Abstract
Cancer is a complex and highly dynamic process. Genetically engineered mouse models (GEMs) that develop cancer are essential systems for dissecting the processes that lead to human cancer. These animal models provide a means to determine the causes of malignancy and to develop new treatments, thus representing a resource of immense potential for medical oncology. The sophistication of modeling cancer in mice has increased to the extent that now we can induce, study and manipulate the cancer disease process in a manner that is impossible to perform in human patients. However, all GEMs described so far have diverse shortcomings in mimicking the hierarchical structure of human cancer tissues. In recent years, a more detailed picture of the cellular and molecular mechanisms determining the formation of cancer has emerged. This Commentary addresses new experimental approaches toward a better understanding of carcinogenesis and discusses the impact of new animal models.
Introduction: the need to reproduce human cancer in the mouse
The dilemma of current cancer therapies is that, although most cancer patients respond to therapy, only a few are definitely cured (Etzioni et al., 2003). Current cancer therapies are designed to target proliferating tumor cells. Although such strategies eliminate the visible portion of the tumor, namely the tumor mass, they mostly fail to eliminate the unseen root of cancer (Sanchez-Garcia et al., 2007). In order to study and accurately solve the complex host-tumor interactions that occur during tumor development, it is necessary to perform experiments in an in vivo setting in which the neoplasm emerges in the appropriate microenvironment. Research in mice integrates the complexity of the organs and their different cell types within the context of the global physiological status of the organism. Certain strains of mice develop cancer spontaneously (Hardisty, 1985). However, such models develop a restricted subset of tumor types that do not reflect the common forms of human cancer and do not allow the systematic investigation of tumor genetics and gene-environment interactions. Since the discovery that human tumors contain activated oncogenes (Fig. 1A), many efforts have been made to develop organ-specific cancer mouse models where tumors arise from normal cells that are resident in their natural tissue microenvironments in the context of intact immune systems. The ultimate goal is to be able to mimic, in the mouse, the entire molecular, cellular, tissular and organic features of human cancers, including their initiation, progression, evolution, response to therapy, and eventual cure or relapse. Of course, this is a vicious circle, since there are many things about human cancer that we still do not understand, so how can we possibly try to reproduce them? However, we believe that the quest for the best animal models will be precisely where the answers to many of the unsolved questions about cancer will be found, and that the vicious circle will become a virtuous one, since animal models will provide an invaluable feedback to our understanding of cancer in the human.
Fig. 1.
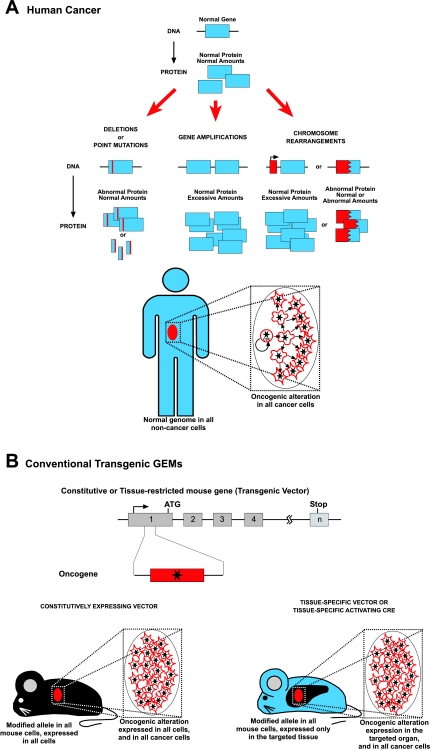
Main molecular mechanisms of human cancer and traditional mouse cancer models. (A) Human cancer is a genetic disease that can originate from several possible types of alterations affecting the structure and/or number of oncogenes or tumor suppressor genes. Independently of the nature of the oncogenic insult, all human tumor cells carry the oncogenic alteration, from the cell of origin to the more differentiated cancer cells, although the role of this oncogene may be different at different stages of tumor differentiation, and these mutations might become carrier mutations rather than driving ones depending on the cellular context. CSC, cancer stem cells. (B) In the classical transgenic mouse models of cancer, the oncogene is expressed under the control of a gene that can be either constitutively expressed or, alternatively, tissue restricted. In both cases, all of the cells in the mouse are genetically modified. In the first case, all of the cells express the oncogene. In the second case, the oncogene is expressed in all the cells of a certain chosen tissue. This rather uncontrolled oncogenic expression leads to the appearance of tumors that do not necessarily reproduce the hierarchical structure of human cancers.
Transgenic mice as model systems: the beginnings
The introduction of transgenic methodology in the cancer field showed that human oncogenes produce tumors when introduced into mouse genomic DNA from the germ line (Steward et al., 1982; Stewart et al., 1984; Adams et al., 1985; Hanahan, 1985; Leder et al., 1986). These seminal works showed that oncogene expression is not only required for the initiation of cancer, but also for the maintenance of the disease, which disappears again when the inducing stimulus is switched off (Chin et al., 1999; Huettner et al., 2000; Boxer et al., 2004; Perez-Caro et al., 2007). This has kept oncogenes firmly in focus as therapeutic targets (Fig. 1). However, in these early transgenic experiments, the phenotype was highly influenced by the choice of the attached expression cassette that regulates when and where the transgene is going to be expressed. Specifically, in the case of tissue-directed cassettes, they are used under the assumption that the main bulk of the cellular population that forms the tumor mass is also the relevant population in terms of tumor origin. This intuitive observation does not need to be true: erythrocytes are the most abundant cells in the blood, but they do not contribute at all to blood regeneration, nor do they carry any genetic information that is relevant to their function or origin. So, targeting oncogenes to specific differentiated cell types just because these cell types are the most abundant ones in the tumor mass does not need to recapitulate the ontogeny or even the structure of the tumor (Fig. 1B). Another technical artifact is that, unlike the human oncogenes, which occur sporadically in single cells during prenatal or postnatal development, these transgenic mice express the oncogene in all developing and/or adult cells in which the expression cassette is active.
Introducing oncogenes into embryonic stem (ES) cells to generate dominant mouse mutants: knock-in mouse models
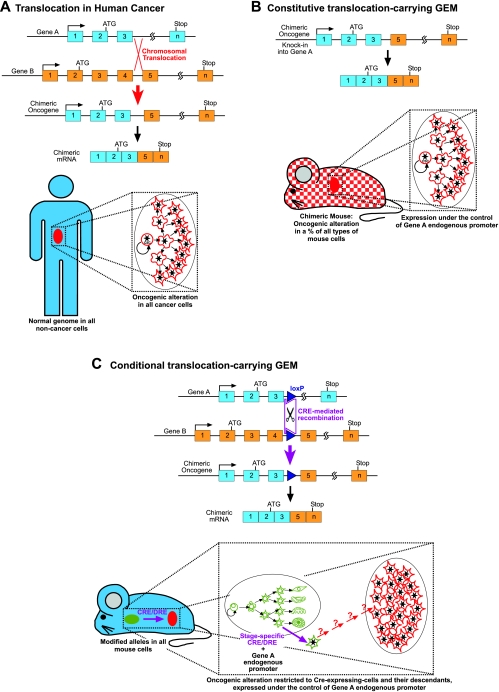
In order to express the initial oncogenic event in the correct cell type, one possibility would be to introduce the alteration in the specific locus of the genome where the wild-type version of the proto-oncogene or suppressor gene is located, using homologous recombination in embryonic stem (ES) cells followed by blastocyst injections to create chimeric mice (Fig. 2). In this way, only a single copy of the oncogene is expressed and a non-directed restriction for genome alteration is obtained, so that a limited number of cells in the organism undergo the genomic alteration, but they can be any type of cell. Therefore, if the mutant ES cells have a biased contribution to the embryo or animal, it can be very informative about the nature of the defect caused by the cancer gene. Chimera studies have also been useful in answering the question of whether the initial oncogenic mutation is sufficient in nature to induce the tumor phenotype (Castilla et al., 1996; Castellanos et al., 1997; Yergeau et al., 1997; Okuda et al., 1998; Castilla et al., 1999; Dobson et al., 1999). A chimera approach was used to investigate the biological role of the Bcr-ABLp190 and Mll-AF9 oncogenes (Fig. 2B) (Corral et al., 1996; Castellanos et al., 1997). Both studies demonstrated oncogenicity and lineage specificity in the chimeric mice. Despite the activity of the Bcr and Mll endogenous promoters in a variety of lineages, these mice only developed leukemias, the specific pathologies that these fusion genes are associated with in humans (Corral et al., 1996; Castellanos et al., 1997). Thus, these findings indicated that Bcr-ABLp190 and Mll-AF9 were sufficient to induce the tumor phenotype when expressed from the correct endogenous promoters. Similar studies were carried out with the Aml1-ETO and Cbfb-MYH11 fusions that are associated with myeloid leukemia (Castilla et al., 1996; Yergeau et al., 1997; Okuda et al., 1998; Castilla et al., 1999). However, in these cases, the modified ES cells could not contribute to the hematopoietic lineages and leukemia did not develop in the chimeric mice (Castilla et al., 1996; Castilla et al., 1999). Furthermore, all these knock-in models also presented other complications, mainly the fact that only the chimeras are viable, and the attempts to obtain heterozygous descendants through chimera germ line transmission systematically failed. Altogether, these data demonstrated that the oncogenicity of some of these fusion genes is restricted to the context of sporadically acquired mutations and cannot be reproduced through inherited germ line events.
Fig. 2.
Example of the mimicking of a complex human oncogenic alteration in the mouse. (A) Molecular mechanism of a human chromosomal translocation resulting in a chimeric oncogene. (B) Knock-in mouse model of chromosomal translocation. By homologous recombination in ES cells, one allele of gene A is modified to introduce the 3′-elements of gene B in order to mimic the rearrangement seen in humans. These ES cells are injected into wild-type (WT) blastocysts to generate chimeric mice that are composed of WT and genetically modified cells. Therefore, a percentage of the cells in every organ of the chimera carry the oncogenic alteration, which is expressed under the regulatory sequences of gene A, thus generating a model that very closely mimics the human case where tumoral cells are mixed in a background of normal cells. Unfortunately, most of these chimeric mice cannot produce viable knock-in offspring, indicating that fusion proteins are toxic for development. (C) Conditional, Cre-inducible translocation model: genes A and B are modified separately by homologous recombination in ES cells, and loxP sites (or the recently developed Dre-rox sites) are introduced at the precise points where chromosomal translocation happens in humans. F1 mice heterozygous for these modified genes are generated and crossed with a tissue-specific Cre (or Dre) recombinase. These mice carry the modified alleles in all cells and have no phenotype in the absence of recombinase. The oncogene is expressed under the regulatory sequences of gene A in all the cells expressing recombinase and their potential descendants. (The expression of the recombinase under differentiated cell promoters in differentiated cells leads to the appearance of tumors that do not necessarily reproduce the hierarchical structure of human cancers.)
Conditional knock-in mouse models
Overall, these studies suggested that the leukemia-initiating genetic events might occur regularly at the stem cell/progenitor level, irrespective of the phenotypic make-up of the bulk population of leukemic blasts. An explanation could be that the oncogene itself determines the differentiation program of the affected cell clone, which contrasts with the opinion that the leukemic phenotype is a reflection of the level of the hematopoietic hierarchy at which the genetic defect occurs. However, as mentioned previously, germ line mutations do not allow the correct modeling of sporadic cancer. A solution could be to restrict the genome alteration, either by limiting the type and/or number of cells that carry it, or by introducing the genetic alteration in a silent way that can be activated in a spatial- or temporal-specific manner. One way to achieve such a model is the use of an inducible and lineage-specific recombinase (Fig. 2C). The Cre recombinase of the P1 bacteriophage and the FLP recombinase of yeast have been the systems of choice for experiments in mammalian systems. Also, the recently developed Dre-rox system adds another set of efficient tools that will enable the generation of more sophisticated mouse models (Anastassiadis et al., 2009). Using these recombinase-based systems, recombination/excision results in the creation of specific inter- or intra-chromosomal rearrangements (Fig. 2C). Thus, completely normal mice carrying this altered allele in a heterozygous form can be established. If a transgene expressing the recombinase under the control of a tissue/cell type-specific promoter is introduced into this homozygous animal, it will rearrange both genes in the specifically designed tissue, rendering the cancer-inducing alteration functional. But, once again, the final cancer phenotype in these conditional knock-in mouse models (Johnson et al., 2001; Forster et al., 2003; Grippo et al., 2003; Coste et al., 2007; Guerra et al., 2007) is influenced by the tissue-specific nature of the cassette expressing the recombinase (Fig. 2C).
Stem cells as the cancer-initiating/propagating population
So clearly, until recently, the main weight of the efforts attempting to mimic cancer in the mouse have been put on the oncogene’s side, greatly overlooking the cellular origin of the tumor. This aspect has been largely taken for granted, always assuming that the phenotype of the mature tumor cells already implied that the closest non-pathological relatives to them would be the cells of origin. It is well established that cancer is a clonal disease that initiates in a single cell whose progeny make up the tumor. However, the nature of the cell in which the initiating mutation occurred in human cancer has received little attention during the last decades. In recent years, there is growing evidence that stem cells are the cells of origin for several types of cancer (Bonnet and Dick, 1997; Cobaleda et al., 2000; Reya et al., 2001; Weissman, 2005; Tan et al., 2006; Ailles and Weissman, 2007; Sanchez-Garcia et al., 2007; Cobaleda et al., 2008; Vicente-Duenas et al., 2009). An example is provided by chronic myelogenous leukemia (CML), a granulocytic disease. However, the BCR-ABL translocation, which is pathognomonic of this disease, does not arise in a granulocyte, but rather in a cell at the top of the hematopoietic differentiation tree (Jamieson et al., 2004). In agreement with this idea, recent findings suggest that a stem cell constitutes the target cell in an increasing number of human solid tumors (Al-Hajj et al., 2003; Singh et al., 2004; Wang et al., 2009).
Much of our current conceptualization of how tumorigenesis occurs in humans is influenced strongly by mouse models of cancer development (Sanchez-Martin et al., 2002; Quigley et al., 2009). Therefore, studies in mice in which the oncogenic alteration(s) is not directed to the specific cells of origin, as it normally occurs in most current mouse models, should be interpreted cautiously. The genetic alterations found in human cancer seem to occur during specific periods of time and are restricted to a few specific cells. In several cases, such as in the case of CML, the cancer cell of origin is a stem/progenitor cell, and this explains the stem cell properties that allow the cancer stem cells to maintain the tumor mass. However, there are also many cancers where, most probably, the cancer cell of origin is a differentiated cell (Cobaleda et al., 2007). In these cases, the combination of the reprogramming capabilities of the oncogenic alteration and the intrinsic plasticity of the target cell (i.e. its susceptibility to the reprogramming) determine the final outcome of a cancer stem cell. Since not all of the cells present the same susceptibility to reprogramming, and not all of the oncogenes possess the same reprogramming capacities (i.e. the ability to confer stem cell features to the target cell), the targeting of the oncogenic alteration to the wrong cellular compartment is likely to be a cause of failure in the generation of accurate mouse models of human cancer.
Potential solutions: stem cell-activated conditional knock-in mouse models
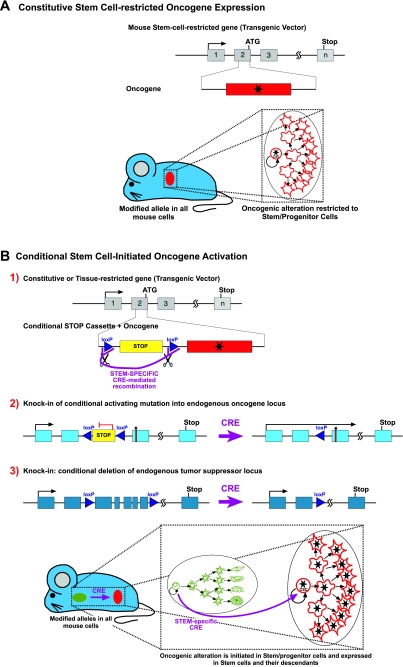
Considering these facts, three independent groups have already shown that the genotype-phenotype correlations found in human cancer can be established in mice by specific targeting of the stem cells (Barker et al., 2009; Perez-Caro et al., 2009; Zhu et al., 2009). In addition to this, it has also been shown that, in the hematopoietic (Eminli et al., 2009) and nervous (Kim et al., 2009) systems, the susceptibility of cells to reprogramming is inversely proportional to their degree of differentiation, and that hematopoietic stem cells (HSC) are 300 times more prone to be reprogrammed than B or T cells (Eminli et al., 2009). This stem cell reprogramming is indeed possible in the case of BCR–ABL-induced CML, showing that cancer stem cells arise through a reprogramming-like mechanism and suggesting that the oncogenes that initiate tumor formation might be dispensable for tumor progression (Fig. 3A) (Perez-Caro et al., 2009). Using the Sca-1 promoter as a stem cell-restricted transgenic expression system, the expression of the oncogene in the reprogramming-prone stem cells and progenitors allows the development of all of the cells that compose the tumor mass by a ‘hands-off’ mechanism. The modified gene is present in all of the mouse cells, but the oncogene expression is limited to the stem/progenitor compartment. This model is very informative with respect to the fact that the oncogenic mutations can have different roles in cancer stem cells versus differentiated cancer cells, and explains why targeted therapies like imatinib can eliminate the latter without affecting the former. However, once again these GEMs differ from the real human situation in the fact that, in the human, all the tumoral cells carry the oncogenic alteration (independently of the role that this alteration is playing at every stage) (Fig. 1A). So, clearly, refinements are required. In order to express cancer-initiating genetic defects randomly in the same target stem/progenitor cells in which the cancer mutations take place in humans, we should take advantage once more of conditional gene targeting approaches but, in this case, in combination with stem cell-specific promoters (Fig. 3B). Using different conditional modifications of oncogenes or tumor suppressor genes, in combination with a stem/progenitor-restricted recombinase, the oncogenic anomaly is initiated in stem cells and maintained in all their descendants, in a manner very similar to how it happens in humans. However, we should be cautious in interpreting the data, because mouse cells that are used to mimic human disease are more prone to transformation than human cells and, thus, one mutation can lead to full-blown cancer in the mouse transgenic model but not in humans. Furthermore, the regulation of certain genes/pathways might differ between mouse and human.
Fig. 3.
New approaches to reproduce the hierarchical structure of human cancer in the mouse. (A) Based on the reprogramming nature of oncogenes, it has been proven that restricting the expression of the oncogenic alterations to the stem cell compartment is all that is needed to recapitulate all the tumoral heterogeneity. Using a stem cell-restricted transgenic expression system, the expression of the oncogene in the reprogramming-prone stem cells and progenitors allows the development of all the cells that compose the tumor mass by a ‘hands-off’ mechanism. The modified gene is present in all the mouse cells but expression of the oncogene is limited to the stem/progenitor compartment. (B) Conditional activation of an oncogenic alteration from the stem cell onwards: by using a conventional transgene that can be activated by recombinase, with the regulatory sequences of a constitutive or tissue-restricted gene (B1); by modifying the locus of an oncogene by introducing a recombinase-inducible activating mutation (B2) or, by modifying the locus of a tumor suppressor to achieve a recombinase-mediated deletion (B3). In these three cases, in combination with a stem/progenitor-restricted recombinase, the oncogenic anomaly is initiated in stem cells and maintained in all their descendants in a manner that is very similar to how it happens in humans.
Outlook
The recent discoveries that crucial genetic events take place within somatic primitive cells in some human cancers have led to enthusiasm within the scientific community for generating cancer mouse models that accurately reflect the genotype-phenotype correlation seen in human cancer. These future mouse cancer models are needed as a source for dissecting the genomic pathways that feed these cancers and for the discovery of new therapeutic leads. The challenge of the next decade is to define cancers according to their unique molecular alterations and to treat them accordingly. These recent discoveries will allow the translation from modern genetic laboratory tools to advances that will improve the lives of cancer patients.
Acknowledgments
We thank all members of lab 13 at IBMCC for their helpful comments and constructive discussions on this project. Research in I.S.-G.’s group is supported partially by FEDER and by MICINN (SAF2006-03726 and SAF2009-08803), and by Junta de Castilla y León (CSI13A08 and proyecto Biomedicina 2009–2010), MEC OncoBIO Consolider-Ingenio 2010 (Ref. CSD2007-0017), an NIH grant (2R01 CA109335-04A1) and by a Group of Excellence Grant (GR15) from Junta de Castilla y León. Research in C.C.’s lab is supported partially by FEDER and by Fondo de Investigaciones Sanitarias (PI080164) and Junta de Castilla y León (SA060A09 and proyecto Biomedicina 2009–2010). Research at J.P.-L.’s lab is supported partially by FEDER and by Fondo de Investigaciones Sanitarias (PI070057), and by MICINN (PLE2009-O119), Junta de Castilla y León (SA078A09 and Proyecto Biomedicina 2009–2010) and Sandra Ibarra Foundation. Deposited in PMC for release after 12 months.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. (1985). The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318, 533–538 [DOI] [PubMed] [Google Scholar]
- Ailles LE, Weissman IL. (2007). Cancer stem cells in solid tumors. Curr Opin Biotechnol. 18, 460–466 [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. (2003). Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100, 3983–3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastassiadis K, Fu J, Patsch C, Hu S, Weidlich S, Duerschke K, Buchholz F, Edenhofer F, Stewart AF. (2009). Dre recombinase, like Cre, is a highly efficient site-specific recombinase in E. coli, mammalian cells and mice. Dis Model Mech. 2, 508–515 [DOI] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. (2009). Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611 [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. (1997). Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 3, 730–737 [DOI] [PubMed] [Google Scholar]
- Boxer RB, Jang JW, Sintasath L, Chodosh LA. (2004). Lack of sustained regression of c-MYC-induced mammary adenocarcinomas following brief or prolonged MYC inactivation. Cancer Cell 6, 577–586 [DOI] [PubMed] [Google Scholar]
- Castellanos A, Pintado B, Weruaga E, Arevalo R, Lopez A, Orfao A, Sanchez-Garcia I. (1997). A BCR-ABL(p190) fusion gene made by homologous recombination causes B-cell acute lymphoblastic leukemias in chimeric mice with independence of the endogenous bcr product. Blood 90, 2168–2174 [PubMed] [Google Scholar]
- Castilla LH, Wijmenga C, Wang Q, Stacy T, Speck NA, Eckhaus M, Marin-Padilla M, Collins FS, Wynshaw-Boris A, Liu PP. (1996). Failure of embryonic hematopoiesis and lethal hemorrhages in mouse embryos heterozygous for a knocked-in leukemia gene CBFB-MYH11. Cell 87, 687–696 [DOI] [PubMed] [Google Scholar]
- Castilla LH, Garrett L, Adya N, Orlic D, Dutra A, Anderson S, Owens J, Eckhaus M, Bodine D, Liu PP. (1999). The fusion gene Cbfb-MYH11 blocks myeloid differentiation and predisposes mice to acute myelomonocytic leukaemia. Nat Genet. 23, 144–146 [DOI] [PubMed] [Google Scholar]
- Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N, Shen Q, O’Hagan R, Pantginis J, Zhou H, et al. (1999). Essential role for oncogenic Ras in tumour maintenance. Nature 400, 468–472 [DOI] [PubMed] [Google Scholar]
- Cobaleda C, Gutierrez-Cianca N, Perez-Losada J, Flores T, Garcia-Sanz R, Gonzalez M, Sanchez-Garcia I. (2000). A primitive hematopoietic cell is the target for the leukemic transformation in human philadelphia-positive acute lymphoblastic leukemia. Blood 95, 1007–1013 [PubMed] [Google Scholar]
- Cobaleda C, Jochum W, Busslinger M. (2007). Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature 449, 473–477 [DOI] [PubMed] [Google Scholar]
- Cobaleda C, Cruz JJ, Gonzalez-Sarmiento R, Sanchez-Garcia I, Perez-Losada J. (2008). The emerging picture of human breast cancer as a stem cell-based disease. Stem Cell Rev. 4, 67–79 [DOI] [PubMed] [Google Scholar]
- Corral J, Lavenir I, Impey H, Warren AJ, Forster A, Larson TA, Bell S, McKenzie AN, King G, Rabbitts TH. (1996). An Mll-AF9 fusion gene made by homologous recombination causes acute leukemia in chimeric mice: a method to create fusion oncogenes. Cell 85, 853–861 [DOI] [PubMed] [Google Scholar]
- Coste I, Freund JN, Spaderna S, Brabletz T, Renno T. (2007). Precancerous lesions upon sporadic activation of beta-catenin in mice. Gastroenterology 132, 1299–1308 [DOI] [PubMed] [Google Scholar]
- Dobson CL, Warren AJ, Pannell R, Forster A, Lavenir I, Corral J, Smith AJ, Rabbitts TH. (1999). The mll-AF9 gene fusion in mice controls myeloproliferation and specifies acute myeloid leukaemogenesis. EMBO J. 18, 3564–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eminli S, Foudi A, Stadtfeld M, Maherali N, Ahfeldt T, Mostoslavsky G, Hock H, Hochedlinger K. (2009). Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat Genet. 41, 968–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, Radich J, Anderson G, Hartwell L. (2003). The case for early detection. Nat Rev Cancer 3, 243–252 [DOI] [PubMed] [Google Scholar]
- Forster A, Pannell R, Drynan LF, McCormack M, Collins EC, Daser A, Rabbitts TH. (2003). Engineering de novo reciprocal chromosomal translocations associated with Mll to replicate primary events of human cancer. Cancer Cell 3, 449–458 [DOI] [PubMed] [Google Scholar]
- Grippo PJ, Nowlin PS, Demeure MJ, Longnecker DS, Sandgren EP. (2003). Preinvasive pancreatic neoplasia of ductal phenotype induced by acinar cell targeting of mutant Kras in transgenic mice. Cancer Res. 63, 2016–2019 [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. (2007). Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11, 291–302 [DOI] [PubMed] [Google Scholar]
- Hanahan D. (1985). Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature 315, 115–122 [DOI] [PubMed] [Google Scholar]
- Hardisty JF. (1985). Factors influencing laboratory animal spontaneous tumor profiles. Toxicol Pathol. 13, 95–104 [DOI] [PubMed] [Google Scholar]
- Huettner CS, Zhang P, Van Etten RA, Tenen DG. (2000). Reversibility of acute B-cell leukaemia induced by BCR-ABL1. Nat Genet. 24, 57–60 [DOI] [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, et al. (2004). Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 351, 657–667 [DOI] [PubMed] [Google Scholar]
- Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. (2001). Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 410, 1111–1116 [DOI] [PubMed] [Google Scholar]
- Kim JB, Sebastiano V, Wu G, Arauzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, et al. (2009). Oct4-induced pluripotency in adult neural stem cells. Cell 136, 411–419 [DOI] [PubMed] [Google Scholar]
- Leder A, Pattengale PK, Kuo A, Stewart TA, Leder P. (1986). Consequences of widespread deregulation of the c-myc gene in transgenic mice: multiple neoplasms and normal development. Cell 45, 485–495 [DOI] [PubMed] [Google Scholar]
- Okuda T, Cai Z, Yang S, Lenny N, Lyu CJ, van Deursen JM, Harada H, Downing JR. (1998). Expression of a knocked-in AML1-ETO leukemia gene inhibits the establishment of normal definitive hematopoiesis and directly generates dysplastic hematopoietic progenitors. Blood 91, 3134–3143 [PubMed] [Google Scholar]
- Perez-Caro M, Gutierrez-Cianca N, Gonzalez-Herrero I, Lopez-Hernandez I, Flores T, Orfao A, Sanchez-Martin M, Gutierrez-Adan A, Pintado B, Sanchez-Garcia I. (2007). Sustained leukaemic phenotype after inactivation of BCR-ABLp190 in mice. Oncogene 26, 1702–1713 [DOI] [PubMed] [Google Scholar]
- Perez-Caro M, Cobaleda C, Gonzalez-Herrero I, Vicente-Duenas C, Bermejo-Rodriguez C, Sanchez-Beato M, Orfao A, Pintado B, Flores T, Sanchez-Martin M, et al. (2009). Cancer induction by restriction of oncogene expression to the stem cell compartment. EMBO J. 28, 8–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley DA, To MD, Perez-Losada J, Pelorosso FG, Mao JH, Nagase H, Ginzinger DG, Balmain A. (2009). Genetic architecture of mouse skin inflammation and tumour susceptibility. Nature 458, 505–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. (2001). Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 [DOI] [PubMed] [Google Scholar]
- Sanchez-Garcia I, Vicente-Duenas C, Cobaleda C. (2007). The theoretical basis of cancer-stem-cell-based therapeutics of cancer: can it be put into practice? BioEssays 29, 1269–1280 [DOI] [PubMed] [Google Scholar]
- Sanchez-Martin M, Rodriguez-Garcia A, Perez-Losada J, Sagrera A, Read AP, Sanchez-Garcia I. (2002). SLUG (SNAI2) deletions in patients with Waardenburg disease. Hum Mol Genet. 11, 3231–3236 [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. (2004). Identification of human brain tumour initiating cells. Nature 432, 396–401 [DOI] [PubMed] [Google Scholar]
- Steward TA, Wagner EF, Mintz B. (1982). Human beta-globin gene sequences injected into mouse eggs, retained in adults, and transmitted to progeny. Science 217, 1046–1048 [DOI] [PubMed] [Google Scholar]
- Stewart TA, Pattengale PK, Leder P. (1984). Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell 38, 627–637 [DOI] [PubMed] [Google Scholar]
- Tan BT, Park CY, Ailles LE, Weissman IL. (2006). The cancer stem cell hypothesis: a work in progress. Lab Invest. 86, 1203–1207 [DOI] [PubMed] [Google Scholar]
- Vicente-Duenas C, Perez-Caro M, Abollo-Jimenez F, Cobaleda C, Sanchez-Garcia I. (2009). Stem-cell driven cancer: “hands-off” regulation of cancer development. Cell Cycle 8, 1314–1318 [DOI] [PubMed] [Google Scholar]
- Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM. (2009). A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461, 495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman IL. (2005). Normal and neoplastic stem cells. Novartis Found Symp. 265, 35–50; discussion 50,–54, 92–97 [PubMed] [Google Scholar]
- Yergeau DA, Hetherington CJ, Wang Q, Zhang P, Sharpe AH, Binder M, Marin-Padilla M, Tenen DG, Speck NA, Zhang DE. (1997). Embryonic lethality and impairment of haematopoiesis in mice heterozygous for an AML1-ETO fusion gene. Nat Genet. 15, 303–306 [DOI] [PubMed] [Google Scholar]
- Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. (2009). Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 457, 603–607 [DOI] [PMC free article] [PubMed] [Google Scholar]