Abstract
Iron deficiency is a common cause of anemia. In end-stage renal disease (ESRD), iron deficiency impairs the therapeutic efficacy of recombinant erythropoietin. Oral or parental iron supplements usually are effective in treating iron deficiency anemia (IDA). Some patients, however, respond poorly to iron supplements and are diagnosed as having iron-refractory iron deficiency anemia (IRIDA). The disease represents a medical challenge but its underlying mechanism was unclear. Hepcidin is a central player in iron homeostasis. It down-regulates the iron exporter ferroportin, thereby inhibiting iron absorption, release and recycling. In ESRD, plasma hepcidin levels are elevated, which contributes to iron deficiency in patients. Matriptase-2, a liver transmembrane serine protease, has been found to have a major role in controlling hepcidin gene expression. In mice, defects in the Tmprss6 gene encoding matriptase-2 result in high hepcidin expression and cause severe microcytic anemia. Similarly, mutations in the human TMPRSS6 gene have been identified in patients with IRIDA. Thus, matriptase-2 is critical for iron homeostasis and may play a role in renal disease.
Keywords: matriptase-2, TMPRSS6, hepcidin, end-stage renal disease, EPO resistance
ANEMIA IN END-STAGE RENAL DISEASE
Anemia is a common complication in patients with end-stage renal disease (ESRD), as a result of progressive loss of erythropoietin production in the kidney. Recombinant human erythropoietin (rh-EPO) is used to treat anemia in ESRD patients. In many ESRD patients, however, high doses of rh-EPO fail to boost red blood cell counts to an adequate level1. The poor response to EPO therapy, or EPO resistance, is a major problem, which increases the mobility and mortality in ESRD patients2.
Iron deficiency is one of the most common causes of EPO resistance. Up to ~40% of patients with EPO-resistance were found to have low levels of serum ferritin or transferrin saturation3. Iron is an essential element in the heme group of hemoglobin subunits in red blood cells. When iron is in short supply, hemoglobin production is impaired. Thus, in ESRD patients EPO resistance often reflects an underlying defect in iron homeostasis. In fact, ESRD patients suffer significant iron loss during hemodialysis. The abnormal iron metabolism may worsen when inflammation occurs in these patients.
Most patients with iron deficiency anemia (IDA) can be treated by oral or parental iron supplements. In ESRD patients, intravenous iron supplements significantly improve the therapeutic efficacy of rh-EPO4. However, some patients with IDA respond poorly to iron therapy. The condition is known as iron-refractory iron deficiency anemia (IRIDA), which is characterized by a hypochromic and microcytic anemia with a low mean corpuscular erythrocyte volume, low transferrin saturation, and abnormal iron absorption. The disease represents a medical challenge but, for many years, its cause was unclear. Most recently, genetic studies have provided new insights into its underlying mechanisms.
IRON ABSORPTION AND BALANCE
Plasma and cellular iron levels are tightly regulated by mechanisms that control iron absorption, storage, recycling and release5. To absorb iron, the insoluble ferric iron (Fe3+) from vegetables and grains needs to be converted into the ferrous form (Fe2+) by a brush border ferric reductase in the duodenum and upper jejunum. The ferrous iron then is transferred across the enterocyte membrane by divalent metal transporter (DMT) 1. The heme iron from red meat has a better bioavailability than that of the inorganic iron, and its absorption appears to be mediated by a different molecular mechanism.
In blood, iron is carried by transferrin to various tissues to be taken by cells in a transferrin receptor-mediated endocytotic process5. Once inside the cell, iron is released from transferrin in the acidic endosome and stored in the form of ferritin. A significant portion, up to ~75%, of plasma iron is taken by bone marrow to make hemoglobin in red blood precursors. Several other cell types including enterocytes, hepatocytes, and reticuloendothelial macrophages also serve as major iron storage sites.
When plasma iron levels are low, iron release is increased from enterocytes, hepatocytes and macrophages to meet the physiological demand. Conversely, when plasma iron levels are high, iron will remain stored in these cells. The body loses iron when cells are shed from the gastrointestinal tract or blood is lost, for example, during hemodialysis. Curiously, our body lacks a way to excrete iron from the kidney, which differs from how plasma sodium levels are adjusted. As a result, the regulated iron release from the cellular storage sites becomes a major mechanism to control plasma iron levels.
FERROPORTIN AND HEPCIDIN IN IRON HOMEOSTASIS
The release of iron from the cell is mediated by an exporter called ferroportin5, a transmembrane protein on the surface of the iron-storing cells. Ferroportin exports iron from the intracellular storage pool, thereby increasing plasma iron levels.
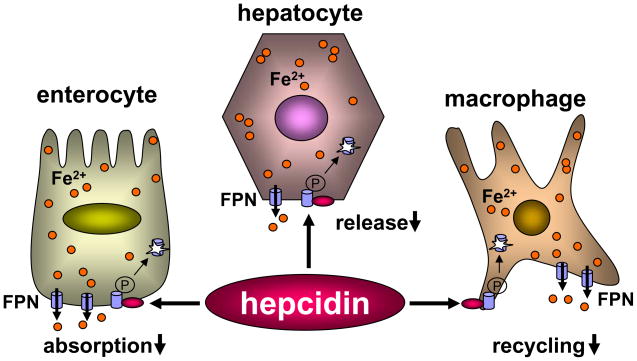
Hepcidin, a peptide of 25 amino acids made in the liver, plays a key role in iron homeostasis by interacting with ferroportin6. When it binds to ferroportin, hepcidin induces phosphorylation of ferroportin, causing its internalization and subsequent degradation in lysosomes7,8 (Fig. 1). As a result, hepcidin lowers ferroportin protein expression on the cell surface, thereby inhibiting iron export from intracellular pools. Thus, hepcidin controls plasma iron level by reducing iron absorption in the gut, lowering iron release from hepatocytes, and preventing iron recycling by macrophages (Fig. 1).
Figure 1. Hepcidin down-regulates ferroportin expression on the cell surface.
Ferroportin (FPN) is expressed on the surface of enterocytes, hepatocytes and tissue macrophages. The binding of hepcidin to FPN leads to its phosphorylation, internalization, and degradation. Low levels of FPN expression reduce iron absorption in the gut, lower iron release from the liver, and prevent iron recycling by tissue macrophages.
The importance of hepcidin in iron metabolism has been demonstrated in genetic studies. In mice, for example, disrupting the hepcidin gene caused iron accumulation in the liver, pancreas, and heart, increased serum iron levels, and reduced iron content in the spleen9. Conversely, overexpression of hepcidin in transgenic mice resulted in decreased body iron levels and severe microcytic anemia10. Similar findings also have been reported in humans. Some patients with large hepatic adenomas had significantly high levels of hepcidin expression and decreased serum transferrin saturation11. The patients developed severe anemia that responded poorly to iron therapy.
HEPCIDIN IN PATIENTS WITH RENAL DISEASE
High levels of plasma or serum hepcidin have been detected in patients with chronic or ESRD12–15. The data are consistent with the impaired iron absorption and low hemoglobin levels in these patients. In addition to its role in iron metabolism, hepcidin may have a direct effect on erythropoiesis. In cell culture, hepcidin was shown to antagonize EPO-mediated erythroid colony formation, suggesting a possibility of hepcidin in inhibiting erythroid progenitor growth and/or survival16. This potential function of hepcidin needs to be verified in in vivo studies, and its significance in EPO resistance in ESRD patients remains to be determined.
HEPCIDIN GENE REGULATION
The Hamp gene, encoding hepcidin, is expressed primarily in the liver. Bone morphogenetic proteins (BMPs) are major activators for Hamp gene expression17,18. The binding of BMP to its cell surface receptor activates the SMAD signaling pathway and induces Hamp gene expression19. Hemojuvelin, a glycosylphosphatidylinositol (GPI)-anchored membrane protein, acts as a co-receptor for BMP to promote Hamp gene expression. In humans and mice, mutations in the hemojuvelin gene reduce hepcidin expression and cause iron overload in the liver, pancreas and heart, but reduced iron levels in tissue macrophages20.
In addition to BMP and hemojuvelin, other molecules also regulate hepcidin expression. For example, IL-6 was reported to stimulate hepcidin expression, possibly through the JAK/STAT3 signaling pathway21. On the other hand, growth differentiation factor (GDF) 15 and hypoxia-inducible transcription factors (HIFs) were reported to suppress hepcidin expression21. It remains to be determined how different signaling pathways act in concert in regulating hepcidin expression.
MATRIPTASE-2/TMPRSS6 IN IRON METABOLISM AND ANEMIA
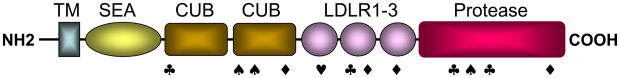
Matriptase-2, also called TMPRSS6, is a type II transmembrane serine protease22. It consists of a short cytoplasmic tail at the N-terminus, a transmembrane domain and an extracellular region that includes a SEA (sea urchin sperm protein, enteropeptidase, and agrin) domain, 2 CUB (complement factor C1s/C1r, urchin embryonic growth factor, and bone morphogenetic protein) domains, 3 LDL receptor repeats and a trypsin-like protease domain at the C-terminus (Fig. 2). Matriptase-2 is highly expressed in the liver. Low levels of matriptase-2 mRNA were detected in the kidney, uterus, brain, adrenal gland, and testis.
Figure 2. Matriptase-2 domain structure and mutants identified in patients with IRIDA.
Matriptase-2 consists of an N-terminal transmembrane domain (TM), one SEA domain, two CUB domains, three LDL receptor repeats (LDLR), and a C-terminal trypsin-like protease domain. Relative positions of nonsense (♠), missense (◆), splicing junction (♣) and frameshift (♥) mutations identified in patients with IRIDA are indicated.
Recent studies revealed a critical role of matriptase-2 in iron metabolism. In a chemically induced mutant mouse strain, mask characterized by extensive loss of body hair and microcytic anemia, the Tmprss6 gene encoding matriptase-2 was found to be disrupted by a premature stop codon23. In these mice, dietary iron absorption was significantly reduced as a result of markedly increased hepcidin levels. Similar findings of alopecia and severe IDA were reported in Tmprss6−/− mice created by gene knockout24. Tmprss6−/− mice also had reduced ferroportin expression and iron accumulation in enterocytes. These studies show an important role of matriptase-2 in suppressing hepcidin expression.
Mutations in the human TMPRSS6 gene have been found in patients with IRIDA. Several groups have identified nonsense, missense, frameshift, and splice junction mutations in the TMPRSS6 gene in patients with familial or sporadic IRIDA25–27 (Fig. 2). In all families analyzed to date, the disease appeared to be inherited in an autosomal recessive manner. In these patients, high levels of hepcidin were present in their urine samples, consistent with the idea that TMPRSS6 gene mutations led to higher hepcidin expression, thereby preventing iron absorption and causing IRIDA. These new findings prompted further studies to understand how matriptase-2 inhibits hepcidin expression.
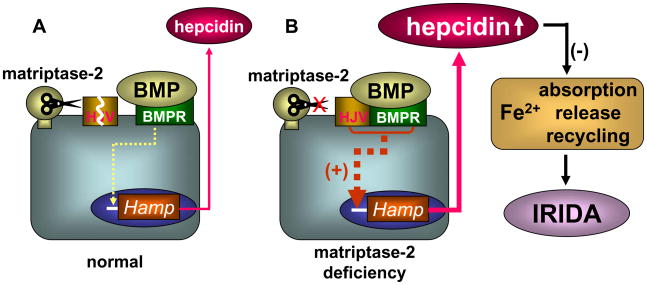
Matriptase-2 is a trypsin-like enzyme22. A possible explanation for matriptase-2-mediated hepcidin inhibition is that the enzyme degrades hepcidin proteolytically. This hypothesis, however, was unlikely because in mice disrupting the Tmprss6 gene increased hepcidin mRNA levels, suggesting that matriptase-2 acted at the transcriptional level23,24. Consistently, overexpression of matriptase-2 inhibited the Hamp promoter activity23. Most recently, matriptase-2 was shown to degrade hemojuvelin in cell membrane28. As hemojuvelin is a co-receptor for BMP to promote hepcidin expression, matriptase-2 seems to inhibit hepcidin expression by degrading hemojuvelin (Fig. 3A). In patients and mutant mice, therefore, matriptase-2 deficiency elevates hemojuvelin and, subsequently, hepcidin levels. This leads to impaired iron absorption and in turn causes IRIDA (Fig. 3B). Together, the latest studies have elucidated the biological function of matriptase-2 and its role in iron homeostasis.
Figure 3. Regulation of hepcidin expression by matriptase-2.
Matriptase-2 prevents hepcidin overexpression by degrading hemojuvelin (HJV), which acts as a co-receptor for BMP to promote Hamp gene expression (panel A). In matriptase-2 deficiency (panel B), high levels of HJV enhances the BMP signaling pathway, leading to overexpression of hepcidin. Hepcidin inhibits iron absorption, release and recycling, thereby causing IRIDA.
PERSPECTIVE
Iron is essential for life. Regulatory mechanisms have been evolved, allowing the body to obtain sufficient amounts of iron from the environment and yet preventing its possible toxic effect of overload. Hepcidin has been identified as a central regulator in iron homeostasis. Now a critical role of matriptase-2 has been discovered in regulating hepcidin expression and in IRIDA. Membrane serine proteases are known to be involved in maintaining homeostasis. The transmembrane protease corin in the heart, for example, activates atrial natriuretic peptide, a cardiac hormone that regulates sodium homeostasis and blood pressure29,30. Matriptase-2 shares structural similarities with corin but instead functions in the liver to regulate iron homeostasis. It will be important to determine if additional structurally related membrane proteases are involved in regulating other metal elements that are of metabolic importance.
The finding of the matriptase-2 function in regulating hepcidin expression encourages more research to understand its role in renal disease, in which abnormal iron metabolism is common. How are matriptase-2 expression and activity regulated in ESRD patients? Is it possible that a decreased matriptase-2 activity contributes to elevated plasma hepcidin levels and exacerbates anemia in these patients? Can we take pharmacological approaches to enhance matriptase-2 expression and/or activity to improve iron absorption and prevent anemia? As iron deficiency and anemia are important complications in renal disease, studies to answer these and many other questions about matriptase-2 and hepcidin may lead to better diagnosis and treatment for ESRD patients.
Acknowledgments
We thank our colleagues in the field of membrane proteases for their support and helpful discussions. This work was supported in part by grants from the Ralph Wilson Medical Research Foundation, the Bakken Heart-Brain Institute, and the NIH (R01 HL089298).
Footnotes
DISCLOSURE
The authors declare no competing interests.
References
- 1.Elliott J, Mishler D, Agarwal R. Hyporesponsiveness to erythropoietin: causes and management. Adv Chronic Kidney Dis. 2009;16:94–100. doi: 10.1053/j.ackd.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Regidor DL, Kopple JD, Kovesdy CP, et al. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol. 2006;17:1181–1191. doi: 10.1681/ASN.2005090997. [DOI] [PubMed] [Google Scholar]
- 3.Horl WH, Jacobs C, Macdougall IC, et al. European best practice guidelines 14–16: inadequate response to epoetin. Nephrol Dial Transplant. 2000;15 (Suppl 4):43–50. [PubMed] [Google Scholar]
- 4.Besarab A, Amin N, Ahsan M, et al. Optimization of epoetin therapy with intravenous iron therapy in hemodialysis patients. J Am Soc Nephrol. 2000;11:530–538. doi: 10.1681/ASN.V113530. [DOI] [PubMed] [Google Scholar]
- 5.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 6.Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- 7.De Domenico I, Ward DM, Langelier C, et al. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18:2569–2578. doi: 10.1091/mbc.E07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 9.Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolas G, Bennoun M, Porteu A, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci U S A. 2002;99:4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100:3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 12.Ashby DR, Gale DP, Busbridge M, et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009;75:976–981. doi: 10.1038/ki.2009.21. [DOI] [PubMed] [Google Scholar]
- 13.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297. doi: 10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 14.Malyszko J, Malyszko JS, Pawlak K, Drozdowska-Rams L, Brzosko S, Mysliwiec M. Hepcidin is linked to anemia and inflammation in peritoneal dialysis patients. Perit Dial Int. 2008;28:418–421. [PubMed] [Google Scholar]
- 15.Tomosugi N, Kawabata H, Wakatabe R, et al. Detection of serum hepcidin in renal failure and inflammation by using ProteinChip System. Blood. 2006;108:1381–1387. doi: 10.1182/blood-2005-10-4043. [DOI] [PubMed] [Google Scholar]
- 16.Dallalio G, Law E, Means RT., Jr Hepcidin inhibits in vitro erythroid colony formation at reduced erythropoietin concentrations. Blood. 2006;107:2702–2704. doi: 10.1182/blood-2005-07-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babitt JL, Huang FW, Wrighting DM, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 18.Truksa J, Peng H, Lee P, Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci U S A. 2006;103:10289–10293. doi: 10.1073/pnas.0603124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang RH, Li C, Xu X, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 20.De Domenico I, Ward DM, Kaplan J. Hepcidin regulation: ironing out the details. J Clin Invest. 2007;117:1755–1758. doi: 10.1172/JCI32701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang AS, Enns CA. Iron homeostasis: recently identified proteins provide insight into novel control mechanisms. J Biol Chem. 2009;284:711–715. doi: 10.1074/jbc.R800017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hooper JD, Campagnolo L, Goodarzi G, Truong TN, Stuhlmann H, Quigley JP. Mouse matriptase-2: identification, characterization and comparative mRNA expression analysis with mouse hepsin in adult and embryonic tissues. Biochem J. 2003;373:689–702. doi: 10.1042/BJ20030390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320:1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folgueras AR, de Lara FM, Pendas AM, et al. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood. 2008;112:2539–2545. doi: 10.1182/blood-2008-04-149773. [DOI] [PubMed] [Google Scholar]
- 25.Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA) Nat Genet. 2008;40:569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillem F, Lawson S, Kannengiesser C, Westerman M, Beaumont C, Grandchamp B. Two nonsense mutations in the TMPRSS6 gene in a patient with microcytic anemia and iron deficiency. Blood. 2008;112:2089–2091. doi: 10.1182/blood-2008-05-154740. [DOI] [PubMed] [Google Scholar]
- 27.Melis MA, Cau M, Congiu R, et al. A mutation in the TMPRSS6 gene, encoding a transmembrane serine protease that suppresses hepcidin production, in familial iron deficiency anemia refractory to oral iron. Haematologica. 2008;93:1473–1479. doi: 10.3324/haematol.13342. [DOI] [PubMed] [Google Scholar]
- 28.Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dries DL, Victor RG, Rame JE, et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–2410. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 30.Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int. 2009;75:142–146. doi: 10.1038/ki.2008.418. [DOI] [PMC free article] [PubMed] [Google Scholar]