Abstract
Although the role of emotion in social economic decision-making has been increasingly recognized, the impact of mood disorders, such as depression, on such decisions has been surprisingly neglected. To address this gap, fifteen depressed and twenty-three nondepressed individuals completed a well-known economic task, in which they had to accept or reject monetary offers from other players. Although depressed individuals reported a more negative emotional reaction to unfair offers, they accepted significantly more of these offers than did controls. A positive relationship was observed in the depressed group, but not in controls, between acceptance rates of unfair offers and resting cardiac vagal control, a physiological index of emotion regulation capacity. The discrepancy between depressed individuals’ increased emotional reactions to unfair offers and their decisions to accept more of these offers contrasts with recent findings that negative mood in nondepressed individuals can lead to lower acceptance rates. This suggests distinct biasing processes in depression, which may be related to higher reliance on regulating negative emotion.
Keywords: decision-making, depression, cardiac vagal control
Introduction
Cognitive biases or distortions are well-documented in depression (Beck, 2008) and are often the focus of therapeutic intervention with cognitive behavioral therapy (Whisman, Miller, Norman, & Keitner, 1991). Much of the empirical literature focuses on alteration in attributions, but comparatively little research has examined how such cognitive alterations in depression influence decision-making. Outside of treatment decisions, very few studies have actually examined the degree to which decision-making is altered in depression, and whether any such disturbances lead to sub-optimal outcomes. As the role of both task-related and incidental emotion in decision-making is increasingly incorporated in general economic models of decision-making (Loewenstein & Lerner, 2003), social decision-making (i.e. involving interactions of two or more individuals) has been shown to engage an ensemble of neural systems relevant to emotion, reward valuation, and planning (Sanfey, 2007). Therefore, mood disturbances, such as those observed in depression, may well lead to decision biases in a social context (Strack & Coyne, 1983). These types of decisions may in fact have the greatest influence on the day-to-day lives of patients, and thus such research may contribute to practical efforts to improve depressed individuals’ confidence, self-esteem, and social connectedness.
Reward in depression
The limited decision-making research with unmedicated patients suggests that depression is associated with decreased approach-related behavior and reduced sensitivity to reward, which appears to underlie a failure to maximize potential monetary earnings (Henriques & Davidson, 2000; Pizzagali, Iosifescu, Hallett, Ratner,& Fava, 2008). These findings are consistent with both anhedonia and the tendency to neglect pleasurable stimuli often found in depression, as well as with research showing that sad affect may focus attention more on threatening cues (Forgas, 2003) than on opportunities to profit (Lerner, Small, & Lowenstein, 2004). Recent neuroimaging research further suggests that depressed individuals’ decreased sensitivity to reward may stem more from a relative increase in affective conflict and monitoring efforts than failure to engage dopaminergic reward systems (Holmes & Pizzagali, 2008; Knutson, Bhanji, Cooney, Atlas, & Gotlib, 2008). Although these investigations do not directly touch on social contexts, they do provide evidence of distinct patterns of decision-making in depression.
Social decision-making
In order to examine the impact of depression on social decision-making, we employed a well-known economic task, the Ultimatum Game (UG; Guth et al., 1982), in which one player (the “proposer”) makes an offer to another player (the “responder”) regarding how to split an amount of money between them. The responder can either accept the offer, in which case the money is split as proposed, or reject the offer, in which case neither player receives anything. Whereas standard economic models would predict that responders should accept any non-zero offers (still preferable to no gain at all), individuals typically accept about 50% of unfair offers (defined as 30% or less of the pot; Camerer, 2003), and experience a negative emotional response and increased arousal when receiving unfair offers (Sanfey, Rilling, Aronson, Nystrom, & Cohen, 2003; Van ’t Wout, Kahn, Sanfey, & Aleman, 2006). Although social decision-making has been extensively studied, the use of this task in clinical populations is still in its infancy (Agay, Kron, Carmel, Mendlovic,& Levkovitz, 2008). Two recent studies, however, suggest that characteristics associated with depression, sad affect and serotonin depletion, may lead to more aggressiveness and retaliation in the UG. Our group recently reported that induced sad mood resulted in lower acceptance rates of unfair UG offers, with sad participants also reporting significantly more anger than neutral participants when receiving unfair offers (Harlé & Sanfey, 2007). Another study (Crockett, Clark, Tabibnia, Lieberman, & Robbins, 2008) found that nondepressed subjects undergoing tryptophan depletion, which leads to decreased brain serotonin and has been associated with more social aggressiveness, exhibited lower acceptance rates of unfair offers as compared to a placebo control group. Because sadness and disruption of serotonergic neurotransmission have been implicated in clinical depression (Porter, Mulder, Joyce, Miller, Kennedy, 2008), one hypothesis is that depressed individuals may process unfair offers as more offensive and thus might be more sensitive and aggressive towards negative social signals. Compared to controls, the depressed group might then on average accept fewer unfair offers and report a more negative emotional reaction when receiving these offers.
Alternatively, some research has shown that depressed individuals are more accurate than nondepressed in estimating contingencies between behavior and external events, and that such estimation is not affected by the valence of such prediction outcomes (e.g. reward vs. loss). Thus depressed individuals may be more realistic about their degree of control over certain transaction outcomes (Alloy & Abramson, 1979). If depressed individuals are indeed more realistic in assessing unfair offers, they may be less likely than controls to think that their decisions will affect either their partners or the subsequent offers they will receive, and thus may expect lower offers in the first place (i.e. being more realistic regarding the opportunistic nature of proposers). Therefore, an alternative hypothesis is that depressed individuals may exhibit higher acceptance rates of unfair offers compared to controls. These higher rates may be independent of their emotional reaction to unfair offers (e.g. they may still react more negatively to unfairness), particularly if they more realistically assess the lack of impact of their decisions.
Emotion Regulation
In addition to assessing behavioral performance and emotion, the present study examined the role of physiologically-driven emotion regulation processes in such decisions, as research suggests that brain regions subserving one’s ability to regulate emotion are involved in responders’ ability to accept unfair UG offers (Koenigs & Tranel, 2007). Numerous studies have suggested that parasympathetically-driven cardiac vagal control (CVC; i.e. respiratory-linked changes in heart rate), may index one’s ability to regulate emotion and respond adaptively to various stressors, with higher CVC reflecting a stronger ability to self-regulate (Porges, 2007; Thayer & Lane, 2000). Moreover, there is evidence that, compared to nondepressed individuals, depressed individuals’ CVC may be reduced (Booij et al., 2006), suggesting emotion regulation may be impaired in depression, although others have failed to show such group differences (Lehofer et al., 1997). Thus, it is of interest to examine whether individual differences in CVC are related to UG decisions, potentially due to CVC’s putative influence on emotion regulation.
Methods
Participants
Participants were recruited from among students who participated in a 4-session study of psychophysiological indicators of risk for depression, and which examined individuals with a wide range of depression, ranging from nondepressed to clinical severity. A total of 38 participants (15 depressed; 23 controls) aged 18–24 consented to complete the UG at the conclusion of the fourth session. We derived two groups based on participants’ scores on the Beck Depression Inventory (BDI-II, Beck, Steer, & Garbin, 1988) on the day of the UG task. The ‘depressed’ group was defined as those with BDI scores greater than 16 and included 11 meeting DSM-IV criteria for MDD and 4 having subthreshold MDD, defined as meeting at least 4 out of 5 DSM-IV symptoms for MDD or scoring >30 on the BDI on the UG day. The ‘control’ group was defined as those with no current or past MDD diagnostic and a BDI score below 5. MDD diagnostics were based on intake interviews with the Structured Clinical Interview (SCID) for the DSM-IV (First, Spitzer, Gibbon, & Williams, 1994) conducted by Masters- or Ph.D.-level clinical psychology graduate students (Kappa=.81) about two weeks prior the UG session. Exclusion criteria for the study included: any other current Axis I diagnosis as assessed by the SCID, any current psychotropic pharmacological treatment (e.g. antidepressant medication), history of psychosis or mania, substance abuse/dependence within the past 4 months, any medical disorder or CNS history that could affect emotional function1. All procedures were approved by the Human Subjects Protection Program at the University of Arizona.
Experimental Procedures
In addition to the SCID and BDI measures, participants were administered the Hamilton Rating Scale for Depression (HRSD, Hamilton, 1967), to obtain a clinician-based measure of depression, and completed the State-Trait Anxiety Inventory (STAI, Spielberger, Vagg, Barker, Donham, & Westberry, 1995) at the intake session, in order to assess the relationship between anxiety and CVC. In addition, the Positive and Negative Affect Scale (PANAS, Watson, Clark, & Tellegen, 1988) was administered at the start of the fourth experimental session to assess the potential mediating role of negative affect in participants’ emotional reaction to unfair offers.
Cardiac Activity
Resting electrocardiographic (ECG) activity was recorded for two 8-minute periods before participants played the UG. ECG was recorded using silver-silver chloride electrodes placed on the left clavicle and digitized at 2000 Hz. Participants were instructed to rest quietly. Interbeat interval (IBI) series were derived from the ECG and were hand-corrected for artifacts and ectopic beats. In addition to heart rate, Respiratory Sinus Arrhythmia (RSA), a vagal-based measure of heart rate variability in the high frequency band (0.12–0.4 Hz), was extracted using CMetX software (Allen, Chambers,& Towers, 2007). This program converts the IBI series to a timeseries sampled at 10 Hz, filters the series using a 0.12–0.4 Hz finite impulse response filter, and then takes the natural log of the variance of this filtered waveform as the estimate of RSA.
Decision-making
Participants first filled out a short instructional handout about the UG summarizing the basic rules (mentioned above) and asking them about their expectations in the game (e.g. range of offers expected, etc). They were told they would play as responders and receive one-time offers from various proposers. After completing two practice trials and indicating that they fully understood the game, participants played the UG, receiving 24 different offers presented in a randomized order. Each offer involved a $10 split, and participants were informed they would be playing for real money and would be paid in cash based on a percentage of their earnings in the game. A computerized version of the UG was used, and participants were told that they would be playing the game over a computer network with partners located at other universities. The pictures that participants saw were selected from a pool of actual UG players’ photographs with equal proportion of males and females, and with emotionally neutral expressions (Harlé & Sanfey, 2007; Sanfey et al., 2003). On each trial, participants saw a picture of their proposer partner for 4 seconds. They then saw the proposer’s offer, at which point they were instructed to choose from two options (Accept or Reject) by way of a button press. They had a maximum of 10 seconds to decide to either accept or reject this offer. After the decision, the outcome (e.g. how much each player received) was presented for 4 seconds. Based on the assumption that proposers would behave sensibly (i.e. not offer more than half of the pot), proposer offers ranged from 50 cents to $5 and included 6 fair offers (3x$5, 3x$4), 6 slightly unfair offers (3x$3, 3x$2.50), 6 moderately unfair offers (3x$2, 3x$1.50), and 6 highly unfair offers (3x$1 and 3x$0.50). At the end of the task, participants completed a brief questionnaire asking them to rate the extent to which they felt each of twelve basic emotions “when receiving unfair offers (e.g. $1 or $2 out of $10)”, each rated using an 8-point Likert scale from (Harlé & Sanfey, 2007).
Results
Clinical Profile
The depressed group (mean BDI=27.8) included 11 (73%) individuals diagnosed with current MDD. The depressed group had higher HRSD scores (M=14.5) than the control group (M=1.6, t=5.3, p<.001). Depressed participants also reported higher state (M=56.4, t=9.2, p<.001) and trait (M=56.1, t=9.1, p<.001) anxiety than controls (M=29.6 and M=32.0, respectively), as measured by the STAI. Groups did not differ in age (M=19.0, t=.98, ns). No significant gender group difference was observed (χ2=2.5, ns), although the depressed group had more females (78%) than did the control group (52%). However, gender did not relate to the dependent variables in the present study and did not affect the main analyses results when added as a predictor or moderator. Data analyses of cardiac vagal control (RSA) were conducted after removing three subjects with ectopic cardiac patterns (2 controls and 1 depressed), as well as one (depressed) outlier based on Cook’s distance. RSA in the control group (M=6.83) did not differ significantly from RSA in the depressed group (M=6.76, t=.26, ns). Nonetheless, within the depressed sample, BDI scores were negatively related to RSA (r=−.56, p<.05). This relationship, however, was mediated by trait anxiety (R2=.78; using the hierarchical regression method advocated by Baron and Kenny (1986). After accounting for anxiety scores (beta=−.66, t=−3.56, p<.05), depression severity (measured by BDI scores) no a longer significantly predicted RSA (beta=−.33, t=−2.15, ns), consistent with partial mediation.
Decision-Making
The primary metric of interest in the UG was the proportion of offers accepted for each offer amount. Two aggregate acceptance rates were also computed for “fair” (i.e. $4–$5) and “unfair” (i.e. $0.50–$3) offers respectively. These categories were based on questionnaire data confirming that $4 and $5 offers were consistently considered fair by most participants, as in previous UG studies (Camerer, 2003; Harlé & Sanfey, 2007). Depressed and control participants did not differ in their pre-task perceived cutoff between unfair and fair offers (M=$4.10, SD=$0.80), or in the offer they would typically make as a proposer (M=$4.20, SD=$1.10). Based on debriefing results, no participants indicated any suspicion of deception with regards to the use of virtual partners.
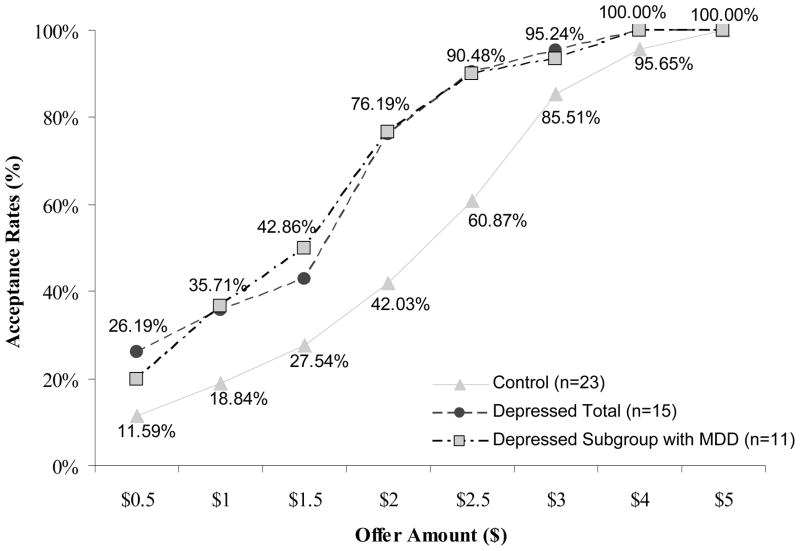
After mean-centering all independent variables, a linear mixed model (LMM; West, Welch, & Galecki, 2007) was fit to the data using offer acceptance rate as the dependent variable, offer amount as a within-subject (level 1) factor, and clinical status as a between-subject factor (level 2). Subject was modeled as a random factor and a diagonal matrix structure was specified to model residual variance across offer amounts (allowing the model to fit a different variance component at each level). Significant main effects of offer amount (F(1,104)=393.0, p<.001) and clinical status (F(1,53)=4.3, p<.05), as well as a significant offer by clinical status interaction (F(1,104)=13.6, p<.001) were obtained. More specifically, the depressed group accepted significantly more $0.5, $1.0, $1.5, $2.0 and $2.5 offers than the nondepressed group (p<.05 with Bonferroni corrections), whereas groups did not differ in accepting $3.0, $4.0, and $5.0 offers.
In terms of aggregate acceptance rates, and thus consistent with our alternative hypothesis, groups did not differ in their acceptance rates of fair offers (average acceptance rate = 99%, SEM= 0.8%), but depressed participants accepted significantly more unfair offers (61%, SEM= 7.1%) than controls (41%, SEM=5.7%; t=2.2, p<.05; Cohen d=0.74, see Figure 1. Total earnings in the game were $50.30 for the depressed group and $43.02 for the control group (t=2.4, p<.05; d=.87).
Figure 1. Acceptance rates by offer amount.
Depressed Total includes participants with and without a current DSM-IV diagnosis of MDD, whereas Depressed with MDD includes only those with a DSM-IV diagnosis of MDD.
Emotional Reaction to Unfair Offers
Following the UG, participants rated their subjective emotional state for unfair offers. Twelve basic emotions, including both positive and negative emotions, were rated using an 8-point Likert scale: anger, arousal, amusement, confusion, contentment, disgust, fear, happiness, pain, sadness, surprise, and tension. Compared with the controls, depressed participants reported significantly higher levels of disgust (t=−2.33, p<.05, d=.78), as well as surprise (t=−2.58, p<.05, d=.71). Depressed participants also showed a trend in reporting greater levels of anger (p=.07, d=.59). No group differences emerged regarding the other emotions.
Regression analyses were further conducted to assess whether the clinical status had still an impact on these emotion ratings above and beyond the generally more negative affect observed in depressed individuals. Clinical status significantly predicted disgust (F=6.6, p<.05; adjusted R2 =.14) and surprise (F=10.4, p<.05 adjusted R2 =.21) in response to unfair offers, with depressed status resulting in higher level of these negative emotions. Clinical status remained a statistically significant predictor in models that included participants’ negative reported affect (from the PANAS) as an additional continuous independent variable. Squared semi-partial correlations for clinical status were 0.12 and 0.11 when predicting disgust and surprise, respectively, while simultaneously accounting for negative affect.
Cardiac Vagal Control (RSA) & Acceptance Rates
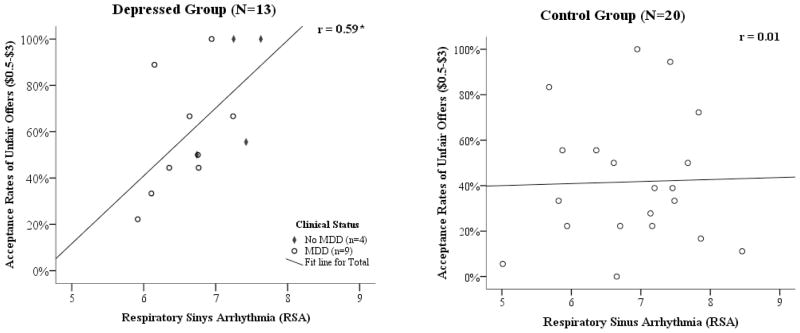
Using regression analysis, cardiac vagal control, indexed by RSA, was examined as a predictor of acceptance rates of unfair UG offers, with clinical status as a potential moderator. A moderated regression model was statistically significant (F=3.13, p<.05, adjusted R2=.17), with a significant effect of clinical status (beta=.38, t=2.37, p<.05) and a marginally significant clinical status X RSA interaction (beta=.43, p=.05). More specifically, a statistically significant positive relationship was observed between RSA and acceptance rates of unfair offers in the depressed group (r=.59, p<.05), but was not evident in the control group (r=.01, ns; see Figure 2).
Figure 2.
Acceptance rates of unfair offers as a function of respiratory sinus arrhythmia (RSA) by clinical group.
Discussion
This sample of depressed, un-medicated participants demonstrated significantly altered social decision-making patterns compared to controls, accepting more unfair monetary offers than control participants in a well-studied social decision-making task. Interestingly, such increased acceptance rates in depressed individuals would appear more “rational” from a standard economic standpoint (i.e. maximizing financial gain), and indeed this group made more money in the task. However, despite higher acceptance rates, the depressed group actually reported higher levels of disgust, anger, and surprise upon receiving unfair offers.
The finding of greater disgust, surprise, and anger in the depressed group upon receiving the offers appears consistent with recent empirical findings showing that both transient sad mood manipulations (Harlé & Sanfey, 2007) and acute tryptophan depletion (Crockett et al., 2008) prompt a similar emotional reaction to unfairness using the same task. Such findings raise the possibility that the same reaction of anger in both the depressed group and the transiently sad nondepressed group (Harlé & Sanfey, 2007) may involve similar neural systems. One hypothesis is that a depressed state or a sad mood may engage the anterior insula, a neural region associated with the processing of bodily emotions, and also previously implicated when responders receive unfair UG offers (Sanfey et al., 2003). Thus, depression, like sad mood, may result in an increased negative perception of the social signal underlying unfair offers, mediated by increased activity in anterior insula. In addition, serotonergic reserves may be lower in depressed individuals than in nondepressed adults (Porter et al., 2008), which may contribute to a more aggressive emotional reaction to unfairness (Crockett et al. 2008).
Despite this, we observed higher acceptance rates of unfair offers among the depressed participants, which contrast with the findings of the aforementioned studies. Thus, while the depth of emotional reactivity may be similar across depressed and sad but nondepressed groups, it appears that in clinical depression distinct processes may intervene prior to the decision itself. One possibility for such behavioral discrepancy is that the increased acceptance of unfair offers observed in depressed individuals reflects more realistic expectations in the UG task (Alloy & Abramson, 1979). Though depressed participants did not differ from controls in terms of their expectations of offers and fairness in the task, they may still have been more realistic (perhaps resulting from a more analytic processing style or negative cognitive bias) about the impact their decisions have on their partners.
Another more plausible potential explanation for the higher acceptance rates observed in the depressed group relates to emotion regulation processes, with psychophysiological data indicating a possible relationship between cardiac vagal control and the ability to manage one’s emotional reaction to unfair offers in order to maximize one’s economic gain. Although the depressed and control groups did not differ in terms of average RSA, a positive relationship between RSA and acceptance rates was observed in the depressed group, but not in the control group. These findings, suggest that depressed individuals’ larger negative emotional responses to unfair offers may prompt a stronger reliance on regulating these emotions, as compared with nondepressed participants (who are not as indignant about lower offers). Thus, independent of trait or baseline capacity to regulate emotion, depressed individuals may be more likely to use emotion regulation processes when making these social interactive decisions, which may in fact help them in managing emotional reactions, and in turn lead to more acceptances. Additionally, nondepressed individuals may have various strategies available to regulate their emotional responses to unfairness besides RSA driven mechanisms (e.g. more global, optimistic framing), whereas such alternative processes may be impaired or insufficient in depressed individuals, leaving vagal control as a primary option to self-regulate. Nonetheless, caution is warranted in interpreting these results, as the present study did not measure phasic changes in RSA during the task itself. Future research should assess for group differences in RSA suppression in response to unfair UG offers.
The similar resting levels of cardiac vagal control (RSA) between depressed and control participants may appear inconsistent with research reporting lower heart rate variability in depressed groups (Booij et al, 2006). Other work, however, has shown no difference in vagal control between depressed and control groups (Lehofer et al. 1997). Some have also shown that anxiety symptoms, and not depression severity, are typically more strongly associated with lower cardiac vagal control (Friedman, 2007), which is further consistent with the presently observed negative relationship between RSA and trait anxiety in the depressed group. Moreover, to control for confounding variables of a clinical nature, participants in the present study were excluded on the basis of clinical conditions other than unipolar depression, including anxiety disorders. Thus, the range of state and trait anxiety measures within the present sample may be more constrained and lower than in other depressed groups described in the literature, and thus less inclusive of high anxiety/low cardiac vagal control individuals. This may in turn explain why the depressed sample did not have lower average RSA than the control group.
The present study has some limitations, including a small sample size (particularly for depressed individuals), stringent exclusion criteria, the use of recalled post-task emotion ratings, and the use of an undergraduate student sample, limiting the generalizability of our results. This study also used BDI scores to establish depression status as opposed to MDD diagnosis based DSM-IV criteria to maximize sample size and favor depression severity on the day of the decision-making task, which limits generalizability to a pure MDD population. However, most individuals in the depressed sample (73%) had a current diagnosis of MDD and effect sizes were similar when including only those with current MDD in the analyses. In addition, internal validity is increased by the use of a non-medicated sample.
In conclusion, the present study revealed a nuanced emotional and behavioral pattern in unmedicated depressed individuals when they make simple interactive financial decisions. These results suggest that the impact of clinical depression on social decision-making may be more complex than the impact of sad mood or even serotonin deficiency in nondepressed individuals. In fact, despite a well-documented pattern of negative cognitive framing in depression, depressed individuals actually ended the task monetarily better off than nondepressed controls. Thus the present study emphasizes the importance of studying decision-making within a realistic and ecologically valid context, for instance using socially interactive tasks with real financial contingencies. These findings underscore the need to refine our understanding of higher-order cognitive processes in depression.
Acknowledgments
This work was supported by funding from the National Institutes for Health (R03 MH077058) (AGS), and in part by grants from the National Institutes of Health (R01 MH066902) and the National Alliance for Research on Schizophrenia and Depression (JJBA). The authors wish to thank Eliza Fergerson, Jamie Velo, Dara Halpern, Craig Santerre, Eynav Accortt, Andrew Bismark, and Jay Hegde for assistance with subject recruitment, and Luke Chang and Mascha van ’t Wout for helpful comments.
Footnotes
44 (54%) were excluded during the recruitment period; UG and excluded participants did not differ in average BDI (t=.64, ns), in proportion of individuals with current MDD (χ2=.89, ns) and in gender distribution (χ2=3.1, ns).
Data analyses were redone defining the depressed group to include only MDD. The LMM offerXgroup interaction remained statistically significant (p<.005), with similar effect sizes for group mean differences in acceptance rates of unfair offers (p=.06, d=.74), reported disgust (p<.05, d=.84), surprise (p=.07, d=.71) and anger (p<.05, d=.91) when receiving unfair offers, and in the correlation between RSA and acceptance rates of unfair offers (r=.53, p=.11)
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/abn.
References
- Agay N, Kron S, Carmel Z, Mendlovic S, Levkovitz Y. Ultimatum bargaining behavior of people affected by schizophrenia. Psychiatry Research. 2007;157:39–46. doi: 10.1016/j.psychres.2006.03.026. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Allen JJB, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: A pragmatic primer and a brief comparison of metrics. Biological Psychology. 2007;74:243–262. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY. Judgment of contingencies in depressed and non- depressed students: sadder but wiser? Journal of Experimental Psychology:General. 1979;108:441–485. doi: 10.1037//0096-3445.108.4.441. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Booij L, Swenne CA, Brosschot JF, Haffmans J, Thayer JF, Van der Does W. Tryptophan depletion affects heart rate variability and impulsivity in remitted depressed patients with a history of suicidal ideation. Biological Psychiatry. 2006;60:507–514. doi: 10.1016/j.biopsych.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Camerer CF. Behavioral Game Theory: Experiments in Strategic Interaction. New York: Russell Sage Foundation; 2003. pp. 46–117. [Google Scholar]
- Crockett MJ, Clark L, Tabibnia G, Lieberman MD, Robbins TW. Serotonin modulates behavioral reactions to unfairness. Science. 2008;320:1739. doi: 10.1126/science.1155577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon MG, Williams JBW. The Structured Clinical Interview for DSM-IV. Unpublished manuscript. Biometrics Research Department, New York State Psychiatric Institute; New York: 1994. [Google Scholar]
- Forgas JP. In: Handbook of affective sciences. Davidson RJ, Scherer KJ, Goldsmith HH, editors. New York: Oxford; 2003. pp. 596–618. [Google Scholar]
- Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology. 2007;74:185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Guth W, Schmittberger R, Schwarze B. An experimental analysis of ultimatum bargaining. Journal of Economical Behavior. 1982;3:367–388. [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Harlé KM, Sanfey AG. Incidental sadness biases social economic decisions in the Ultimatum Game. Emotion. 2007;7:876–881. doi: 10.1037/1528-3542.7.4.876. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cognition & Emotion. 2000;14:711–724. [Google Scholar]
- Holmes AJ, Pizzagalli DA. Response conflict and frontocingulate dysfunction in unmedicated participants with major depression. Neuropsychologia. 2008;46:2904–13. doi: 10.1016/j.neuropsychologia.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biological Psychiatry. 2008;63:686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the Ultimatum Game. Journal of Neuroscience. 2007;27:951–6. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehofer M, Moser M, Hoehn-Saric R, McLeod D, Liebmann P, Drnovsek B, et al. Major depression and cardiac autonomic control. Biological Psychiatry. 1997;42:914–919. doi: 10.1016/S0006-3223(96)00494-5. [DOI] [PubMed] [Google Scholar]
- Lerner JS, Small DA, Loewenstein G. Heart Strings and Purse Strings: Carryover effects of emotions on economic decisions. Psychological Science. 2004;15:337–341. doi: 10.1111/j.0956-7976.2004.00679.x. [DOI] [PubMed] [Google Scholar]
- Loewenstein G, Lerner J. The role of affect in decision-making. In: Davidson RJ, Goldsmith HH, Scherer KR, editors. Handbook of Affective Sciences. Oxford, England: Oxford University Press; 2003. [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. Journal of Psychiatric Research. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Porter RJ, Mulder RT, Joyce PR, Miller AL, Kennedy M. Tryptophan hydroxylase gene (TPH1) and peripheral tryptophan levels in depression. Journal of Affective Disorders. 2008;109:209–212. doi: 10.1016/j.jad.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The Neural Basis of Economic decision-Making in the Ultimatum Game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Sanfey AG. Social decision-making: Insights from Game Theory and Neuroscience. Science. 2007;318:598–602. doi: 10.1126/science.1142996. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Vagg PR, Barker LR, Donham GW, Westberry LG. Factor structure of the State–Trait Anxiety Inventory. In: Sarason IG, Spielberger CD, editors. Stress and anxiety. Washington, DC: Hemisphere; 1980. pp. 95–109. [Google Scholar]
- Strack S, Coyne JC. Social confirmation of dysphoria: shared and private reactions to depression. Journal of Personality and Social Psychology. 1983;44:798–806. doi: 10.1037//0022-3514.44.4.798. [DOI] [PubMed] [Google Scholar]
- Van ’t Wout M, Kahn RS, Sanfey AG, Aleman A. Affective state and decision- making in the Ultimatum Game. Experimental Brain Research. 2006;169:564–568. doi: 10.1007/s00221-006-0346-5. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of Positive and Negative Affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- West BT, Welch KB, Galecki AT. Linear mixed models: a practical guide using statistical software. Chapman & Hall/CRC; Boca Raton. FL: 2007. [Google Scholar]
- Whisman MA, Miller IW, Norman WH, Keitner GI. Cognitive therapy with depressed inpatients: Specific effects on dysfunctional cognitions. Journal of Consulting and Clinical Psychology. 1991;59:282–288. doi: 10.1037//0022-006x.59.2.282. [DOI] [PubMed] [Google Scholar]