Abstract
Oxidative stress is considered a major contributor to the etiology of both “normal” senescence and severe pathologies with serious public health implications. Several cellular sources, including mitochondria, are known to produce significant amounts of reactive oxygen species (ROS) that may contribute to intracellular oxidative stress. Mitochondria possess at least 10 known sites that are capable of generating ROS, but they also feature a sophisticated multilayered ROS defense system that is much less studied. This review summarizes the current knowledge about major components involved in mitochondrial ROS metabolism and factors that regulate ROS generation and removal at the level of mitochondria. An integrative systemic approach is applied to analysis of mitochondrial ROS metabolism, which is “dissected” into ROS generation, ROS emission, and ROS scavenging. The in vitro ROS-producing capacity of several mitochondrial sites is compared in the metabolic context and the role of mitochondria in ROS-dependent intracellular signaling is discussed.
Keywords: ROS sink, ROS targets, ROS sources, nutraceutics, succinate, malate, hypoxia-induced ROS increase
Introduction
Ever-accumulating evidence supports multiple important roles of reactive oxygen species (ROS) in cell metabolism and signaling. The significance of ROS as aggravating or primary factors in numerous pathologies and senescence is firmly established, widely recognized, and extensively reviewed elsewhere (e.g., see Refs. 1–13). More recent data strongly suggest that ROS are involved in physiological signaling cascades regulating various cellular and organ functions,9,14–16 with H2O2 being a chief messenger molecule. This is a relatively new and rapidly expanding research field so it is not surprising that many aspects of cellular ROS metabolism are not yet well understood. Mitochondrial ROS metabolism represents one of such difficult issues. This minireview summarizes the current knowledge about major components involved in mitochondrial ROS metabolism and factors that regulate ROS generation and removal at the level of mitochondria. We also present some of the most recently published and unpublished experimental data obtained in our and other laboratories. Some of this material has been earlier presented by the author at The New York Academy of Science 2007 meeting Mitochondria & Oxidative Stress in Neurodegenerative Disorders held in honor of Dr. John P. Blass.
Are Mitochondria a Source, a Sink, or a Target of ROS?
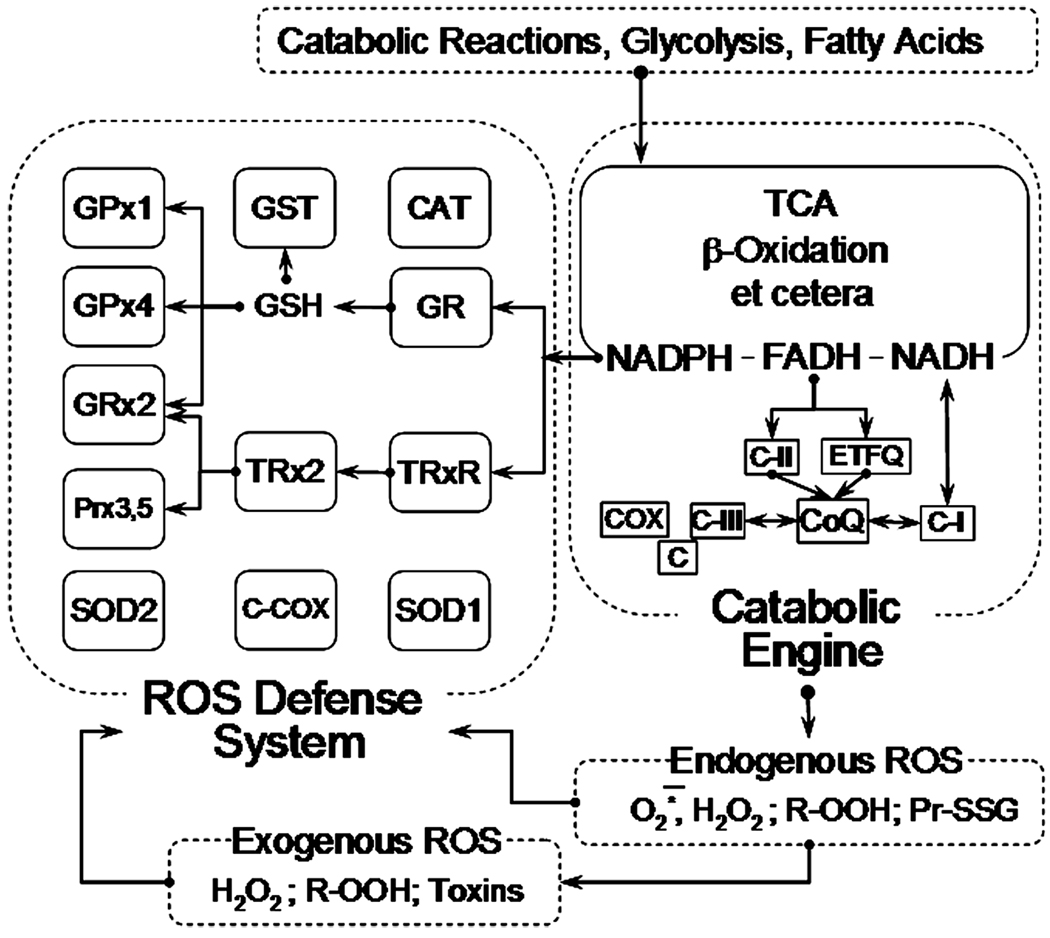
For the purpose of this review, mitochondria in all their complexity can be viewed as being constructed of two functionally distinct “blocks,” the “Catabolic engine” (CE) and the “ROS defense system” (RDS) (Fig. 1).17 The former includes all enzymatic systems catalyzing the oxidation of various substrates generated inside and outside mitochondria, such as the tricarboxylic acid cycle, β-oxidation of fatty acids, and other enzymes. All the reducing equivalents generated in these reactions reduce pyridine and flavin nucleotides NAD and FAD, which in turn are oxidized by coenzyme Q (CoQ) in reactions catalyzed by several enzyme complexes located in the inner membrane of mitochondria. The flux of electrons from substrates through various redox carriers and centers in these enzymes is ultimately terminated in a four-electron reduction of molecular oxygen to water, catalyzed by the cytochrome c oxidase (Fig. 1). The current paradigm holds that en route to water some of the substrate-derived electrons are diverted from the flow and participate in a single-electron reduction of oxygen, thereby converting it into superoxide, a progenitor ROS. This renders mitochondria a source of ROS.
Figure 1.
Mitochondrial catabolic engine and reactive oxygen species (ROS) defense system. See text and Ref. 18 for further detail. Abbreviations: GPx1, mitochondrial glutathione peroxidase; GPx4, mitochondrial phospholipid hydroperoxide glutathione peroxidase; Grx2, glutaredoxin-2; Prx3,5, peroxiredoxins 3 and 5; SOD2, mitochondrial manganese superoxide dismutase; GST, glutathione-S-transferase; GSH, reduced glutathione; TRx2, thioredoxin-2; CCOX, cytochrome c plus cytochrome c oxidase; Cat, catalase; GR, gluthatione reductase; TRxR, mitochondrial thioredoxin-2 reductase; SOD1, CuZn superoxide dismutase; TCA, tricarboxylic acid cycle; C-II, succinate dehydrogenase; ETFQ, electron transferring flavoprotein:CoQ reductase; CoQ, coenzyme Q; CI, Complex I of the respiratory chain; C-III, Complex III of the respiratory chain; C, cytochrome c; COX, cytochrome c oxidase.
The RDS comprises several enzymes specialized in removal of superoxide, H2O2, and organic hydroperoxides (Fig. 1). Most of these enzymes are ubiquitously present in all mammalian mitochondria; the expression level of these enzymes exhibits tissue and species specificity (see Ref. 18 for details about individual enzymes pictured in Fig. 1). A unique feature of RDS is that almost all its enzymes rely on NADPH as a source of reducing equivalents needed for their activity. The NADPH reduction is carried on by three intramitochondrial enzymes: isocitrate dehydrogenase (NADPH linked), malic enzyme, and transhydrogenase.19 These enzymes utilize substrates that are either shared, or generated, or both in the reactions catalyzed by CE. To note, the intramitochondrial pools of NADPH and GSH are rather large (ca. 3–5 mM NADPH20,21 and 2–14 mM GSH22–25). Due to this, transient changes in the activity of CE would not immediately affect the amounts of NADPH and GSH available to RDS and its ability to extinguish short bursts in ROS concentration, its “shock-absorbing capacity.” However, a prolonged activity of RDS, its “endurance,” ultimately depends on the supply of NADPH and GSH, thus depending on the ability of CE to regenerate these compounds. Another unique feature of RDS is that it is not specific toward dealing with intramitochondrial ROS; exogenous ROS species capable of crossing the inner membrane of mitochondria are also detoxified by RDS. Moreover, it is done at the expense of some reducing equivalents generated in CE. This renders mitochondria a sink of ROS.
A feedback relation between the two systems is further amplified by the fact that some crucial components of CE, such as isocitrate-generating aconitase26 and a key trichloroacetic acid (TCA) enzyme ketoglutarate dehydrogenase complex,27 are very sensitive to the damage caused by ROS. Their damage could result in the overall impairment in the catabolic flux paralleled by a decrease in the efficiency of RDS because of the lack of NADPH and an increase in the intramitochondrial ROS. Recalling that mtDNA is particularly vulnerable to ROS-induced damage (e.g., Refs. 28–30) and that it encodes all mitochondrial tRNAs and ribosomal RNAs and 13 of the mitochondrial proteins crucial to the proper functioning of the mitochondrial CE, which in turn supplies ATP to the cell and performs numerous other tasks crucial for sustaining cell viability, one might also think about mitochondria as primary targets of ROS-induced cellular damage.
There of course is a fourth possibility; pertinent to circumstances, mitochondria might behave as a source, sink, and target of ROS simultaneously or switch between these three modes depending on the spatial and temporal metabolic conditions in their surroundings. Further insight on the role of mitochondria in ROS metabolism may be gained by comparing the capacities of CE and RDS toward ROS production and removal and the regulation of these systems by metabolic signals.
Characteristics of the Mitochondrial ROS-producing System
Recalling that superoxide/O2 couple has a moderate redox potential (E1/2 = −0.16 V31), the reaction of one-electron reduction of oxygen is thermodynamically favorable for numerous mitochondrial oxidoreductases.32 Therefore, determining a “main” source of ROS in CE appears to be difficult, if possible at all. Nevertheless, an evaluation of ROS-producing capacity of individual parts of CE is possible, to a degree, in experiments with isolated intact mitochondria and their subfractionated components. By using this approach, 10 potential sources of ROS have so far been identified in mammalian mitochondria (Fig. 2 and see Refs. 18, 33 for details). Among these sources, the highest ROS-producing capacity has been demonstrated for Complex I and Complex III (Table 1) of the mitochondrial respiratory chain and the enzyme dihydrolipoamide dehydrogenase. The latter enzyme is the common component of pyruvate, α-ketoglutarate, and branched-chain ketoacid dehydrogenase complexes and also participates in the glycine-cleavage system.34 In intact mitochondria, their activities are linked to each other and to the rest of CE through the common pools of intermediates, such as NADH and CoQ. A change in electron flux through one of them changes the flux through the other electron carriers in CE. In other words, the degree of reduction of electron carriers in these enzymes in intact mitochondria is determined by the overall flux of reducing equivalents through the CE. An important ramification of this is that ROS production capacity of mitochondria should be controlled by the factors affecting and reflecting the metabolic state of intact mitochondria. It has been found that the most important factors controlling the ROS production in mitochondria are the chemical nature of the substrates fuelling CE, the amplitude of the membrane potential in mitochondria, the pH of the matrix of mitochondria, and the oxygen tension in their surroundings.
Figure 2.
Reported mitochondrial sites capable of ROS generation. Known ROS-generating enzymes are shown in a context of their location within mitochondria. See text and Ref. 18 for further detail. Abbreviations: OM, outer mitochondrial membrane; IM, inner mitochondrial membrane; MAO, mono amine oxidases A and B; b5, cytochrome b5 reductase; DHOH, dihydroorotate dehydrogenase; αGDH, α-glycerophosphate dehydrogenase; C-I, Complex I of the respiratory chain; CoQ, coenzyme Q; C-III, Complex III of the respiratory chain; C, cytochrome c; COX, cytochrome c oxidase; SDH, succinate dehydrogenase; ACO, aconitase; KGDHC, α-ketoglutarate dehydrogenase complex; PDHC, pyruvate dehydrogenase complex; e, electrons. Arrows indicate the direction of electron flux between the enzymes and CoQ.
TABLE 1.
A Comparison of the Reactive Oxygen Species (ROS)-Generating Capacities in the Various Mitochondrial Sites
| ROS- producing site |
H2O2 generation range, nmol/ min/mg |
Conditions |
|---|---|---|
| RET | 1.0–3.0 | Succinate or α-glycerophosphate, State 4 respiration |
| FET | 0.06–0.4 | glutamate + malate, State 4 respiration |
| Max C-I + Matrix | 0.1–0.4 | NAD-linked substrates + rotenone |
| Dehydrogenases | ||
| C-I | 0.3–0.6 | NADH + rotenone, permeabilized mitochondria |
| C-III | 0–0.2 | Succinate + rotenone + antimycin A, intact mitochondria |
| Max C-III | 0–2.0 | Succinate + fumarate + antimycin A, permeabilized mitochondria |
| MAO | 0.7–1.5 | 100 µM Kynurenin, no oxidative substrates |
This table presents a compilation of data obtained by the author of this review in numerous (done over about a 5-year period) experiments with isolated rat brain mitochondria (except for the MAO data, which were obtained with isolated nonsynaptic mouse brain mitochondria, C57/Bl6 strain, n = 6).
Abbreviations: RET, reverse electron transfer, represents H2O2 generation by isolated rat brain mitochondria incubated under conditions described in Figure 3. Note that although Complex I is frequently considered as the site of ROS generation under these conditions, this concept has no stringent experimental support because ROS generation in other NAD-linked intramitochondrial sites, such as dihydrolipoamide dehydrogenase cannot be excluded (discussed in Ref. 18); FET, forward electron transfer, represents H2O2 generation supported by the oxidation of NAD-linked substrates; State 4 describes a nonphosphorylating resting respiration state when all the energy expenditures experienced by mitochondria originate from an inherent proton leak through the inner mitochondrial membrane; max C-I + matrix dehydrogenases is H2O2 emission measured with isolated mitochondria supplied with NAD-linked oxidative substrates in the presence of Complex I (C-I) inhibitor, rotenone, which blocks the net-electron transfer through the respiratory chain. As in the case of FET, an involvement of various NAD-linked enzymes located in the mitochondrial matrix in the generation of ROS cannot be excluded under these conditions; C-I, mitochondria were permeabilized with a pore-forming peptide, alamethicin, washed out of low-molecular weight matrix components, and supplemented with 100 µM NADH; max C-III corresponds to the maximum generation of ROS at the level of Complex III of the respiratory chain. See Ref. 86 for details.
Metabolic Factors Affecting Mitochondrial ROS Production
Although many substrate dehydrogenases comprising CE use a common pool of NAD as their electron acceptor, they exhibit quite different structural, physical, and chemical properties, such as the midpoint redox potentials and the rates and equilibrium constants of the catalyzed reactions. Because of this, a degree of reduction of NAD pool (NADH/NAD+ ratio) under a steady-state condition depends on the chemical nature of the oxidized substrates. In turn, it affects the steady-state reduction level of other electron carriers linked to this pool in CE and the rate of ROS emission. It has been demonstrated that the rate of ROS emission by isolated mitochondria oxidizing NAD-linked substrates obligatory depends on the choice of substrate and almost linearly correlates with the characteristic NADH reduction level set by the oxidation of a selected substrate.35,36 The rate of ROS production increases at high NADH/NAD+ ratios.18,33
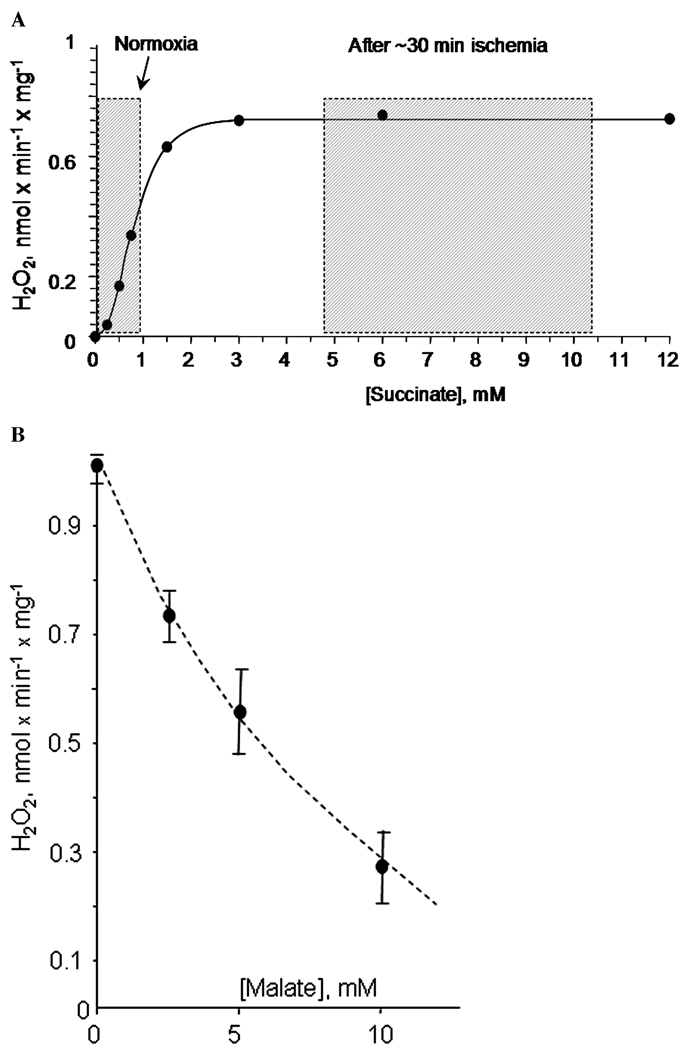
Succinate represents a special case of all the metabolites oxidized in CE. It has long been known that succinate oxidation can support the highest rate of ROS production in non-phosphorylating mitochondria (Fig. 2; Ref. 18 and references therein). However, many researchers dismissed the physiological significance of this fact on the grounds of both the perceived molecular mechanism of the effect and low concentration of succinate in most tissues. We consider the first reason irrelevant as mechanistic explanations of an empirical fact could be many and changing with advancing of the knowledge in the field. The second argument deserves an extended consideration. In vitro, an oxidation of millimolar concentrations of succinate can easily result in the generation of a supraphysiological level of protonmotive force,37 which would result in artificially high membrane potential in mitochondria. As the latter strongly affects the rate of ROS production (discussed below), it also would be artificially high, exaggerating the rates of ROS production occurring in cells.38 Indeed, the concentration of succinate in most tissues is believed to be low, in the 0.2–0.5 mM range, much less than 2–5 mM typically used in experiments with isolated mitochondria. However, succinate concentration increases several fold to the millimolar range in ischemic and hypoxic tissue.39–41 Even under normal conditions, succinate concentration exhibits several-fold fluctuations from 0.3 to 1 mM in perfused heart.42 Succinate also accumulates in muscle tissue during exercise.43 Figure 3A shows that ROS production by mitochondria incubated under nonphosphorylating conditions was strongly enhanced even by a twofold to threefold increase in succinate concentration.44 More recent study demonstrated that succinate strongly enhances mitochondrial ROS emission even in the presence of NAD-linked substrates, which is a more physiological situation.45 Therefore, dismissing succinate-supported ROS generation as unphysiological is unreasonable. Insofar as intermittent hypoxia is a physiological feature in many tissues, accumulation of succinate during the hypoxic period might also contribute to an increased mitochondrial ROS generation upon reoxygenation.
Figure 3.
Features of succinate-supported ROS emission in rat brain mitochondria. (A) Concentration dependence of succinate-supported ROS emission in rat brain mitochondria incubated under nonphosphorylating conditions. Shaded boxes indicate the range of tissue succinate concentrations typically observed under normoxic conditions (normoxia) and after 20–30 min of hypoxia ischemia. See text for further detail. (B) Malate inhibits succinate-supported ROS emission in rat brain mitochondria incubated under nonphosphorylating conditions. Experimental conditions: incubation buffer was composed of 125 mM KCl, 2 mM KH2PO4, 1 mM MgCl2, 0.2 mg/ml of fatty acids-free BSA, 20 mM HEPES (pH 7.2), 0.2 mM EGTA, and (A) pictured concentrations of succinate or (B) 5 mM succinate. Mitochondria were added at 0.25 mg/ml; t = 37°. The emission of H2O2 was estimated from the changes in fluorescence using an H2O2 detection mixture of 4 U/ml of horseradish peroxidase, 40 U/ml SOD, and 10 µM Amplex Red (Molecular Probes, Oregon, USA).
A Digression: Nutraceutics As a Way to Control Mitochondrial ROS Production
Another very interesting feature of succinate-supported ROS production is its sensitivity to inhibition with another physiological TCA metabolite. Earlier we demonstrated that malate caused a dose-dependent inhibition of ROS production in nonphosphorylating brain mitochondria oxidizing succinate (Fig. 3B, from Ref. 46). Recently, this finding was confirmed by Muller et al.47 who hypothesized that malate effect can be explained by the oxaloacetate-induced inhibition of succinate dehydrogenase, which is a well-known mechanism where malate in the mitochondrial matrix serves as a precursor for oxaloacetate, which in turn strongly inhibits48,49 succinate dehydrogenase. The fact that one metabolite can significantly suppress mitochondrial ROS production caused by another metabolite is remarkable. In our opinion, it lends strong support to the validity of “nutraceutics” as an approach to manipulate mitochondrial ROS production in vivo in a whole organism. Nutraceutics as an approach to improve mitochondrial functioning is well commercialized in the form of various food supplements, such as vitamins and antioxidants, to be ingested to maintain the health of one’s mitochondria. There is a strong scientific basis to back up this approach.50 We believe that it may also be possible to control the mitochondrial ROS production by simply selecting food sources rich in certain mitochondrial TCA substrates, such as malate. While this approach awaits its rigorous experimental assessment, it should be noted that there is at least one pioneering example indirectly supporting its validity. In 2001, Prof. John P. Blass reported that giving a mixture containing malate to Alzheimer’s disease sufferers resulted in a noticeable improvement of their mental functions (Challenging Views of Alzheimer’s Disease Conference Cincinnati, Ohio, July 27–29, 2001). To note, mitochondria-associated oxidative stress is thought to significantly contribute to neurodegeneration in Alzheimer’s disease.51–54
Back to the Metabolic Factors Affecting Mitochondrial ROS Production
The magnitude of the membrane potential in mitochondria reflects their metabolic workload. When mitochondria are actively phosphorylating ADP, their membrane potential is approximately 15–20% lower than that in the resting state when no phosphorylation occurs. Other energy-dissipating reactions, such as Ca2+ uptake, may induce even stronger decline in the membrane potential. The rate of ROS emission is strongly controlled by the magnitude of the membrane potential. With NAD-linked oxidative substrates, a decrease in the membrane potential associated with an onset of the active phosphorylation results in approximately 30% decrease in the ROS emission rate; a similar decrease in the membrane potential inhibits ROS emission by 90% in mitochondria oxidizing succinate.18 The membrane potential reflects and affects the redox potential of the electron carriers of the respiratory chain. In the absence of respiratory chain inhibitors, the lower the potential, the lower is the reduction level of electron carriers; it is therefore not surprising that ROS production is decreased at lower membrane potential (see Refs. 18, 33 for a more detailed discussion).
Another metabolic factor known to control the rate of ROS emission is the acidity of the medium. It is well established that mitochondrial ROS emission is inhibited at acidic pH and stimulated at alkaline pH;55–57 however, there is yet no consensus on the mechanism of this effect.
Oxygen Tension and Mitochondrial ROS
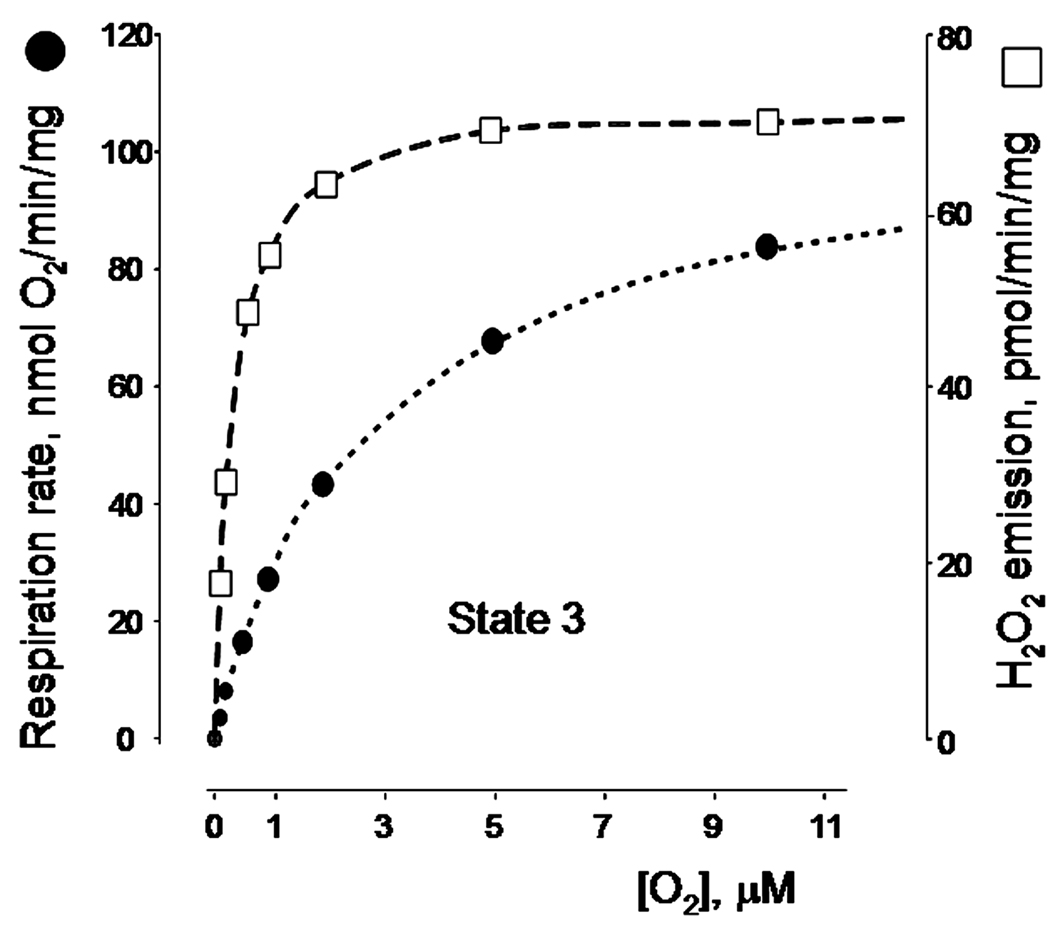
The effect of the oxygen tension on the rate of ROS production has long been unclear. Whereas earlier work by Britton Chance’s group58 demonstrated that mitochondrial ROS production is enhanced under hyperoxic conditions, the effect of the physiological oxygen concentration had not been explored. It has long been known that tissue oxygen tension is much less than that in ambient air and that the oxygen gradient can be readily observed at the intracellular level.59 It was suggested that, at low intracellular levels of oxygen, mitochondrial ROS production should decline dramatically, whereas the amount of oxygen would still be enough to saturate cytochrome oxidase and maintain normal mitochondrial respiration.60,61 However, recent experiments by Paul Brookes’ group do not support this hypothesis.62 These authors found that ROS emission was almost unaffected by changes in oxygen tension ranging from 250 µM (approximately ambient oxygen) to as low as approximately 5–7 µM (intracellular range). Moreover, the apparent affinity of ROS-producing sites toward oxygen was actually much higher than that of cytochrome oxidase, so the ROS emission remained steady at the low levels of oxygen when the respiration rate was becoming inhibited (Fig. 4, the graph is rederived from the data reported in Ref. 62).
Figure 4.
Effect of the oxygen tension on the rates of phosphorylating respiration and H2O2 emission in rat liver mitochondria. The graph was rederived from the data reported in Ref. 62. See text for the detail.
An important aspect of these findings is that it provides a strong argument against the role of mitochondria in so-called hypoxic increase in ROS production. This phenomenon was reported by many groups as a moderate increase in a steady-state level of intracellular ROS occurring at low oxygen tension as compared to normoxic levels of ROS. A dominating explanation of this phenomenon implicated an increase in mitochondrial ROS emission at low oxygen tensions because of some real and perceived properties of the mitochondrial organization that would be irrelevant to discuss here (see Ref. 62 for the references and detailed discussion). However, no increase in ROS emission has been observed with isolated mitochondria at any level of oxygen tension (Fig. 4), meaning that the hypoxic increase in ROS production cannot be attributed to mitochondria per se.62
Regarding the hypoxic increase in ROS production, we would like to note that it might be an experimental artifact. Most if not all authors reporting this effect made use of H2DCF-DA fluorescence indicator to detect the levels of intracellular ROS. This molecule can be oxidized, albeit slowly, by various ROS into a fluorescent form, fluorescein, thereby reflecting intracellular ROS levels. However, the reaction of H2DCF oxidation by the superoxide and H2O2 is complex and unspecific toward these ROS; it is known to be affected by various intracellular factors that are very difficult (if at all possible) to control experimentally (see an excellent recent review63 by Peter Wardman for a thorough discussion of pitfalls and advantages of this and other ROS probes). Moreover, H2DCF oxidation is strongly enhanced by various hemoproteins and by the reduced cytochrome c.64–66 Therefore, it is not a suitable ROS indicator under conditions when significant changes in the reduction of cytochrome c are expected, such as hypoxia. Another example of a seemingly paradoxical enhancement of an oxygen-requiring reaction in hypoxia comes from classical studies on the liver toxicity of CCl4 and several other organic compounds. The toxicity of these compounds is attributed to the lipid peroxidation enhancement by their carbon radicals. A propagation of lipid peroxidation requires oxygen; however the toxicity of CCl4 peaks at some hypoxic levels of oxygen. This had been explained by the fact that CCl4 is activated to its radical form in a reaction with microsomal cytochrome P450 at a site where O2 usually becomes activated during the monooxygenase cycle of the enzyme. This results in a competition between xenobiotics and oxygen for electrons and a higher rate of radical production at lower oxygen tension.67 The reactivity of the fluorescent ROS indicators toward similar cellular enzymes is generally not known. Therefore, any results demonstrating an enhancement of ROS production in hypoxia should be considered with caution when fluorescent indicators are involved.
Although the rate of ROS production is apparently not controlled by the oxygen availability in its physiological range, the oxygen is still being consumed to produce ROS and the electrons for the ROS production are derived from oxidative substrates. It is therefore interesting to evaluate how much of the total oxygen consumed by mitochondria is diverted toward ROS production. An earlier and frequently cited evaluation is that about 1–2% of the total consumed oxygen is diverted to ROS production.58 It is frequently misinterpreted as “1–2% of oxygen consumed by cells or tissue is converted to ROS.” It however means that 1–2% of total oxygen consumed by isolated pigeon heart mitochondria in resting state is diverted to the production of H2O2. This rate is expected to be different in mitochondria from other tissues and species and to depend on the experimental conditions. For example, Table 2 lists the rates of ROS production in percent of the total oxygen consumption by isolated rat brain mitochondria under two major metabolic conditions, the resting and the phosphorylating respiration. A percentage of oxygen diverted to ROS production depends upon both the metabolic condition and the oxidative substrate. It may be safely assumed that an amount of the total electron (and oxygen) flux diverted to ROS formation in mitochondria is tissue and species specific simply because the expression levels of the ROS–producing enzymes exhibit such specificity.18,68,69
TABLE 2.
Substrate-Specific Mitochondrial ROS Emission Expressed in Percent to the Rate of Oxygen Consumption
| H2O2 production (% from the respiration rate) |
||
|---|---|---|
| Substrate | Resting respiration |
Phosphorylating respiration |
| Citrate | 0.71 | 0.19 |
| Glutamate | 0.33 | 0.05 |
| Malate | 0.76 | 0.07 |
| Malate + Glutamate | 0.79 | 0.08 |
| α-Ketoglutarate | 1.07 | 0.07 |
| Pyruvate | 0.89 | 0.19 |
| Succinate | 3.15 | 0.04 |
Experimental conditions were as described in Figure 3. The phosphorylating respiration rates were recorded under the same conditions except that the incubation buffer was supplemented with 400 µM ADP. All substrates were added at 5 mM concentration except for the malate plus glutamate, which were added at 1 and 5 mM, respectively.
Properties of Mitochondrial RDS
Although the presence of many RDS enzymes in mitochondria has long been acknowledged, the capacity and the regulation of RDS in its integrity are little studied. Mammalian mitochondria are well equipped to scavenge the superoxide generated either internally or externally.
The manganese superoxide dismutase (MnSOD, SOD2) is located in the mitochondrial matrix where it protects mitochondrial targets against the internally generated superoxide (reviewed in Ref. 18,70).
At least two other mitochondrial enzymes are capable of efficient scavenging of the externally generated superoxide, CuZn superoxide dismutase (SOD1) and cytochrome c plus cytochrome c oxidase system (C-COX, Fig. 2). Although most of SOD1 is found in cytosol, some amount of this enzyme can be found in the space between the outer and the inner mitochondrial membranes71–73 where it can participate in the removal of the superoxide generated outside mitochondria, at least in theory. However, its contribution to the superoxide-scavenging capacity of mitochondria has not been thoroughly investigated. Inarrea et al. reported that SOD1 in intact rat liver mitochondria did not participate in the removal of the exogenous superoxide unless the mitochondrial outer membrane was disrupted selectively by digitonin.72 To note, intact rat liver mitochondria neither scavenged nor dismutated externally generated superoxide at all.72 This is at variance with other reports documenting quite prominent scavenging of externally produced superoxide by heart,74,75 liver,76 and yeast76 mitochondria.
The second system capable of efficient superoxide scavenging is cytochrome c plus cytochrome c oxidase. The intermembrane space of mitochondria contains approximately 0.7 mM cytochrome c,77 which can be alternatively reduced by the respiratory chain or superoxide78 and regenerated (oxidized) by cytochrome c oxidase. The oxidation of superoxide-reduced cytochrome c by cytochrome c oxidase generates proton-motive force that mitochondria can use to produce ATP.75 The antioxidant properties of cytochrome c were confirmed in experiments with isolated mitochondria,74 but the physiological role and in vivo efficiency of this RDS remain to be explored.
Some known properties of RDS will be discussedhereonthe example of H2O2 removal by rodent brain mitochondria. It should be clearly understood that these properties of RDS may not be the same in mitochondria from other tissues and species as the expression levels of the RDS enzymes are tissue and species specific.68,69
A recent study by Alexandre’s group demonstrated that rat brain mitochondria remove H2O2 at a very high rate of approximately 0.3–6.7 nmol/min/mg protein.79 The rate of H2O2 removal exhibited complex dependence on the metabolic state of mitochondria and the nature of oxidative substrate used; the lowest rate (0.3 nmol/min/mg) was observed with deenergized mitochondria incubated in the absence of exogenous substrates. The highest rate of H2O2 removal (6.7 nmol/min/mg) was observed in mitochondria energized by NAD-linked substrates glutamate and malate incubated in the presence of Ca2+ chelator ethyleneglycolbis(β-aminoethyl ether)-N,N’-tetra-acetic acid (EGTA). It should be noted that the highest rate of H2O2 removal is approximately two to three times higher than a maximum rate of H2O2 production typically reported with isolated rodent brain mitochondria (e.g., Fig. 2). Authors concluded that H2O2 removal in brain mitochondria is negatively controlled by Ca2+ via inhibition of the glutathione reductase/glutathione peroxidase (GR/GPx) system and that brain mitochondria function as intracellular Ca2+-modulated peroxide sinks. Another conclusion that can be drawn from this study is that mitochondrial GR and GPx are the major players in removing exogenous H2O2.79
To note, the rate of H2O2 removal in experiments by Alexandre’s group was measured by following the disappearance of a rather high amount of added H2O2 bolus (8 nmol in 1.6 ml, 5 µM H2O2 concentration). The highest reported level of H2O2 in brain stands as approximately 100 µM as measured by microdialysis in rat striatum after ischemia/reperfusion.80 However, the steady state levels of H2O2 in naïve rat brain yielded much lower values, approximately 0.008 µM of H2O2,81 whereas typical steady-state H2O2 concentrations in other tissues and cell cultures have been reported to be in the 0.01–0.1 µM range.82,83 It would therefore be of interest to assess the ability of mitochondria to remove H2O2 at these low physiological concentrations. We did such experiments using 0.5–0.8 nmol H2O2 introduced as a bolus; we also used a H2O2 generation system comprised of xanthine + xanthine oxidase co-incubated with isolated brain mitochondria and adjusted to generate H2O2 at 0.005–0.07 nmol/min, which is within a range of H2O2 emission characteristic for experiments with isolated mitochondria. Our preliminary data agree well with those reported79 in that the rate of H2O2 removal is affected by the nature of oxidative substrates and the metabolic state of mitochondria. We also found two important features of RDS that have not yet been reported.
First, brain mitochondria isolated from GPx1 knockout mice devoid of glutathione peroxidase activity84,85 exhibited exactly the same rates of H2O2 removal as wild-type mouse brain mitochondria. Thus, it appears that GPx1 is not essential in removing low concentrations of H2O2. To note, these results should be interpreted with caution as the GPx1 knockout mice might harbor compensatory increases in other H2O2 scavenging mechanisms thereby obscuring the role of the GPx1 per se.
Second, we found that when H2O2 was generated by xanthine + xanthine oxidase, there was no continuous accumulation of H2O2 in the incubation medium. A few tens of seconds after an initiation of H2O2 generation, its level in the incubation medium reached a steady state and did not change through the duration of the experiment for over 20 min. As anticipated, the steady-state level of H2O2 was proportional to the rate of H2O2 production and the content of mitochondria in the medium. Moreover, it was also affected by the nature of oxidative substrate present in the incubation medium, with succinate producing the highest level and NAD-linked substrates the lowest steady-state levels of H2O2. Therefore, mitochondria essentially “clamp” or buffer the steady-state levels of H2O2 (A.A. Starkov et al., manuscript in preparation).
These experiments explain a puzzling earlier observation made by us and by other researchers86 and discussed in Ref. 18. When intact mitochondria were co-incubated with a H2O2 detection system comprising horseradish peroxidase and its fluorogenic substrate, the rate of ROS emission was readily detectable. However, when the H2O2 detection mix was added to the incubation medium after removing mitochondria, very little of accumulated H2O2 was detected, grossly disproportional to the expected levels calculated from ROS emission rates measured in the presence of H2O2 detection mix. A time-dependent accumulation of H2O2 was observed only when mitochondria were structurally compromised.18 When succinate was used as an oxidative substrate, an accumulation of H2O2 was also observed but it was not proportional to the time of incubation prior to removal of mitochondria and adding the H2O2 detection system (A.A. Starkov et al., manuscript in preparation). The latter is not surprising considering that mitochondria clamp the steady-state level of H2O2 at a value proportional to the rate of its generation and that succinate supports the highest rate of H2O2 production in intact brain mitochondria (Fig. 2). On the other hand, structural damage to mitochondria renders their RDS inefficient because of the leakage of GSH and NADPH from the matrix, thereby allowing time-dependent accumulation of H2O2.
Summarizing, it appears that at least brain mitochondria function as a sink and a source of H2O2 simultaneously. Their RDS is extremely efficient at higher concentrations of H2O2, which makes mitochondria a bona fide sink of H2O2. However, RDS is much less efficient at low physiological concentrations ofH2O2,and because of that it buffers the steady state level of H2O2 at a value proportional to its generation rate and the mass and the metabolic state of mitochondria.
Role of Mitochondria in Cellular ROS Metabolism and Signaling
Although mitochondria are frequently named the major source of intracellular ROS in recent literature, they are definitely not the only source. Numerous other cellular sources of ROS have been reported, for example, enzymes, such as membrane-bound NADH and NADPH oxidases, xanthine oxidase, eNOS, lipoxygenases, cyclooxygenases, microsomal metabolism of xenobiotics, and peroxisomal β-oxidation of fatty acid (reviewed in Ref. 14 and numerous reviews cited therein). Although many of these sources are capable of substantial ROS production, the latter is either limited by the availability of their substrates or requires a specific activation by other factors. In other words, the ROS generation by nonmitochondrial sources exhibit spatial and temporal discontinuity pertinent to metabolic fluctuations in a particular cell in a particular moment of time. On the other hand, mitochondria are ubiquitously present in cells and generate ROS continuously as long as they have access to oxygen and oxidative substrates. However, the amount and protein composition of mitochondria vary among tissues and the level of ROS they generate also reflects metabolic fluctuations. Moreover, many of nonmitochondrial ROS-producing enzymes are also ubiquitously expressed and may contribute to the ROS production in parallel with mitochondria. Currently, it is not technically possible to systematically compare their ROS-producing capacity with that of mitochondria under realistic metabolic conditions. Multiple sources of ROS in mitochondria co-exist with a powerful multileveled RDS system. The contribution of each of those systems in the overall cellular ROS metabolism is expected to exhibit the species and tissue specificity and therefore it has to be determined on a case-by-case basis. Questions, such as “are mitochondria a major source/sink/target of ROS in cells” could not be answered in general.
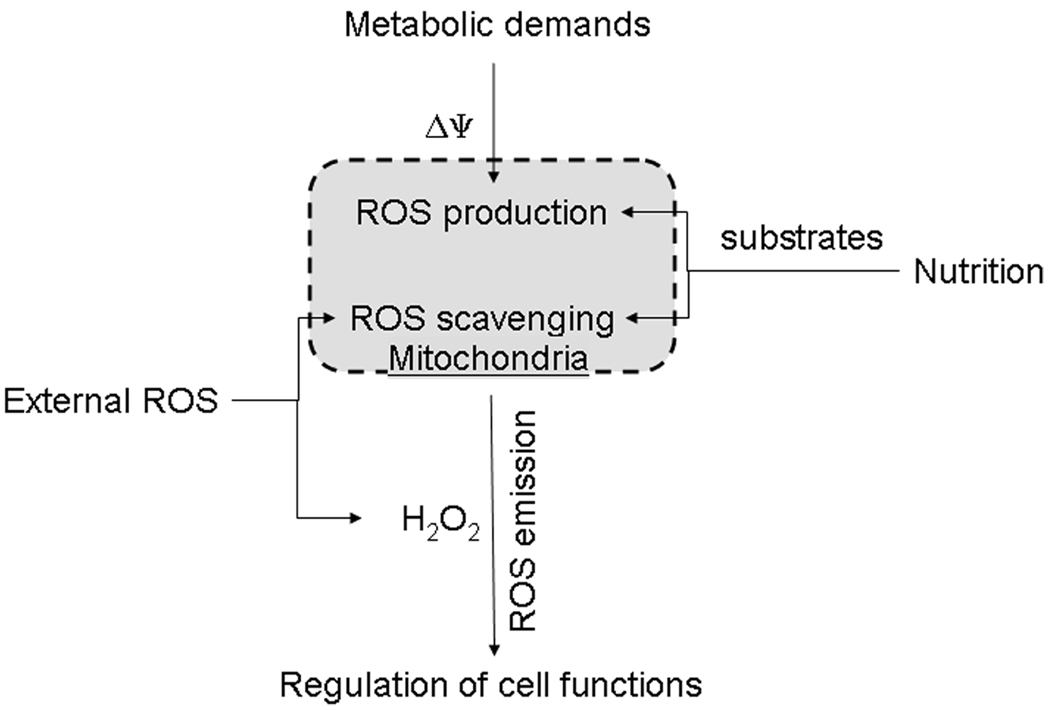
Overall, it may be suggested that mitochondria serve as an important ROS-stabilizing feedback node in the cellular ROS-signaling network. Being a source and a sink of ROS at the same time, they “sense” changes in the intracellular metabolic demands, nutrition supply, their own abundance and functional and structural integrity, and the external ROS “pressure,” and “report” the integrated signal back to the cell in the form of the steady-state level of H2O2 set forth by a balance between their H2O2 emission and removal rates (Fig. 5).
Figure 5.
Mitochondria sense changes in metabolism and ROS pressure and respond with an integrated signal in the form of H2O2. The magnitude of the mitochondrial membrane potential (ΔΨ) changes following fluctuations in the demands, such as ATP synthesis, which are imposed by cellular metabolism on the mitochondrial catabolic engine. Changes in cell nutrition are reflected by changes in the pattern of available oxidative substrates, monoamine oxidase substrates, and other factors. This results in changes in ROS generation in various mitochondrial sites. Internally generated ROS are combined with extramitochondrial ROS and get filtered through the mitochondrial ROS-scavenging system. The resulting level of the H2O2 emission serves as an integrated feedback signal from mitochondria to other intracellular H2O2-sensing systems.
Conclusion
Irrespectively of whether or not mitochondria are the major source of ROS in cells, strategies toward diminishing their contribution to intracellular oxidative stress are of great practical interest. However, considering various strategies aiming to decrease mitochondrial ROS, there is one important question to be answered. This question is “what exactly should be decreased?” Indeed, one should distinguish between the generated ROS, the emitted ROS, and the scavenged ROS. The generated ROS is an amount of ROS primarily produced in mitochondrial sites. Insofar as we can deduce from in vitro experiments with isolated mitochondria (which contributed approximately 99% of all that we know about mitochondrial functions), it should be possible to decrease this “innate” ROS generation by either changing a content/properties of the ROS-generating sites genetically or by targeting mitochondrial metabolism through nutraceutical or pharmacological approaches. While the former approach appears to be rather dubious (at this time), the second approach seems to be workable, to some extent, through metabolic interventions, such as caloric restriction or changing an intracellular pattern of mitochondrial oxidative substrates through nutrition supply. The emitted ROS is a difference between the generated ROS and the ROS scavenged by the mitochondrial RDS. Theoretically, it should be possible to change the emission of ROS by either manipulating the ROS generation or by changing the affinity of ROS-scavenging reactions in RDS to the mitochondria-generated ROS. However, the physiological level of ROS emission from mitochondria is negligible (as discussed in this review) and unlikely to be of any significance except as a signal. Therefore, it is not clear whether diminishing the ROS emission below its physiological level would be beneficial. It might, of course, be desirable in a pathology associated with a severe oxidative stress caused by a supraphysiological ROS production from damage to mitochondria.
Alternatively, increasing the capacity of mitochondrial RDS toward the faster removal of supraphysiological levels of ROS seems quite attractive under all circumstances. It might be achieved by enhancing the mitochondrial RDS with nonenzymatic antioxidants87 and metabolites supplying the reducing equivalents to RDS (e.g., malate, isocitrate), or by a selective stimulation of expression of RDS components. Taking into account the properties of mitochondrial RDS as discussed above, it seems unlikely that increasing its capacity toward removal of high levels of ROS would change the physiological ROS signaling mediated by the ROS emission from mitochondria. However, it would provide a better guard against any abrupt increase in the steady-state ROS levels caused by extracellular or intracellular events.
Acknowledgments
The author is very grateful to Dr. M. Flint Beal for the benevolent support of the author’s research and to Steven F. Zhang for his skilled and enthusiastic assistance in conducting H2O2 scavenging experiments. This study was funded by National Institute on Aging grant AG014930.
Footnotes
Conflicts of Interest
The author declares no conflicts of interest.
References
- 1.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 2.Orrenius S. Reactive oxygen species in mitochondria-mediated cell death. Drug. Metab. Rev. 2007;39:443–455. doi: 10.1080/03602530701468516. [DOI] [PubMed] [Google Scholar]
- 3.Droge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newsholme P. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J. Physiol. 2007;583:9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim GJ, Chandrasekaran K, Morgan WF. Mitochondrial dysfunction, persistently elevated levels of reactive oxygen species and radiation-induced genomic instability: a review. Mutagenesis. 2006;21:361–367. doi: 10.1093/mutage/gel048. [DOI] [PubMed] [Google Scholar]
- 6.Di Filippo C. Oxidative stress as the leading cause of acute myocardial infarction in diabetics. Cardiovasc. Drug. Rev. 2006;24:77–87. doi: 10.1111/j.1527-3466.2006.00077.x. [DOI] [PubMed] [Google Scholar]
- 7.Okayama Y. Oxidative stress in allergic and inflammatory skin diseases. Curr. Drug. Targets Inflamm. Allergy. 2005;4:517–519. doi: 10.2174/1568010054526386. [DOI] [PubMed] [Google Scholar]
- 8.Storz P. Reactive oxygen species in tumor progression. Front. Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 9.Valko M. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishigori C, Hattori Y, Toyokuni S. Role of reactive oxygen species in skin carcinogenesis. Antioxid. Redox. Signal. 2004;6:561–570. doi: 10.1089/152308604773934314. [DOI] [PubMed] [Google Scholar]
- 12.Schiller J. Contribution of reactive oxygen species to cartilage degradation in rheumatic diseases: molecular pathways, diagnosis and potential therapeutic strategies. Curr. Med. Chem. 2003;.10:2123–2145. doi: 10.2174/0929867033456828. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003;79:829–843. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 14.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 15.Giorgio M. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 16.Afanas’ev IB. Signaling functions of free radicals superoxide & nitric oxide under physiological & pathological conditions. Mol. Biotechnol. 2007;37:2–4. doi: 10.1007/s12033-007-0056-7. [DOI] [PubMed] [Google Scholar]
- 17.Starkov AA. Protein-mediated energy-dissipating pathways in mitochondria. Chem. Biol. Interact. 2006;163:133–144. doi: 10.1016/j.cbi.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70:200–224. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 19.Vogel R. The regeneration of reduced glutathione in rat forebrain mitochondria identifies metabolic pathways providing the NADPH required. Neurosci. Lett. 1999;275:97–100. doi: 10.1016/s0304-3940(99)00748-x. [DOI] [PubMed] [Google Scholar]
- 20.Tischler ME, Hecht P, Williamson JR. Effect of ammonia on mitochondrial and cytosolic NADH and NADPH systems in isolated rat liver cells. FEBS Lett. 1977;76:99–104. doi: 10.1016/0014-5793(77)80129-4. [DOI] [PubMed] [Google Scholar]
- 21.Williamson JR, Corkey BE. Assay of citric acid cycle intermediates and related compounds-update with tissue metabolite levels and intracellular distribution. Methods Enzymol. 1979;55:200–222. doi: 10.1016/0076-6879(79)55025-3. [DOI] [PubMed] [Google Scholar]
- 22.Wahllander A. Hepatic mitochondrial and cytosolic glutathione content and the subcellular distribution of GSH-S-transferases. FEBS Lett. 1979;97:138–140. doi: 10.1016/0014-5793(79)80069-1. [DOI] [PubMed] [Google Scholar]
- 23.Griffith OW, Meister A. Origin and turnover of mitochondrial glutathione. Proc. Natl. Acad. Sci. USA. 1985;82:4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free. Radic. Biol. Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebrin I, Sohal RS. Comparison of thiol redox state of mitochondria and homogenates of various tissues between two strains of mice with different longevities. Exp. Gerontol. 2004;39:1513–1519. doi: 10.1016/j.exger.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardner PR. Superoxide radical and iron modulate aconitase activity in mammalian cells. J. Biol. Chem. 1995;270:13399–13405. doi: 10.1074/jbc.270.22.13399. [DOI] [PubMed] [Google Scholar]
- 27.Chinopoulos C, Tretter L, Adam-Vizi V. Depolarization of in situ mitochondria due to hydrogen peroxide-induced oxidative stress in nerve terminals: inhibition of alpha-ketoglutarate dehydrogenase. J. Neurochem. 1999;73:220–228. doi: 10.1046/j.1471-4159.1999.0730220.x. [DOI] [PubMed] [Google Scholar]
- 28.Kang D, Hamasaki N. Mitochondrial oxidative stress and mitochondrial DNA. Clin. Chem. Lab. Med. 2003;41:1281–1288. doi: 10.1515/CCLM.2003.195. [DOI] [PubMed] [Google Scholar]
- 29.LeDoux SP. Mitochondrial DNA repair: a critical player in the response of cells of the CNS to genotoxic insults. Neuroscience. 2007;145:1249–1259. doi: 10.1016/j.neuroscience.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Houten B, Woshner V, Santos JH. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair (Amst) 2006;5:145–152. doi: 10.1016/j.dnarep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Sawyer DT, Valentine JS. How super is superoxide? Acc. Chem. Res. 1981;14:393–400. [Google Scholar]
- 32.Turrens JF. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol. Sci. 2006;27:639–645. doi: 10.1016/j.tips.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Carothers DJ, Pons G, Patel MS. Dihydrolipoamide dehydrogenase: functional similarities and divergent evolution of the pyridine nucleotide-disulfide oxidoreductases. Arch. Biochem. Biophys. 1989;268:409–425. doi: 10.1016/0003-9861(89)90309-3. [DOI] [PubMed] [Google Scholar]
- 35.Tretter L, Adam-Vizi V. Generation of re-active oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J. Neurosci. 2004;24:7771–7778. doi: 10.1523/JNEUROSCI.1842-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starkov AA. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J. Neurosci. 2004;24:7779–7788. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholls DG. The effective proton conductance of the inner membrane of mitochondria from brown adipose tissue. Dependency on proton electrochemical potential gradient. Eur. J. Biochem. 1977;77:349–356. doi: 10.1111/j.1432-1033.1977.tb11674.x. [DOI] [PubMed] [Google Scholar]
- 38.Nicholls DG. Mitochondrial membrane potential and aging. Aging Cell. 2004;3:35–40. doi: 10.1111/j.1474-9728.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 39.Wiesner RJ, Rosen P, Grieshaber MK. Pathways of succinate formation and their contribution to improvement of cardiac function in the hypoxic rat heart. Biochem. Med. Metab. Biol. 1988;40:19–34. doi: 10.1016/0885-4505(88)90100-4. [DOI] [PubMed] [Google Scholar]
- 40.Kakinuma Y. Myocardial metabolic markers of total ischemia in vitro. Nagoya. J. Med. Sci. 1994;57:35–42. [PubMed] [Google Scholar]
- 41.Benzi G, Pastoris O, Dossena M. Relationships between gamma-aminobutyrate and succinate cycles during and after cerebral ischemia. J. Neurosci. Res. 1982;7:193–201. doi: 10.1002/jnr.490070210. [DOI] [PubMed] [Google Scholar]
- 42.Sato K. Insulin, ketone bodies, and mitochondrialenergytransduction. FASEB J. 1995;9:651–658. doi: 10.1096/fasebj.9.8.7768357. [DOI] [PubMed] [Google Scholar]
- 43.Hochachka PW, Dressendorfer RH. Succinate accumulation in man during exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1976;35:235–242. doi: 10.1007/BF00423282. [DOI] [PubMed] [Google Scholar]
- 44.Starkov AA, Fiskum G. On the mechanism of reactive oxygen species production by rat heart and brain mitochondria relevant to ischemia/reperfusion injury. 3rd Albany Conference on Frontiers of Mitochondrial Research; September 14–17; Rensselaerville, New York. 2000. [Google Scholar]
- 45.Zoccarato F. Succinate modulation of H2O2 release at NADH:ubiquinone oxidoreductase (Complex I) in brain mitochondria. Biochem. J. 2007;406:125–129. doi: 10.1042/BJ20070215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Starkov AA, Fiskum G. Succinate and Ca2+ accumulation enhance reactive oxygen species production by rat brain mitochondria (Abstract). Annual Meeting of the Society for Neuroscience. Vol 27 prog. 328.8; Nov. 10–15; San Diego California. 2001. [Google Scholar]
- 47.Muller FL. High rates of superoxide production in skeletal muscle mitochondria respiring on both Complex I and Complex II linked substrates. Biochem. J. 2008;409:491–499. doi: 10.1042/BJ20071162. [DOI] [PubMed] [Google Scholar]
- 48.Zeyelmaker WP, Slater EC. The inhibition of succinate dehydrogenase by oxaloacetate. Biochim. Biophys. Acta. 1967;132:210–212. doi: 10.1016/0005-2744(67)90214-8. [DOI] [PubMed] [Google Scholar]
- 49.Wojtczak L, Wojtczak AB, Ernster L. The inhibition of succinate dehydrogenase by oxalacetate. Biochim. Biophys. Acta. 1969;191:10–21. doi: 10.1016/0005-2744(69)90310-6. [DOI] [PubMed] [Google Scholar]
- 50.Liu J, Ames BN. Reducing mitochondrial decay with mitochondrial nutrients to delay and treat cognitive dysfunction, Alzheimer’s disease, and Parkinson’s disease. Nutr. Neurosci. 2005;8:67–89. doi: 10.1080/10284150500047161. [DOI] [PubMed] [Google Scholar]
- 51.Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann. Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- 52.Beal MF. Mitochondrial dysfunction and oxidative damage in Alzheimer’s and Parkinson’s diseases and coenzyme Q10 as a potential treatment. J. Bioenerg Biomembr. 2004;36:381–386. doi: 10.1023/B:JOBB.0000041772.74810.92. [DOI] [PubMed] [Google Scholar]
- 53.Reddy PH, Beal MF. Are mitochondria critical in the pathogenesis of Alzheimer’s disease? Brain Res Brain Res Rev. 2005;49:618–632. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Gibson GE. The alpha-ketoglutarate-dehydrogenase complex: a mediator between mitochondria and oxidative stress in neurodegeneration. Mol. Neurobiol. 2005;31:43–63. doi: 10.1385/MN:31:1-3:043. [DOI] [PubMed] [Google Scholar]
- 55.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem. J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeshige K, Minakami S. NADH- and NADPH-dependent formation of superoxide anions by bovine heart submitochondrial particles and NADH-ubiquinone reductase preparation. Biochem. J. 1979;180:129–135. doi: 10.1042/bj1800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lambert AJ, Brand MD. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem.J. 2004;384:511–517. doi: 10.1042/BJ20040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones DP. Intracellular diffusion gradients of O2 and ATP. Am. J. Physiol. 1986;250:C663–C675. doi: 10.1152/ajpcell.1986.250.5.C663. [DOI] [PubMed] [Google Scholar]
- 60.Skulachev VP. Nonphosphorylating respiration as the mechanism preventing the formation of active forms of oxygen. Mol. Biol. (Mosk) 1995;29:1199–1209. [PubMed] [Google Scholar]
- 61.Skulachev VP. Role of uncoupled and noncoupledoxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q. Rev. Biophys. 1996;29:169–202. doi: 10.1017/s0033583500005795. [DOI] [PubMed] [Google Scholar]
- 62.Hoffman DL, Salter JD, Brookes PS. Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: implications for hypoxic cell signaling. Am. J. Physiol. Heart. Circ. Physiol. 2007;292:H101–H108. doi: 10.1152/ajpheart.00699.2006. [DOI] [PubMed] [Google Scholar]
- 63.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free. Radic. Biol. Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 64.Burkitt MJ, Wardman P. Cytochrome C is a potent catalyst of dichlorofluorescin oxidation: implications for the role of reactive oxygen species in apoptosis. Biochem. Biophys. Res. Commun. 2001;282:329–333. doi: 10.1006/bbrc.2001.4578. [DOI] [PubMed] [Google Scholar]
- 65.Ohashi T. Rapid oxidation of dichlorodi-hydrofluorescin with heme and hemoproteins: formation of the fluorescein is independent of the generation of reactive oxygen species. FEBS Lett. 2002;511:21–27. doi: 10.1016/s0014-5793(01)03262-8. [DOI] [PubMed] [Google Scholar]
- 66.Burkitt M. Activation of cytochrome c to a peroxidase compound I-type intermediate by H2O2 relevance to redox signalling in apoptosis. Biochem. Soc. Symp. 2004;71:97–106. doi: 10.1042/bss0710097. [DOI] [PubMed] [Google Scholar]
- 67.De Groot H, Sies H. Cytochrome P-450, reductive metabolism, and cell injury. Drug. Metab. Rev. 1989;20:275–284. doi: 10.3109/03602538909103543. [DOI] [PubMed] [Google Scholar]
- 68.Johnson DT. Tissue heterogeneity of the mammalian mitochondrial proteome. Am. J. Physiol. Cell Physiol. 2007;292:C689–C697. doi: 10.1152/ajpcell.00108.2006. [DOI] [PubMed] [Google Scholar]
- 69.Johnson DT. Functional consequences of mitochondrial proteome heterogeneity. Am. J. Physiol. Cell Physiol. 2007;292:C698–C707. doi: 10.1152/ajpcell.00109.2006. [DOI] [PubMed] [Google Scholar]
- 70.Robinson BH. The role of manganese superoxide dismutase in health and disease. J. Inherit. Metab. Dis. 1998;21:598–603. doi: 10.1023/a:1005427323835. [DOI] [PubMed] [Google Scholar]
- 71.Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J. Biol. Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 72.Inarrea P. Redox activation of mitochondrial intermembrane space Cu,Zn-superoxide dismutase. Biochem. J. 2005;387:203–209. doi: 10.1042/BJ20041683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hervias I, Beal MF, Manfredi G. Mitochondrial dysfunction and amyotrophic lateral sclerosis. Muscle. Nerve. 2006;33:598–608. doi: 10.1002/mus.20489. [DOI] [PubMed] [Google Scholar]
- 74.Korshunov SS. The antioxidant functions of cytochrome c. FEBS Lett. 1999;462:192–198. doi: 10.1016/s0014-5793(99)01525-2. [DOI] [PubMed] [Google Scholar]
- 75.Mailer K. Superoxide radical as electron donor for oxidative phosphorylation of ADP. Biochem. Biophys. Res. Commun. 1990;170:59–64. doi: 10.1016/0006-291x(90)91240-s. [DOI] [PubMed] [Google Scholar]
- 76.Guidot DM. Mitochondrial respiration scavenges extramitochondrial superoxide anion via a nonenzymatic mechanism. J. Clin. Invest. 1995;96:1131–1136. doi: 10.1172/JCI118100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hackenbrock CR, Chazotte B, Gupte SS. The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J. Bioenerg. Biomembr. 1986;18:331–368. doi: 10.1007/BF00743010. [DOI] [PubMed] [Google Scholar]
- 78.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 79.Zoccarato F, Cavallini L, Alexandre A. Respiration-dependent removal of exogenous H2O2 in brain mitochondria: inhibition by Ca2+ J. Biol. Chem. 2004;279:4166–4174. doi: 10.1074/jbc.M308143200. [DOI] [PubMed] [Google Scholar]
- 80.Hyslop PA. Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: correlation with the cytotoxic potential of H2O2in vitro. Brain Res. 1995;671:181–186. doi: 10.1016/0006-8993(94)01291-o. [DOI] [PubMed] [Google Scholar]
- 81.Yusa T. Hyperoxia increases H2O2 production by brain in vivo. J. Appl. Physiol. 1987;63:353–358. doi: 10.1152/jappl.1987.63.1.353. [DOI] [PubMed] [Google Scholar]
- 82.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 83.Boveris A, Cadenas E. Cellular sources and steady-state levels of reactive oxygen species. In: In: Clerch LB, Massaro DJ, editors. Oxygen, Gene Expression and Cellular Function. Marcel Dekker; New York: 1997. pp. 1–25. [Google Scholar]
- 84.Cheng WH. Cellular glutathione peroxidase knockout mice express normal levels of selenium-dependent plasma and phospholipid hydroperoxide glutathione peroxidases in various tissues. J. Nutr. 1997;127:1445–1450. doi: 10.1093/jn/127.8.1445. [DOI] [PubMed] [Google Scholar]
- 85.Ho YS. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J. Biol. Chem. 1997;272:16644–16651. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- 86.Staniek K, Nohl H. H(2)O(2) detection from intact mitochondria as a measure for one-electron reduction of dioxygen requires a non-invasive assay system. Biochim. Biophys. Acta. 1999;1413:70–80. doi: 10.1016/s0005-2728(99)00083-3. [DOI] [PubMed] [Google Scholar]
- 87.Cocheme HM. Mitochondrial targeting of quinones: therapeutic implications. Mitochondrion. 2007;7 Suppl:S94–S102. doi: 10.1016/j.mito.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 88.Starkov AA, Fiskum G. Myxothiazol induces H(2)O(2) production from mitochondrial respiratory chain. Biochem. Biophys. Res. Commun. 2001;281:645–650. doi: 10.1006/bbrc.2001.4409. [DOI] [PubMed] [Google Scholar]