Abstract
We demonstrate color and monochrome on-chip imaging of Caenorhabditis elegans samples over a wide field-of-view using incoherent lensless in-line holography. Digital reconstruction of the recorded lensless holograms rapidly creates the C. elegans images within <1 s over a field-of-view of >24 mm2. By digitally combining the reconstructed images at three different wavelengths (red, green and blue), color images of dyed samples are also acquired. This wide field-of-view and compact on-chip imaging modality also permits straightforward integration with microfluidic systems.
Caenorhabditis elegans (C. elegans) has been extensively studied as a model organism to better understand the underlying mechanisms of various human diseases.1 It has several important features that justify this widespread use. First, C. elegans is easy to culture in laboratory environments, growing and reproducing rapidly and cost-effectively. Further, it is a transparent, optically accessible organism with well developed nervous and reproductive systems, intestine, skin and muscles. As a result, high-throughput phenotypical characterization of C. elegans samples, which primarily involves optical imaging in the form of conventional microscopy, has led to important discoveries in biomedical research and specifically in drug discovery, significantly impacting genetics,2 oncology3 and neurobiology.4,5 Motivated by these advances, various high-throughput platforms were demonstrated for phenotypical screening of this model organism.6–9 In these existing systems, phenotype characterization relies on conventional lens-based light microscopy, which has a limited imaging field-of-view, despite the use of bulky and expensive objective-lenses.
Here we demonstrate color and monochrome on-chip imaging of C. elegans samples using an alternative optical microscopy platform that is especially suitable for high-throughput screening applications, also offering straightforward integration with microfluidics. When compared to the state of the art, our approach does not utilize any lenses, lasers or other bulky optical components or any mechanical scanning, making it highly compact and simple to use. Our on-chip imaging approach is based on incoherent lensless in-line holography10 (see Fig. 1) which provides a significantly larger field-of-view (FOV), permitting simultaneous imaging of C. elegans samples over an area of >24 mm2, i.e., ~10 fold larger than a typical 10× objective-lens FOV. Digital reconstruction of the recorded lensless holograms rapidly creates the C. elegans images within less than 1 s; and by digitally combining these reconstructed images at three different wavelengths (red, green and blue) color images of the samples that are labeled with functional dyes are also acquired. Furthermore, this lensless holographic microscope requires almost no sensitive optical alignment, making it quite easy to operate, even for non-technical personnel. Through its integration with micro-fluidic systems, this lensless on-chip imaging platform, being much simpler and more compact than conventional optical microscopes, together with a significantly larger FOV, could especially be important for high-throughput imaging of C. elegans samples providing a new tool for biomedical research in various fields including genetics, oncology and neurobiology.
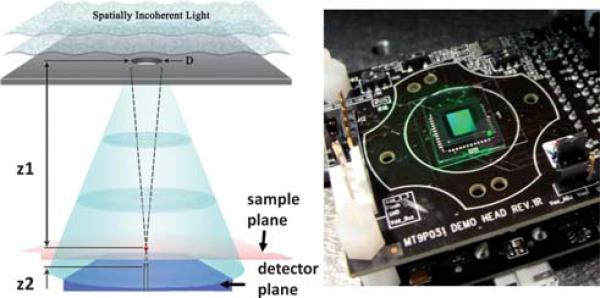
Fig. 1.
(left) Schematic diagram of the incoherent lensfree holographic microscope that is used for imaging of C. elegans samples on a chip. A pinhole with a diameter of D (50–100 μm) is illuminated with a spatially incoherent light source. z1 (5–10 cm) and z2 (~1 mm) are the aperture to sample and sample to sensor distances, respectively, where z1 >> z2. (right) A monochrome CMOS sensor array with a pixel size of 2.2 μm was used for recording lensless holograms presented in this paper.
Fig. 1 illustrates the schematic diagram of our lensfree holographic imaging system. A spatially incoherent light source (from a monochromator with a relatively broad bandwidth of ~15 nm) is filtered through an aperture (50–100 μm), situated z1 = 5–10 cm above the sample plane. C. elegans samples, either located within a microfluidic channel or simply on a cover-glass, are placed with z2 < 1–2 mm distance to the sensor-array. The incoherent illumination light at the pinhole plane picks up partial spatial coherence as it propagates adistanceof z1, and attains a coherence diameter of 0.5–1 mm at the sample plane.11 As a result of this, the scattered waves from the worms can interfere with the unperturbed background wave (i.e. reference wave), which permits digital recording of lensless in-line holograms of each C. elegans worm, individually. The fact that the coherence diameter at the sample plane is much smaller than the entire FOV (>24 mm2) does not pose a limitation, since each C. elegans worm is within a coherence diameter, hence is “effectively” illuminated by a coherent plane wave. When compared to existing in-line holographic imaging platforms,12 the presented incoherent approach has a rather un-conventional hologram recording geometry that has orders of magnitude larger z1/z2 ratio, a unit fringe magnification and an unusually large source aperture (~50 to 100 μm). These striking differences form the key to achieve lensless holographic imaging over a wide FOV of >24 mm2, while using an incoherent source without the need for any mechanical alignment or light-coupling optics. This also enables significant reduction of speckle noise and undesired interference effects among worms of the same FOV. The trade-off for these advantages is that the pixel size of the sensor chip now starts to be the bottleneck for spatial resolution, resulting in a resolution of ~1.5 μm.10 However, as we illustrate in this manuscript, the reconstructed lensfree C. elegans images still permit an imaging performance comparable to a 10× objective-lens (NA ≈ 0.2), while improving the FOV by ~10 fold, without the use of any lenses or mechanical scanning.
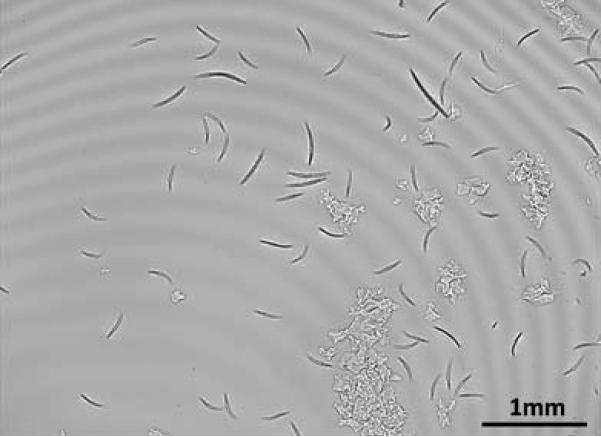
To illustrate its proof of concept, Fig. 2 shows a full FOV digital hologram recorded with this lensless on-chip imaging system. Through digital reconstruction of the captured holograms, all the C. elegans worms within this large FOV can be simultaneously imaged and tracked for long-term observation of the animals. This reconstruction process involves propagating the holographic fields back and forth between the sensor and sample planes using iterative phase recovery methods;10,13,14 and with a state of the art graphics processing unit (GPU; e.g., NVIDIA GeForce GTX 285), we have made this digital computation >40× faster than a typical CPU, making the reconstructions converge within <1 s over the entire FOV of >24 mm2.
Fig. 2.
A lensfree hologram that is captured with the incoherent holographic microscope of Fig. 1 is shown. Digital processing of this raw hologram permits simultaneous on-chip imaging of C. elegans samples over >24 mm2 FOV—see Fig. 3. Using a state of the art GPU, this digital reconstruction process takes <1 s over the entire imaging FOV.
Fig. 3 illustrates the imaging performance of the system for two different live C. elegans worms. For the details of the imaging conditions refer to Fig. 1 and 2. For sample preparation, a small volume of nematode growth medium (NGM) containing the worms was extracted from the culture. The NGM was suspended in DI water. After a gentle vortex and centrifugation step, the worms were then extracted with a pipette, and sandwiched between two cover glasses. This step ensured immobilization of the worms due to mechanical pressure, without any treatment with immobilizing drugs. To avoid image blur in our images, similar to a conventional microscope, such an immobilization step was utilized.
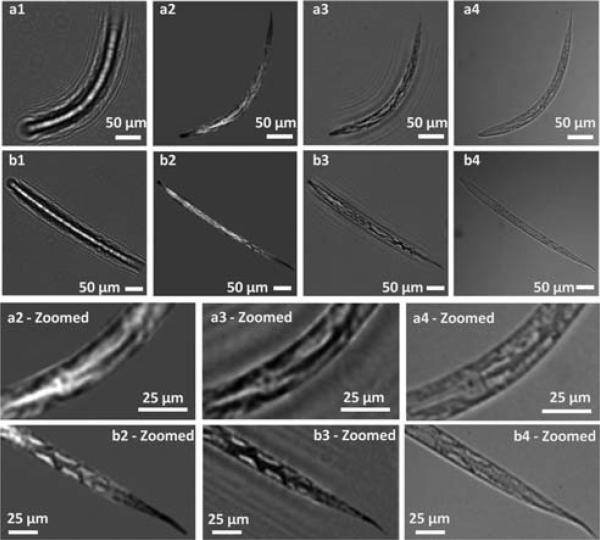
Fig. 3.
Incoherent lensfree on-chip imaging of two different C. elegans samples. (a1 and b1) Raw lensless holograms captured by the sensorarray. (a2 and b2) Intensity images of the reconstructed holograms using method 1. (a3 and b3) Intensity images of the reconstructed holograms using method 2. (a4 and b4) Conventional 10× (NA = 0.2) transmission microscope images of the same samples for comparison. The bottom two rows show the zoomed-in images to better illustrate the comparison. Experimental parameters: z1 = 10 cm, z2 = 1 mm, D = 50 μm, spatially incoherent light source at λ = 500 nm with a bandwidth of ~15 nm. Exposure time ≈ 30 ms.
In Fig. 3, (a1) and (b1) show the raw holograms that are digitally cropped from the larger FOV, whereas (a2), (b2) and (a3), (b3) show the intensity of the reconstructed images using two different digital reconstruction methods. Both of these methods recover the lost hologram phase through iterative constraint algorithms. Method #1 is based on a twin-image elimination algorithm developed for inline holography13 that utilizes the fact that the object information is present in two different planes, namely the real and virtual image planes. On the other hand, method #2 is a more general phase recovery technique,14 which does not necessarily require a hologram as input, and can work for the recovery of any complex optical field. As illustrated in Fig. 3, method #1 has stronger twin-image elimination capabilities while method #2 is more reliable for recording geometries with large Fresnel numbers (>10). To better illustrate the imaging performance of the system, Fig. 3 also provides zoomed images of the worms. These reconstructed images agree very well with transmission microscope images (a4) and (b4) that are obtained by a 10× (NA = 0.2) objective-lens using a regular microscope.
Fig. 4 further illustrates the imaging performance of the lensless holographic on-chip imaging system under different illumination wavelengths and spectral bandwidths (BWs). The results are shown only for method #1 since similar results are also obtained for method #2. The reconstructed images exhibit quite strong contrast to resolve the fine spatial features of the C. elegans samples in all cases. This figure illustrates that our imaging modality is not hungry for temporal coherence of the source, such that a narrow-band low-power light-emitting diode (LED) would also yield similar reconstruction results. Moreover, Fig. 4 also suggests that our system performs equally well under different illumination colors. This brings a quite valuable flexibility and functionality to the presented setup, especially for color imaging of stained C. elegans samples under multi-wavelength illumination as will be demonstrated next.
Fig. 4.
Reconstruction results (based on method 1) of the C. elegans sample shown in Fig. 3(b) are illustrated with respect to two different illumination wavelengths and two different spectral bandwidths (BWs). All the other imaging conditions are the same as in Fig. 3. For comparison purposes, a microscope image of the same sample is shown in Fig. 3(b4). Exposure times of the raw holographic images are ~200 ms and ~30 ms for 5 nm and 15 nm bandwidth illumination, respectively. Scale bars 50 μm.
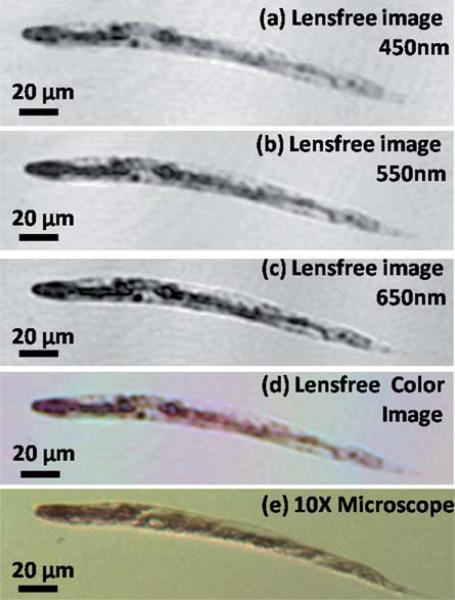
For various applications, the phenotype characterization is achieved by staining of the C. elegans samples with functional dyes,15 and therefore color imaging is significant for C. elegans imaging. Towards this end, we also demonstrate the color imaging capability of our lensless on-chip imaging platform using C. elegans samples stained with Ponceau S red stain, which mostly marks the proteins in the middle and tail sections of the worms. After the staining steps, by sequentially illuminating the samples with incoherent light at 450 nm (blue), 550 nm (green) and 650 nm (red), three holograms were obtained using the same lensfree set-up of Fig. 1. Fig. 5(a), (b) and (c) show the separately reconstructed intensity images at each one of these wavelengths for a given worm. By digitally fusing these reconstructed images at blue, green and red illumination wavelengths, a pseudo-color image is obtained as shown in Fig. 5(d), which clearly reveals the red stained regions and compares successfully to the microscope image given in Fig. 5(e).
Fig. 5.
(a, b, and c) Digitally reconstructed holographic images of a stained C. elegans sample using Ponceau S red stain, captured with illumination wavelengths at 450 nm, 550 nm and 650 nm, respectively (FWHM ≈ 15 nm in each case). (d) Lensfree color image obtained by fusing the reconstructions at each wavelength. (e) Brightfield microscope image obtained with a 10× objective-lens for comparison purposes.
In conclusion, we have demonstrated color and monochrome lensfree on-chip imaging of C. elegans samples over a large FOV. Using incoherent lensless in-line holography, diffraction holograms of C. elegans samples are recorded on a chip, where digital reconstruction of these lensless holograms rapidly creates the sample image over an FOV of >24 mm2, which constitutes ~10 fold improvement with respect to the FOV of a typical 10× objective-lens. Employing multi-wavelength illumination, we also demonstrated the color imaging performance of the system using C. elegans samples dyed with Ponceau S red stain.
Acknowledgements
We acknowledge the support of the Okawa Foundation, Vodafone Americas Foundation, DARPA DSO (under 56556-MS-DRP), NSF (under awards # 0754880 and 0930501), NIH (under 1R21EB009222-01 and the NIH Director's New Innovator Award—award number DP2OD006427 from the Office of the Director, National Institutes of Health), AFOSR (under Project # 08NE255). A. Ozcan also gratefully acknowledges the support of the Office of Naval Research (ONR) under Young Investigator Award 2009. Finally, we also acknowledge Askin Kocabas of Harvard University for his kind assistance with the samples.
References
- 1.Kaletta T, Hengartner MO. Nat. Rev. Drug Discovery. 2006;5:387–399. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- 2.Barr MM, Sternberg PW. Nature. 1999;401:386–389. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- 3.Hara M, Han M. Proc. Natl. Acad. Sci. U. S. A. 1995;92:3333–3337. doi: 10.1073/pnas.92.8.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H, Rogers MJ, Richmond JE, McIntire SL. Nature. 2004;430:891–896. doi: 10.1038/nature02798. [DOI] [PubMed] [Google Scholar]
- 5.Yanik MF, Cinar H, Cinar HN, Chisholm AD, Jin Y, Yakar AB. Nature. 2004;432:822. doi: 10.1038/432822a. [DOI] [PubMed] [Google Scholar]
- 6.Rohde CB, Zeng F, Rubio RG, Angel M, Yanik MF. Proc. Natl. Acad. Sci. U. S. A. 2007;104:13891–13895. doi: 10.1073/pnas.0706513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crane MM, Chung K, Lu H. Lab Chip. 2009;9:38–40. doi: 10.1039/b813730g. [DOI] [PubMed] [Google Scholar]
- 8.Burns AR, Kwok TCY, Howard A, Houston E, Johanson K, Chan A, Cutler SR, McCourt P, Roy PJ. Nat. Protoc. 2006;1:1906–1914. doi: 10.1038/nprot.2006.283. [DOI] [PubMed] [Google Scholar]
- 9.Dupuy D, Bertin N, Hidalgo CA, Venkatesan K, Tu D, Lee D, Rosenberg J, Svrzikapa N, Blanc A, Carnec A, Carvunis AR, Pulak R, Shingles J, Hoyes JR, Newbury RH, Viveiros R, Mohler WA, Tasan M, Roth FP, Le Peuch C, Hope IA, Johnsen R, Moerman DG, Barabasi AL, Baillie D, Vidal M. Nat. Biotechnol. 2007;25:663–668. doi: 10.1038/nbt1305. [DOI] [PubMed] [Google Scholar]
- 10.Isikman SO, Seo S, Sencan I, Erlinger A, Ozcan A. Lensfree cell holography on a chip: From holographic cell signatures to microscopic reconstruction. IEEE-LEOS Annual Meeting Conference Proceedings; Antalya, Turkey. 2009; pp. 404–405. DOI: 10.1109/LEOS.2009.5343233. [Google Scholar]
- 11.Brady D. Optical Imaging and Spectroscopy. Wiley-OSA; Hoboken, NJ, USA: 2009. pp. 190–193. ch. 6. [Google Scholar]
- 12.Xu W, Jericho MH, Meinertzhagen IA, Kreuzer JH. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11301–11305. doi: 10.1073/pnas.191361398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koren G, Polack F, Joyeux D. J. Opt. Soc. Am. A. 1993;10:423–433. [Google Scholar]
- 14.Fienup JR. Opt. Lett. 1978;3:27–29. doi: 10.1364/ol.3.000027. [DOI] [PubMed] [Google Scholar]
- 15.Joung JK, Ramm EI, Pabo CO. Proc. Natl. Acad. Sci. U. S. A. 2000;97:7382–7387. doi: 10.1073/pnas.110149297. [DOI] [PMC free article] [PubMed] [Google Scholar]