Abstract
Epidermal keratinocytes overgrow in response to ultraviolet-B (UVB), which may be associated with skin photoaging and cancer development. Recently, we found that HIF-1α controls the keratinocyte cell cycle and thereby contributes to epidermal homeostasis. A further study demonstrated that HIF-1α is down-regulated by UVB and that this process is involved in UVB-induced skin hyperplasia. Therefore, we hypothesized that the forced expression of HIF-1α in keratinocytes would prevent UVB-induced keratinocyte overgrowth. Among several agents known to induce HIF-1α, pyrithione-zinc (Py-Zn) overcame the UVB suppression of HIF-1α in cultured keratinocytes. Mechanistically, Py-Zn blocked the degradation of HIF-1α protein in keratinocytes, while it did not affect the synthesis of HIF-1α. Moreover, the p21 cell cycle inhibitor was down-regulated after UVB exposure, but was robustly induced by Py-Zn. In mice repeatedly irradiated with UVB, the epidermis became hyperplastic and HIF-1α disappeared from nuclei of epidermal keratinocytes. However, a cream containing Py-Zn effectively prevented the skin thickening and up-regulated HIF-1α to the normal level. These results suggest that Py-Zn is a potential agent to prevent UVB-induced photoaging and skin cancer development. This work also provides insight into a molecular target for treatment of UVB-induced skin diseases.
Keywords: Ultraviolet, Skin, Hyperplasia, Hypoxia-inducible factor-1α, Pyrithione-zinc
INTRODUCTION
Ultraviolet radiation is the most important cause of skin photoaging and cancer. After the skin is irradiated with ultraviolet, epidermal DNAs are damaged and form abnormal DNA adducts, leading to apoptosis, growth arrest, DNA mutation, and cell transformation [1]. Ultraviolet light is classified into three types (UVA, UVB, and UVC) according to its wavelength. Of these ultraviolet wavelengths, UVB (290~320 nm) is primarily responsible for breaking DNA strands. Immediately after keratinocytes are exposed to UVB, the cell cycle is arrested, and this response to UVB is regarded as a protective process because it allows recovery time for damaged cells [2]. At 48 h after UVB exposure, however, mouse skins were thickened with increased PCNA (a proliferation marker) in epidermal keratinocytes [3-5]. This epidermal proliferation at a subacute phase may be a regeneration process in damaged skin. If the skin repeatedly undergoes UVB injury and regeneration, the cellular processes to control epidermal homeostasis could be deregulated, which may initiate skin photoaging or carcinogenesis.
HIF-1α, a member of the basic-helix-loop-helix (bHLH) Per-Arnt-Sim (PAS) family, transactivates numerous genes that enable cells to adapt to hypoxia [6]. The hypoxic adaptation includes increased erythropoiesis, neovascularization, activated glycolysis, increased glucose uptake, and up-regulation of pro-survival factors [7]. Many of the hypoxia-induced genes possess the conserved sequence 5-ACGTG-3 at their hypoxia response elements, and HIF-1α associates with ARNT to target the elements and activate the genes. In addition to gene expression, HIF-1α plays a key role in controlling cancer cell growth under hypoxia; that is, HIF-1α sequesters MAX from the c-Myc/MAX complex, antagonizes the p21 gene-repressive action of c-Myc/MAX, and induces the p21 cell cycle inhibitor [8]. Likewise, HIF-1α plays a role in epidermal homeostasis by controlling the keratinocyte cell cycle through p21 expression [9]. Epidermal keratinocytes undergo cell cycle arrest in the G1 phase when they are cultured at high cell densities. Concomitantly, HIF-1α and p21 are both induced in a cell density-dependent manner in cultured keratinocytes, and are also normally expressed in human and mouse skins. In vitro and in vivo experiments using HIF-1α siRNAs further suggest that HIF-1α is responsible for keratinocyte cell cycle arrest and that it is involved in epidermal homeostasis by controlling epidermal overgrowth. Furthermore, keratinocyte HIF-1α is suppressed after UVB exposure and is involved in UVB-induced keratinocyte overgrowth [10]. In mice exposed to UVB, their epidermises became hyperplastic, and HIF-1α disappeared from the skin. Based on these results, we suggest that the deregulation of HIF-1α is associated with UVB-induced epidermal hyperplasia.
Given these reports, epidermal growth is controlled by HIF-1α, but is uncontrolled under UVB irradiation due to HIF-1α suppression. Then, is UVB-induced epidermal hyperplasia prevented by an HIF-1α inducer? In this study, we addressed this question and searched for small molecules to protect the skin from UVB effects by inducing HIF-1α.
METHODS
Materials
Pyrithione-zinc (Py-Zn), cycloheximide (CHX), polyethylene glycol (PEG), bafilomycin A1, baicalein, (-)-epigallocatechin-3-gallate (EGCG), and other chemicals were purchased from Sigma-Aldrich Corp. (St. Louis, MO). Culture media and fetal bovine serum (FBS) were purchased from Invitrogen Corp. (Carlsbad, CA). Anti-human HIF-1α antiserum was generated in rabbits against bacterially expressed fragments encompassing amino acids 418-698 of human HIF-1α, as previously described [11]. Antibodies against p21 and β-tubulin and HRP-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA).
Cell culture and UVB irradiation
HaCaT cells (an immortalized human epidermal keratinocyte cell line) were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 100 units/ml penicillin, and 100 µg/ml streptomycin. The cells were grown in a humidified 5% CO2 atmosphere at 37℃, and cell numbers were adjusted to reach 80~100% confluence. Cultured cells were exposed to UVB in a chamber (Vilber Lourmat, Torcy, France) equipped with five UVB lamps (peak wavelength of 312 nm, 8 Watts) and stainless steel walls. To irradiate cells with UVB, we opened the lids of the culture dishes and replaced the media with phosphate-buffered saline (PBS). Immediately after cells were irradiated with 5~20 mJ/cm2 of UVB, PBS was replaced with RPMI-1640 plus 10% FBS medium that had been equilibrated with 5% CO2.
UVB irradiation to mice
Male nude mice (BALB/cAnNCrj/nu/nu) were purchased from Charles River Japan Inc. (Shin-Yokohama, Japan) and housed in a specific pathogen-free room under controlled temperature and humidity. All animal procedures were performed according to the procedures stipulated in the Seoul National University Laboratory Animal Maintenance Manual. At 4 hours prior to UVB irradiation, a 1% Py-Zn PEG cream was applied to the upper back of the mice and a PEG cream to the lower back. During UVB irradiation, the left backs were open and the right backs were screened with aluminum foil as the control skins. Mice were irradiated once a day with 460 mJ/cm2 of UVB for four days. Left and right back skins were excised, fixed with 4% paraformaldehyde, and embedded into paraffin blocks.
Immunohistochemistry
HIF-1α was analyzed by immunohistochemical staining in paraffin-embedded specimens of mouse skin. Six-micrometer serial sections were cut from paraffin block, deparaffinized in 100% xylene, and rehydrated in a graded alcohol series. HIF-1α antigen was retrieved by heating the sections in a microwave for 5 min in 10 mM sodium citrate (pH 6.0). After blocking nonspecific sites, sections were incubated overnight at 4℃ with anti-HIF-1α antiserum (1 : 100). To visualize HIF-1α, the sections were incubated with secondary antibody (1 : 400). Avidin-biotin-HRP complex was used to localize bound antibodies, and diaminobenzidine was used as the final chromogen.
Immunoblotting
Proteins were electrophoresed on 8% or 12% SDS/polyacrylamide gels and then transferred to Immobilon-P membranes (Millipore, Bedford, MA). The membranes were blocked with 5% skim milk in Tris-buffered saline containing 0.1% Tween-20 (TTBS) at room temperature for 1 h, and incubated overnight at 4℃ with primary antibodies (1 : 1,000) in the blocking solution. The membranes were incubated for 1 h with HRP-conjugated secondary antibodies (1 : 5,000) in the blocking solution. Immune complexes were visualized using an Enhanced Chemiluminescence Plus kit (Amersham Biosciences, Piscataway, NJ).
Statistical analysis
All data were analyzed using Microsoft Excel 2007 software, and results are expressed as means and standard deviations. The Mann-Whitney U test (http://faculty.vassar.edu/lowry/utest.html) was used to compare epidermal thickness and HIF-1α-positive cell number. Differences were considered statistically significant at the p<0.05 level. All statistical tests were two-tailed.
RESULTS
Test compounds
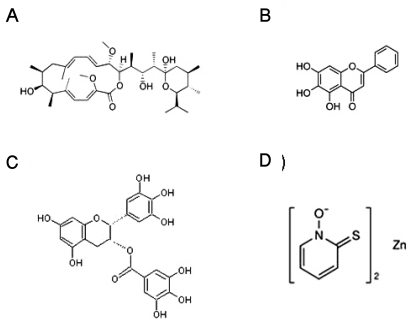
Given the previous reports, we tested four compounds as HIF-1α inducer candidates, namely, bafilomycin A1 [12], baicalein [13], EGCG [14], and Py-Zn [15]. The molecular structures of the test compounds are illustrated in Fig. 1. Based on the cytotoxicity results (data not shown) and doses reported previously, we determined the optimal doses of the test compounds; bafilomycin A1, 5 nM; baicalein, 200 µM; EGCG, 500 µM; Py-Zn, 2 µM.
Fig. 1.
Chemical structures of the test compounds. (A) Bafilomycin A1, (B) baicalein, (C) epigallocatechin 3-gallate (EGCG), (D) pyrithione-zinc (Py-Zn).
Pyrithione-zinc induces HIF-1α in UVB-irradiated keratinocytes
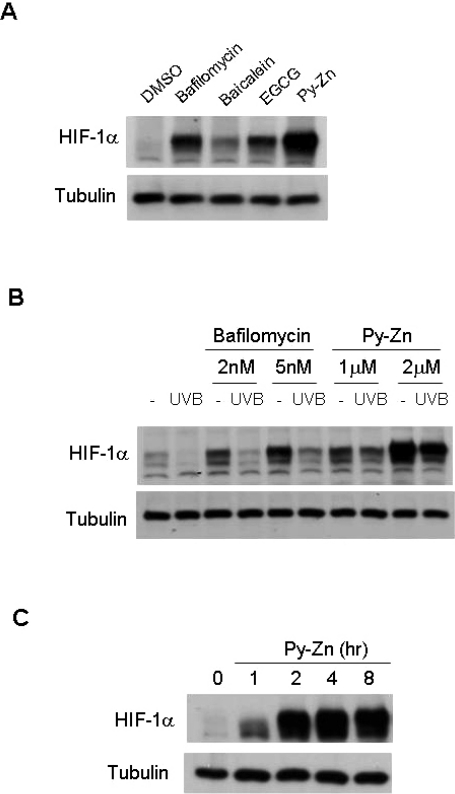
As was observed previously, all compounds tested induced HIF-1α expression in normoxic keratinocytes (Fig. 2A). In terms of HIF-1α levels, bafilomycin A1 and Py-Zn were more effective inducers than the other compounds. To be a good candidate as a UVB-protective agent, the compound should recover HIF-1α expression even after UVB exposure. Therefore, we examined whether the compounds induced HIF-1α in UVB-irradiated keratinocytes. Py-Zn was found to induce HIF-1α substantially in such conditions, whereas bafilomycin A1 failed to rescue HIF-1α (Fig. 2B). In a time course, HIF-1α was induced as early as 1 hour after Py-Zn treatment, and fully expressed after 4 to 8 hours (Fig. 2C). Based on these results, we decided to use Py-Zn as a UVB-protective agent in keratinocytes.
Fig. 2.
HIF-1α levels in keratinocytes. (A) Effects of test compounds on HIF-1α expression. After HaCaT cells had been cultured to a cell density of 80~100%, the cells were treated with various compounds for 4 hours. The cells were harvested and subjected to immunoblotting for HIF-1α and β-tubulin. Concentration: bafilomycin A1, 5 nM; baicalein, 200 µM; EGCG, 500 µM; pyrithione-zinc (Py-Zn), 2 µM. (B) HIF-1α levels after UVB irradiation. HaCaT cells were pretreated with bafilomycin A1 or Py-Zn at the concentrations indicated 2 hours before UVB irradiation, and transiently irradiated with 15 mJ/cm2 of UVB. Two hours later, HaCaT cells were harvested for immunoblotting. (C) Time course of Py-Zn-induced HIF-1α expression. HaCaT cells were treated with 2 µM Py-Zn, harvested at the indicated time, and then subjected to immunoblotting.
Pyrithione-zinc stabilizes HIF-1α in keratinocytes
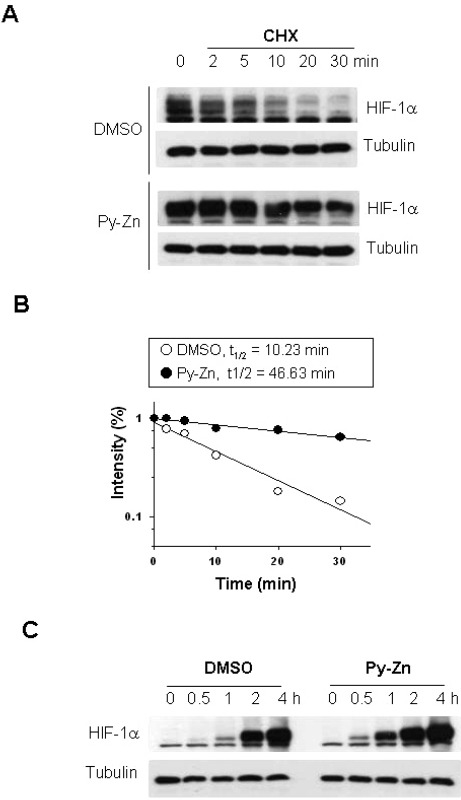
To understand the mechanism underlying the induction of HIF-1α by Py-Zn, we first examined whether Py-Zn stabilizes HIF-1α in keratinocytes. Keratinocytes were treated with cycloheximide (CHX) to block de novo synthesis of HIF-1α protein. In untreated keratinocytes, HIF-1α level declined rapidly after CHX treatment and almost disappeared after CHX treatment for 20 min. However, Py-Zn noticeably delayed the decay of HIF-1α, and thus a substantial amount of HIF-1α remained even after CHX treatment for 30 min (Fig. 3A). To calculate the half-life of HIF-1α protein, we quantified the protein band intensities using the ImageJ 1.36b program and plotted the first-order kinetics. The half-life of HIF-1α was 10.23 min in untreated keratinocytes and was prolonged to 46.63 min in Py-Zn-treated keratinocytes (Fig. 3B). We next analyzed the de novo synthesis of HIF-1α protein. HIF-1α was first eliminated by treating keratinocytes with CHX. To initiate HIF-1α synthesis, cells were washed with PBS and incubated in fresh media containing 10 µM MG132 to prevent the degradation of newly synthesized HIF-1α. Fig. 3C shows that there was no difference in HIF-1α synthesis between untreated and Py-Zn-treated keratinocytes. These results indicate that Py-Zn up-regulates HIF-1α by stabilizing it.
Fig. 3.
Py-Zn stabilizes HIF-1α in keratinocytes. (A) HIF-1α protein stability. HaCaT cells were pretreated with DMSO vehicle or 2 µM Py-Zn for 1 hour, and then further treated with 60 µg/ml of cycloheximide (CHX). Cells were harvested at the indicated time, and HIF-1α levels were analyzed by immunoblotting. (B) Half-lives of HIF-1α proteins. The protein band densities were quantified using ImageJ and plotted as a function of time (first-order kinetics). (C) HIF-1α protein synthesis. HaCaT cells were treated with 60 µg/ml of cycloheximide for 30 min to remove remaining HIF-1α. To initiate HIF-1α synthesis, cells were washed with PBS and incubated in fresh media containing 10 µM MG132, and HIF-1α levels were determined at the indicated time by immunoblotting.
Pyrithione-zinc induces p21 in UVB-irradiated keratinocytes
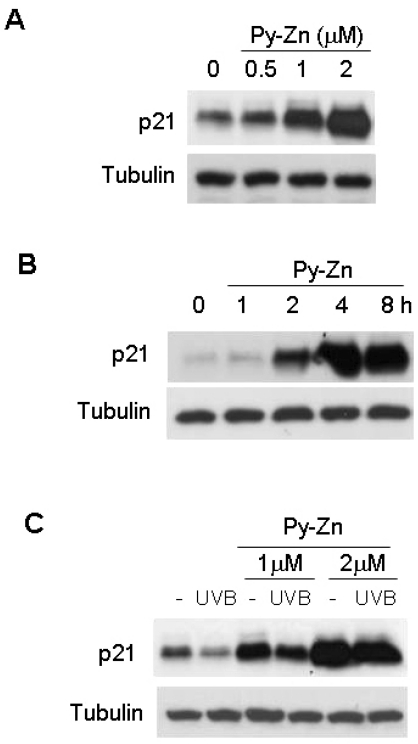
We previously demonstrated that HIF-1α induces cell cycle arrest in keratinocytes by inducing p21 [9]. Thus, we examined whether Py-Zn induced p21 through HIF-1α expression in keratinocytes, and found such an effect by Py-Zn (Fig. 4A). We also found that p21 is induced on the same time course as HIF-1α is (Fig. 4B). Furthermore, the basal level of p21 was significantly reduced after UVB, but Py-Zn overcame the UVB suppression of p21 (Fig. 4C). Based on these results, Py-Zn is expected to inhibit the re-growth of UVB-irradiated keratinocytes.
Fig. 4.
p21 levels in keratinocytes. (A) Effect of Py-Zn on p21 expression in keratinocytes. HaCaT cells were treated with Py-Zn at the concentrations indicated for 4 hours and then harvested for immunoblotting. (B) Time course of Py-Zn-induced p21 expression. HaCaT cells were treated with 2 µM Py-Zn, harvested at the indicated time, and then subjected to immunoblotting with anti-p21 antiserum. (C) p21 levels after UVB irradiation. HaCaT cells were pretreated with Py-Zn at the indicated concentrations 2 hours before UVB irradiation, and irradiated with 15 mJ/cm2 of UVB. Two hours later, HaCaT cells were harvested for immunoblotting.
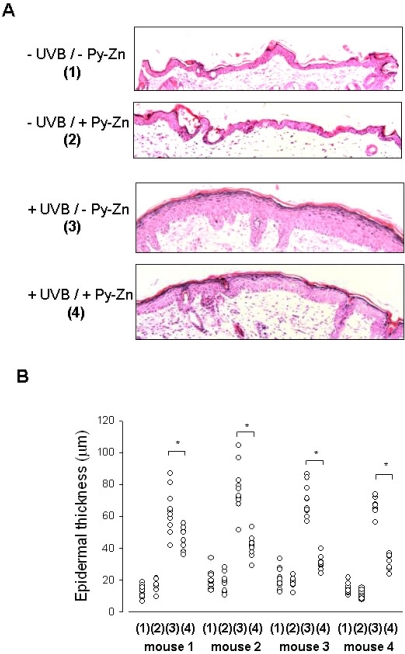
Pyrithione-zinc prevents UVB-induced skin hyperplasia and HIF-1α suppression in vivo
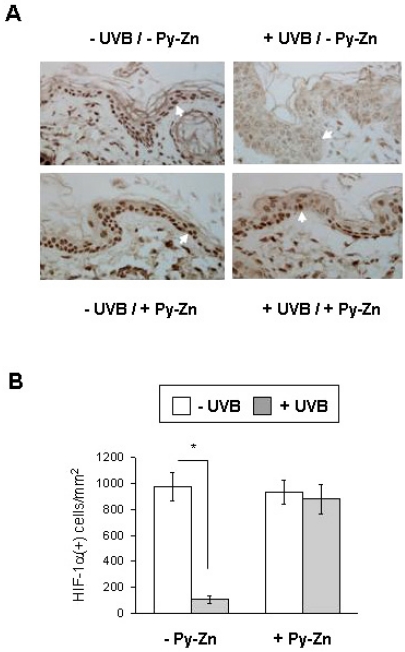
To examine the in vivo effect of Py-Zn on UVB-induced epidermal hyperplasia, PEG or Py-Zn-containing PEG cream was applied to the backs of four nude mice, which were then irradiated with UVB once a day for four days. The back skins were then excised and prepared for immunohistochemistry. When the skin samples were stained with H&E, the epidermal layer in UVB-exposed skin looked thicker than that in the contralateral non-exposed skin. However, the epidermal thickness in Py-Zn-treated skin was noticeably reduced compared to that in untreated skins after UVB irradiation (Fig. 5A). To evaluate the effect of Py-Zn on skin hyperplasia, we measured the epidermal thickness at 10 different skin regions per mouse and plotted the data in Fig. 5B. Although skin thickness was somewhat variable within the observed zones, skin treated with Py-Zn was generally thinner than untreated skin. Statistical analyses showed significant differences between the two groups (Fig. 5B). We next analyzed HIF-1α expression by immunohistochemistry using anti-HIF-1α antiserum. As expected, HIF-1α was clearly detected in nuclei of the control epidermis, but disappeared after UVB irradiation. When treated with Py-Zn, the skin expressed substantial levels of HIF-1α even after UVB irradiation (Fig. 6A). We counted HIF-1α-positive cells and plotted the data in Fig. 6B. HIF-1α expression in Py-Zn-treated skin was not significantly altered by UVB exposure, but was markedly attenuated in untreated skins. These in vivo findings suggest that Py-Zn prevents UVB-induced skin hyperplasia by maintaining keratinocyte HIF-1α.
Fig. 5.
In vivo effect of Py-Zn on UVB-induced skin hyperplasia. The back skin of each mouse was divided into four parts: the right lower quadrant for -UVB/-Py-Zn (1), the right upper quadrant for -UVB/+Py-Zn (2), the left lower quadrant for +UVB/-Py-Zn (3), the left upper quadrant for +UVB/+Py-Zn (4). Four mice were pretreated with PEG or 1% Py-Zn PEG cream 4 hours before UVB irradiation and then exposed to 460 mJ/cm2 of UVB once a day for four days. Back skins were excised, fixed, and embedded into paraffin blocks. (A) H&E staining of mouse skin. Skin specimens were obtained from 10 different sites per quadrant of each mouse and stained with H&E. (B) The thickness of the epidermis was analyzed at a magnification of 100×. The results were obtained from four mice (mouse 1~4). *Denotes p<0.05 between two groups.
Fig. 6.
In vivo effect of Py-Zn on UVB-induced HIF-1α suppression. (A) HIF-1α staining. The skin specimens were obtained as described in Fig. 5 and stained with anti-HIF-1α antibody. Nuclear HIF-1α was identified at a magnification of 100×. (B) Numbers of HIF-1α-positive keratinocytes. Three different slides per quadrant were stained and counted, and the data were collected from tissues of four mice (12 slides per group). *Denotes p<0.05 between two groups.
DISCUSSION
In the present study, we sought an HIF-1α-inducing compound that could prevent development of post-UVB epidermal hyperplasia. Initially, we tried four compounds known to induce HIF-1α and found that Py-Zn was the most effective of these compounds. Mechanistically, Py-Zn induced HIF-1α by blocking the degradation of HIF-1α. This compound also strongly induced p21 expression. To examine the in vivo effect of Py-Zn on UVB-induced epidermal hyperplasia, nude mice were pretreated with a Py-Zn PEG cream and then irradiated with UVB. After intermittent exposure to UVB, mouse skin became thicker and lost HIF-1α within the cell nuclei. A Py-Zn-containing cream prevented the skin hyperplasia and recovered HIF-1α to almost control level. These results suggest that Py-Zn could be developed as a novel HIF-1α-targeting agent to prevent UVB-induced skin diseases.
The expression of HIF-1α is regulated at multiple steps, namely, proteolysis, translation, and protein interactions. The middle portion of HIF-1α contains the Pro-Ser-Thr-rich oxygen-dependent degradation domain (ODDD; aa. 401-603), which is involved in proteolysis [16]. In aerobic conditions, HIF-1-prolyl hydroxylases (PHD1-3) hydroxylate two proline residues (P402 and P564) located at both termini of the ODDD. Then, von Hippel-Lindau tumor suppressor protein (pVHL) binds to the hydroxylated sites, leading to the ubiquitination and proteasomal degradation of HIF-1α [17]. In oxygen-deficient conditions, the oxygen-dependent hydroxylation process is limited, thereby stabilizing HIF-1α. With respect to the regulation of HIF-1α translation, two distinct mechanisms have been reported: cap-dependent translation and IRES-dependent translation [18,19]. The HIF-1α 5'-UTR segment is the target site in both types of translational regulation. In the cap-dependent pathway, HIF-1α translation is facilitated by the receptor tyrosine kinases-PI3K-Akt-mTOR pathway [20]. In addition, HIF-1α interacts with a variety of proteins, some of which are known to regulate the stability of HIF-1α positively or negatively. For example, HSP90 and HSP70 associate with HIF-1α and induce the stabilization and the degradation of HIF-1α, respectively [21,22].
In most mammalian cells, HIF-1α is scarcely detected under normoxia by Western blotting. However, we previously found that epidermal keratinocytes uniquely express substantial amounts of HIF-1α even in normoxia. We also demonstrated that mitochondrial reactive oxygen species and the MEK/ERK pathway are involved in HIF-1α stabilization in keratinocytes [9]. In another study, we showed that UVB down-regulates HIF-1α by inhibiting the cap-dependent translation of HIF-1α [10]. As the translation step precedes protein stabilization, the keratinocyte's ability to stabilize HIF-1α might be in vain after UVB irradiation. For this reason, we searched for an HIF-1α inducer strong enough to overcome the UVB effect. Fortunately, we successfully found a compound that satisfied our criteria, namely, Py-Zn.
Then, how does Py-Zn stabilize HIF-1α? In fact, we previously reported the HIF-1α-inducing activity of Py-Zn in various cell lines, such as PC3, DU145, MKN28, and HEK293 cells [15]. In that study, we used Py-Zn as a zinc ionophore to transport Zn2+ into cells. HIF-1α is normally expressed in human and mouse prostates, which contain extremely high concentration of zinc. Indeed, Zn2+ induces HIF-1α expression in prostate cells even under normoxic conditions. Mechanistically, Zn2+ inactivates PHD activity and precludes pVHL binding to HIF-1α. Given that Py-Zn increases the intracellular concentration of Zn2+, it is expected that Py-Zn stabilizes HIF-1α due to the increased Zn2+ concentration. However, we can not rule out the possibility that pyrithione per se is responsible for HIF-1α stabilization. The precise mechanism underlying the Py-Zn effect thus remains an open question.
Py-Zn is a coordination complex composed of two pyrithiones and a Zn2+ ion. It is well-known as an active ingredient in shampoos for treating dandruff and seborrheic dermatitis [23,24]. As it also has antifungal and antibacterial properties [25], Py-Zn is used topically to treat various skin diseases, such as athlete's foot, eczema, ringworm, tinea, atypical dermatitis, and vitiligo. Py-Zn is used worldwide in many approved over-the-counter (OTC) topical drugs and shampoos. These creams or shampoos usually include 1% (~30 mM) to 2% (~60 mM) of Py-Zn. In the present study, we tested Py-Zn as a UVB-protective cream and found that the 1% Py-Zn cream effectively prevented UVB-induced epidermal hyperplasia in mouse skin. To the best of our knowledge, this is the first report suggesting the UVB-protective effect of Py-Zn in the skin. Therefore, we propose that Py-Zn could be used as an active ingredient in sunscreen. However, Py-Zn-containing sunscreen must be validated clinically.
In conclusion, UVB irradiation suppresses HIF-1α in cultured keratinocytes and in mouse skin and is also associated with proliferation of keratinocytes and with epidermal thickening in mice. This skin response to UVB is believed to underlie UVB-induced hyperproliferative skin diseases, such as premature photoaging and skin cancer. Based on this scenario, HIF-1α expression could offer a means of preventing UVB-induced skin diseases; this hypothesis is supported by this work. Therefore, we propose that HIF-1α is a therapeutic target for preventing skin hyperplasia and, furthermore, that Py-Zn is a promising compound for developing a new ultraviolet-protective sunscreen.
ACKNOWLEDGEMENTS
This work was supported by a grant (# A080139) from Korea Health Industry Development Institute.
ABBREVIATIONS
- PCNA
proliferating cell nuclear antigen
- bHLH
basic-helix-loop-helix
- PAS
Per-Arnt-Sim
- HIF-1α
hypoxia-inducible factor-1α
- ARNT
aryl hydrocarbon receptor nuclear translocator
- siRNA
small interfering RNA
- Py-Zn
pyrithione-zinc
- CHX
cycloheximide
- PEG
polyethylene glycol
- H&E
hematoxylin & eosin
- EGCG
(-)-epigallocatechin-3-gallate
- FBS
fetal bovine serum
- RPMI
Roswell Park Memorial Institute
- ODDD
oxygen-dependent degradation domain
- PHD
HIF-1-prolyl hydroxylase
- pVHL
von Hippel-Lindau tumor suppressor protein
- IRES
internal ribosome entry site
- 5'-UTR
5'-untranslated region
- PI3K
phosphoinositide-3-kinase
- mTOR
mammalian target of rapamycin
- Akt
serine/threonine protein kinase
- HSP
heat shock protein
- MEK/ERK
mitogen-activated protein kinase kinase/extracellular signal-regulated kinase
- HEK293
human embryonic kidney cell line 293
- OTC
over-the-counter
References
- 1.Melnikova VO, Ananthaswamy HN. Cellular and molecular events leading to the development of skin cancer. Mutat Res. 2005;571:91–106. doi: 10.1016/j.mrfmmm.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Marrot L, Meunier JR. Skin DNA photodamage and its biological consequences. J Am Acad Dermatol. 2008;58:S139–S148. doi: 10.1016/j.jaad.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Ouhtit A, Muller HK, Davis DW, Ullrich SE, McConkey D, Ananthaswamy HN. Temporal events in skin injury and the early adaptive responses in ultraviolet-irradiated mouse skin. Am J Pathol. 2000;156:201–207. doi: 10.1016/S0002-9440(10)64720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JK, Kim JH, Nam KT, Lee SH. Molecular events associated with apoptosis and proliferation induced by ultraviolet-B radiation in the skin of hairless mice. J Dermatol Sci. 2003;32:171–179. doi: 10.1016/s0923-1811(03)00094-x. [DOI] [PubMed] [Google Scholar]
- 5.El-Abaseri TB, Putta S, Hansen LA. Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis. 2006;27:225–231. doi: 10.1093/carcin/bgi220. [DOI] [PubMed] [Google Scholar]
- 6.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 7.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1α induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho YS, Bae JM, Chun YS, Chung JH, Jeon YK, Kim IS, Kim MS, Park JW. HIF-1α controls keratinocyte proliferation by up-regulating p21(WAF1/Cip1) Biochim Biophys Acta. 2008;1783:323–333. doi: 10.1016/j.bbamcr.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Cho YS, Kim CH, Park JW. Involvement of HIF-1α in UVB-induced epidermal hyperplasia. Mol Cells. 2009;28:537–543. doi: 10.1007/s10059-009-0148-2. [DOI] [PubMed] [Google Scholar]
- 11.Chun YS, Choi E, Kim GT, Lee MJ, Lee MJ, Lee SE, Kim MS, Park JW. Zinc induces the accumulation of hypoxia-inducible factor (HIF)-1, but inhibits the nuclear translocation of HIF-1α, causing HIF-1β inactivation. Biochem Biophys Res Commun. 2000;268:652–656. doi: 10.1006/bbrc.2000.2180. [DOI] [PubMed] [Google Scholar]
- 12.Lim JH, Park JW, Kim MS, Park SK, Johnson RS, Chun YS. Bafilomycin induces the p21-mediated growth inhibition of cancer cells under hypoxic conditions by expressing hypoxia-inducible factor-1α. Mol Pharmacol. 2006;70:1856–1865. doi: 10.1124/mol.106.028076. [DOI] [PubMed] [Google Scholar]
- 13.Cho H, Lee HY, Ahn DR, Kim SY, Kim S, Lee KB, Lee YM, Park H, Yang EG. Baicalein induces functional hypoxia-inducible factor-1α and angiogenesis. Mol Pharmacol. 2008;74:70–81. doi: 10.1124/mol.107.040162. [DOI] [PubMed] [Google Scholar]
- 14.Weinreb O, Amit T, Youdim MB. A novel approach of proteomics and transcriptomics to study the mechanism of action of the antioxidant-iron chelator green tea polyphenol (-)-epigallocatechin-3-gallate. Free Radic Biol Med. 2007;43:546–556. doi: 10.1016/j.freeradbiomed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Park SE, Park JW, Cho YS, Ryu JH, Paick JS, Chun YS. HIF-1α promotes survival of prostate cells at a high zinc environment. Prostate. 2007;67:1514–1523. doi: 10.1002/pros.20641. [DOI] [PubMed] [Google Scholar]
- 16.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 18.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang KJ, Kappel A, Goodall GJ. Hypoxia-inducible factor-1α mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Mol Biol Cell. 2002;13:1792–1801. doi: 10.1091/mbc.02-02-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arsham AM, Plas DR, Thompson CB, Simon MC. Phosphatidylinositol 3-kinase/Akt signaling is neither required for hypoxic stabilization of HIF-1α nor sufficient for HIF-1-dependent target gene transcription. J Biol Chem. 2002;277:15162–15170. doi: 10.1074/jbc.M111162200. [DOI] [PubMed] [Google Scholar]
- 21.Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1α-degradative pathway. J Biol Chem. 2002;277:29936–29944. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- 22.Luo W, Zhong J, Chang R, Hu H, Pandey A, Semenza GL. Hsp70 and CHIP selectively mediate ubiquitination and degradation of hypoxia-inducible factor (HIF)-1α but Not HIF-2α. J Biol Chem. 2010;285:3651–3663. doi: 10.1074/jbc.M109.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierard-Franchimont C, Goffin V, Decroix J, Pierard GE. A multicenter randomized trial of ketoconazole 2% and zinc pyrithione 1% shampoos in severe dandruff and seborrheic dermatitis. Skin Pharmacol Appl Skin Physiol. 2002;15:434–441. doi: 10.1159/000066452. [DOI] [PubMed] [Google Scholar]
- 24.Bailey P, Arrowsmith C, Darling K, Dexter J, Eklund J, Lane A, Little C, Murray B, Scott A, Williams A, Wilson D. A doubleblind randomized vehicle-controlled clinical trial investigating the effect of ZnPTO dose on the scalp vs. antidandruff efficacy and antimycotic activity. Int J Cosmet Sci. 2003;25:183–188. doi: 10.1046/j.1467-2494.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 25.Guthery E, Seal LA, Anderson EL. Zinc pyrithione in alcohol-based products for skin antisepsis: persistence of antimicrobial effects. Am J Infect Control. 2005;33:15–22. doi: 10.1016/j.ajic.2004.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]