cardiovascular disease. Am J Manag Care. 2002;8:S691–S700. [PubMed] [Google Scholar]
- 2.Toyoda K, Yasaka M, Iwade K, Nagata K, Koretsune Y, Sakamoto T, Uchiyama S, Gotoh J, Nagao T, Yamamoto M, Takahashi JC, Minematsu K Bleeding with Antithrombotic Therapy (BAT) Study Group. Dual antithrombotic therapy increases severe bleeding events in patients with stroke and cardiovascular disease: a prospective, multicenter, observational study. Stroke. 2008;39:1740–1745. doi: 10.1161/STROKEAHA.107.504993. [DOI] [PubMed] [Google Scholar]
- 3.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–782. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 4.King SB, III, Smith SC, Jr, Hirshfeld JW, Jr, Jacobs AK, Morrison DA, Williams DO, Feldman TE, Kern MJ, O'Neill WW, Schaff HV, Whitlow PL, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee. Circulation. 2008;117:261–295. doi: 10.1161/CIRCULATIONAHA.107.188208. [DOI] [PubMed] [Google Scholar]
- 5.Steinhubl SR, Bhatt DL, Brennan DM, Montalescot G, Hankey GJ, Eikelboom JW, Berger PB, Topol EJ. Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med. 2009;150:379–386. doi: 10.7326/0003-4819-150-6-200903170-00006. [DOI] [PubMed] [Google Scholar]
- 6.Ohman EM, Bhatt DL, Steg PG, Goto S, Hirsch AT, Liau CS, Mas JL, Richard AJ, Rother J, Wilson PW REACH Registry Investigators. The REduction of Atherothrombosis for Continued Health (REACH) Registry: an international, prospective, observational investigation in subjects at risk for atherothrombotic events-study design. Am Heart J. 2006;151:786.e1–710. doi: 10.1016/j.ahj.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.Austin PC, Tu JV. Automated variable selection methods for logistic regression produced unstable models for predicting acute myocardial infarction mortality. J Clin Epidemiol. 2004;57:1138–1146. doi: 10.1016/j.jclinepi.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 10.Austin PC, Tu JV. Bootstrap methods for developing predictive models. Am Stat. 2004;58:131–137. [Google Scholar]
- 11.Sauerbrei W, Schumacher M. A bootstrap resampling procedure for model building: application to the Cox regression model. Stat Med. 1992;11:2093–2109. doi: 10.1002/sim.4780111607. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry-Ribaudo L, Booth J, Topol EJ CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 13.The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 14.Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, Radford MJ. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006;151:713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT, Pollack CV, Jr, Peterson ED, Alexander KP. Baseline risk of major bleeding in non-ST-segment elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) Bleeding Score. Circulation. 2009;119:1873–1882. doi: 10.1161/CIRCULATIONAHA.108.828541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikolsky E, Mehran R, Dangas G, Fahy M, Na Y, Pocock SJ, Lincoff AM, Stone GW. Development and validation of a prognostic risk score for major bleeding in patients undergoing percutaneous coronary intervention via the femoral approach. Eur Heart J. 2007;28:1936–1945. doi: 10.1093/eurheartj/ehm194. [DOI] [PubMed] [Google Scholar]
- 17.Rothwell PM. External validity of randomised controlled trials: ‘to whom do the results of this trial apply? Lancet. 2005;365:82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 18.Steg PG, López-Sendón J, Lopez de Sa E, Goodman SG, Gore JM, Anderson FA, Jr, Himbert D, Allegrone J, Van de Werf F GRACE Investigators. External validity of clinical trials in acute myocardial infarction. Arch Intern Med. 2007;167:68–73. doi: 10.1001/archinte.167.1.68. [DOI] [PubMed] [Google Scholar]
- 19.Pfisterer M, Brunner-La Rocca HP, Buser PT, Rickenbacher P, Hunziker P, Mueller C, Jeger R, Bader F, Osswald S, Kaiser C BASKET-LATE Investigators. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006;48:2584–2591. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Smith SC, Jr, Feldman TE, Hirshfeld JW, Jr, Jacobs AK, Kern MJ, King SB, III, Morrison DA, O'Neil WW, Schaff HV, Whitlow PL, Williams DO, Antman EM, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B American College of Cardiology/American Heart Association Task Force on Practice Guidelines; ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention) Circulation. 2006;113:e166–e286. doi: 10.1161/CIRCULATIONAHA.106.173220. [DOI] [PubMed] [Google Scholar]
- 21.Maisel WH. Unanswered questions - drug-eluting stents and the risk of late thrombosis. N Engl J Med. 2007;356:981–984. doi: 10.1056/NEJMp068305. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FK, Furberg CD, Johnson DA, Mahaffey KW, Quigley EM American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2008;118:1894–1909. doi: 10.1161/CIRCULATIONAHA.108.191087. [DOI] [PubMed] [Google Scholar]
- 23.Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, Rumsfeld JS. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301:937–944. doi: 10.1001/jama.2009.261. [DOI] [PubMed] [Google Scholar]
- 24.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 25.Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]