Abstract
The metabolic syndrome (MetS) is characterized by obesity concomitant with other metabolic abnormalities such as hypertriglyceridemia, reduced high-density lipoprotein levels, elevated blood pressure and raised fasting glucose levels. The precise definition of MetS, the relationships of its metabolic features, and what initiates it, are debated. However, obesity is on the rise worldwide, and its association with these metabolic symptoms increases the risk for diabetes and cardiovascular disease (among many other diseases). Research needs to determine the mechanisms by which obesity and MetS increase the risk of disease. In light of this growing epidemic, it is imperative to develop animal models of MetS. These models will help determine the pathophysiological basis for MetS and how MetS increases the risk for other diseases. Among the various animal models available to study MetS, mice are the most commonly used for several reasons. First, there are several spontaneously occurring obese mouse strains that have been used for decades and that are very well characterized. Second, high-fat feeding studies require only months to induce MetS. Third, it is relatively easy to study the effects of single genes by developing transgenic or gene knockouts to determine the influence of a gene on MetS. For these reasons, this review will focus on the benefits and caveats of the most common mouse models of MetS. It is our hope that the reader will be able to use this review as a guide for the selection of mouse models for their own studies.
Introduction
The clustering of several metabolic abnormalities within an individual was first discussed by Dr Reaven in his Banting lecture in 1988 (Reaven, 1988). Although this clinical phenotype has been given different names over the years (insulin resistance syndrome, Syndrome X), it is now most commonly referred to as the metabolic syndrome (MetS). The constellation of metabolic abnormalities and their ‘cut-off’ points are still under discussion; however, the most recent consensus statement provided by the International Diabetes Federation (IDF) defines MetS as central obesity PLUS any two of the following: elevated plasma triglyceride (TG) levels (≥150 mg/dl), reduced high-density lipoproteins (HDL) (<40 mg/dl for men and <50 mg/dl for women), increased blood pressure (≥130 mmHg systolic or ≥85 mmHg diastolic), or increased fasting plasma glucose (≥100 mg/dl). By this definition, 20–25% of the world’s population has MetS. Individuals with MetS have a twofold elevated risk of having a heart attack or stroke, and a fivefold increased risk of developing diabetes. Thus, it is crucially important that we combat MetS both in the clinic and at the bench.
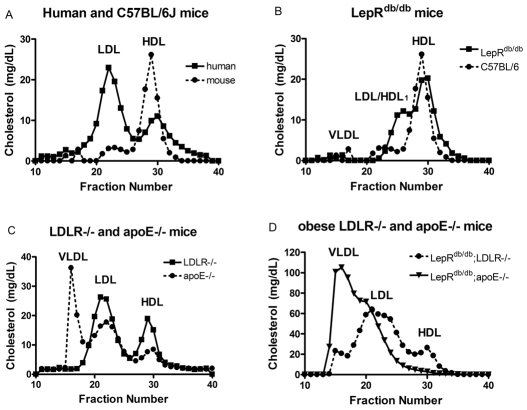
This review article will focus on the primary mouse models of MetS in use today that are available commercially. Clearly, mouse models differ from human disease in many different aspects. Cutoff points such as waist circumference cannot be transferred from humans to mice. In addition, normal mouse lipoprotein profiles have primarily atheroprotective HDL, whereas normal human lipoprotein profiles contain primarily atherogenic low-density lipoproteins (LDL) (Fig. 1A). Finally, the presence of hypertension in obese mice is inconsistent. Thus, no one mouse model can exactly mimic all aspects of human MetS. For the purpose of this review, we will define rodent MetS as obesity combined with dyslipidemia, hypertension and/or elevated glucose levels. Other things that might be of interest to consider in mouse models of MetS are systemic inflammation, hepatic steatosis and albuminuria; however, these characteristics will not be covered in the current review. In general, obesity and insulin resistance (IR) coincide in mouse models; however, there are some exceptions to this, i.e. lipodystrophy, which will also be discussed. We have attempted to include more extensive descriptions of the most commonly used models. We would also like to refer readers to the Jackson Laboratory Mouse Phenome Database (http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=docs/home), which contains invaluable phenotypic data on many different mouse strains.
Fig. 1.
Lipoprotein profiles different models. Lipoproteins were separated from plasma by running 100 μl over a Superose 6 column at a flow rate of 0.5 ml/min in buffer containing 0.15 M NaCl, 0.01 M Na2HPO4 and 1 mM EDTA. Forty 0.5 ml fractions were collected, and the cholesterol concentration in fractions 10–40 was assessed. Typically, VLDL elutes in fractions 15–20, LDL in fractions 21–27, and HDL in fractions 28–34. (A) Lipoprotein profiles comparing a ‘normal’ human profile with a C57BL/6J profile. (B) C57BL/6J and LepRdb/db mice. The LDL/HDL1 peak elutes in fractions 23–27. (C) Lipoprotein profiles of lean LDLR−/− and apoE−/− mice. (D) Lipoprotein profiles of obese LDLR−/− and apoE−/− mice.
Models of obesity and insulin resistance
The IDF criteria for defining MetS requires the presence of visceral obesity, with a waist circumference of >94 cm in males and >80 cm in females. In humans, central obesity is positively associated with peripheral IR leading to hyperinsulinemia (Banerji et al., 1999). Furthermore, a fasting plasma glucose concentration of greater than 100 mg/dl is also recognized by the IDF as a defining characteristic of MetS. Through the use of mouse models, the pathophysiology by which obesity leads to the development of IR can be investigated. Similar to humans, elevations in abdominal fat and IR have been demonstrated in diet-induced and genetic mouse models of MetS.
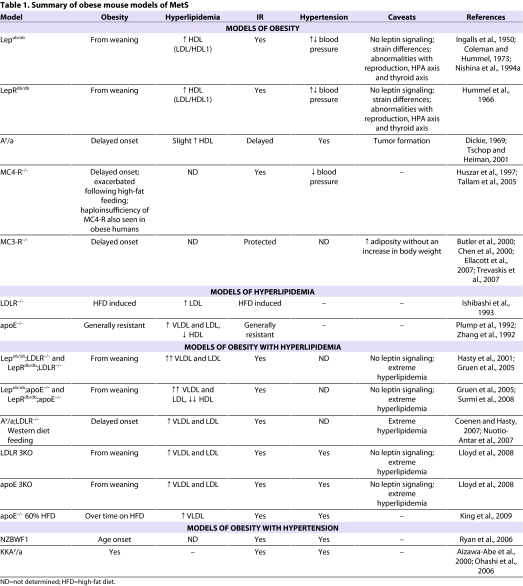
Many of the mouse models most commonly recognized in the study of obesity arose from spontaneous mutations. Among these are leptin-deficient (Lepob/ob), leptin receptor-deficient (LepRdb/db) and lethal yellow agouti (Ay/a) mice (Table 1). The abbreviations Lepob/ob and LepRdb/db will be used in this article as they bring in elements of their traditional phenotypic, and their more recently discovered genetic, designations. Although their primary phenotype is obesity, these mice also display IR and some degree of dyslipidemia and, thus, can be considered models of MetS. Other spontaneous monogenic forms of obesity found in mice are the tub and fat mutations (for a review, see Carroll et al., 2004); however, these mice are less well characterized and are not as commonly used for studies of MetS. We will also discuss two genetic knockout models of obesity, the melanocortin 4 receptor (MC4-R) and melanocortin 3 receptor (MC3-R) knockout mice.
Table 1.
Summary of obese mouse models of MetS
Leptin-deficient mice
The Lepob/ob model arose from a spontaneous mutation at the Jackson Laboratory (Ingalls et al., 1950), but it wasn’t until 1994 that the gene mutated in Lepob/ob mice was identified and named ‘leptin’ by Dr Friedman’s group (Zhang et al., 1994). The Lepob/ob mice are visually obese by 4 weeks of age, and their growth curves do not plateau even at 12 months of age. On a chow diet, Lepob/ob mice can weigh greater than 100 grams, which is four times that of their littermate controls. The genetic background is an extremely important consideration for the use of Lepob/ob mice in studying MetS. On all backgrounds, leptin deficiency results in hyperphagia, reduced energy expenditure and extreme obesity. On the C57BL/6J background, Lepob/ob mice have mild hyperglycemia that is apparent transiently from 8 to 12 weeks of age. At this point, pancreatic β-cell compensation occurs and increased insulin levels bring glucose homeostasis under control (Genuth et al., 1971). By contrast, on the C57BL/KsJ background, Lepob/ob mice develop hyperglycemia and diabetes, with blood glucose levels being sustained at about 400 mg/dl, accompanied by only temporary elevations in insulin levels and followed by β-cell failure (Coleman and Hummel, 1973). Because of the development of overt diabetes, Lepob/ob mice on the C57BL/KsJ background reach their maximum weight at 3–4 months of age, after which they gradually lose weight and often die by 6 months of age (Coleman and Hummel, 1973). More recent studies of strain differences have shown that Lepob/ob mice on the BALB/cJ background have a 35–40% reduction in weight gain with elevations in fed insulin and plasma TG levels, whereas Lepob/ob mice on the FVB/N background have exacerbated IR compared with those on the C57BL/6J background (Qiu et al., 2001; Haluzik et al., 2004).
Lepob/ob mice have elevated plasma cholesterol levels; however, the elevation is in HDL rather than very low-density lipoprotein (VLDL) or LDL (Fig. 1B; please note that the figure represents a LepRdb/db mouse, however, the Lepob/ob mice have a similar profile). The excess HDL in Lepob/ob mice is larger than normal HDL, appears as a shoulder on the HDL peak (Nishina et al., 1994a), and has been labeled ‘LDL/HDL1’ (Silver et al., 1999). This LDL/HDL1 does not promote atherosclerotic lesion formation (Plummer and Hasty, 2008) and Lepob/ob mice are actually protected from diet-induced atherosclerosis compared with C57BL/6J controls (Nishina et al., 1994b; Plummer and Hasty, 2008). Therefore, when using Lepob/ob mice as a model for MetS, lipoprotein profiles should always be analyzed to determine whether changes in total plasma cholesterol levels are due to modulation of VLDL, LDL or HDL.
Lepob/ob mice also have pronounced metabolic abnormalities related to reproduction, the hypothalamic-pituitary-adrenal (HPA) axis and the thyroid axis. For example, Lepob/ob mice are infertile owing to the deficiency of leptin and can thus be difficult to obtain in sufficient numbers for metabolic studies. The role of leptin in regulating the HPA axis is well established (reviewed in Malendowicz et al., 2007), and the absence of leptin in Lepob/ob mice significantly impacts this important metabolic regulation system. These alterations in different neuroendocrine axes should be taken into account when selecting this animal model.
Leptin receptor-deficient mice
LepRdb/db mice are deficient in the leptin receptor. As with Lepob/ob mice, the obese and insulin-resistant phenotype of LepRdb/db mice depends upon their genetic background, with mice on the C57BL/KsJ background developing diabetes and mice on the C57BL/6J background only becoming insulin resistant (Hummel et al., 1966; Coleman, 1978). The metabolic profiles of LepRdb/db and Lepob/ob mice are nearly identical. Both models are obese, hyperinsulinemic, hyperglycemic (depending on the age and strain), and have elevated total cholesterol levels with a predominance of LDL/HDL1 particles (see Fig. 1B and details in section above). In addition, the abnormalities with reproduction, HPA axis and thyroid axis are seen in both models. The primary difference between the two models is that the LepRdb/db mice have dramatic elevations in circulating leptin concentrations that are proportional to their degree of adiposity, whereas the Lepob/ob mice are absent in circulating leptin. This difference can be capitalized upon in certain studies where exogenous leptin can be added. In addition, tissue or bone marrow transplants can be performed to study the effect of leptin on other cell types (Bodary et al., 2002; Bodary et al., 2005; Surmi et al., 2008).
Agouti lethal yellow mice
Several spontaneous mutations leading to placement of the agouti (a) gene under the control of constitutively active promoters result in ectopic ubiquitous expression of the agouti protein. These mice then display various coat colors and obesity phenotypes (Dickie, 1969). In the brain, the agouti protein competes against the anorexigenic factor α-melanocyte stimulating hormone (MSH) for binding to the melanocortin 4 receptor (MC4-R), acting as an antagonist of this signaling pathway (Yen et al., 1994). Thus, agouti mice display adult-onset obesity and IR owing to hyperphagia and hypoactivity (Tschop and Heiman, 2001), similar to what is seen in MC4-R−/− mice (see below) (Lu et al., 1994; Fan et al., 1997; Huszar et al., 1997). The agouti yellow obese (Ay/a) mouse model is one of the most commonly used and is generally on the C57BL/6J or KK backgrounds, with the phenotype being more extreme in the KK strain. Lipoprotein profiles of Ay/a mice are similar to those of Lepob/ob and LepRdb/db mice, although the LDL/HDL1 peak is not as pronounced (unpublished data). Obese Ay/a mice are hypertensive, possibly owing to the ability of the agouti protein to inhibit α-MSH action in the brain (Mark et al., 1999).
The Ay/a mice express a number of characteristics that are ideal for MetS studies. They are fertile until about 4 months of age, making it easier to obtain offspring in large numbers. Also, the delayed onset of obesity and the presence of intact leptin signaling are useful traits for MetS. However, as with the Lepob/ob and LepRdb/db mice, they are resistant to atherosclerotic lesion formation on a high-fat diet (Nishina et al., 1994b). It should also be noted that these mice are susceptible to tumor formation.
Melanocortin 4 receptor (MC4-R) null mouse
It is becoming clear that changes to homeostatic networks in the central nervous system (CNS) can have profound effects not only on weight regulation, but on other aspects of metabolism. For example, studies by Stafford and colleagues showed that modulation of neuropeptide Y (NPY) signaling in the brain profoundly influences hepatic TG production rates, resulting in an increase in VLDL secretion (Stafford et al., 2008). It has also been shown that an increase in brain glucose concentration can reduce plasma TG levels (Lam et al., 2007). The mechanism by which changes in CNS signaling modulate hepatic TG storage versus secretion may be mediated by the central melanocortin system (Nogueiras et al., 2007).
The MC4-R is expressed in a number of nuclei in the rodent brain that are associated with autonomic and neuroendocrine pathways (Mountjoy et al., 1994). The central melanocortin system is known to mediate many of the actions of the adipokine leptin and plays a crucial role in the central regulation of energy homeostasis (for a review, see Cone, 2005). Strikingly, alterations in the MC4R gene are the most common monogenic cause of obesity known in humans (Vaisse et al., 1998; Yeo et al., 1998; Hinney et al., 1999; Mergen et al., 2001; Marti et al., 2003), including recent genome-wide association studies (Meyre et al., 2009; Willer et al., 2009). Furthermore, mice that are deficient in MC4-R expression show many of the same phenotypic characteristics as humans with MC4R gene mutations (Farooqi et al., 2000; Farooqi et al., 2003; Greenfield et al., 2009). The MC4-R-deficient mouse has a behavioral obesity syndrome characterized by hyperphagia, hyperglycemia, hyperinsulinemia, hypometabolism, and increased lean mass and linear growth (Huszar et al., 1997). Circumventing the hyperphagia by restricting the food intake of the MC4-R−/− mice to the same level as wild-type controls (pair-feeding), reduces the adiposity in these animals; however, they still remain significantly heavier than their wild-type counterparts (Ste Marie et al., 2000) highlighting the role of the hypometabolism in this phenotype. The hyperinsulinemia in this model is partially independent of obesity, as young MC4-R-deficient animals have been shown to have elevated circulating insulin levels prior to the onset of obesity (Fan et al., 2000). Despite their profound obesity in adulthood, the MC4-R-deficient animals are not hypertensive, but tend to be hypotensive (Tallam et al., 2005). Finally, another notable feature of the MC4-R null mouse is a profound sensitivity to high-fat feeding, which exacerbates the hyperphagia, obesity and hyperinsulinemia (Sutton et al., 2006).
Although MC4-R null mice develop obesity and hyperglycemia/hyperinsulinemia typical of MetS, little is known about dyslipidemia in this model. Circulating TGs and non-esterified fatty acids (NEFAs) appear normal (Albarado et al., 2004); however, as might be expected for an animal with such profound obesity, these animals show ectopic storage of fat in the liver, i.e. hepatic steatosis (Sutton et al., 2006). To the best of our knowledge, the serum lipoprotein profile of these animals has not been established, but one might anticipate that they would show a pattern similar to the Ay/a mouse, as both models share the MC4-R signaling deficiency.
Melanocortin 3 receptor (MC3-R) null mouse
In addition to the MC4-R, the MC3-R is the other centrally expressed melanocortin receptor that plays a role in regulating energy homeostasis, although its function is not as well studied. Genetic deficiency of the MC3-R results in a unique metabolic phenotype in mice, which is characterized by an increase in adiposity in the absence of substantial increases in body weight, food intake or alterations in glucose homeostasis (Butler et al., 2000; Chen et al., 2000). As with the MC4-R−/− mouse, little work has focused on the lipoprotein profile in these animals. Despite the elevated adiposity seen in this model, animals are relatively resistant to many of the negative consequences of obesity, such as IR and hepatic steatosis, even when high-fat feeding further increases their adiposity. The resistance to the development of MetS in this model despite obesity is mediated, at least in part, by a reduced inflammatory response to obesity (Ellacott et al., 2007; Trevaskis et al., 2007); thus, this model may be useful for the study of obesity in the absence of MetS.
Summary of obese mouse models of MetS
The Lepob/ob, LepRdb/db and Ay/a mice are the three most commonly used spontaneously mutant obese mouse models. They display IR and can even develop diabetes depending on the background strain. In addition, Ay/a mice have intact leptin signaling and display a delayed onset obesity that can be amplified by high-fat feeding, making them a good model for human obesity. The MC4-R-deficient mouse model is similar to the Ay/a mice and is important because obesity in humans can be a result of mutations in the MC4R gene. However, with regards to plasma lipid levels and blood pressure, all three models fall short of an ideal model for MetS, and investigators should recognize this before choosing them for MetS studies.
Models of hyperlipidemia
According to the IDF, plasma TG levels of >150 mg/dl, or plasma HDL levels of <40 mg/dl in males and <50 mg/dl in females, are two components of MetS. Visceral obesity and IR are strongly correlated with the development of dyslipidemia (Semenkovich, 2006). Increased adiposity along with elevated insulin levels can lead to an elevation of NEFAs, which impact lipoprotein metabolism. With the advent of gene targeting strategies in mice, it has been possible to generate mice which are deficient in genes that are known to influence lipoprotein metabolism. Most mouse models are hyperlipidemic (elevated VLDL and LDL) rather than dyslipidemic (elevated TG and reduced HDL); however, they are still useful for studies of MetS. Many different hyperlipidemic models have been developed; however, this review will focus on the two that are most commonly used for studies of hyperlipidemia and atherosclerosis.
Low-density lipoprotein receptor-deficient mice
One of the most severe forms of human hyperlipidemia, familial hypercholesterolemia, occurs due to mutations in the low-density lipoprotein receptor (LDLR), which results in elevated levels of the atherogenic lipoprotein LDL. Thus, the first mouse model of hyperlipidemia developed was the LDLR−/− mouse (Ishibashi et al., 1993). These mice develop moderate hypercholesterolemia (total cholesterol ~250 mg/dl) on a chow diet with lipoprotein profiles similar to humans (i.e. elevated LDL) (Fig. 1C). On a high-fat/high-cholesterol ‘Western-type’ diet containing 21% fat and 0.15% added cholesterol, LDLR−/− mice develop severe hyperlipidemia and extensive atherosclerosis (Ishibashi et al., 1993). Furthermore, when LDLR−/− mice are placed on a diet with greater than 20% fat content they also become obese and display IR (Wu et al., 2006). Thus, the LDLR−/− mouse model can be particularly useful when studying diet-induced obesity and IR in the presence of hyperlipidemia.
Apolipoprotein E (apoE)-deficient mice
The apolipoprotein E (apoE)-deficient mouse is another commonly used model of hyperlipidemia. ApoE is a ligand found on remnant lipoproteins that is recognized by various receptors in the liver. In humans, apoE deficiency, or the presence of mutant forms of apoE, results in type III hyperlipidemia characterized by the presence of elevated VLDL lipoproteins and an early age onset of atherosclerosis (Mahley, 1988). Unlike their LDLR−/−counterparts, apoE−/− mice develop a more severe hyperlipidemia (total cholesterol ~350 mg/dl), characterized by elevations in VLDL and reductions in HDL (Fig. 1C), which leads to spontaneous atherosclerosis on a chow diet (Plump et al., 1992; Zhang et al., 1992). In many cases, apoE−/− mice do not become obese, nor do they develop IR, even on a high-fat diet (Gao et al., 2007; Hofmann et al., 2008). The reason that apoE−/− mice are protected against obesity and IR in some studies is not entirely clear; however, there is some evidence that apoE may modulate adipocyte TG storage (Yue et al., 2004; Huang et al., 2006).
Despite other studies showing that apoE deficiency can protect against obesity, our laboratory has shown that specific dietary modulations may potentially make apoE−/− mice useful for studies of MetS. Specifically, we found that apoE−/− mice fed a high-fat diet (with 60% of the calories from fat) for 17 weeks have increased body weight and atherosclerosis (King et al., 2009). These high-fat-fed apoE−/− mice are glucose intolerant and also display elevated systemic inflammation, as measured by increased serum amyloid A concentrations in the plasma. Thus, apoE−/− mice can be used as a model of MetS under the proper dietary conditions. In addition, other genetic modulations, such as placing them on the Lepob/ob or LepRdb/db background (described below), also allow for their use in MetS studies.
Models of obesity with hyperlipidemia
Obese mouse models such as Ay/a, Lepob/ob and LepRdb/db have increased total plasma cholesterol levels; however, this is the result of increased HDL rather than increased VLDL and LDL levels (Nishina et al., 1994a; Silver et al., 1999; Silver et al., 2000; Gruen et al., 2005; Gruen et al., 2006; Coenen and Hasty, 2007). The increase in HDL probably accounts for the resistance of these obese mice to atherosclerotic lesion formation (Nishina et al., 1994b). By contrast, human MetS is associated with obesity, increased levels of TG-rich VLDL and reduced levels of HDL. To generate obese mouse models with hyperlipidemia, our laboratory and others have crossed the Lepob/ob, LepRdb/db and Ay/a mice onto LDLR−/− and apoE−/− backgrounds. The models described below are all on the C57BL/6J strain.
Lepob/ob;LDLR−/− and LepRdb/db;LDLR−/− mice
To develop a model that better reflects MetS-related hyperlipidemia, we crossed Lepob/ob mice onto an LDLR−/−background (Lepob/ob;LDLR−/−) (Hasty et al., 2001). These mice are obese and develop dramatic hypercholesterolemia characterized by elevated VLDL and LDL (Fig. 1D; please note that the figure represents a LepRdb/db;LDLR−/− mouse, however, the Lepob/ob;LDLR−/− mice have a similar profile), as well as hypertriglyceridemia. Furthermore, these mice spontaneously develop atherosclerotic lesions, and are thus very useful for studying the role of obesity in cardiovascular disease. Our group and others have used this model to study therapeutic treatments for MetS, as well as to study mechanisms of obesity-related hyperlipidemia (Mertens et al., 2003; Verreth et al., 2004; Hasty et al., 2006; Verreth et al., 2006; Coenen et al., 2007a). LepRdb/db;LDLR−/− mice have a phenotype identical to that of the Lepob/ob;LDLR−/− mice with the exception that they have very high circulating leptin levels (Gruen et al., 2006). We have also crossed the Lepob/ob and LepRdb/db mice onto an apoE−/− background, and these mice are also obese, insulin resistant and hyperlipidemic (Gruen et al., 2006; Atkinson et al., 2008). The Lepob/ob;apoE−/−and LepRdb/db;apoE−/− mice have extreme elevations in VLDL and almost no HDL (Fig. 1D). Although these mice provide a better model for MetS than do Lepob/ob and LepRdb/db mice, because of their hyperlipidemia and susceptibility to atherosclerosis, the hyperlipidemia is quite extreme and the caveat remains that they are completely deficient in leptin signaling.
LDLR 3KO and apoE 3KO mice
Recently, Lloyd et al. crossed Lepob/ob;LDLR−/− and Lepob/ob;apoE−/−mice onto an apoB100 only background (named LDLR 3KO and apoE 3KO, respectively) (Lloyd et al., 2008). These mice are obese (>40 grams), hyperinsulinemic (>30 ng/ml), hyperlipidemic (total cholesterol >750 mg/dl and TGs >250 mg/dl) and hypertensive (systolic pressure >150 mmHg), as measured by the tail cuff method. Interestingly, the apoE 3KO mice are diabetic by 9–10 weeks of age, whereas the LDLR 3KO mice are not. This may be because of their apoE versus LDLR genotype, or the fact that the apoE 3KO mice were only 74.6% C57BL/6J, whereas the LDLR 3KO mice were 94.7% C57BL/6J in the original report. One unique aspect to these mice is that they only express apoB100 (and not apoB48) from their livers. In humans, apoB48 is expressed solely from the intestines and apoB100 is expressed solely from the liver, whereas, in rodents, both forms of apoB are expressed in the liver. Thus, expression of only apoB100 from the livers of these mice provides a lipoprotein metabolism setting which is similar to that seen in humans.
Ay/a;LDLR−/− and Ay/a;apoE−/− mice
To develop a model of MetS with delayed onset obesity and hyperlipidemia, we crossed the Ay/a mice onto an LDLR−/−background (Coenen et al., 2007b; Coenen and Hasty, 2007). In contrast to LDLR−/− mice, the Ay/a;LDLR−/− mice are obese, have an increased fat mass, and have slightly elevated plasma cholesterol and TG levels. Furthermore, when these mice are placed on a Western diet they become even more obese and hyperlipidemic, and also develop IR. The hyperlipidemia appears to be the result of both increased hepatic TG production and decreased VLDL clearance. The Ay/a;LDLR−/− mice do not develop overt hypertension; however, on the Western-type diet they develop a fatty liver. Thus, these mice seem to have many different aspects of MetS and, to date, appear to be one of the best models for use.
The Ay/a and apoE−/− mice have also been crossed to generate Ay/a;apoE−/− mice (Gao et al., 2007). Interestingly, the deficiency of apoE protects Ay/a mice from obesity and hepatic steatosis, and improved their insulin sensitivity. Thus, the phenotype of Ay/a mice differs in the presence of LDLR versus apoE deficiency.
Summary of obese hyperlipidemic mouse models of the MetS
By crossing models of hyperlipidemia onto obesity-prone backgrounds, it is possible to study several aspects of MetS simultaneously. The mouse models listed above are the most commonly used in this regard, and their use has shed light on the mechanisms by which obesity influences lipoprotein metabolism. In addition, these models have been useful in testing various agents for their therapeutic potential. One disappointment in the studies of these models is that the presence of IR does not appear to impact atherosclerotic lesion formation in most models unless there are concomitant changes in plasma lipid levels (Merat et al., 1999; Wu et al., 2006; Coenen and Hasty, 2007; and reviewed in Goldberg and Dansky, 2006).
Models of hypertension
Blood pressure is regulated by a complex interaction between the nervous system, kidney, and a variety of humoral and mechanical factors. All individuals with MetS do not have hypertension, and the mechanisms contributing to hypertension in specific patients with MetS are not well defined. However, clinical studies have demonstrated that individuals with hypertension as a component of MetS have increased atherosclerosis (Irace et al., 2005; Kawamoto et al., 2005). This section will focus on blood pressure effects in mouse models of MetS and diet-induced obesity. However, to date, the majority of studies investigating the role of obesity in hypertension have been performed in rat models of the disease, and few studies have investigated the role of hypertension in mouse models of obesity. Other issues include limitations in the methods used to measure moderate increases in blood pressure in mice, which will be addressed below. Given its role in hypertension, we have focused on the renin-antiogtensin system (RAS) and then summarized the effects of alterations in the major adipokines, leptin and adiponectin, on hypertension as a component of MetS.
Models related to the renin-angiotensin system
In humans, a role for the RAS in the development of obesity, obesity-related hypertension and other metabolic perturbations is well established. Furthermore, angiotensin II (AngII), a potent vasoconstrictor, and other components of the RAS are present in adipose tissue (for a review, see Cassis et al., 2008). Clinical data suggest that angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) should be considered as the first line treatment for hypertension in obese patients (Masuo et al., 2001; Kintscher et al., 2007). Moreover, clinical data also suggest that ARBs improve insulin sensitivity, further demonstrating their utility in treating MetS in humans as well as in animal models of MetS.
The mRNA for the precursor of AngII, angiotensinogen, is increased in the visceral adipose tissue of mice fed a high-fat diet (Rahmouni et al., 2004). Moreover, C57BL/6J mice with diet-induced obesity and hypertension exhibit a reduction in visceral adipose tissue expression of angiotensin converting enzyme 2, an enzyme that catabolizes AngII (Gupte et al., 2008). Blockade of the AngII type 1 (AT1) receptor in these mice abolishes hypertension, as determined by telemetry (Gupte et al., 2008). Similarly, AT1 receptor deficiency decreases diet-induced increases in body weight and adiposity, and improves IR with a concomitant reduction in blood pressure, as measured by the tail cuff method (Kouyama et al., 2005). AngII also mediates biological effects through the AT2 receptor, which has been suggested to decrease AT1 receptor-mediated increases in blood pressure. Interestingly, mice that are deficient in the AT2 receptor are also protected against the development of diet-induced obesity and insulin resistance, as well as hypertension as measured by tail cuff (Yvan-Charvet et al., 2005). Thus, both clinical and animal models demonstrate an important role of the RAS in many aspects of MetS, as well as a role in obesity-related hypertension.
Models of obesity with hypertension
Diet-induced obese models
Decisions regarding the use of models of hypertension can be complicated, primarily owing to inconsistencies in demonstrating hypertension in different experimental obese mouse models. This is most likely to arise from differences in background strain, age, gender, modes of inducing obesity (e.g. diet versus monogenic), and the method of measuring hypertension. For example, in C57BL/6J mice that are fed the same high-fat diet, diet-induced obesity can have differential effects on systolic blood pressure when measured by radiotelemetry and the tail cuff method (Gupte et al., 2008; Police et al., 2009; Symons et al., 2009). It is possible that the magnitude of the blood pressure increases (10–15 mmHg systolic pressure) in studies using radiotelemetry in obese mice may not be within the range of sensitivity of tail cuff methods.
Lepob/ob and LepRdb/db mice
Clinical data demonstrate increasing leptin concentrations with increasing blood pressure in non-obese individuals with essential hypertension (Agata et al., 1997). However, although the chronic elevations in leptin observed in obese individuals would be expected to result in enhanced sympathetic activity, which contributes to hypertension, obese individuals are generally resistant to the actions of leptin. Leptin correlates positively with adiposity and increases sympathetic activity and blood pressure when infused chronically into lean rodents (Shek et al., 1998). Thus, mouse models with the absence of intact leptin signaling pathways would be expected to have low blood pressure. In fact, Lepob/ob mice (on the C57BL/6J background) are hypotensive (Mark et al., 1999), whereas studies in LepRdb/db mice are conflicting, reporting that the mice are both hypotensive and hypertensive, as measured by telemetry in both reports (Bodary et al., 2007; Su et al., 2008). Transgenic mice that overexpress leptin are protected from obesity; however, they are hyperleptinemic and hypertensive. Thus, these animal models suggest that there may be selective leptin resistance, i.e. resistance to the metabolic effects while preserving the effects of leptin to enhance sympathetic activity.
NZBWF1 mice
NZBWF1 mice with systemic lupus erythematosus are a model of MetS with hyperleptinemia. These mice have age-onset obesity, increased visceral adiposity, increased plasma leptin concentrations, altered glucose tolerance and hypertension, suggesting that they are also a model of MetS with hypertension (Ryan et al., 2006). Interestingly, treatment of the NZBWF1 mice with a thiazolidinedione (TZD) reduced blood pressure, however it did not alter insulin sensitivity (Venegas-Pont et al., 2009).
KKAy/a mice
The KKAy/a mouse is a model of MetS with age-onset obesity, hypertension and IR (Aizawa-Abe et al., 2000; Ohashi et al., 2006). Interestingly, overexpression of adiponectin in KKAy/a mice results in decreased blood pressure without altering IR. Treatment of KKAy/a mice with bis(allixinato)oxovanadium, an antidiabetic drug, also decreased blood pressure, as measured by tail cuff and enhanced insulin sensitivity; however, it did not alter adiponectin concentrations (Adachi et al., 2006). Clinical studies have demonstrated that administration of the AngII receptor blocker, telmisartan, increases adiponectin, decreases body weight, and improves insulin sensitivity with a concomitant reduction in blood pressure in patients (Makita et al., 2008; Yamada et al., 2008). Although telmisartan is an ARB, it is also a partial agonist for peroxisome proliferator-activated receptor (PPAR)-γ. Taken together, these data suggest that adiponectin may play a central role in obesity-related hypertension in humans with MetS and in mouse models of MetS. Other studies have also demonstrated that treatment with ACE inhibitors and other ARBs increases adiponectin concentrations in individuals with MetS, however, the effects on blood pressure were not presented (Tian et al., 2009).
Models of metabolic syndrome without obesity (lipodystrophy)
Adipose tissue functions primarily to store TGs and release NEFAs, as well as to secrete an array of adipokines and hormones. In humans and animals, dysregulation of adipose tissue function can lead not only to obesity, but also to lipodystrophy, resulting in increased circulation of lipids and inflammatory adipokines. This can result in progression of IR and the storage of fatty acids in organs such as the liver or muscle. Several mouse models have been developed with a focus on disruption of adipocyte function leading to lipodystrophy (Table 2). These models exhibit a number of markers of MetS, including hyperlipidemia, IR and ectopic lipid accumulation.
Table 2.
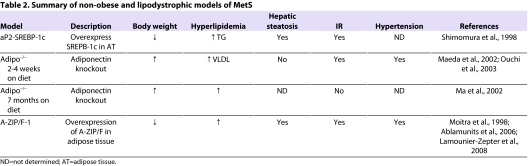
Summary of non-obese and lipodystrophic models of MetS
Adiponectin-deficient mice
Adiponectin is synthesized and secreted by adipocytes. Low circulating levels of adiponectin are strongly associated with MetS in obese humans (Arita et al., 1999). Adiponectin/ACRP30-knockout (adipo−/−) mice on a C57BL/6J background have relatively normal metabolic parameters on a normal chow diet; however, they do display an increase in VLDL-TG levels (Maeda et al., 2002; Oku et al., 2007). The phenotype of adipo−/− mice varies in different reports. When placed on a high-fat/high-sucrose diet for 2 weeks, adipo−/− mice develop elevated plasma levels of tumor necrosis factor-α (TNF-α), glucose, insulin and free fatty acids levels (Maeda et al., 2002). By contrast, adipo−/− mice on a high-fat/high-sucrose diet for 7 months do not develop IR because of enhanced beta oxidation in muscle and liver (Ma et al., 2002). Moreover, following a high-fat/high sucrose diet for 4 weeks, adipo−/− mice have a higher systolic blood pressure and impaired endothelium-dependent vascular relaxation (Ouchi et al., 2003). Similarly, when maintained on a high-salt diet for 3 weeks, these mice develop hypertension, which is prevented by adiponectin treatment (Ohashi et al., 2006). When fed a 50% high-fat diet (Zhou et al., 2008), or on a choline-deficient L-amino-defined diet (Kamada et al., 2007), adipo−/− mice develop liver steatosis that is reversed by adiponectin treatment (Zhou et al., 2008). In a separate study, Nawrocki and colleagues also developed adipo−/− mice and, in their studies, these animals developed hepatic but not peripheral IR when placed on a high-fat diet (Nawrocki et al., 2006). In a third adipo−/− model, Kubota et al. described increased plasma insulin, glucose and serum TG levels following feeding a 32% high-fat diet for 10 weeks. These mice also developed an increased thickness of the femoral arteries, suggesting that they are prone to develop cardiovascular disease (Kubota et al., 2002). Thus, in general, adipo−/− mice display some aspects of MetS when placed on a high-fat diet in the presence or absence of obesity.
Transgenic aP2-SREBP-1c mice
Sterol regulatory element binding proteins (SREBP) are a family of nuclear transcription factors that regulate cholesterol and fatty acid metabolism. In mammals there are three isoforms of SREBP: SREBP-1a, SREBP-1c and SREBP-2. Transgenic aP2-SREBP-1c mice that overexpress SREBP-1c in adipose tissue have a reduced body weight and elevated plasma TG, glucose and insulin levels; they also have very low levels of adipokines such as leptin and adiponectin (Shimomura et al., 1998). They develop liver steatosis owing to an influx of plasma TG (Horton et al., 2003). Treatment of these mice with leptin prevents liver steatosis (Shimomura et al., 1999; Asilmaz et al., 2004). Currently there is no data on how a high-fat diet affects other metabolic parameters in these mice.
A-ZIP/F-1 mice
A-ZIP/F-1 mice were generated to investigate the role of the transcription factors CCAAT/enhancer binding protein (C/EBP) and activator protein-1 (AP-1) in the development and function of white adipose tissue (Moitra et al., 1998). C/EBP α, β and δ are a family of transcription factors that play a role in the growth and differentiation of adipocytes. A-ZIP/F-1 mice were developed by enhancing the expression of A-ZIP/F in adipocytes by using an aP2 enhancer promoter. Overexpression of A-ZIP/F inhibits the binding and function of B-ZIP proteins to both the C/EBP and AP-1 transcription factors. Adult A-ZIP/F-1 mice have an increased body weight compared with littermates. These mice are devoid of white adipose tissue and have reduced levels of brown adipose tissue, and thus are a model of lipodystrophy. A-ZIP/F-1 mice develop liver steatosis, which accounts for the increased body weight. These mice are also hyperglycemic, hyperinsulinemic, hyperlipidemic and hypertensive (Moitra et al., 1998; Ablamunits et al., 2006; Lamounier-Zepter et al., 2008).
Conclusions
There are many different naturally occurring and gene-targeted mutations in mice that lead to obesity and other metabolic defects associated with human MetS. Care should be taken when choosing an animal model for MetS studies, taking into account the diet used, as well as the degree to which they develop obesity, hyperlipidemia, IR and hypertension. Although there is no perfect animal model of the human disease, each of the mouse models described have specific attributes that make them useful for studying both the mechanisms of development of MetS, as well as potential therapies.
Acknowledgments
We are grateful to Marnie Gruen, and to Dr Larry Swift and Dr Amy Major for careful reading of our manuscript. A.J.K. is supported by the Vanderbilt Molecular Endocrinology Training Program (NIH T32DK07563); K.L.J.E. is supported by NIH grant DK078850; and V.L.K. is supported by NIH grant P20 RR021954. A.H.H. is supported by an NIH grant R01HL089466 and by a Career Development Award from the American Diabetes Association (1-07-CD-10). Deposited in PMC for release after 12 months.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Ablamunits V, Cohen Y, Brazee IB, Gaetz HP, Vinson C, Klebanov S. (2006). Susceptibility to induced and spontaneous carcinogenesis is increased in fatless A-ZIP/F-1 but not in obese ob/ob mice. Cancer Res. 66, 8897–8902 [DOI] [PubMed] [Google Scholar]
- Adachi Y, Yoshikawa Y, Yoshida J, Kodera Y, Katoh A, Takada J, Sakurai H. (2006). Improvement of diabetes, obesity and hypertension in type 2 diabetic KKAy mice by bis(allixinato)oxovanadium(IV) complex. Biochem Biophys Res Commun. 345, 945–950 [DOI] [PubMed] [Google Scholar]
- Agata J, Masuda A, Takada M, Higashiura K, Murakami H, Miyazaki Y, Shimamoto K. (1997). High plasma immunoreactive leptin level in essential hypertension. Am J Hypertens. 10, 1171–1174 [DOI] [PubMed] [Google Scholar]
- Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, et al. (2000). Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest. 105, 1243–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarado DC, McClaine J, Stephens JM, Mynatt RL, Ye J, Bannon AW, Richards WG, Butler AA. (2004). Impaired coordination of nutrient intake and substrate oxidation in melanocortin-4 receptor knockout mice. Endocrinology 145, 243–252 [DOI] [PubMed] [Google Scholar]
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, et al. (1999). Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 257, 79–83 [DOI] [PubMed] [Google Scholar]
- Asilmaz E, Cohen P, Miyazaki M, Dobrzyn P, Ueki K, Fayzikhodjaeva G, Soukas AA, Kahn CR, Ntambi JM, Socci ND, et al. (2004). Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest. 113, 414–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RD, Coenen KR, Plummer MR, Gruen ML, Hasty AH. (2008). Macrophage-derived apolipoprotein E ameliorates dyslipidemia and atherosclerosis in obese apolipoprotein E-deficient mice. Am J Physiol Endocrinol Metab. 294, E284–E290 [DOI] [PubMed] [Google Scholar]
- Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. (1999). Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 84, 137–144 [DOI] [PubMed] [Google Scholar]
- Bodary PF, Westrick RJ, Wickenheiser KJ, Shen Y, Eitzman DT. (2002). Effect of leptin on arterial thrombosis following vascular injury in mice. JAMA 287, 1706–1709 [DOI] [PubMed] [Google Scholar]
- Bodary PF, Gu S, Shen Y, Hasty AH, Buckler JM, Eitzman DT. (2005). Recombinant leptin promotes atherosclerosis and thrombosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 25, E119–E122 [DOI] [PubMed] [Google Scholar]
- Bodary PF, Shen Y, Ohman M, Bahrou KL, Vargas FB, Cudney SS, Wickenheiser KJ, Myers MG, Jr, Eitzman DT. (2007). Leptin regulates neointima formation after arterial injury through mechanisms independent of blood pressure and the leptin receptor/STAT3 signaling pathways involved in energy balance. Arterioscler Thromb Vasc Biol. 27, 70–76 [DOI] [PubMed] [Google Scholar]
- Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. (2000). A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 141, 3518–3521 [DOI] [PubMed] [Google Scholar]
- Carroll L, Voisey J, van Daal A. (2004). Mouse models of obesity. Clin Dermatol. 22, 345–349 [DOI] [PubMed] [Google Scholar]
- Cassis LA, Police SB, Yiannikouris F, Thatcher SE. (2008). Local adipose tissue renin-angiotensin system. Curr Hypertens Rep. 10, 93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, et al. (2000). Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 26, 97–102 [DOI] [PubMed] [Google Scholar]
- Coenen KR, Hasty AH. (2007). Obesity Potentiates Development of Fatty Liver and Insulin Resistance, but Not Atherosclerosis in High Fat Diet-Fed Agouti LDLR Deficient Mice. Am J Physiol Endocrinol Metab. 293, E492–E499 [DOI] [PubMed] [Google Scholar]
- Coenen KR, Gruen ML, Hasty AH. (2007a). Obesity causes very low density lipoprotein clearance defects in low-density lipoprotein receptor-deficient mice. J Nutr Biochem. 18, 727–735 [DOI] [PubMed] [Google Scholar]
- Coenen KR, Gruen ML, Chait A, Hasty AH. (2007b). Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes 56, 564–573 [DOI] [PubMed] [Google Scholar]
- Coleman DL. (1978). Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 14, 141–148 [DOI] [PubMed] [Google Scholar]
- Coleman DL, Hummel KP. (1973). The influence of genetic background on the expression of the obese (Ob) gene in the mouse. Diabetologia 9, 287–293 [DOI] [PubMed] [Google Scholar]
- Cone RD. (2005). Anatomy and regulation of the central melanocortin system. Nat Neurosci. 8, 571–578 [DOI] [PubMed] [Google Scholar]
- Dickie MM. (1969). Mutations at the agouti locus in the mouse. J Hered. 60, 20–25 [DOI] [PubMed] [Google Scholar]
- Ellacott KL, Murphy JG, Marks DL, Cone RD. (2007). Obesity-induced inflammation in white adipose tissue is attenuated by loss of melanocortin-3 receptor signaling. Endocrinology 148, 6186–6194 [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. (1997). Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385, 165–168 [DOI] [PubMed] [Google Scholar]
- Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. (2000). The central melanocortin system can directly regulate serum insulin levels. Endocrinology 141, 3072–3079 [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T, O’Rahilly S. (2000). Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 106, 271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. (2003). Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 348, 1085–1095 [DOI] [PubMed] [Google Scholar]
- Gao J, Katagiri H, Ishigaki Y, Yamada T, Ogihara T, Imai J, Uno K, Hasegawa Y, Kanzaki M, Yamamoto TT, et al. (2007). Involvement of apolipoprotein e in excess fat accumulation and insulin resistance. Diabetes 56, 24–33 [DOI] [PubMed] [Google Scholar]
- Genuth SM, Przybylski RJ, Rosenberg DM. (1971). Insulin resistance in genetically obese, hyperglycemic mice. Endocrinology 88, 1230–1238 [DOI] [PubMed] [Google Scholar]
- Goldberg IJ, Dansky HM. (2006). Diabetic vascular disease: an experimental objective. Arterioscler Thromb Vasc Biol. 26, 1693–1701 [DOI] [PubMed] [Google Scholar]
- Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, et al. (2009). Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 360, 44–52 [DOI] [PubMed] [Google Scholar]
- Gruen ML, Plummer MR, Zhang W, Posey KA, Linton MF, Fazio S, Hasty AH. (2005). Persistence of high density lipoprotein particles in obese mice lacking apolipoprotein A-I. J Lipid Res. 46, 2007–2014 [DOI] [PubMed] [Google Scholar]
- Gruen ML, Saraswathi V, Nuotio-Antar AM, Plummer MR, Coenen KR, Hasty AH. (2006). Plasma insulin levels predict atherosclerotic lesion burden in obese hyperlipidemic mice. Atherosclerosis 186, 54–64 [DOI] [PubMed] [Google Scholar]
- Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, English VL, Cassis LA. (2008). ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol. 295, R781–R788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluzik M, Colombo C, Gavrilova O, Chua S, Wolf N, Chen M, Stannard B, Dietz KR, Le Roith D, Reitman ML. (2004). Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology 145, 3258–3264 [DOI] [PubMed] [Google Scholar]
- Hasty A, Shimano H, Osuga J, Namatame I, Takahashi A, Yahagi N, Perrey S, Iizuka Y, Tamura Y, Amemiya-Kudo M, et al. (2001). Severe hypercholesterolemia, hypertriglyceridemia, and atherosclerosis in mice packing both leptin and the low density lipoprotein receptor. J Biol Chem. 276, 37402–37408 [DOI] [PubMed] [Google Scholar]
- Hasty AH, Gruen ML, Terry ES, Surmi BK, Atkinson RD, Gao L, Morrow JD. (2006). Effects of vitamin E on oxidative stress and atherosclerosis in an obese hyperlipidemic mouse model. J Nutr Biochem. 18, 127–133 [DOI] [PubMed] [Google Scholar]
- Hinney A, Schmidt A, Nottebom K, Heibult O, Becker I, Ziegler A, Gerber G, Sina M, Gorg T, Mayer H, et al. (1999). Several mutations in the melanocortin-4 receptor gene including a nonsense and a frameshift mutation associated with dominantly inherited obesity in humans. J Clin Endocrinol Metab. 84, 1483–1486 [DOI] [PubMed] [Google Scholar]
- Hofmann SM, Perez-Tilve D, Greer TM, Coburn BA, Grant E, Basford JE, Tschop MH, Hui DY. (2008). Defective lipid delivery modulates glucose tolerance and metabolic response to diet in apolipoprotein E-deficient mice. Diabetes 57, 5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Shimomura I, Ikemoto S, Bashmakov Y, Hammer RE. (2003). Overexpression of sterol regulatory element-binding protein-1a in mouse adipose tissue produces adipocyte hypertrophy, increased fatty acid secretion, and fatty liver. J Biol Chem. 278, 36652–36660 [DOI] [PubMed] [Google Scholar]
- Huang ZH, Reardon CA, Mazzone T. (2006). Endogenous ApoE expression modulates adipocyte triglyceride content and turnover. Diabetes 55, 3394–3402 [DOI] [PubMed] [Google Scholar]
- Hummel KP, Dickie MM, Coleman DL. (1966). Diabetes, a new mutation in the mouse. Science 153, 1127–1128 [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, et al. (1997). Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 [DOI] [PubMed] [Google Scholar]
- Ingalls AM, Dickie MM, Snell GD. (1950). Obese, a new mutation in the house mouse. J Hered. 41, 317–318 [DOI] [PubMed] [Google Scholar]
- Irace C, Cortese C, Fiaschi E, Carallo C, Sesti G, Farinaro E, Gnasso A. (2005). Components of the metabolic syndrome and carotid atherosclerosis: role of elevated blood pressure. Hypertension 45, 597–601 [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. (1993). Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 92, 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y, Matsumoto H, Tamura S, Fukushima J, Kiso S, Fukui K, Igura T, Maeda N, Kihara S, Funahashi T, et al. (2007). Hypoadiponectinemia accelerates hepatic tumor formation in a nonalcoholic steatohepatitis mouse model. J Hepatol. 47, 556–564 [DOI] [PubMed] [Google Scholar]
- Kawamoto R, Tomita H, Oka Y, Ohtsuka N, Kamitani A. (2005). Metabolic syndrome and carotid atherosclerosis: role of elevated blood pressure. J Atheroscler Thromb. 12, 268–275 [DOI] [PubMed] [Google Scholar]
- King VL, Hatch NW, Chan HW, de Beer MC, de Beer FC, Tannock LR. (2009). A Murine Model of Obesity With Accelerated Atherosclerosis. Obesity (Silver Spring). 18, 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintscher U, Bramlage P, Paar WD, Thoenes M, Unger T. (2007). Irbesartan for the treatment of hypertension in patients with the metabolic syndrome: a sub analysis of the Treat to Target post authorization survey. Prospective observational, two armed study in 14,200 patients. Cardiovasc Diabetol. 6, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouyama R, Suganami T, Nishida J, Tanaka M, Toyoda T, Kiso M, Chiwata T, Miyamoto Y, Yoshimasa Y, Fukamizu A, et al. (2005). Attenuation of diet-induced weight gain and adiposity through increased energy expenditure in mice lacking angiotensin II type 1a receptor. Endocrinology 146, 3481–3489 [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, et al. (2002). Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 277, 25863–25866 [DOI] [PubMed] [Google Scholar]
- Lam TK, Gutierrez-Juarez R, Pocai A, Bhanot S, Tso P, Schwartz GJ, Rossetti L. (2007). Brain glucose metabolism controls the hepatic secretion of triglyceride-rich lipoproteins. Nat Med. 13, 171–180 [DOI] [PubMed] [Google Scholar]
- Lamounier-Zepter V, Bornstein SR, Kunes J, Zicha J, Krsek M, Ehrhart-Bornstein M, Ziegler CG, Kiessling A, Funk RH, Haluzik M. (2008). Adrenocortical changes and arterial hypertension in lipoatrophic A-ZIP/F-1 mice. Mol Cell Endocrinol. 280, 39–46 [DOI] [PubMed] [Google Scholar]
- Lloyd DJ, McCormick J, Helmering J, Kim KW, Wang M, Fordstrom P, Kaufman SA, Lindberg RA, Veniant MM. (2008). Generation and characterization of two novel mouse models exhibiting the phenotypes of the metabolic syndrome: Apob48-/-Lepob/ob mice devoid of ApoE or Ldlr. Am J Physiol Endocrinol Metab. 294, E496–E505 [DOI] [PubMed] [Google Scholar]
- Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO, et al. (1994). Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 371, 799–802 [DOI] [PubMed] [Google Scholar]
- Ma K, Cabrero A, Saha PK, Kojima H, Li L, Chang BH, Paul A, Chan L. (2002). Increased beta-oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem. 277, 34658–34661 [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, et al. (2002). Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 8, 731–737 [DOI] [PubMed] [Google Scholar]
- Mahley RW. (1988). Type III hyperlipoproteinemia: role of defective receptor binding of apolipoprotein E in the development of this lipid disorder. Das Arztliche Laboratorium 36, 60–64 [Google Scholar]
- Makita S, Abiko A, Naganuma Y, Moriai Y, Nakamura M. (2008). Effects of telmisartan on adiponectin levels and body weight in hypertensive patients with glucose intolerance. Metabolism 57, 1473–1478 [DOI] [PubMed] [Google Scholar]
- Malendowicz LK, Rucinski M, Belloni AS, Ziolkowska A, Nussdorfer GG. (2007). Leptin and the regulation of the hypothalamic-pituitary-adrenal axis. Int Rev Cytol. 263, 63–102 [DOI] [PubMed] [Google Scholar]
- Mark AL, Shaffer RA, Correia ML, Morgan DA, Sigmund CD, Haynes WG. (1999). Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens. 17, 1949–1953 [DOI] [PubMed] [Google Scholar]
- Marti A, Corbalan MS, Forga L, Martinez JA, Hinney A, Hebebrand J. (2003). A novel nonsense mutation in the melanocortin-4 receptor associated with obesity in a Spanish population. Int J Obes Relat Metab Disord. 27, 385–388 [DOI] [PubMed] [Google Scholar]
- Masuo K, Mikami H, Ogihara T, Tuck ML. (2001). Weight reduction and pharmacologic treatment in obese hypertensives. Am J Hypertens. 14, 530–538 [DOI] [PubMed] [Google Scholar]
- Merat S, Casanada F, Sutphin M, Palinski W, Reaven PD. (1999). Western-type diets induce insulin resistance and hyperinsulinemia in LDL receptor-deficient mice but do not increase aortic atherosclerosis compared with normoinsulinemic mice in which similar plasma cholesterol levels are achieved by a fructose-rich diet. Arterioscler Thromb Vasc Biol. 19, 1223–1230 [DOI] [PubMed] [Google Scholar]
- Mergen M, Mergen H, Ozata M, Oner R, Oner C. (2001). A novel melanocortin 4 receptor (MC4R) gene mutation associated with morbid obesity. J Clin Endocrinol Metab. 86, 3448. [DOI] [PubMed] [Google Scholar]
- Mertens A, Verhamme P, Bielicki JK, Phillips MC, Quarck R, Verreth W, Stengel D, Ninio E, Navab M, Mackness B, et al. (2003). Increased low-density lipoprotein oxidation and impaired high-density lipoprotein antioxidant defense are associated with increased macrophage homing and atherosclerosis in dyslipidemic obese mice: LCAT gene transfer decreases atherosclerosis. Circulation 107, 1640–1646 [DOI] [PubMed] [Google Scholar]
- Meyre D, Delplanque J, Chevre JC, Lecoeur C, Lobbens S, Gallina S, Durand E, Vatin V, Degraeve F, Proenca C, et al. (2009). Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet. 41, 157–159 [DOI] [PubMed] [Google Scholar]
- Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, et al. (1998). Life without white fat: a transgenic mouse. Genes Dev. 12, 3168–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. (1994). Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 8, 1298–1308 [DOI] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, et al. (2006). Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 281, 2654–2660 [DOI] [PubMed] [Google Scholar]
- Nishina PM, Lowe S, Wang J, Paigen B. (1994a). Characterization of plasma lipids in genetically obese mice: the mutants obese, diabetes, fat, tubby, and lethal yellow. Metabolism 43, 549–553 [DOI] [PubMed] [Google Scholar]
- Nishina PM, Naggert JK, Verstuyft J, Paigen B. (1994b). Atherosclerosis in genetically obese mice: the mutants obese, diabetes, fat, tubby, and lethal yellow. Metabolism 43, 554–558 [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, et al. (2007). The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 117, 3475–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuotio-Antar AM, Hachey DL, Hasty AH. (2007). Carbenoxolone treatment attenuates symptoms of metabolic syndrome and atherogenesis in obese, hyperlipidemic mice. Am J Physiol Endocrinol Metab. 293, E1517–E1528 [DOI] [PubMed] [Google Scholar]
- Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, et al. (2006). Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension 47, 1108–1116 [DOI] [PubMed] [Google Scholar]
- Oku H, Matsuura F, Koseki M, Sandoval JC, Yuasa-Kawase M, Tsubakio-Yamamoto K, Masuda D, Maeda N, Ohama T, Ishigami M, et al. (2007). Adiponectin deficiency suppresses ABCA1 expression and ApoA-I synthesis in the liver. FEBS Lett. 581, 5029–5033 [DOI] [PubMed] [Google Scholar]
- Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, Kumada M, Ohashi K, Okamoto Y, Nishizawa H, et al. (2003). Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension 42, 231–234 [DOI] [PubMed] [Google Scholar]
- Plummer MR, Hasty AH. (2008). Atherosclerotic lesion formation and triglyceride storage in obese apolipoprotein AI-deficient mice. J Nutr Biochem. 19, 664–673 [DOI] [PubMed] [Google Scholar]
- Plump AS, Smith JD, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. (1992). Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71, 343–353 [DOI] [PubMed] [Google Scholar]
- Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. (2009). Obesity Promotes Inflammation in Periaortic Adipose Tissue and Angiotensin II-Induced Abdominal Aortic Aneurysm Formation. Arterioscler Thromb Vasc Biol. 29, 1458–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Ogus S, Mounzih K, Ewart-Toland A, Chehab FF. (2001). Leptin-deficient mice backcrossed to the BALB/cJ genetic background have reduced adiposity, enhanced fertility, normal body temperature, and severe diabetes. Endocrinology 142, 3421–3425 [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Mark AL, Haynes WG, Sigmund CD. (2004). Adipose depot-specific modulation of angiotensinogen gene expression in diet-induced obesity. Am J Physiol Endocrinol Metab. 286, E891–E895 [DOI] [PubMed] [Google Scholar]
- Reaven GM. (1988). Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37, 1595–1607 [DOI] [PubMed] [Google Scholar]
- Ryan MJ, McLemore GR, Jr, Hendrix ST. (2006). Insulin resistance and obesity in a mouse model of systemic lupus erythematosus. Hypertension 48, 988–993 [DOI] [PubMed] [Google Scholar]
- Semenkovich CF. (2006). Insulin resistance and atherosclerosis. J Clin Invest. 116, 1813–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shek EW, Brands MW, Hall JE. (1998). Chronic leptin infusion increases arterial pressure. Hypertension 31, 409–414 [DOI] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, Brown MS. (1998). Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 12, 3182–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Bashmakov Y, Horton JD. (1999). Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 274, 30028–30032 [DOI] [PubMed] [Google Scholar]
- Silver DL, Jiang X-C, Tall AR. (1999). Increased high density lipoprotein (HDL), defective hepatic catabolism of apoA-I and apoA-II, and decreased apoA-I mRNA in ob/ob mice. J Biol Chem. 274, 4140–4146 [DOI] [PubMed] [Google Scholar]
- Silver DL, Wang N, Tall AR. (2000). Defective HDL particle uptake in ob/ob hepatocytes causes decreased recycling, degradation, and selective lipid uptake. J Clin Invest. 105, 151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford JM, Yu F, Printz R, Hasty AH, Swift LL, Niswender KD. (2008). Central nervous system neuropeptide Y signaling modulates VLDL triglyceride secretion. Diabetes 57, 1482–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. (2000). A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci USA 97, 12339–12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Guo Z, Randall DC, Cassis L, Brown DR, Gong MC. (2008). Hypertension and disrupted blood pressure circadian rhythm in Type 2 diabetic db/db mice. Am J Physiol Heart Circ Physiol. 295, H1634–H1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmi BK, Atkinson RD, Gruen ML, Coenen KR, Hasty AH. (2008). The role of macrophage leptin receptor in aortic root lesion formation. Am J Physiol Endocrinol Metab. 294, E488–E495 [DOI] [PubMed] [Google Scholar]
- Sutton GM, Trevaskis JL, Hulver MW, McMillan RP, Markward NJ, Babin MJ, Meyer EA, Butler AA. (2006). Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology 147, 2183–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, et al. (2009). Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 104, 1085–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallam LS, Stec DE, Willis MA, da Silva AA, Hall JE. (2005). Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension 46, 326–332 [DOI] [PubMed] [Google Scholar]
- Tian F, Luo R, Zhao Z, Wu Y, Ban D. (2009). Blockade of the RAS increases plasma adiponectin in subjects with metabolic syndrome and enhances differentiation and adiponectin expression of human preadipocytes. Exp. Clin. Endocrinol. Diabetes October 23 [Epub ahead of print] [doi: 10.1055/s-0029-1237706]. [DOI] [PubMed] [Google Scholar]
- Trevaskis JL, Gawronska-Kozak B, Sutton GM, McNeil M, Stephens JM, Smith SR, Butler AA. (2007). Role of adiponectin and inflammation in insulin resistance of Mc3r and Mc4r knockout mice. Obesity (Silver Spring) 15, 2664–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop M, Heiman ML. (2001). Rodent obesity models: an overview. Exp Clin Endocrinol Diabetes 109, 307–319 [DOI] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Guy-Grand B, Froguel P. (1998). A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 20, 113–114 [DOI] [PubMed] [Google Scholar]
- Venegas-Pont M, Sartori-Valinotti JC, Maric C, Racusen LC, Glover PH, McLemore GR, Jr, Jones AV, Reckelhoff JF, Ryan MJ. (2009). Rosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosus. Am J Physiol. 296, R1282–R1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verreth W, De Keyzer D, Pelat M, Verhamme P, Ganame J, Bielicki JK, Mertens A, Quarck R, Benhabiles N, Marguerie G, et al. (2004). Weight-loss-associated induction of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation 110, 3259–3269 [DOI] [PubMed] [Google Scholar]
- Verreth W, Ganame J, Mertens A, Bernar H, Herregods MC, Holvoet P. (2006). Peroxisome proliferator-activated receptor-alpha,gamma-agonist improves insulin sensitivity and prevents loss of left ventricular function in obese dyslipidemic mice. Arterioscler Thromb Vasc Biol. 26, 922–928 [DOI] [PubMed] [Google Scholar]
- Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, et al. (2009). Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 41, 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Vikramadithyan R, Yu S, Pau C, Hu Y, Goldberg IJ, Dansky HM. (2006). Addition of dietary fat to cholesterol in the diets of LDL receptor knockout mice: effects on plasma insulin, lipoproteins, and atherosclerosis. J Lipid Res. 47, 2215–2222 [DOI] [PubMed] [Google Scholar]
- Yamada S, Ano N, Toda K, Kitaoka A, Shiono K, Inoue G, Atsuda K, Irie J. (2008). Telmisartan but not candesartan affects adiponectin expression in vivo and in vitro. Hypertens Res. 31, 601–606 [DOI] [PubMed] [Google Scholar]
- Yen TT, Gill AM, Frigeri LG, Barsh GS, Wolff GL. (1994). Obesity, diabetes, and neoplasia in yellow A(vy)/- mice: ectopic expression of the agouti gene. FASEB J. 8, 479–488 [DOI] [PubMed] [Google Scholar]
- Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S. (1998). A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 20, 111–112 [DOI] [PubMed] [Google Scholar]
- Yue L, Rasouli N, Ranganathan G, Kern PA, Mazzone T. (2004). Divergent effects of peroxisome proliferator-activated receptor gamma agonists and tumor necrosis factor alpha on adipocyte ApoE expression. J Biol Chem. 279, 47626–47632 [DOI] [PubMed] [Google Scholar]
- Yvan-Charvet L, Even P, Bloch-Faure M, Guerre-Millo M, Moustaid-Moussa N, Ferre P, Quignard-Boulange A. (2005). Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes 54, 991–999 [DOI] [PubMed] [Google Scholar]
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N. (1992). Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 258, 468–471 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. (1994). Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 [DOI] [PubMed] [Google Scholar]
- Zhou M, Xu A, Tam PK, Lam KS, Chan L, Hoo RL, Liu J, Chow KH, Wang Y. (2008). Mitochondrial dysfunction contributes to the increased vulnerabilities of adiponectin knockout mice to liver injury. Hepatology 48, 1087–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]