Abstract
Background
Chronic administration of ethanol increases plasma prolactin levels and enhances estradiol’s mitogenic action on the lactotropes of the pituitary gland. The present study was conducted to determine the changes in the pituitary levels of G proteins during the tumor development following alcohol and ethanol treatments.
Methods
Using ovariectomized Fischer-344 female rats, we have determined ethanol and estradiol actions at 2 and 4 weeks on pituitary weight and pituitary cell contents of prolactin, Gs. Gq11, Gi1, Gi2, and Gi3 proteins. Western blots were employed to measure protein contents.
Results
Ethanol increased basal and estradiol-enhanced wet weight and the prolactin content in the pituitary in a time-dependent manner. Chronic exposure of estradiol increased the levels of Gs protein in the pituitary. Unlike estradiol, ethanol exposure did not show significant effect on the basal level of Gs protein, but moderately increased the estradiol-induced levels of this protein. Estradiol exposure enhanced Gq11 protein levels in the pituitary after 2 and 4 weeks, while ethanol treatment failed to alter these protein levels in the pituitary in control-treated or estradioltreated ovariectomized rats. In the case of Gi1, estradiol but not ethanol increased the level of this protein at 4 weeks of treatment. However, estradiol and ethanol alone reduced the levels of both Gi2 and Gi3 proteins at 2 and 4 weeks of treatment. Ethanol also significantly reduced the estradiol-induced Gi2 levels at 4 weeks and Gi3 level at 2 and 4 weeks.
Conclusions
These results confirm ethanol’s and estradiol’s growth-promoting and prolactin stimulating actions on lactotropes of the pituitary and further provide evidence that ethanol and estradiol may control lactotropic cell functions by altering expression of specific group of G proteins in the pituitary.
Keywords: Prolactinomas, Prolactin, Estradiol, Ethanol, G Proteins, Female Rats
G proteins belong to the super family of guanine tri-phosphate (GTP) binding proteins and function as transducers of information across the cell membrane by coupling diverse receptors to intracellular effectors (Gilman, 1987;Ross, 1989; Simon et al., 1991). All the G proteins are heterotrimer, consisting of α, β, and γ subunits. The α subunit has been shown to play critical role in regulation of certain intracellular effectors. To date, approximately 20 mammalian α subunit genes have been identified and divided into four major subfamilies (Gs, Gi, Gq, and G12) based on the degree of amino acid identity (Wilkie et al., 1992). Gs and Gq mediate stimulatory processes, such as hormone secretion, by activating adenylyl cyclases and phospholipase C, respectively. The members of Gi subfamily including Gi1, Gi2, and Gi3 generally transduce inhibitory signals by reducing adenylyl cyclase activity and intracellular calcium (Ca2+) levels via potassium(K+) channel activation and Ca2+ channel blockade (Derwahl et al., 1996; Vallar and Meldolesi, 1989; Vallar et al., 1987). The G12 subfamily comprised of the subunits G12 and G13 are known to be involved in various cellular processes ranging from cytoskeletal changes to cell growth and oncogenesis (Dhanasekaran and Dermott, 1996). Abnormal expression of G proteins in different target tissues has been described in numerous pathophysiological states including human pituitary adenomas (Ballare et al., 1997; Landis et al., 1989; Spiegel et al., 1993; Vallar et al., 1987). It is possible specific types or amounts ofG protein available for receptor activation may be relevant to stimulatory or inhibitory effects of hormone or drugs.
In the pituitary, the secretion and growth of prolactin secreting lactotropes are controlled by hypothalamic hormones that act via G protein-coupled receptors, sharing the common structural and functional motif characterized by 7 transmembrane domains (Baldwin, 1994; Ben-Jonahan and Hnasko, 2001; Freeman et al., 2000, Gregerson et al., 2001; Gershengorn and Osman, 1996). Lactotropes functions are also controlled by estradiol which modulates the action of many of these hypothalamic hormones (Sarkar, 2006). Recently, we have shown that like estradiol, ethanol exposure also increases pituitary weight, plasma prolactin levels, and proliferation of pituitary lactotropes (De et al., 2002). We have also shown that both agents alter the level of the dopamine D2 receptor in the pituitary. Dopamine plays a key role in maintaining the normal function of lactotropes in the pituitary gland. Abnormalities in dopamine receptors and dopamine transporter function lead to hyperplasia of lactotropes (Bosse et al., 1997; Kelly et al., 1997; Laccarino et al., 2002; Saiardi et al., 1997). It is well known that estradiol inhibits dopamine release from the hypothalamus and downregulates dopamine D2 receptor activity in lactotropes (Guivarc’h et al., 1998; Oomizu et al., 2003; Sarkar et al., 1982). The D2 receptor is a 7 transmembrane segment protein with a long third intracellular loop and a short intracellular C-terminus. The sixth exon of the D2 receptor gene is often excluded in the mature transcript, resulting in a short (29 amino acids shorter) isoform (D2S). Estradiol strongly favors the expression of the long isoform (D2L) mRNA over the short isoform D2S (Guivarc’h et al., 1998; Oomizu et al., 2003), thus causing uncoupling to the G protein (Guiramand et al., 1995). It is interesting to note that ethanol, which increases lactotropic cell proliferation, also favors production of the D2L over the D2S isoform (Oomizu et al., 2003). As dopamine receptor is G protein-coupled receptor, so the possibility arises that the down stream action of ethanol or estradiol may involve alteration in expression of these G proteins. The aim of the current study was to compare the effects of ethanol and estradiol on G protein levels and to correlate the changes in the levels of G proteins and prolactin.
MATERIALS AND METHODS
Animals
Female Fischer 344 rats with a body weight of 160 to 200 g were obtained from Simonsen Laboratories and were housed in a controlled environment (temperature 22°C; lights on, 05:00 to 19:00 hours) and provided with rodent chow meal and water ad libitum. Animals were ovariectomized bilaterally and subcutaneously implanted with a 1 cm estradiol-17β-filled silastic capsule (Dow Corning Corp., Midland, MI) or an empty silastic capsule under pentobarbital anesthesia. The estradiol-capsule maintained plasma levels of estradiol 17-β between 120 and 150 pg/ml (De et al., 1995). After 1 week of implants, animals were ad libitum-fed rodent chow meal (ad lib-fed), pair-fed isocaloric liquid diet (pair-fed) or fed with ethanol-containing liquid diet (alcohol-fed) for 14 or 28 days using the procedure as described by us previously (De et al., 2002). The concentration of ethanol in the diet was 8.7% v/v that provided about 37% of the total dietary calories. The ethanol treatment regiment used has shown to maintain blood alcohol levels within the range of 115 to 123 mg/dl between days 10 and 30 (De et al., 2002). Anterior pituitaries of these animals were obtained, wet weight of these tissues were recorded, and tissues were extracted and used for measurements of G proteins using western blots. Animal surgery and care were performed in accordance with institutional guidelines and complied with NIH policy. The animal protocol was approved by the Rutgers Animal Care and Facilities Committee.
Western Blot
For determining the levels of various proteins, pituitaries were homogenized in lysis buffer on ice, and protein levels were determined using the Bio-Rad protein assay (Bio-Rad, Irvine, CA). Equal amounts of protein from each sample were resolved on 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred overnight to immobilon-P polyvinylidene fluoride membranes. Membranes were incubated with primary antibody for 18 hours at 4°C in blocking buffer. Membranes were then washed and incubated with peroxidase conjugated secondary antibody for 1 hours. Afterwards membranes were washed and then incubated with enzymatic chemiluminescence Western blot chemiluminescence reagent. Membranes were exposed to X-ray films and developed using X-ray developer. For quantification of protein levels, band intensities of proteins were determined using Scion Image software (Sunnyvale, CA) and normalized to the corresponding actin band intensities. The primary antibodies used were rabbit anti-Gs antibody, rabbit anti-Gq11 antibody, mouse anti-Gi1 antibody, mouse anti-Gi2 antibody, rabbit anti-Gi3 antibody, and mouse anti-actin antibody. All the antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), except for anti-Gi3 and actin antibody which were bought from Millipore (Billerica, MA) and Oncogene Research Products (San Diego, CA), respectively. The specificity of the antisera used for the western blot were assessed using the specific blocking peptide. Hundred- fold more concentrations of the blocking peptides prevented the binding of the antibodies with the G proteins (data not shown).
Statistics
The mean and standard error (SEM) of the data were determined and are presented in the text and figures. Data were analyzed using 2-way ANOVA. The differences between groups were determined using the Benferroni post-tests. A value of p < 0.05 was considered a significant difference.
RESULTS
Pituitary Weight and Prolactin Contents
The estrogen treatment regimen employed in this study has been used previously to demonstrate hyperprolactinemic and pituitary tumorigenic effects of estradiol on the pituitary. The ethanol-feeding paradigm used in this study also has been shown by us previously to be effective in maintaining blood alcohol levels within the range of 115 to 123 mg/dl (De et al., 2002) and in elevating plasma levels of prolactin and in potentiating estrogen miogenic action on the pituitary (De et al., 1995; De et al., 2002). In this study, we have used pair-feeding liquid diets as a control for feeding alcohol-containing liquid diet and did not include ad lib-feeding to determine the caloric influence on pituitary weight prolactin level, because we have previously found no significant differences in the levels of plasma prolactin or in the DNA content in the pituitary between pair-fed and ad lib-fed rats (De et al., 2002).
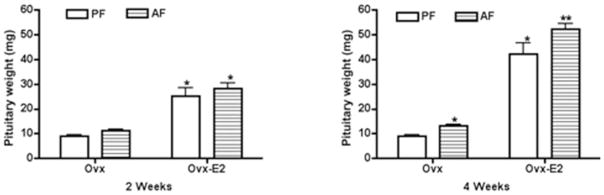
In this study, we show that estradiol-treated ovariectomized rats had significantly higher wet weight of the pituitary and pituitary contents of prolactin at 2 and 4 weeks compared with control-treated ovariectomized rats (Figs 1 and 2). Ethanol treatment increased the pituitary weight and the content of pituitary prolactin both in ovariectomized rats and in estradiol- treated ovariectomized rats.
Fig. 1.
Effect of ethanol and estradiol on pituitary wet weight in ovariectomized (Ovx) and estradiol-treated ovariectomized animals (Ovx-E2) at 2 and 4 weeks. Rats were fed an ethanol diet (AF) or an isocaloric control diet (PF) for 2 or 4 weeks. The anterior pituitary glands were collected from the rats, washed in PBS and weighed. Data are mean ± SE obtained from four to six rats. *p < 0.05, compared with PF-fed Ovx group; **p < 0.05, compared with AF-fed E2 group.
Fig. 2.
Effect of ethanol and estradiol on pituitary prolactin contents in ovariectomized (Ovx) and estradiol-treated ovariectomized animals (Ovx-E2). Rats were fed an ethanol diet (AF) or an isocaloric control diet (PF) for 2 or 4 weeks. The pituitary level of prolacitn was measured by Western blot. The tissue level of actin was used as loading control. Upper panel shows the representative blots. Lower panel shows the ratio of band intensities of prolactin and actin. Each bar represents mean ± SEM of 4 to 6 different animals. *p < 0.05, compared with PF-fed Ovx group; **p < 0.05, compared with AF-fed E2 group.
Pituitary G Protein Contents
The present study focused on the determination of the changes of G proteins that are known to regulate the function of lactotropes of the anterior pituitary (Albert, 2002); therefore, the changes in levels of prolactin stimulatory Gs and Gq11 (Bouvier et al., 1991; Yajima et al., 1997) and prolactin inhibitory Gi subfamily including Gi1, Gi2, and Gi3 (Albert et al., 1997; Guiramand et al., 1995; Vallar and Meldolesi, 1989) were measured following estradiol and ethanol treatments. The changes of G protein levels following ethanol with or without estradiol treatment for 2 and 4 weeks are shown in Figs 3 to 7.
Fig. 3.
Effect of ethanol and estradiol on pituitary Gs protein contents in ovariectomized (Ovx) and estradiol-treated ovariectomized animals (Ovx-E2). Rats were fed an ethanol diet (AF) or an isocaloric control diet (PF) for 2 or 4 weeks. The pituitary level of Gs proteins was determined by Western blot. The tissue level of actin was used as loading control. Upper panel shows the representative blots. Lower panel shows the ratio of band intensities of G proteins and actin. Each bar represents mean ± SEM of 4 to 6 different animals. *p < 0.05, compared with respective PF group.
Fig. 7.
Effect of ethanol and estradiol on pituitary Gi23 protein contents in ovariectomized (Ovx) and estradiol-treated ovariectomized animals (Ovx-E2). Rats were fed an ethanol diet (AF) or an isocaloric control diet (PF) for 2 or 4 weeks. The pituitary level of Gi3 was measured by Western blot. The tissue level of actin was used as loading control. Upper panel shows the representative blots. Lower panel shows the ratio of band intensities of Gi3 protein and actin. Each bar represents mean ± SEM of 4 to 6 different animals. *p < 0.05, compared with PF-fed Ovx group; **p < 0.05, compared with AF-fed E2 group.
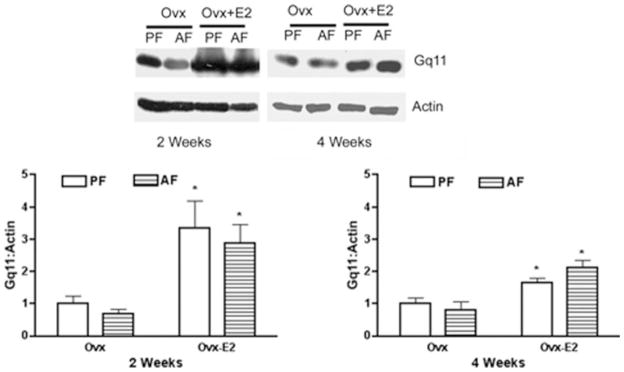
The levels of all the G protein studied were similar in ovariectomized rats at 2 and 4 weeks. Although there were no significant interaction between ethanol feeding and estradiol action on stimulatory Gs and Gq11, chronic exposure of estradiol significantly increased the levels of Gs protein in the pituitary after 2 and 4 weeks when compared with control treated ovariectomized rats. Unlike estradiol, ethanol exposure did not show significant effect on the Gs protein levels either at 2 or 4 weeks in ovariectomized rats. Estradiol exposure also enhanced Gq11 protein levels in the pituitary after 2 and 4 weeks when compared with control-treated ovariectomized rats (Fig. 4). Estradiol stimulatory effect was more pronounced at 2 weeks than at 4 weeks. Ethanol treatment did not affect pituitary levels of Gαq11 either in control-treated or estradiol-treated ovariectomized rats.
Fig. 4.
Effect of ethanol and estradiol on pituitary Gq11 protein contents in ovariectomized (Ovx) and estradiol-treated ovariectomized animals (Ovx-E2). Rats were fed an ethanol diet (AF) or an isocaloric control diet (PF) for 2 or 4 weeks. The pituitary level of Gq11 was measured by Western blot. The tissue level of actin was used as loading control. Upper panel shows the representative blots. Lower panel shows the ratio of band intensities of Gq11 proteins and actin. Each bar represents mean ± SEM of 4 to 6 different animals. *p < 0.01, compared with respective PF group.
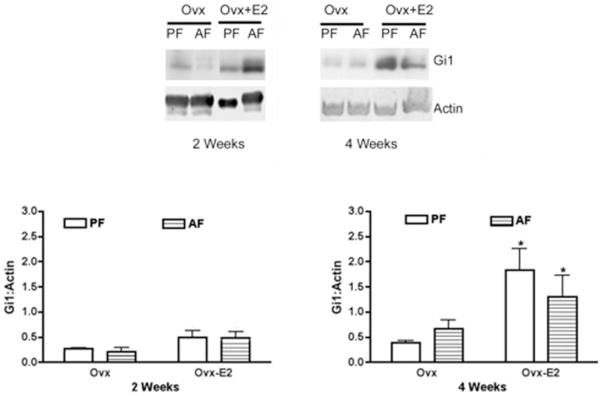
Effects of estradiol and ethanol on the Gi subfamily of proteins in the pituitary varied. There was significant interaction between alcohol feeding (alcohol feeding and control feeding) and estradiol treatment (estradiol and control) for Gi3 (2 weeks, p < 0.02, F = 6.646, df = 21 and 4 weeks, p < 0.02, F = 7.01, df = 19) and Gi2 (2 weeks, p < 0.001, F = 23.22, df = 21 and 4 weeks, p < 0.04, F = 5.44, df = 19), In the case of Gi1, estradiol increased the level of this protein in the pituitary only after 4 weeks of treatment, while ethanol produced no significant effect on the basal or the estradiol-induced level of Gi1 protein in the pituitary at 2 or 4 weeks (Fig. 5). Both ethanol and estradiol alone reduced the levels of Gi2 protein after 2 and 4 weeks of treatment (Fig. 6). Ethanol also significantly reduced the estradiolinduced Gi2 level at 4 weeks. Ethanol and estradiol alone significantly suppressed the levels of Gi3 protein after 2 and 4 weeks of treatment (Fig. 7). Ethanol also reduced the estradiol- induced Gi3 protein level in the pituitary at 2 and 4 weeks.
Fig. 5.
Effect of ethanol and estradiol on pituitary Gi1 protein contents in ovariectomized (Ovx) and estradiol-treated ovariectomized animals (Ovx-E2). Rats were fed an ethanol diet (AF) or an isocaloric control diet (PF) for 2 or 4 weeks. The pituitary level of Gi1 was measured by Western blot. The tissue level of actin was used as loading control. Upper panel shows the representative blots. Lower panel shows the ratio of band intensities of Gi1 proteins and actin. Each bar represents mean ± SEM of 4 to 6 different animals. *p < 0.01, compared with respective PF group.
Fig. 6.
Effect of ethanol and estradiol on pituitary Gi2 protein contents in ovariectomized (Ovx) and estradiol-treated ovariectomized animals (Ovx-E2). Rats were fed an ethanol diet (AF) or an isocaloric control diet (PF) for 2 or 4 weeks. The pituitary level of Gi2 was measured by Western blot. The tissue level of actin was used as loading control. Upper panel shows the representative blots. Lower panel shows the ratio of band intensities of Gi2 proteins and actin. Each bar represents mean ± SEM of 4 to 6 different animals. *p < 0.05, compared with PF-fed Ovx group; **p < 0.05, compared with AF-fed E2 group.
DISCUSSION
Abnormal expression of G proteins in different target tissues has been described in numerous pathophysiological conditions (Spiegel et al., 1993; Speigel, 1996). The data available suggest that human pituitary tumors, such as hypothyroidism, hypoadrenalism and hypogonadism are associated with a reduction in Gs and an increase in Gi expression (Bouvier et al., 1991; Milligan et al., 1987). However, human prolactinomas is associated with increased Gs and decreased Gi expression (Ballare et al., 1997). In this study, we found an increase in the Gs protein levels after estradiol treatment. The changes in the Gs protein levels after estradiol treatment followed similar patterns with the changes in the pituitary weight and pituitary prolactin content. Using primary cultures of prolactin-producing lactotropes, it has been show that estradiol treatments did not affect the expression of Gs protein in lactotropes (Livingstone et al., 1998). However, prolactinsecreting GH3 cells expressing constitutively active Gs show enhanced hormone secretion and proliferation (Ham et al., 1997). Additionally, Gs overexpressing GH3 cells show increased cell growth and prolactin secretion (Gaiddon et al., 1995). Hence, an increase in Gs protein levels may favor prolactinoma growth in estrogen and ethanol-treated animal.
Estradiol induced increase of pituitary weight and prolactin content were associated with a significant increase in pituitary Gq11 levels both at 2 and 4 weeks. Previously, a significant amount of Gq11 level was detected in human prolactinomas (Ballare et al., 1997). Gq11, a pertussis toxin-insensitive G protein has been shown to be involved in the exocytotic pathway and hormone release in a prolactin and GH producing GH3 cells (Yajima et al., 1997). It has been shown that hypothalamic neuropeptide thyrotropin-releasing hormone (TRH) stimulates prolactin secretion via activation of receptors coupled to Gq/11 family. Activation of TRH receptors results in the stimulation of phospholipase C activity, leading to calcium mobilization, protein kinase C activation, and prolactin release (Kaiser et al., 1994). As estradiol is known to increase thyrotropin- releasing hormone (TRH) receptors in the pituitary (Kimura et al., 1994) and enhances TRH-induced prolactin release (Majo et al., 1999), it is possible that the estradiol-induced increase in pituitary Gq11 levels might be involved in the steroid-increased lactotropic cell function. Ethanol did not produce any significant effect on Gq11 levels in the pituitary, suggesting that this G protein may not participate in ethanol action on lactotropic cell growth and hormone production.
Both estradiol and ethanol reduced pituitary level of Gi2 and Gi3 but not Gi1, which was increased after long-term treatment with the steroid. The changes in the levels of Gi2 and Gi3 proteins negatively associated with the changes in pituitary weight and in prolactin content following estradiol or ethanol treatment. These data suggest that, both estradiol and ethanol action may depend on inhibition of the function of a negative regulator of lactotropes. Dopamine is one of the major negative regulators of lactotropic cell growth and secretion (reviewed by Ben-Jonahan and Hnasko, 2001). Both estrogen and ethanol has been shown to reduce secretion of dopamine from the tuberoinfundibular dopaminergic neurons (Arita and Kimura, 1987; Boyadjieva and Sarkar, 1995). Dopamine acts via D2 receptors that couple to pertussis toxin-sensitive Gi/Go proteins to inhibit prolactin secretion and growth of lactotropes (Albert et al., 1997; Sarkar, 2006; Vallar and Meldolesi, 1989). Two subtypes of D2 receptors (D2S-short and D2L-long) are generated by alternate splicing of 29 amino acids and display similar receptor pharmacology and coupling properties (Civelli et al., 1993). Both estradiol and ethanol have been shown to increase D2L expression but reduce D2S production (Guivarc’h et al., 1998; Oomizu et al., 2003). Differences in G protein selectivity of D2S and D2L receptors have been reported previously. Gi2 was required for D2L-mediated inhibition of forskolin-stimulated adenylyl cyclase (Montmayeur et al., 1993; Senogles, 1994), but not for D2S-mediated inhibition of Gs-stimulated adenylyl cyclase (Ghahremani et al., 1999; Liu et al., 1994). D2-induced inhibition of DNA synthesis was sensitive to pertussis toxin. In prolactin-producing GH4ZR7 cells, Gi2 did not appear to participate in D2S inhibition of TRH-induced mitogen-activated protein kinase (MAPK), but Go and Gi3 did (Albert, 2002). It is suggested that the D2S receptor may use Go and Gi3 to inhibit MAPK, but other G proteins appear to target different pathways and reduce cell proliferation (Albert, 2002). Depletion of Gi2 blocked inhibition of basal and vasoactive intestinal peptide-stimulated cAMP formation by these receptors (Liu et al., 1994, 1999), suggests that cAMP inhibition is dispensable for inhibition of DNA synthesis. In contrast to secretion, inhibition of [3H]thymidine incorporation was completely, rather than partially, blocked upon depletion of individual G proteins (Albert, 2002). This indicates that D2S-induced inhibition of [3H]thymidine incorporation requires coordinate mediation by several G proteins, whereas inhibition of secretion requires fewer G proteins.
In conclusion, the mechanisms that mediate estrogen or ethanol action on lactotropic cell growth and prolactin production may involve suppression of inhibitory G proteins, like Gi2 and Gi3. Estrogen which is a more potent stimulator of lactotropic cell growth and hormone production simultaneously increases the stimulatory G proteins and hence results in more tumor growth and prolactin secretion in comparison to ethanol. Further studies using lactotropic cell culture are needed to clarify the role of individual G proteins in ethanol and estrogen action on lactotropic cell growth and prolactin production.
Acknowledgments
This investigation was supported by National Institutes of Health Grant AA11591.
References
- Albert PR. G protein preferences for dopamine D2 inhibition of prolactin secretion and DNA synthesis in GH4 pituitary cells. Mol Endocrinol. 2002;16:1903–1911. doi: 10.1210/me.2001-0329. [DOI] [PubMed] [Google Scholar]
- Albert PR, Ghahremani MH, Morris SJ. Mechanisms of dopaminergic regulation of prolactin secretion. In: Neve KA, Neve RL, editors. The dopamine receptors. Humana Press; Totowa: 1997. pp. 359–381. [Google Scholar]
- Arita J, Kimura F. Direct inhibitory effect of long term estradiol treatment on dopamine synthesis in tuberoinfundibular dopaminergic neurons: in vitro studies using hypothalamic slices. Endocrinology. 1987;121:692–698. doi: 10.1210/endo-121-2-692. [DOI] [PubMed] [Google Scholar]
- Baldwin JM. Structure and function of receptors coupled to G proteins. Curr Opin Cell Biol. 1994;6:180–190. doi: 10.1016/0955-0674(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Ballare E, Mantovani S, Bassetti M, Lania A, Spada A. Immunodetection of G proteins in human pituitary adenomas: evidence for a low expression of proteins of the Gi subfamily. Eur J Endocrinol. 1997;137:482–489. doi: 10.1530/eje.0.1370482. [DOI] [PubMed] [Google Scholar]
- Ben-Jonahan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22:724–763. doi: 10.1210/edrv.22.6.0451. [DOI] [PubMed] [Google Scholar]
- Bosse R, Fumagalli F, Jaber M, Giros B, Gainetdinov RR, Wetsel WC, Missale C, Caron MG. Anterior pituitary hypoplasia and dwarfism in mice lacking the dopamine transporter. Neuron. 1997;19:127–138. doi: 10.1016/s0896-6273(00)80353-0. [DOI] [PubMed] [Google Scholar]
- Bouvier C, Lagace G, Collu R. G protein modulation by estrogens. Mol Cell Endocrinol. 1991;79:65–73. doi: 10.1016/0303-7207(91)90096-b. [DOI] [PubMed] [Google Scholar]
- Boyadjieva NI, Sarkar DK. Effects of dopamine D1 and D2 receptor agonists and antagonists on basal and ethanol-modulated beta-endorphin secretion from hypothalamic neurons in primary cultures. J Neuroendocrinol. 1995;17:819–825. doi: 10.1111/j.1365-2826.1995.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Civelli O, Bunzow JR, Grandy DK. Molecular diversity of the dopamine receptors. Annu Rev Pharmacol Toxicol. 1993;33:281–307. doi: 10.1146/annurev.pa.33.040193.001433. [DOI] [PubMed] [Google Scholar]
- De A, Boyadjieva N, Oomizu S, Sarkar DK. Ethanol induces hyperprolactinemia by increasing prolactin release and lactotrope growth in female rats. Alcohol Clin Exp Res. 2002;26:1420–1429. doi: 10.1097/01.ALC.0000030621.35354.E0. [DOI] [PubMed] [Google Scholar]
- De A, Boyadjieva N, Pastorcic M, Sarkar DK. Potentiation of the mitogenic effect of estrogen on the pituitary gland by alcohol consumption. Int J Oncology. 1995;7:643–648. doi: 10.3892/ijo.7.3.643. [DOI] [PubMed] [Google Scholar]
- Derwahl M, Hamacher C, Russo D, Broecker M, Manole D, Schatz H, Kopp P, Filetti S. Constitutive activation of the Gs alpha protein-adenylate cyclase pathway may not be sufficient to generate toxic thyroid adenomas. J Clin Endocrinol Metab. 1996;81:1898–1904. doi: 10.1210/jcem.81.5.8626855. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran N, Dermott JM. Signaling by the G12 class of G proteins. Cell Signal. 1996;8:235–245. doi: 10.1016/0898-6568(96)00048-4. [DOI] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin structure, function and regulation of secretion. Physiol Rev. 2000;76:175–191. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Gaiddon C, Mercken L, Bancroft C, Loeffier JP. Transcriptional effects in GH3 cells of Gs alpha mutants associated with human pituitary tumors: stimulation of adenosine 3′,5′-monophosphate response element-binding protein-mediated transcription and of prolactin and growth hormone promoter activity via protein kinase A. Endocrinology. 1995;136:4331–4338. doi: 10.1210/endo.136.10.7664652. [DOI] [PubMed] [Google Scholar]
- Gershengorn MC, Osman R. Molecular and cellular biology of thyrotropin- releasing hormone receptors. Physiol Review. 1996;76:175–191. doi: 10.1152/physrev.1996.76.1.175. [DOI] [PubMed] [Google Scholar]
- Ghahremani MH, Cheng P, Lembo PM, Albert PR. Distinct roles for Gαi2, Gαi3, and Gβγ in modulation of forskolin- or Gs-mediated cAMP accumulation and calcium mobilization by dopamine D2S receptors. J Biol Chem. 1999;274:9238–9245. doi: 10.1074/jbc.274.14.9238. [DOI] [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Ann Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gregerson KA, Flagg TP, O’Neill TJ, Anderson M, Lauring O, Horel JS, Welling PA. Identification of G protein-coupled, inward rectifier potassium channel gene products from the rat anterior pituitary gland. Endocrinology. 2001;142:2820–2832. doi: 10.1210/endo.142.7.8236. [DOI] [PubMed] [Google Scholar]
- Guiramand J, Montmayeur J-P, Crealine J, Bhatia M, Borrelli E. Alternative splicing of the dopamine D2 receptor directs specificity of coupling to G-proteins. J Biol Chem. 1995;270:7354–7358. doi: 10.1074/jbc.270.13.7354. [DOI] [PubMed] [Google Scholar]
- Guivarc’h D, Vincent J-D, Vernier P. Alternative splicing of the D2 dopamine receptor messenger ribonucleic acid is mediated by activated sex steroid receptors in the MMQ prolactin cell line. Endocrinology. 1998;139:4213–4221. doi: 10.1210/endo.139.10.6246. [DOI] [PubMed] [Google Scholar]
- Ham J, Ivan M, Wynford-Thomas D, Scanlon MF. GH3 cells expressing constitutively active Gs alpha (Q227L) show enhanced hormone secretion and proliferation. Mol Cell Endocrinol. 1997;127:41–47. doi: 10.1016/s0303-7207(96)03987-1. [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Katzenellenbogen RA, Conn PM, Chin WW. Evidence that signaling pathways by which thyrotropin-releasing hormone and gonadotropin- releasing hormone act are both common and distinct. Mol Endocrinol. 1994;8:1038–1048. doi: 10.1210/mend.8.8.7527898. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Asa SL, Zhang G, Saez C, Bunzow JR, Allen RG, Hnasko R, Ben-Jonathan N, Grandy DK, Low MJ. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor- deficient mice. Neuron. 1997;19:103–113. doi: 10.1016/s0896-6273(00)80351-7. [DOI] [PubMed] [Google Scholar]
- Kimura N, Arai K, Sahara Y, Suzuki H, Kimura N. Estradiol transcriptionally and posttranscriptionally up-regulates thyrotropin-releasing hormone receptor messenger ribonucleic acid in rat pituitary cells. Endocrinology. 1994;134:432–440. doi: 10.1210/endo.134.1.8275956. [DOI] [PubMed] [Google Scholar]
- Laccarino C, Samad TK, Mathis C, Kercret H, Picetti R, Borrelli E. Control of lactotrop proliferation by dopamine: essential role of signaling through D2 receptors ERKs. Proc Soc Natl Acad Sci USA. 2002;99:14530–14535. doi: 10.1073/pnas.222319599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis C, Masters SB, Spada A, Pace AM, Bourne HR, Vallar I. GTPase inhibiting mutation activate the alpha chain of Gs and stimulate adenylate cyclase in human pituitary tumors. Nature. 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- Liu YF, Ghahremani MH, Rasenick MM, Jakobs KH, Albert PR. Stimulation of cAMP synthesis by Gi-coupled receptors upon ablation of distinct Gαi protein expression. Gi subtype specificity of the 5-HT1A receptor. J Biol Chem. 1999;274:16444–16450. doi: 10.1074/jbc.274.23.16444. [DOI] [PubMed] [Google Scholar]
- Liu YF, Jakobs KH, Rasenick MM, Albert PR. G protein specificity in receptor-effector coupling. Analysis of the roles of Go and Gi2 in GH4C1 pituitary cells. J Biol Chem. 1994;269:13880–13886. [PubMed] [Google Scholar]
- Livingstone JD, Lerant A, Freeman ME. Ovarian steroids modulate responsiveness to dopamine and expression of G-proteins in lactotropes. Neuroendocrinology. 1998;68:172–179. doi: 10.1159/000054363. [DOI] [PubMed] [Google Scholar]
- Majo G, Lorenzo MJ, Blasi J, Aguado F. Exocytotic protein components in rat pituitary gland after long-term estrogen administration. J Endocrinol. 1999;161:323–331. doi: 10.1677/joe.0.1610323. [DOI] [PubMed] [Google Scholar]
- Milligan G, Spiegel Am, Unson CG, Sagerson ED. Chemically induced hypothyroidism produces elevated amounts of the alpha subunit of the inhibitory guanine nucleotide binding protein (Gi) and the beta subunit common to all G-proteins. Biochem J. 1987;247:223–227. doi: 10.1042/bj2470223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montmayeur JP, Guiramand J, Borrelli E. Preferential coupling between dopamine D2 receptors and G proteins. Mol Endocrinol. 1993;7:161–170. doi: 10.1210/mend.7.2.7682286. [DOI] [PubMed] [Google Scholar]
- Oomizu S, Boyadjieva N, Sarkar DK. Ethanol and estradiol modulate alternative splicing of dopamine D2 receptor mRNA and abolish the inhibitory action of bromocriptine on prolactin release from the pituitary gland. Alcohol Clin Exp Res. 2003;27:975–980. doi: 10.1097/01.ALC.0000071743.57855.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross EM. Signal sorting and amplification through G protein-coupled receptors. Neuron. 1989;3:141–152. doi: 10.1016/0896-6273(89)90027-5. [DOI] [PubMed] [Google Scholar]
- Saiardi A, Bozzi Y, Baik JH, Borrelli E. Antiproliferative role of dopamine: loss of D2 receptors causes hormonal dysfunction and pituitary hyperplasia. Neuron. 1997;19:115–126. doi: 10.1016/s0896-6273(00)80352-9. [DOI] [PubMed] [Google Scholar]
- Sarkar DK. Genesis of prolactinomas: studies using estrogen-treated animals. Front Horm Res. 2006;35:32–49. doi: 10.1159/000094307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar DK, Gottschall PE, Meites J. Damage to hypothalamic dopaminergic neurons is associated with development of prolactin-secreting tumors. Science. 1982;218:684–686. doi: 10.1126/science.7134966. [DOI] [PubMed] [Google Scholar]
- Senogles SE. The D2 dopamine receptor isoforms signal through distinct Giα proteins to inhibit adenylyl cyclase. A study with site-directed mutant Giα proteins. J Biol Chem. 1994;269:23120–23127. [PubMed] [Google Scholar]
- Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Spiegel AM. Mutation in G protein-coupled receptor in endocrine disease. J Clin EndocrinolMetab. 1996;8:2434–2442. doi: 10.1210/jcem.81.7.8675557. [DOI] [PubMed] [Google Scholar]
- Spiegel AM, Weinstein LS, Shenker A. Abnormalities in G protein-coupled signal transduction pathways in human disease. J Clin Invest. 1993;92:1119–1125. doi: 10.1172/JCI116680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar L, Meldolesi J. Mechanisms of signal transduction at the dopamine D2 receptor. Trends Pharmacol Sci. 1989;10:74–77. doi: 10.1016/0165-6147(89)90082-5. [DOI] [PubMed] [Google Scholar]
- Vallar L, Spada A, Giannattasio G. Altered Gs and adenylate cyclase activity in human GH-secreting pituitary adenomas. Nature. 1987;330:566–568. doi: 10.1038/330566a0. [DOI] [PubMed] [Google Scholar]
- Wilkie TM, Gilbert DJ, Olsen AS, Chen XN, Amatruda TT, Korenberg JR, Trask BJ, de Jong P, Reed RR, Simon MI, Jenkins NA, Copeland NG. Evolution of the mammalian G protein α subunit multigene family. Nat Genet. 1992;2:85–91. doi: 10.1038/ng0592-85. [DOI] [PubMed] [Google Scholar]
- Yajima Y, Uchino K, Ito H, Kawashima S. Mastoparan-stimulated prolactin secretion in rat pituitary GH3 cells involves activation of Gq/11 proteins. Endocrinology. 1997;138:1949–1958. doi: 10.1210/endo.138.5.5111. [DOI] [PubMed] [Google Scholar]