Abstract
Impairment in spinal inhibition caused by quantitative alteration of GABAergic elements following peripheral nerve injury has been postulated to mediate neuropathic pain. In the present study, we tested whether neuropathic pain could be induced or reversed by pharmacologically modulating spinal GABAergic activity, and whether quantitative alteration of spinal GABAergic elements after peripheral nerve injury was related to the impairment of GABAergic inhibition or neuropathic pain. To these aims, we first analyzed the pain behaviors following the spinal administration of GABA antagonists (1 µg bicuculline/rat and 5 µg phaclofen/rat), agonists (1 µg muscimol/rat and 0.5 µg baclofen/rat) or GABA transporter (GAT) inhibitors (20 µg NNC-711/rat and 1 µg SNAP-5114/rat) into naïve or neuropathic animals. Then, using Western blotting, PCR or immunohistochemistry, we compared the quantities of spinal GABA, its synthesizing enzymes (GAD65, 67) and its receptors (GABAA and GABAB) and transporters (GAT-1, and -3) between two groups of rats with different severity of neuropathic pain following partial injury of tail-innervating nerves; the allodynic and non-allodynic groups. Intrathecal administration of GABA antagonists markedly lowered tail-withdrawal threshold in naïve animals, and GABA agonists or GAT inhibitors significantly attenuated neuropathic pain in nerve-injured animals. However, any quantitative changes in spinal GABAergic elements were not observed in both the allodynic and non-allodynic groups. These results suggest that although the impairment in spinal GABAergic inhibition may play a role in mediation of neuropathic pain, it is not accomplished by the quantitative change in spinal elements for GABAergic inhibition and therefore these elements are not related to the generation of neuropathic pain following peripheral nerve injury.
Keywords: GABA, GAD65, GAD67, GAT-1, GAT-3, Peripheral nerve injury, Neuropathic pain
INTRODUCTION
Neuropathic pain has been defined as "pain initiated or caused by a primary lesion or dysfunction in the nervous system" [1] and is characterized by spontaneous burning pain, hyperalgesia and allodynia [2-4]. However, this pain is not observed in all the individuals with nerve injury. Patients even with an identical nerve injury, such as limb amputation, may or may not suffer from neuropathic pain [5-7]. This is also true for neuropathic pain in experimental animal studies [8-11]. Therefore, the identification of change(s) or factor(s) associated with individual difference in the manifestation of neuropathic pain may provide critical clues for the mechanisms of neuropathic pain.
Several lines of evidence suggest that peripheral nerve injury-induced loss of GABAergic inhibition in the spinal dorsal horn may be responsible for neuropathic pain [12-14]. This is very likely, because pharmacological antagonism of GABAergic inhibitory transmission in the spinal cord of naive animals induces neuropathic pain-like behaviors [15-18]. This mechanism for neuropathic pain has been supported by the following evidence; reduced levels of spinal GABA [19-22], its synthesizing enzymes [12,20] and its receptors [23,24], which are thought to result from excitotoxic apoptosis of spinal GABAergic interneuron [12]. Reduction of spinal GABA transporters (GAT) after nerve injury has also been suggested as a contributor to neuropathic pain [25]. On the other hand, however, some studies showed no significant loss in the content of GABA synaptosome preparations [14], GABA receptors [26] or spinal GABAergic interneurons in neuropathic animals [27-29]. Furthermore, there is an evidence of increase, rather than decrease, in the level of GABA in the ipsilateral dorsal horn [30] or in GABAergic inhibitory activity of dorsal horn neurons [31] following peripheral nerve injury.
As for the discrepancy in the above findings, it is quite possible that differential changes in spinal GABAergic elements following peripheral nerve injury might be correlated with individual difference in the manifestation of neuropathic pain; the elements are decreased in the allodynic animals and increased in the non-allodynic ones. Therefore, in the present study, we examined 1) whether pharmacological reduction of spinal GABAergic activity by intrathecal application of antagonists in naïve animals could induce neuropathic pain-like behavior, 2) whether increase of GABAergic activity by spinal administration of GABA agonists or GAT inhibitors might reverse neuropathic pain behavior, and 3) whether quantitative changes in the spinal GABAergic elements were related to the generation of neuropathic pain by comparing the quantities of spinal GABA, its synthesizing enzymes, its receptors and transporters between two groups of rats that showed different severity of neuropathic pain following partial injury of tail-innervating nerves; one group (allodynic group) exhibited robust neuropathic pain such as mechanical, cold and warm allodynia after nerve injury, whereas the other group (non-allodynic group) showed poorly developed signs of neuropathic pain although they underwent the same nerve injury.
METHODS
Animals
Sprague-Dawley male rats, weighing 150~200 g, were housed in suspended wire mesh cages in a room maintained at a 12 h light/dark cycle (lights on at 07:00 h) and 22~25℃, with free access to food and water. All experiments were in accordance with the Korean law on animal care guidelines (8282-13, revised 2007. 1.26) and the guidelines set by the Korea University College of Medicine Animal Research Policies Committee.
Neuropathic surgery
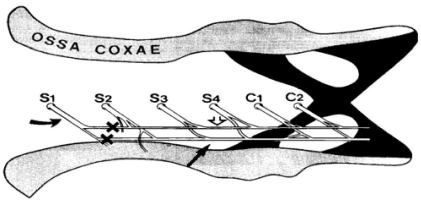
The neuropathic surgery was based on the procedure previously described by Back et al. [32]. Briefly, under enflurane anesthesia (0.5~2%) animals were subjected to unilateral transection of the inferior and superior caudal trunks at the level between the S1 and S2 spinal nerves (Fig. 1). To prevent possible rejoining of proximal and distal ends of the severed trunks, pieces of nerve about 2 mm in length were removed from the distal nerve ends. This procedure severed the S1 spinal nerve innervating the tail.
Fig. 1.
A schematic diagram illustrating how the inferior (black arrow) and superior (open arrow) caudal trunks are composed and the level of the transection (X) of nerve trunks. The curved arrow indicates the S1 spinal nerve.
Behavioral tests for neuropathic pain
Mechanical allodynia was assessed by the tail-withdrawal threshold in response to a series of calibrated von Frey filaments (3.92, 5.88, 9.80, 19.60, 39.20, 58.80, 78.40 and 147.00 mN, Stoeling, Wood Dale, IL, USA; equivalent in grams to 0.4, 0.6, 1.0, 2.0, 4.0, 6.0, 8.0 and 15.0). The 50% withdrawal threshold was determined using the up-down method [33]. In brief, testing was initiated with a filament whose bending force was 19.60 mN in the middle of the series, and the most sensitive spot of the tail was first determined by probing various areas with this filament. In the absence of a tail withdrawal response to this filament, this process for searching the sensitive spot was repeated with the next stiffer filament. However, if the sensitive spot whose positive response had been elicited was determined, this spot was touched just once with one of a series of 8 von Frey filaments in a up-down fashion, as follows; a next weaker stimulus was applied in the presence of a withdrawal response, whereas a next stronger stimulus was applied in the absence of withdrawal. Stimuli were presented at intervals of several seconds. A brisk tail withdrawal to von Frey filament application was regarded as a positive response.
Testing for cold or warm allodynia was performed by measuring tail withdrawal latency to cold (4℃) or warm (40℃) water stimulation, respectively. After immersing the tail into cold or warm water bath, the investigator continuously observed the tail to find out whether it moved abruptly, and measured the latency of the tail movement within a cut-off time of 15 sec. An abrupt tail movement within the cut-off time was considered as a positive withdrawal response, whereas lack of tail movement until the cut-off time or slow tail movement within the cut-off time was not considered to be a positive response. The testing was repeated five times with 5 min intervals, and the average latency of tail response was calculated.
After behavioral testing on each experimental day, animals were assigned to the allodynic group if they showed robust neuropathic pain (≥70% decrease in withdrawal threshold and ≥30% decrease in latencies), or to the non-allodynic group if they exhibited little allodynia (≤10% decrease in withdrawal threshold and latencies). To exclude the possibility that lack of tail-withdrawal response in the non-allodynic group was caused by motor impairment, we conducted a pinch-withdrawal test in both the alloydynic and non-allodynic groups of rats before sacrificing them or immunohistochemistry, Western blotting or PCR: The tip of the tail was pinched with a small toothed forceps, and the number of withdrawal responses out of 10 trials was calculated as a percentage for each group.
Western blotting
Under sodium pentobarbital (50 mg/kg, i.p.) anesthesia, rats were perfused with cold heparinized saline. The S1 spinal segments and dorsal root ganglia (DRG) were quickly removed. The excised spinal segments were cut sagittally into ipsilateral and contralateral hemi-cords, and then quickly frozen on dry ice. All tissues were stored at -80℃ until the day of tissue preparation. Frozen tissue was allowed to thaw and then homogenized in TRI reagent (Sigma-Aldrich, MO, USA). Protein concentration was determined using the BCA protein assay kit (PIERCE, IL, USA). Protein was aliquoted and stored at -80℃.
Thirty microgram each of total protein/lane was resolved on SDS-PAGE gel (10% polyacrylamide gel) at 100 V for 2 h and transferred to a nitrocellulose membrane. Subsequently, the membrane was blocked with 5% non-fat dry milk in TBST (TBS buffer plus 1% Tween 20) for 1 h at room temperature (RT), and incubated with mouse anti-glutamic acid decarboxylase (GAD) 65 kDa (1:2000; Chemicon) [12,34] or mouse anti-GAD67 (1:5,000; Chemicon) [12,35] in TBST overnight at 4℃. The membrane was rinsed three times with TBST for 10 min each, and then incubated with horseradish peroxidase-conjugated anti-mouse IgG (Sigma-Aldrich, MO, USA) for 1 h at RT. Proteins were detected with the enhanced chemiluminescence system (Amersham Pharmarcia Biotech, UK). The membranes were stripped by submerging them in stripping buffer (100 mM β-mercaptoethanol, 2% sodium deodecyl sulphate, 62.5 mM Tris-HCl, pH 6.7) at 60℃ for 30 min with occasional agitation. Subsequently, the membranes were washed three times in TBST for 10 min each, blocked with 5% non-fat dry milk/1× TBST for 1 hr. After stripping, the membranes were reprobed with anti-β-actin as a loading control. Band intensity was measured densitometrically using Scion Image program (Scion Corp.), normalized to β-actin levels and expressed as a percentage of the band from naïve animals.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
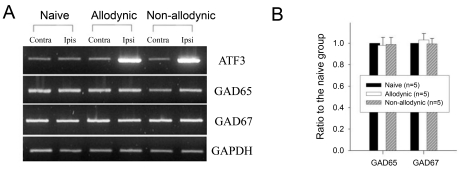
The S1 spinal hemi-cords and DRG were homogenized in TRI reagent (Sigma-Aldrich, MO, USA). RNA samples were spectrophotometrically quantified at 260 nm. A total 20 µl of cDNA was synthesized using 1 µg of total RNA in the mixture of Superscript II (Invitrogen, USA), 5× First-strand buffer, 0.1 M DTT, and 10 mM dNTPs. The PCR was carried out with PCR-premix (Bionner, Korea) and the primer sets for the rat GAD65, GAD67, GAT-1, GAT-3 and activating transcription factor 3 (ATF3) which were adopted as a marker of nerve injury (Table 1). The PCR products were visualized in ethidium bromide containing 1.5% agarose gel using Kodak 1D Image Analysis System (Kodak, USA). The amount of PCR product of each sample was normalized to the amount of GAPDH.
Table 1.
Sequences of primers used for RT-PCR
Immunohistochemistry
According to the result of behavioral testing for 2 weeks following nerve injury, animals were assigned to the allodynic or the non-allodynic groups. The rats were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and perfused with heparinized saline followed by 4% paraformaldehyde and 0.1% picric acid in 0.1 M phosphate buffer (PB, pH 7.4). The S1 spinal segments were harvested by laminectomy. Each segment was post-fixed for 6~8 h in the same fixative and then cryoprotected in 30% sucrose in 0.1 M PB for 48 h at 4℃. The excised segment was sectioned at 14 µm on a cryostat. Spinal cord sections from each group were mounted on the same slides, and were treated under the same condition during immunohistochemical procedure to minimize the among-group variability. Sections were processed for immunostaining with the ABC technique (Vector Elite Kit, Vector, Burlingame, CA, USA). Sections were blocked with 1% bovine serum albumin in 10% normal goat serum (NGS) for 1 h at RT and then incubated for more than two days at 4℃ with mouse anti-GAD65 (1:1,000; Chemicon), mouse anti-GAD67 (1:5,000; Chemicon), rabbit anti-GABA (1:1,000; Chemicon), mouse anti-GABAA receptor β2/β3 subunit (1:50; Chemicon) [12,36], guinea pig anti-GABAB R2 receptor (1:1,000; Chemicon), rabbit anti-GABA transporter (GAT)-1 (1:100; Chemicon) [37], and rabbit anti-GAT-3 (1:500; Chemicon) [37]. Sections were sequentially rinsed with 0.05 M phosphate buffered saline (PBS) and 3% NGS for 30 min each. They were then incubated with biotinylated secondary antibody PBS solution for 1 h, sequentially rinsed with 1% and 3% NGS, and reacted with ABC solution for 1 h. Immunoreactive products were visualized by immersion in a solution of diaminobenzidine (0.05%) with 2% NiCl containing 0.01% hydrogen peroxide. Reacted sections were then dehydrated and coverslipped. For immunofluorescence, the sections were incubated for 1 h at RT with FITC-conjugated secondary antibody (1:200, Chemicon, CA, USA) instead of biotinylated secondary antibody. The tissue from each group was processed in parallel. Control experiments were also carried out by substitution of the primary antibody with PBS.
Ten spinal cord sections were randomly picked from each rat, and were used for measurement of immunostaining intensity in the superficial dorsal horn. To quantify staining intensity, we used a computer-assisted image analysis system (Scion Corp.). An image of a spinal cord section was first captured with a CCD camera through a ×4 objective and converted to digital image composed of pixels having the gray values ranging from 0 to 255. The intensity of immunoreactivity (ir) was expressed as the mean gray-scale value of pixels constituting the image of the superficial dorsal horn. For analyses of lamina in each spinal segment, we first examined the reference sections, which had been stained with toluidine blue, and then recognized the boundaries between laminae I, II and III by the distribution of myelinated nerve fibers. According to this result, in actual image analysis, the approximate outline including lamina I and II was drawn on the digital image of spinal cord. Staining intensity of the ipsilateral or contralateral side was normalized to naïve.
Drug administration
Bicuculline (GABAA antagonist) and muscimol (GABAA receptor agonist) were obtained from Sigma-Adrich (MO, USA). Phaclofen (GABAB receptor antagonist), baclofen (GABAB agonist), NNC-711 (GAT-1 inhibitor) and (S)-SNAP 5114 (GAT-3 inhibitor) were purchased from Tocris Cookson (Bristol, UK). All drugs were dissolved in saline using ultrasonic washer and applied intrathecally. The doses used in the present study were 1 µg of bicuculline [16], 5 µg of phaclofen [16], 1 µg of muscimol [38], 0.5 µg of baclofen [38], 20 µg of NNC-711 and 1 µg of SNAP-5114.
Spinal administration of drugs was carried out by lumbar puncture. Under slight enflurane anesthesia, the rat was held in one hand by the pelvic girdle, and a 30-gauge needle connected to a 25 µl-Hamilton syringe was inserted into the subarachnoid space between the spinous processes of L5 and L6. The syringe was held in position for about 30 sec after the injection of 5 µl/rat.
Behavioral testing was performed 30 min prior to and after administration of drug by an investigator who was unaware of the injection status of the rats.
Statistical analysis
Data are expressed as mean±S.E.M. Paired t-test (or the Wilcoxon Signed Rank Test), unpaired t-test (or the Mann-Whitney Rank Sum Test), and one-way ANOVA (or Kruskal-Wallis ANOVA, depending on normality test) were used wherever appropriate. Statistical analyses of the data obtained from the drug tests were conducted with one-way repeated measures ANOVA test followed by a pairwise comparison of pain behaviors before and after the injection, utilizing Bonferroni t-test. p<0.05 was considered statistically significant.
RESULTS
Pharmacological regulation of spinal GABAergic activity effect of spinal GABA receptor antagonists in naïve animals
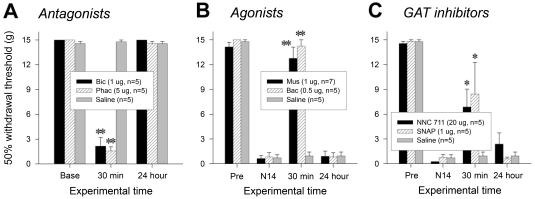
To investigate whether loss of spinal GABAergic activity could induce neuropathic pain-like behavior in naïve animals, we studied the effect of GABA antagonists. As shown in Fig. 2A, bicuculline (1 µg/rat, i.t.) and phaclofen (5 µg/rat, i.t.) induced significant reduction of tail-withdrawal thresholds at 30 min after the injection [**p≤0.001 vs. pre-injection value (Base), one-way repeated measures ANOVA followed by Bonferroni t-test], suggesting the induction of mechanical allodynic sign. These results indicate the probability that nerve injury-induced reduction of spinal GABAergic activity might be a cause of neuropathic pain.
Fig. 2.
Changes in pain behaviors by pharmacological regulation of spinal GABAergic activity. (A) Mechanical allodynia induced by intrathecal administration of GABA antagonists, bicuculline and phaclofen, in naïve rats. (B) Attenuation of nerve injury-induced mechanical allodynia by GABA agonists, muscimol and baclofen, applied spinally. (C) Reversal of nerve injury-induced mechanical allodynia by intrathecally administered GAT inhibitors, NNC 711 for GAT-1 and SNAP 5114 for GAT-3. 'Pre' and 'N14' indicate 1 day before and 14 days after neuropathic surgery, respectively. *p≤0.05, **p≤0.001 vs. pre-injection value (One-way repeated measures ANOVA followed by Bonferroni t-test).
Effect of spinal GABA receptor agonists and GABA transporter inhibitors in neuropathic animals
Next, we asked a question of whether increase in spinal GABAergic activity by GABA receptor agonism or GABA uptake inhibition could attenuate nerve injury-induced pain. As shown in Fig. 2B, muscimol (1 µg/rat, i.t.) and baclofen (0.5 µg/rat, i.t.) significantly increased tail withdrawal thresholds at 30 min after the injection [**p≤0.001 vs. behavioral score at 14 days following the nerve injury (N14), One-way repeated measures ANOVA followed by Bonferroni t-test]. In addition, GAT inhibitors, NNC 711 (20 µg/rat, i.t.) for GAT-1 and SNAP 5114 (1 µg/rat, i.t.) for GAT-3, also relieved mechanical allodynia [Fig. 2C, *p<0.05 vs. behavioral score at 14 days following the nerve injury (N14), One-way repeated measures ANOVA followed by Bonferroni t-test]. These results emphasize an importance of spinal GABAergic activity in the regulation of neuropathic pain processing.
Quantities of spinal GABA synthesizing enzymes (GAD65 and GAD67) after nerve injury
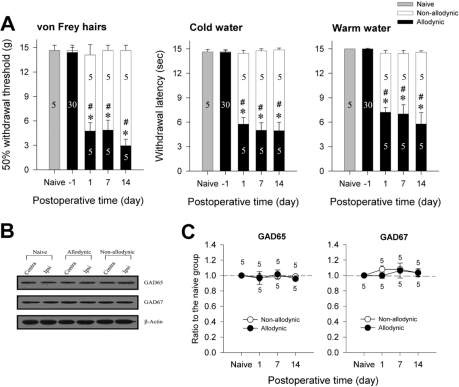
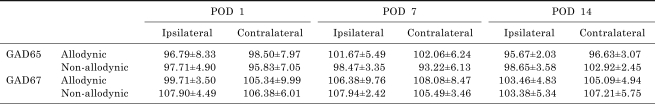
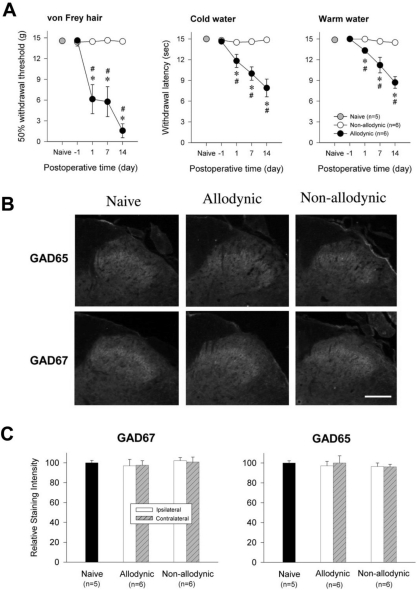
According to the results of behavioral testing on the postoperative day 1, 7 and 14, the rats were assigned to the allodynic (n=5) and non-allodynic groups (n=5). On each postoperative day, tail-withdrawal threshold and latencies of the allodynic group were significantly lower not only than those of the non-allodynic but also the naïve groups (Fig. 3A, *p≤0.001 vs. the naïve group; #p≤0.001 vs. the non-allodynic group, One-way ANOVA followed by all pairwise multiple comparison, utilizing Holm-Sidak method). Despite the same nerve injury, however, tail-withdrawal threshold and latencies of the non-allodynic group did not differ from those of the naïve group. To exclude the possibility that lack of allodynic signs in the non-allodynic group might be due to motor impairment, we carried out a pinch-withdrawal test. The non-allodynic as well as the allodynic group showed 100% withdrawal responses to pinching stimuli, therefore, there was no difference between the two groups (data not shown). The two nerve-injured groups and naïve group were used for immunoblotting and PCR to compare spinal levels of GAD65- and GAD67. Representative immunoblots for two isoforms (GAD65 and GAD67) of the GABA synthesizing enzyme are depicted in Fig. 3B. Quantitative analysis for GAD65- and GAD67-ir revealed no significant difference on any postoperative day between the ipsilateral and contralateral hemi-cords of both the allodynic and non-allodynic groups (Table 2). As illustrated in Fig. 3C, GAD65- and GAD67-ir in the ipsilateral hemi-cords of the both groups were not different from those of the naïve group on any postoperative day. The PCR analysis also revealed that GAD65 and GAD67 mRNA levels in the two nerve-injured groups (allodynic and non-allodynic groups) were not different from those of naïve animals (Fig. 4). In both groups, the explosive increases of ATF3 mRNA in the ipsilateral DRG firmly assured nerve injury (Fig. 4A). Using a different set of animals (Fig. 5A), we conducted immunohistochemistry for GAD65 and GAD67. Fig. 5B shows GAD65- and GAD67-ir in the ipsilateral spinal dorsal horn of the naïve, allodynic and non-allodynic groups at 14 days after neuropathic surgery. As illustrated in Fig. 5C, GAD65- and GAD67-ir in the ipsilateral laminae I and II of the spinal cord in the both nerve-injured groups were not changed, compared not only to the contralateral side but also naïve animals.
Fig. 3.
Western blot analysis for spinal GAD65 and GAD67 among the naïve, allodynic and non-allodynic animals. (A) Difference in the manifestation of neuropathic pain between the allodynic and non-allodynic groups following the same nerve injury, *p≤0.001 vs. the naïve group; #p≤0.001 vs. the non-allodynic group (One-way ANOVA followed by all pairwise multiple comparison, utilizing Holm-Sidak method). (B) Immunoblots of two isoforms of GAD extracted from the S1 spinal segments (injured level) 2 weeks following neuropathic surgery. (C) The temporal courses of immunoreactivities of GAD65 and GAD67 in the ipsilateral hemi-cords. Data are normalized to β-actin and expressed as a ratio to naïve. GAD65- and GAD67-ir in the ipsilateral hemicords of the allodynic and non-allodynic groups are not different from those of the naïve group on any postoperative day.
Table 2.
Postoperative changes in spinal levels of GABA synthesizing enzymes, GAD65 and GAD67. Data are expressed as percentage of naïve animals. POD indicates postoperative day. When compared with naïve animals, significant loss of spinal GAD65 or GAD67 was observed in neither ipsilateral nor contralateral side of both of the allodynic and non-allodynic groups at any postoperative day
Fig. 4.
Reverse transcription-PCR analysis for GAD65 and GAD67 mRNA expression in the naïve, allodynic and non-allodynic groups 2 weeks following neuropathic surgery. Behavioral differences among the groups are illustrated in Fig. 3A. (A) GAD65 and GAD 67 mRNA expression in the S1 spinal segments excised from the naïve, allodynic and non-allodynic animals. ATF3 mRNA expression in the S1 DRG was adopted as a marker of nerve injury. (B) Ipsilateral expression of GAD65 and GAD67 mRNA. Data are normalized to GAPDH and expressed as a ratio to naïve. Expressions of GAD65- and GAD67 mRNA in the ipsilateral hemi-cords of the allodynic and non-allodynic groups are not different from those of the naïve group.
Fig. 5.
Immunohistochemial analysis for spinal GAD65 and GAD67 among the naïve, allodynic and non-allodynic animals 2 weeks following neuropathic surgery. (A) Difference in the manifestation of neuropathic pain between the allodynic and non-allodynic groups, *p<0.05 vs. Presurgical value (One-way repeated ANOVA followed by Bonferroni t-test); #p<0.05 vs. non-allodynic group (Unpaired t-test). (B) Microphotographs illustrating distribution of GAD65- and GAD67-ir in the S1 spinal segment ipsilateral to nerve injury. Scale bar=100 µm. (C) Relative staining intensities of GAD65 and GAD67, compared to the naïve group. GAD65- and GAD67-ir in both the allodynic and non-allodynic groups are not different from those of the naïve group.
Quantities of spinal GABA and its receptors, GABAA and GABAB after nerve injury
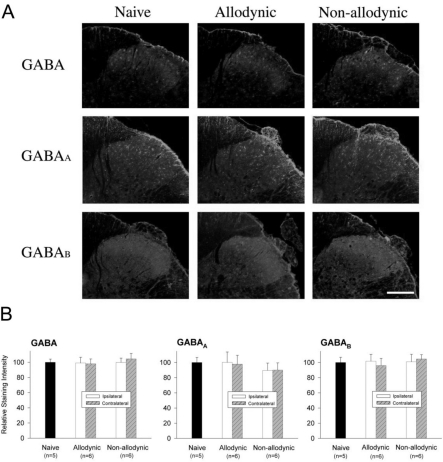
Based on the results of behavioral testing for 2 weeks following the nerve injury, animals were classified into the allodynic (n=6) and non-allodynic groups (n=6) (Fig. 5A). These animals together with naïve ones were then subjected to immunohistochemistry for GABA or its receptors, GABAA and GABAB (Fig. 6A). The quantitative analysis revealed that immunoreactivities of GABA and its receptors in the ipsilateral laminae I and II of the spinal cord in the both groups were not different not only from the contralateral side but also naïve animals (Fig. 6B).
Fig. 6.
Immunohistochemial analysis for spinal GABA and its receptors, GABAA and GABAB, among the naïve, allodynic and non-allodynic animals 2 weeks following neuropathic surgery. Behavioral differences among the groups are illustrated in Fig. 5A. (A) Microphotographs illustrating distribution of GABA-, GABAA and GABAB-ir in the S1 spinal segment ipsilateral to nerve injury. Scale bar=100 µm. (B) Relative staining intensities of GABA, GABAA and GABAB, compared to the naïve group. Immunoreactivities in both the allodynic and non-allodynic groups are not different from those of the naïve group.
Quantities of spinal GABA transporters, GAT-1 and GAT-3 after nerve injury
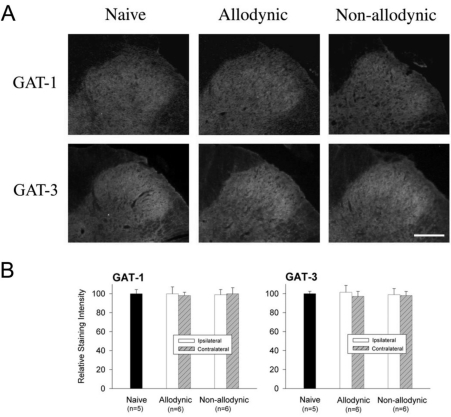
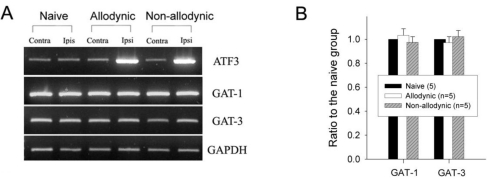
Finally, we compared the levels of spinal GAT-1 and GAT-3 among the allodynic, non-allodynic and naïve animals, using immunohistochemistry and PCR. Despite clear difference in pain behaviors (Fig. 5A), there was no significant difference in the levels of spinal GAT-1- and GAT-3-ir among the groups (Fig. 7). The PCR analysis also revealed that the GAT-1 and GAT-3 mRNA levels in the two nerve-injured groups (allodynic and non-allodynic groups) were not different from those of naïve animals (Fig. 8).
Fig. 7.
Immunohistochemial analysis for spinal GABA transporters, GAT-1 and GAT-3 among the naïve, allodynic and non-allodynic animals 2 weeks following neuropathic surgery. Behavioral differences among the groups are illustrated in Fig. 5A. (A) Microphotographs illustrating distribution of GAT-1- and GAT-3-ir in the S1 spinal segment ipsilateral to nerve injury. Scale bar=100 µm. (B) Relative staining intensities of GAT-1- and GAT-3 as compared to the naïve animals. Immunoreactivities in both the allodynic and non-allodynic groups are not different from those of the naïve group.
Fig. 8.
Reverse transcription-PCR analysis for GABA transporters, GAT-1 and GAT-3, mRNA expression in the naïve, allodynic and non-allodynic groups 2 weeks following neuropathic surgery. Behavioral differences among the groups are illustrated in Fig. 3A. (A) GAT-1 and GAT-3 mRNA expressions in the S1 spinal segments excised from the naïve, allodynic and non-allodynic animals. ATF3 mRNA expression in the S1 DRG was adopted as a marker of nerve injury. (B) Ipsilateral expression of GAT-1 and GAT-3 mRNA. Data are normalized to GAPDH and expressed as a ratio to the naïve animals. Expressions of GAT-1 and GAT-3 mRNA in the ipsilateral hemi-cords of the allodynic and non-allodynic groups are not different from those of the naïve group.
DISCUSSION
In the present study, we examined whether the differential changes of spinal elements for GABAergic transmission after an identical nerve injury are related to individual difference in the manifestation of neuropathic pain, however, we did not find any changes of spinal GABAergic elements irrespective of the induction of neuropathic pain after nerve injury.
First, we examined whether the reduction of spinal GABAergic activity by pharmacological blockade of GABA receptors could induce neuropathic pain. Thus, as described previously [16-18], the tail-withdrawal thresholds in response to mechanical stimulation were monitored in the present study, and we found that intrathecal administration of GABAA and GABAB receptor antagonists induced mechanical allodynic sign in naïve animals. This observation with spinal GABA antagonists indicates that, if the loss of spinal elements for GABAergic transmission was really followed by peripheral nerve injury, the resultant impaired GABAergic inhibition would be responsible for the generation of neuropathic pain [12-14]. Furthermore, any treatment to increase GABAergic activity should reverse the neuropathic pain induced by nerve injury. Consistent with previous studies [16,38,39], spinal administration of GABA agonists in the present study relieved the mechanical allodynia induced by partial injury of tail-innervating nerves. In addition, the inhibition of GABA uptake by GAT inhibitors was also sufficient to suppress neuropathic pain. Therefore, our pharmacological results indicate that spinal GABAergic elements are most likely related to the generation of neuropathic pain.
Next, we asked questions of whether the loss of spinal elements for GABAergic transmission, which has been predicted to reduce GABAergic activity in the spinal dorsal horn [12,19-21,23,24], was actually followed by peripheral nerve injury, and also whether individual difference in the manifestation of neuropathic pain was related to differential changes of the GABAergic elements after same nerve injury. To illuminate these questions, we compared quantities of GABA, its synthesizing enzymes (GAD65 and GAD67), its receptors (GABAA and GABAB receptors) and transporters (GAT-1 and GAT-3) among the naïve, allodynic and non-allodynic animals. Unfortunately, however, we did not find any quantitative changes in these spinal GABAergic elements among the groups.
The hypothesis that nerve injury-induced loss of spinal GABAergic inhibition is related to neuropathic pain has been supported by following observations; the reduction of GABA concentration [19-21], its synthesizing enzymes [12,20] and its receptors [23,24]. All these changes in the spinal cord have been considered to come from excitotoxic apoptosis of GABAergic interneurons within superficial laminae of the spinal dorsal horn [12,40]. However, Polgár et al. found no evidence for significant loss of GABA immunoreactive neurons within superficial laminae in the chronic constriction injury (CCI) [27,29] as well as the spared nerve injury (SNI) models [28] of neuropathic pain. It has also been suggested that axotomy alone is insufficient to produce a loss of dorsal horn neurons. Coggeshall et al. [41] demonstrated that reduction in neuronal numbers in the superficial dorsal horn could be detected only after sciatic nerve transection followed by electrical stimulation of the severed nerve at a stimulus strength sufficient to activate Aβ fibers. Therefore, it is highly likely that impaired spinal GABAergic inhibition after nerve injury might be due to the loss of synaptic connections or the reduced expression of spinal elements for GABAergic transmission [29], rather than the loss of GABAergic neurons. Based on the above consideration, we quantified the spinal GABAergic elements using a densitometrical analysis rather than cell counting. As described above, however, we did not observe any changes in spinal GABAergic elements. Our results are in conflict with previous studies which suggested the nerve injury-induced reduction of spinal GABAergic system [12,16,19,20,24,25]. Nevertheless, we could not presently offer any definitive explanation for the discrepancy. One of the possibilities might be due to the difference in animal (e.g., rats vs. mice, Wistar vs. Sprague-Dawley rats, male vs. female) or neuropathy model (e.g., CCI, SNI, spinal nerve ligation or tail model). Alternatively, as pointed out by Polgár et al. [29], technical problems in the procedures of immunohistochemistry (e.g., choice of fixatives and antibodies) or analysis (simple profile counts, stereological method or densitometrical analysis) might also be associated with the conflicting results. However, in contrast to the previous studies approached just immunohistochemically, we analyzed the expression levels of mRNA for each GABAergic element as well as proteins, which makes our results reliable. Employing the rat tail model of peripheral neuropathy, the quantities of spinal GABAergic elements in the present study were found to be not changed by peripheral nerve injury, therefore, the loss of the elements does not seem to be related to impaired spinal GABAergic activity in this neuropathic state.
It has been suggested that impairment of spinal GABAergic inhibitory activity following nerve injury might be related to an abnormal anion gradient across the membrane of dorsal horn neurons. Coull et al. [42] demonstrated that the reduction of potassium-chloride co-transporter KCC2 after nerve injury led to abnormally high Cl- concentration in dorsal horn neurons, therefore, GABAA receptor activation might reverse GABA action from inhibition to excitation by leading to Cl- efflux rather than Cl- influx. This suggestion sufficiently explains the loss of spinal GABAergic inhibitory action in neuropathic state without any change in spinal GABAergic elements. However, as shown in the present study as well as by Malan et al. [16], spinal application of GABA receptor agonists alleviated nerve injury-induced mechanical allodynia, thus implying that spinal GABAergic effect in neuropathic state remains antinociceptive, but not hyperalgesic.
It is also probable that the change in GAT is related to impairment of GABAergic activity. Because the levels of GAT are increased in inflammatory state [37,43] and GAT is responsible for the rapid termination of GABAergic inhibitory action [44,45], the up-regulation of GAT might impair normal GABAergic inhibitory activity, thereby increasing neuronal excitability. Furthermore, GAT-overexpressing animals show significantly enhanced pain sensitivities, indicating an importance of GAT in pain regulation [43]. However, it remains not clear whether GAT expression is changed also in neuropathic state. Miletic et al. [25] observed decrease of the spinal level of GABA transporter GAT-1 rather than increase, following chronic constriction injury, and suggested that an injury-induced loss of GAT results in depletion of available GABA from its terminals within the spinal dorsal horn. In the present study, however, we did not observe any significant change in the spinal level of GAT following nerve injury. Functional role or quantitative change of GAT in neuropathic state needs to be further studied.
In summary, using the rat tail model of neuropathy in the present study, the levels of spinal GABAergic elements in the allodynic animals were found to be not different from those of the naïve as well as the non-allodynic groups. This indicates that peripheral nerve injury did not affect the amount of spinal GABAergic elements. Therefore, the manifestation of neuropathic pain is not related to the changes in the elements. Although the role of GABAergic system in the generation of neuropathic pain has been underestimated, our present study showed that GABA agonists and GAT inhibitor are very effective in alleviating mechanical allodynia. Therefore, we should revaluate GABAergic elements as alternative targets for therapies.
ACKNOWLEDGEMENTS
This research was supported by a grant (2009K001256) from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, the Republic of Korea.
ABBREVIATIONS
- GABA
γ-aminobutyric acid
- GAD
glutamate decarboxylase
- GAT
gaba transporter
- Bicuculline
(6R)-6-[(5S)-6-methyl-5,6,7,8-tetrahydro[1,3]dioxolo[4,5-g]isoquinolin-5-yl]furo-[3,4-e][1,3]benzodioxol-8(6H)-one
- Phaclofen
(RS)-3-Amono-2-(4-chloropheyl)prophylphosphonic acid
- Bacrofen
(RS)-4-amino-3-(4-chlorophenyl)butanoic acid
- NNC-711
1,2,5,6-Tetrahydro-1-[2-[[(diphenylmethylene)amino]oxy]ethyl]-3-pyridinecaboxylic acid hy-drochloride
- SNAP-5114
1-[2-[tris(4-methoxyphenyl)methoxy]ethyl]-(S)-3-piperid inecabox-ylic acid
References
- 1.Merskey H, Bogduk N. Classification of chronic pain. 2nd ed. Seattle: IASP Press; 1994. p. 211. [Google Scholar]
- 2.Blumberg H, Janig W. Changes of reflexes in vasoconstrictor neurons supplying the cat hindlimb following chronic nerve lesions: a model for studying mechanisms of reflex sympathetic dystrophy? J Auton Nerv Syst. 1983;7:399–411. doi: 10.1016/0165-1838(83)90093-0. [DOI] [PubMed] [Google Scholar]
- 3.Richards RL. The term 'causalgia'. Med Hist. 1967;11:97–99. doi: 10.1017/s0025727300011789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tahmoush AJ. Causalgia: redefinition as a clinical pain syndrome. Pain. 1981;10:187–197. doi: 10.1016/0304-3959(81)90194-9. [DOI] [PubMed] [Google Scholar]
- 5.Boivie J. Central pain: Textbook of pain. In: Wall PD, Melzack R, editors. Textbook of pain. 3rd ed. New York: Churchill Livingstone; 1994. pp. 871–902. [Google Scholar]
- 6.Bonica JJ. Advances in Pain Research and Therapy. New York: Raven Press; 1979. pp. 141–166. [Google Scholar]
- 7.Tasker RR. Pain resulting from central nervous system pathology (Central pain) In: Loeser JD, editor. The management of pain. 1st ed. Philadelphia: Lea and Febiger; 1990. pp. 264–284. [Google Scholar]
- 8.Back SK, Lee J, Hong SK, Na HS. Loss of spinal mu-opioid receptor is associated with mechanical allodynia in a rat model of peripheral neuropathy. Pain. 2006;123:117–126. doi: 10.1016/j.pain.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Drew GM, Siddall PJ, Duggan AW. Mechanical allodynia following contusion injury of the rat spinal cord is associated with loss of GABAergic inhibition in the dorsal horn. . Pain. 2004;109:379–388. doi: 10.1016/j.pain.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Hao JX, Yu W, Xu XJ. Evidence that spinal endogenous opioidergic systems control the expression of chronic pain-related behaviors in spinally injured rats. Exp Brain Res. 1998;118:259–268. doi: 10.1007/s002210050280. [DOI] [PubMed] [Google Scholar]
- 11.Na HS, Kim HJ, Sung B, Back SK, Kim DY, Kim JS, Hong SK. Decrease in spinal CGRP and substance P is not related to neuropathic pain in a rat model. Neuroreport. 2001;12:175–178. doi: 10.1097/00001756-200101220-00042. [DOI] [PubMed] [Google Scholar]
- 12.Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel S, Naeem S, Kesingland A, Froestl W, Capogna M, Urban L, Fox A. The effects of GABA(B) agonists and gabapentin on mechanical hyperalgesia in models of neuropathic and inflammatory pain in the rat. Pain. 2001;90:217–226. doi: 10.1016/S0304-3959(00)00404-8. [DOI] [PubMed] [Google Scholar]
- 14.Somers DL, Clemente FR. Dorsal horn synaptosomal content of aspartate, glutamate, glycine and GABA are differentially altered following chronic constriction injury to the rat sciatic nerve. Neurosci Lett. 2002;323:171–174. doi: 10.1016/s0304-3940(02)00157-x. [DOI] [PubMed] [Google Scholar]
- 15.Hao JX, Xu XJ, Wiesenfeld-Hallin Z. Intrathecal gamma-aminobutyric acidB (GABAB) receptor antagonist CGP 35348 induces hypersensitivity to mechanical stimuli in the rat. Neurosci Lett. 1994;182:299–302. doi: 10.1016/0304-3940(94)90821-4. [DOI] [PubMed] [Google Scholar]
- 16.Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–1167. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72:169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- 18.Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- 19.Castro-Lopes JM, Tavares I, Coimbra A. GABA decreases in the spinal cord dorsal horn after peripheral neurectomy. Brain Res. 1993;620:287–291. doi: 10.1016/0006-8993(93)90167-l. [DOI] [PubMed] [Google Scholar]
- 20.Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K. Changes in GAD- and GABA-immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J Chem Neuroanat. 1998;16:57–72. doi: 10.1016/s0891-0618(98)00062-3. [DOI] [PubMed] [Google Scholar]
- 21.Ibuki T, Hama AT, Wang XT, Pappas GD, Sagen J. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997;76:845–858. doi: 10.1016/s0306-4522(96)00341-7. [DOI] [PubMed] [Google Scholar]
- 22.Ralston DD, Behbehani M, Sehlhorst SC, Meng XW, Ralston HJ. Decreased GABA immunoreactivity in rat dorsal horn is correlated with pain behavior:a light and electron microscopic study. In: Jensen TS, Turner JA, Wiesenfeld-Hallin Z, editors. Preoceedings of the Eighth World Congress on Pain. Seattle: WA: IASP Press; 1997. pp. 547–560. [Google Scholar]
- 23.Bhisitkul RB, Kocsis JD, Gordon TR, Waxman SG. Trophic influence of the distal nerve segment on GABAA receptor expression in axotomized adult sensory neurons. Exp Neurol. 1990;109:273–278. doi: 10.1016/s0014-4886(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 24.Castro-Lopes JM, Malcangio M, Pan BH, Bowery NG. Complex changes of GABAA and GABAB receptor binding in the spinal cord dorsal horn following peripheral inflammation or neurectomy. Brain Res. 1995;679:289–297. doi: 10.1016/0006-8993(95)00262-o. [DOI] [PubMed] [Google Scholar]
- 25.Miletic G, Draganic P, Pankratz MT, Miletic V. Muscimol prevents long-lasting potentiation of dorsal horn field potentials in rats with chronic constriction injury exhibiting decreased levels of the GABA transporter GAT-1. Pain. 2003;105:347–353. doi: 10.1016/s0304-3959(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 26.Engle MP, Gassman M, Sykes KT, Bettler B, Hammond DL. Spinal nerve ligation does not alter the expression or function of GABA(B) receptors in spinal cord and dorsal root ganglia of the rat. Neuroscience. 2006;138:1277–1287. doi: 10.1016/j.neuroscience.2005.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polgar E, Gray S, Riddell JS, Todd AJ. Lack of evidence for significant neuronal loss in laminae I-III of the spinal dorsal horn of the rat in the chronic constriction injury model. Pain. 2004;111:144–150. doi: 10.1016/j.pain.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Polgar E, Hughes DI, Arham AZ, Todd AJ. Loss of neurons from laminas I-III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain. J Neurosci. 2005;25:6658–6666. doi: 10.1523/JNEUROSCI.1490-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polgar E, Hughes DI, Riddell JS, Maxwell DJ, Puskar Z, Todd AJ. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–239. doi: 10.1016/s0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 30.Satoh O, Omote K. Roles of monoaminergic, glycinergic and GABAergic inhibitory systems in the spinal cord in rats with peripheral mononeuropathy. Brain Res. 1996;728:27–36. [PubMed] [Google Scholar]
- 31.Kontinen VK, Stanfa LC, Basu A, Dickenson AH. Electrophysiologic evidence for increased endogenous gabaergic but not glycinergic inhibitory tone in the rat spinal nerve ligation model of neuropathy. Anesthesiology. 2001;94:333–339. doi: 10.1097/00000542-200102000-00024. [DOI] [PubMed] [Google Scholar]
- 32.Back SK, Kim JS, Hong SK, Na HS. Ascending pathways for mechanical allodynia in a rat model of neuropathic pain. Neuroreport. 2003;14:1623–1626. doi: 10.1097/00001756-200308260-00016. [DOI] [PubMed] [Google Scholar]
- 33.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 34.Chang YC, Gottlieb DI. Characterization of the proteins purified with monoclonal antibodies to glutamic acid decarboxylase. J Neurosci. 1988;8:2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufman DL, Houser CR, Tobin AJ. Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem. 1991;56:720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez FJ, Taylor-Blake B, Fyffe RE, De Blas AL, Light AR. Distribution of immunoreactivity for the beta 2 and beta 3 subunits of the GABAA receptor in the mammalian spinal cord. J Comp Neurol. 1996;365:392–412. doi: 10.1002/(SICI)1096-9861(19960212)365:3<392::AID-CNE5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Ng CH, Ong WY. Increased expression of gamma-aminobutyric acid transporters GAT-1 and GAT-3 in the spinal trigeminal nucleus after facial carrageenan injections. Pain. 2001;92:29–40. doi: 10.1016/s0304-3959(00)00468-1. [DOI] [PubMed] [Google Scholar]
- 38.Hwang JH, Yaksh TL. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain. 1997;70:15–22. doi: 10.1016/s0304-3959(96)03249-6. [DOI] [PubMed] [Google Scholar]
- 39.Smith GD, Harrison SM, Birch PJ, Elliott PJ, Malcangio M, Bowery NG. Increased sensitivity to the antinociceptive activity of (+/-)-baclofen in an animal model of chronic neuropathic, but not chronic inflammatory hyperalgesia. Neuropharmacology. 1994;33:1103–1108. doi: 10.1016/0028-3908(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 40.Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]
- 41.Coggeshall RE, Lekan HA, White FA, Woolf CJ. A-fiber sensory input induces neuronal cell death in the dorsal horn of the adult rat spinal cord. J Comp Neurol. 2001;435:276–282. doi: 10.1002/cne.1029. [DOI] [PubMed] [Google Scholar]
- 42.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 43.Hu JH, Yang N, Ma YH, Zhou XG, Jiang J, Duan SH, Mei ZT, Fei J, Guo LH. Hyperalgesic effects of gamma-aminobutyric acid transporter I in mice. J Neurosci Res. 2003;73:565–572. doi: 10.1002/jnr.10677. [DOI] [PubMed] [Google Scholar]
- 44.Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- 45.Mager S, Naeve J, Quick M, Labarca C, Davidson N, Lester HA. Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron. 1993;10:177–188. doi: 10.1016/0896-6273(93)90309-f. [DOI] [PubMed] [Google Scholar]