Abstract
Background and Purpose
Sonic Hedgehog (Shh) protein is required for the maintenance of neural progenitor cells (NPCs) in the embryonic and adult hippocampus. Brain ischemia causes increased proliferation of hippocampal NPCs. We therefore examined whether Shh regulates the increase in proliferation of NPCs after ischemia/hypoxia.
Methods
Male SV129 mice were exposed to a 20-minute middle cerebral artery occlusion (MCAO); hippocampi were then analyzed for Shh mRNA and protein expression by real-time PCR, immunoblot and immunohistochemistry. Primary cell cultures of neurons, astrocytes and NPCs were exposed to 16 hours of hypoxia (1% O2) and analyzed by real-time PCR and immunoblot for Shh expression. Proliferation of NPCs, in vivo and in vitro, was measured by bromodeoxyuridine incorporation.
Results
Among the cell types examined in vitro, only NPC and neurons increased Shh mRNA under hypoxic conditions. Furthermore, hypoxia increased proliferation of NPCs and this proliferation was enhanced by the addition of recombinant Shh or blocked by the pathway specific inhibitor, cyclopamine. MCAO was associated with a transient 2-fold increase in the mRNA encoding both Shh and its transcription factor, Gli1, 0.5 days after ischemia. Within the hippocampus, Shh protein was increased approximately 3-fold, 3 and 7 days after ischemia, and was observed predominantly within cells in the CA3 and hilar regions. Shh was expressed only in mature neurons. In vivo, cyclopamine suppressed ischemia-induced proliferation of subgranular NPCs.
Conclusion
The Shh pathway plays a role in the proliferation of NPCs induced by ischemia/hypoxia and might participate in injury remodeling.
Keywords: Gene Regulation, Hippocampus, Ischemia, Neural Stem Cells, Stem Cells, Trophic Factors, Brain ischemia, Sonic hedgehog, Gene expression, Dentate gyrus
Introduction
It is speculated that brain injury invokes a recapitulation of established developmental programs and the Shh pathway has been implicated. Shh, a secreted glycoprotein, binds to its receptor, patched, and derepresses a transducing protein, smoothened, which in turn activates the transcription factor Gli1. During development, Shh regulates cell proliferation, migration and differentiation, cell survival and axonal guidance.1–6 In the adult rat, overexpression of Shh, in the non-ischemic dentate gyrus, increases neuronogenesis.7 Other investigators have further corroborated Shh’s role in stem cell maintenance in the adult brain,8–10 suggesting that the Shh pathway is functioning in the uninjured adult brain.
Despite this evidence, we are unaware of published data investigating the activation/function of Shh after brain ischemia although, both Shh mRNA and protein are upregulated after ischemia/injury to skeletal muscle11 and airway epithelium.12 In the studies described herein, we found that ischemia/hypoxia to brain and cultured cells upregulated the expression of Shh, particularly in NPCs, and modulated their proliferative response to ischemia/hypoxia.
Materials and Methods
Animals
SV129 male mice 10–12 weeks old were obtained from Taconic (Hudson, NY) for MCAO. SV129 pregnant mice E15, and P1 pups were obtained for cell culture. Our institutional Subcommittee on Research and Animal Care approved all animal use.
Neural Progenitor Culture (NPC)
E15 mouse cortices were minced and incubated at 37°C for 20 minutes in a solution of trypsin, 0.25%, DNase I, 0.01%. The tissue was washed in media containing 10% fetal bovine serum (FBS) and then passed through a 70 µm nylon filter (BD Falcon). Cells were resuspended in defined media containing 1% FBS, DMEM/F12, N2 supplement (Gibco Invitrogen), 0.6% glucose, penicillin/streptomycin/amphotericin B (1:100 dilution, Gibco Invitrogen), 50 mmol/L HEPES , FGF-2 (20 ng/ml) and EGF (20 ng/ml) ( Gibco Invitrogen) and plated at 2 × 105 cells/well into 24-well dishes coated with poly-D-lysine and laminin (PDL/L) (Biocoat, BD Biosciences). After 24 hours, 1% FBS was removed.
Astroglial Culture
The cells harvested as above except that P1 mice pups were used. Cells were plated on 24-well plates at same density as above in DMEM and 10% FBS.
Neuronal Culture
E15–17 cortices were harvested as above and plated at a density of 3 × 105 cells/well. Cells were cultured in Neurobasal with glutamine (2 mmol/L), B27 (2% vol/vol) and penicillin/streptomycin antibiotics at all times, in a 24-well dishes coated with PDL/L (Biocoat). On days 1–3, glutamate (25 µg/ml) and β-mercaptoethanol (10µM) was added. On day 3, cytarabine 10 µmol/L was added for 24 hours.
Hypoxia and Drug Treatment
Cultures were treated in a hypoxia chamber with an oxygen control sensor (Biospherix, Ltd., Redfield, NY) to maintain O2 at 1±0.2% (hypoxia). Controls remained in the incubator in 95% air/5% CO2. Either cyclopamine (Toronto Research Chemicals) and/or recombinant mShh (Stem Cell Technologies) were added to cells at the time of hypoxic exposure.
mRNA Isolation, cDNA Synthesis and Real-time PCR
mRNA from hippocampi was isolated with Trizol Reagent (Invitrogen). mRNA from culture experiments were isolated with RNeasy Mini Kit (Qiagen). cDNA was synthesized according to the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen), using random hexamer primers. Samples were run on an ABI Prism 7000 (Applied Biosystems, Foster City, CA) using Taqman FAM labeled probes (Applied Biosystems). Gene specific amplicons were normalized to an endogenous control of 18S rRNA.
Proliferation Assay
Assay was performed according to manufacturer’s recommendation with some modification (Roche Molecular Biochemicals); additional washing steps and blocking with 1%BSA in PBS, prior antibody incubation, were added. Cells were incubated with BrdU for 16 hours. Plates were read with a Wallac VICTOR3V luminometer (Perkin Elmer Life Sciences, Inc., Boston, MA).
Western blotting
NPCs or hippocampi protein was extracted with urea lysis buffer (8 mol/L urea, 20 mmol/L Tris pH 7.4, 2.5 mmol/L EDTA, 2.5 mmol/L EGTA, 1% DTT, 4% CHAPS, 6% NP-40, protease inhibitor (Roche Applied Science). Total protein (20 µg for Shh or 50µg for HIF1α) was loaded in a 15% or 8%, respectively, Tris-glycine gel. Proteins were transferred onto Hybond ECL nitrocellulose membrane (Amersham Biosciences) or PVDF, respectively, and blocked (1%BSA in PBS). Membrane was incubated overnight with rabbit anti-Shh 1:2500 for cell culture and 1:500 for tissue (SantaCruz Biotechnology) or mouse anti-HIF1α 1:500 (Novus Biological). Membrane was washed and incubated with anti-rabbit/mouse-horseradish peroxidase 1:5000 (Zymed). Bands were detected with ECL plus (Amersham Biosciences). For internal loading control, mouse anti-β-actin 1:10,000 (Sigma-Aldrich) or beta-tubulin 1:5000 (Upstate) and anti-mouse-horseradish peroxidase 1:10,000 (Amersham Biosciences) or anti-mouse-Cy5 1:400 (Jackson ImmunoResearch Laboratories) was used.
Animal Surgery MCAO
Procedure was performed as previously described.13, 14 Briefly, mice were anesthetized with 3% isoflurane and maintained on 1–1.5% isoflurane in 70%N2O/30%O2 using a Fluotec 3 vaporizer (Colonial Medical Supply, Franconia, NH). Regional blood flow was monitored during ischemia and reperfusion by laser-Doppler flowmetry (PF2B; Perimed, Järfälla, Sweden). The MCA was occluded with an 8-0 nylon monofilament (Ethicon), coated with silicone, for 20 minutes. During surgery, body temperature of mice was maintained at 37°C with a heating pad and rectal probe. A total of four to six mice per variable underwent surgery. A control group of mice was subjected to surgery, but the nylon filament was not introduced (sham). In 6 separate animals, an oxygen probe (Cat#BF/OT/E, Oxford-Optronix, Oxford, United Kingdom) was placed in the CA3 region of the hippocampus (−1.7mm bregma, 3mm lateral, 1.8–2 mm depth at a 45 degree angle).
Intraventricular Catheter
The intraventricular catheter with osmotic pump (1007D, Alzet, Durect Corp., Cupertino, CA) was placed into the right lateral ventricle (ipsilateral to ischemia). The catheter placement was 1.4 mm lateral and 0.6 mm caudal from the bregma and 1.8 mm deep. The pump was loaded with either 5 µmol/L cyclopamine, or vehicle (0.1% DMSO in PBS). The delivery rate of the pump was 0.5 µl/hr (cyclopamine delivery, 60 pmol/day).
BrdU injections
Mice received intraperitoneal injections of BrdU (50mg/kg), every four hours for a total of four injections. Mice were sacrificed 30 minutes after last injection.
Tissue Preparation
Brains were removed after transcardial perfusion with 4% paraformaldehyde and fixed overnight then placed in 30% sucrose in PBS for 24 hours. The brains were cut in 20 µm sections on a cryotome. Brains processed for Shh immunostaining paraffin embedded and cut in 8 µm sections.
Immunohistochemistry
Proliferation BrdU studies
Slides were treated with 2N HCl for 30 min for antigen retrieval, then 3% hydrogen peroxide for 10 min. Slides were blocked (10% horse serum). Slides incubated with primary antibody, mouse anti-BrdU (1:100, Becton-Dickson), and secondary antibody, biotinylated horse anti-mouse (1:250, Jackson ImmunoResearch Laboratory), each for 1 hour. Slides were stained with Vectastain elite ABC kit using diaminobenzidine (DAB) (Vector Laboratories).
Shh staining
Slides were processed as described above except that slides were treated with 10mM sodium citrate pH 6 and microwaved instead of 2N HCl. Antibodies used: anti-Shh (1:100, H-160, Santa Cruz Biotechnology), at room temperature for 3 hours; biotinylated anti-rabbit (1:250, Jackson ImmunoResearch Laboratory), at room temperature for 1 hour. Slides were counterstained with hematoxylin. Controls were performed by omitting primary antibody and/or substituting the primary antibody with a control mouse IgG antibody. For double labeling, Shh immunofluorescence was performed as above but streptavidin-Alexa 546 (1:500 dilution, Invitrogen) was used instead of DAB. Other primary antibodies used included mouse anti-NeuN (1:100 dilution, Chemicon), rabbit anti-GFAP-Cy3 (1:1000 dilution, Sigma) and goat anti-doublecortin (1:250 dilution, Santa Cruz Biotechnology).
Data Analysis
Real-time PCR
Analysis of real-time PCR was performed on Q-gene15. Target gene expression is expressed as normalization to internal control 18S rRNA. Statistical comparison of mean normalized expression was performed by ANOVA.
Immunohistochemical analysis
Quantification of BrdU was similar to our previous description.13 Briefly, the modified stereological counting methods involved every 20th section in a series of 80 sections. For measures of proliferation, DAB positive cells, viewed under brightfield with a Nikon Eclipse TE200, 40× objective lens, were counted only at the subgranular zone. The average number of BrdU+ cells was multiplied by the total number of sections. The number of cells/hippocampus was averaged across the group and then differences were compared by ANOVA.
Hypoxia proliferation
Comparisons of conditions were performed by ANOVA with appropriate post-hoc analysis for significance. Significance was considered at a value of p ≤ 0.05.
Results
Shh expression and response to hypoxia in primary cell cultures
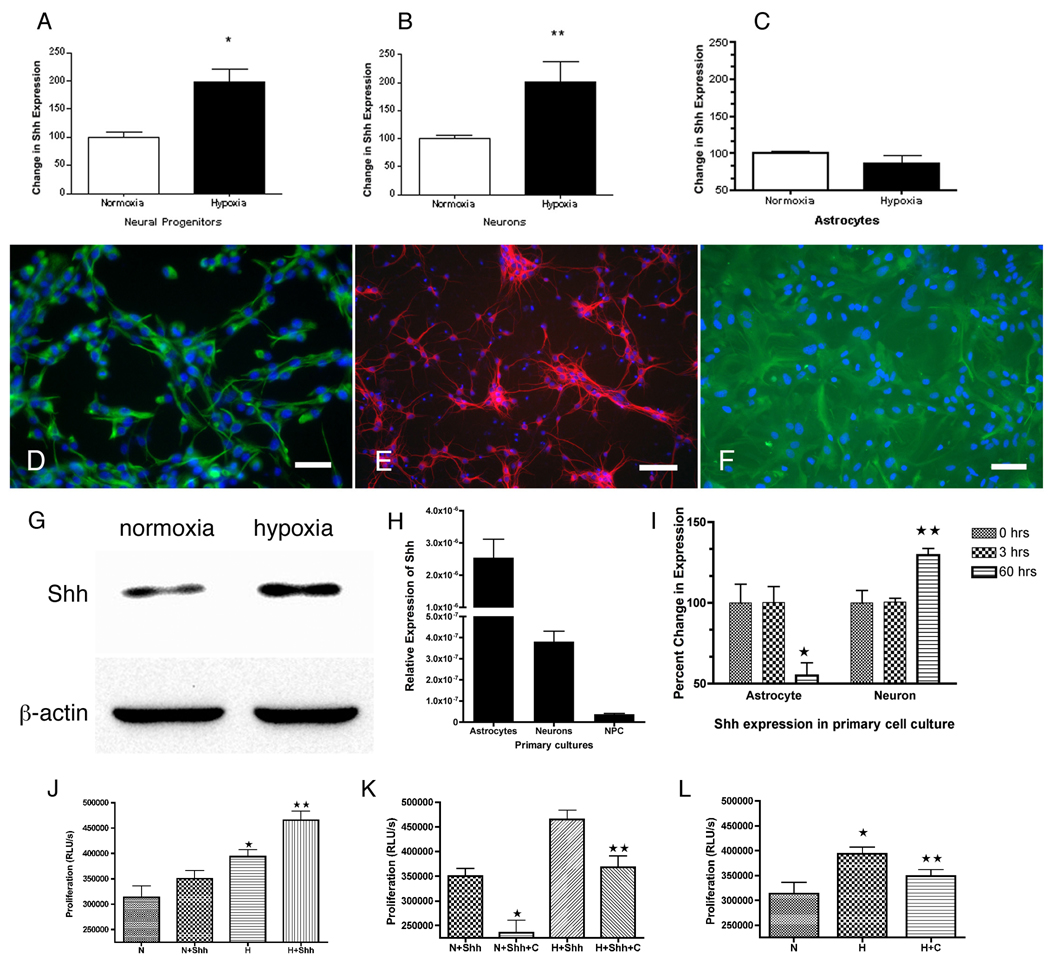
We sought to determine if Shh was upregulated in primary cell cultures exposed to hypoxia. We exposed neurons, astrocytes and NPCs to hypoxia (1% O2) for 4 hours and 16 hours. There were no changes in Shh in any of these cultures over the 4-hour exposure period (data not shown). However, there were significant increases in Shh mRNA in both NPCs and neurons, but not astrocytes, after 16 hours of hypoxia (Fig. 1). A lactate dehydrogenase assay demonstrated no evidence of cell death in hypoxic cultures compared to controls. Shh protein levels were also increased in NPC in response to hypoxia at this time point (Fig. 1G). In addition, neuronal cultures treated with cobalt chloride, a hypoxia-mimicking agent, also increased Shh protein levels (data not shown). When absolute levels of expression were compared, astrocytes expressed levels of Shh at much higher levels than neurons or NPC (Fig. 1H). So we tested whether a more intense hypoxic stimulus (0.1% O2 for 3 hours) would also stimulate increased Shh expression in astrocytes. Our results show that despite high levels of Shh expression in glia, they do not increase Shh expression when exposed to hypoxia (Fig. 1F). These results suggest that hypoxia upregulates Shh in neurons or neural progenitors but not in astrocytes.
Figure 1.
Shh expression in primary cell culture. A–C, Real-time PCR for Shh gene expression after 16hr exposure to 1% oxygen. Hypoxia induces significant increases in Shh in neural progenitor (A) and neurons (B) but not astrocytes (C) (mean ± SEM, *p=0.001, **p=0.01, n=12). Purity of primary cultures >95% for NPC (D: nestin, green), neurons (E: microtubule associated protein-2, red) and glia (F: glial fibrillary acidic protein, green) Bar=20µm. G, Immunoblot demonstrates Shh protein increased in neural progenitors after hypoxia (n=2). Hypoxia increases the Shh band's relative optical density after normalized to actin by 1.9 fold. H, Astrocytes in primary culture express Shh gene at 100 fold greater than NPC (mean ± SEM, n=10). I, Severe short-term hypoxia (0.1% oxygen) for 3 hours does not induce Shh in astrocytes at 3 hours nor at later time point as seen in neurons (mean ± SEM, *p<0.05, **p<0.01, n=6). Effects of hypoxia, Shh, and cyclopamine on the proliferation of NPCs (J–L). NPCs were pulsed with BrdU during exposure to various conditions. BrdU incorporation was measured by ELISA chemiluminescence (relative light units, RLUs). J, Shh and hypoxia additively increase proliferation (p<0.05, * compared to N, ** compared to H). K, Cyclopamine inhibits both Shh and Shh with hypoxia-induced proliferation (p<0.05, * compared to N+Shh, ** compared to H+Shh). L, Cyclopamine inhibits hypoxia-induced proliferation (p<0.05, * compared to N, ** compared to H). N= normoxia, H= 1% oxygen, C= cyclopamine. Shh 20 nmol/L, cyclopamine 5 µmol/L, (mean ± SEM, n= 6 per condition).
Our results above demonstrate that Shh gene expression and protein levels increase in neural progenitors after hypoxia. We then tested whether adding exogenous recombinant Shh increased proliferation of neural progenitors under hypoxia, and whether this hypoxia-induced proliferation could be blocked with the Shh pathway specific inhibitor, cyclopamine. NPCs were exposed to normoxia or hypoxia (1% O2 for 16 hours) and treated with 20 nmol/L Shh and/or 5 µmol/L cyclopamine. Indeed, hypoxia did increase proliferation of neural progenitors. Also, hypoxia and exogenous Shh had an additive effect on proliferation (Fig. 1J–L). Cyclopamine reduced the proliferation stimulus of hypoxia or Shh and their combined treatment.
MCAO induces a transient but functional hypoxia in the hippocampus
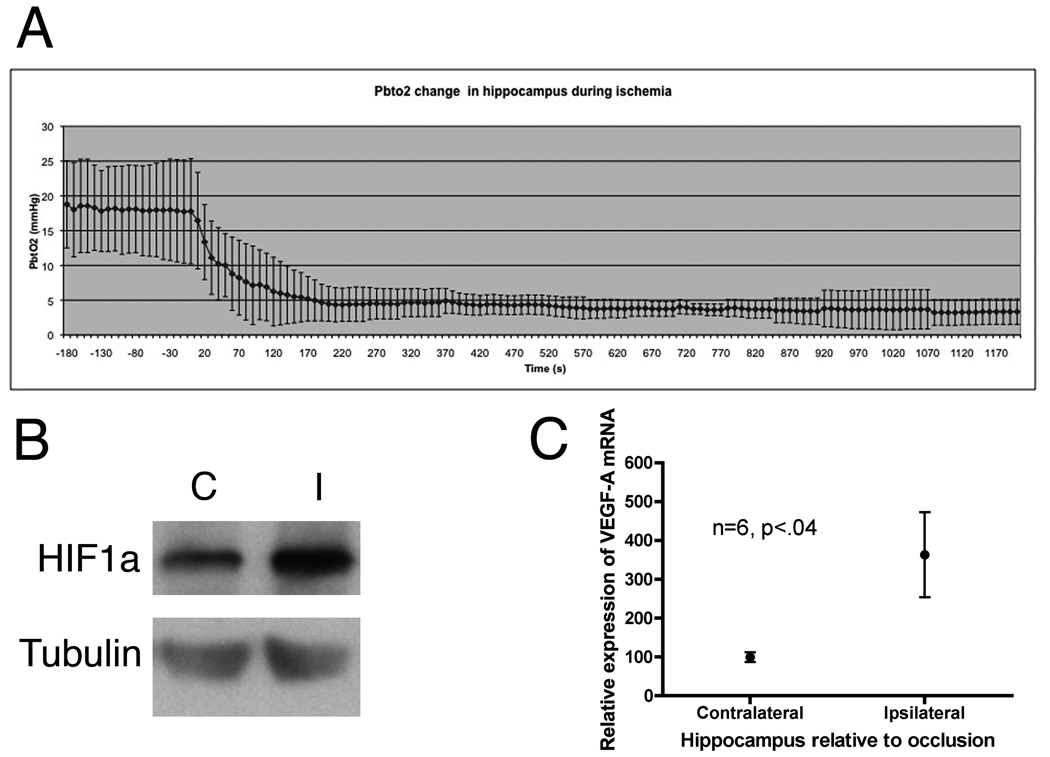
Our above data suggested that in vitro NPC are responsive to hypoxia through upregulation of Shh and that Shh might modulate hypoxia-induced proliferation. We developed a mouse model of mild ischemia, in which the middle cerebral artery is occluded (MCAO) for 20 minutes and then reperfused. Occlusion of the middle cerebral artery in the mouse causes a transient hypoxia as measured with an intra-hippocampal oxygen probe placed at the level of the CA3 and hilus (Fig. 2A). The mean partial pressure of oxygen in brain tissue (PbtO2) at the CA3 prior to MCAO was 17.22 ± 7.1 mmHg, during ischemia the mean PbtO2 was 3.98 ± 2 mmHg. Brain temperature before and during MCAO was unchanged, 36.9 ± 0.8 and 37.54±1.2 °C, respectively. As a measure of functional hypoxia within the hippocampus we analyzed whether hypoxia-inducible factor 1α (HIF1α), a protein, which increases in the setting of low oxygen tension, was altered after hypoxia. After 3 hours, following a 20-min MCAO, the HIF1α increased and average of 2.7 fold in the hippocampus (Fig. 2B). Vascular endothelial growth factor A (VEGF-A) is a classical gene transcribed by the transcription factor HIF1α. We demonstrated that 6 hours after the hypoxic/ischemic event the mRNA levels of VEGF-A increased an average of 360% over the contralateral control hippocampus (Fig. 2C). These results support the concept that the transient ischemia induces a hypoxic stress on the hippocampus in our MCAO model.
Figure 2.
Hippocampal hypoxia. A, oxygen tension in the hippocampus drops from an average baseline of 17mmHg to 4mmHg during occlusion of the middle cerebral artery (PbtO2= brain tissue oxygen). B, 3 hours after reperfusion HIF1α protein in the hippocampus is increased over 3 fold (n=3, C=contralateral, I=ipsilateral). C, VegfA transcription as measured by real-time PCR is increased 6 hours after reperfusion.
MCAO upregulates Shh gene and protein expression in the hippocampus
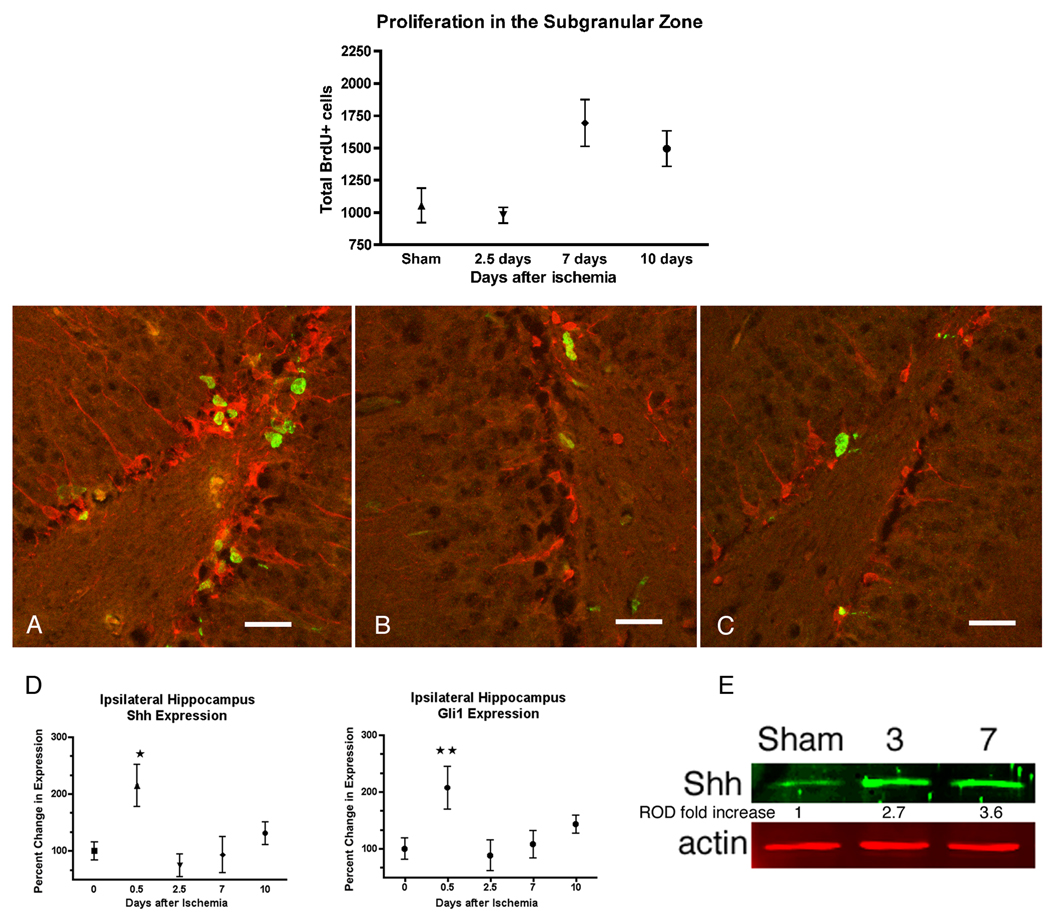
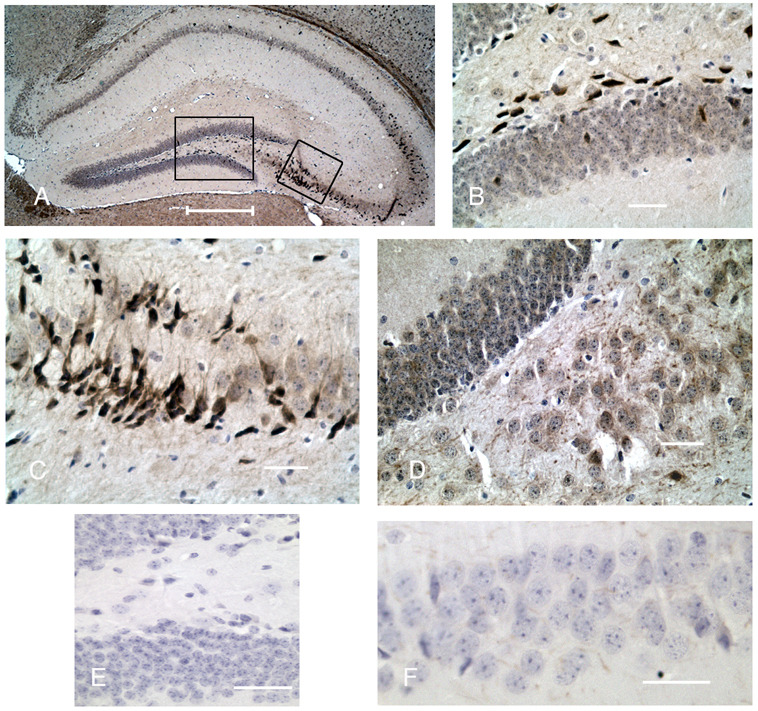
To determine whether the hippocampal hypoxia induced a proliferative response similar to our in vitro model, proliferating hippocampal NPCs were pulse-labeled with BrdU over a 12-hour period, following injury at specified times, then the animals were immediately sacrificed. This method allowed us to quantitate NPC in S-phase in the subgranular zone. In this model there is a significant increase in proliferation in the subgranular zone seen at 7 and 10 days after injury (Fig 3). These proliferating subgranular cells are almost entirely doublecortin positive and represent newly generated migrating neurons (Fig. 3A–C). With this model, we next examined Shh gene expression and its pathway constituents, smoothened (Smo), patched (Ptc), and Gli1 after ischemia within ipsilateral and contralateral hippocampi. We analyzed 4 time periods, 0.5, 2.5, 7 and 10 days after ischemia and compared gene expression to sham operated controls. There was a transient increase in Shh and its transcription factor Gli1 confined to the ipsilateral hippocampus at 12 hours (Fig. 3D). There were no significant changes in Smo or Ptc mRNA in the ipsilateral or contralateral hippocampus (data not shown). Shh protein levels were increased approximately 3-fold in the ipsilateral hippocampi 3 and 7 days after ischemia (Fig. 3E). We confirmed this upregulation by immunohistochemistry and showed that Shh was predominantly expressed in cells within the CA3 and the subgranular region (Fig. 4). We performed double immunohistochemistry for Shh and BrdU to determine if upregulation of Shh occurred in proliferating cells. At 3, 7 (not shown) or 10 days after ischemia, there were no cells that colocalized BrdU and Shh in the subgraular zone or hilus region (Fig. 5). Double immunostaining for NeuN or GFAP demonstrated that almost all Shh positive cells were NeuN positive (only 5% of Shh cells do not label with NeuN) and there was no colocalization with GFAP for any Shh positive cell (Fig. 6). These data suggest that mature neurons are likely the source of ischemia-induced Shh upregulation.
Figure 3.
Ipsilateral subgranular zone proliferation and Shh gene expression after ischemia. Ischemia induces an increase in subgranular zone neural progenitors 7 and 10 days after ischemia as measured by a 12 hour pulse of BrdU 50mg/kg prior to sacrifice (ANOVA p<0.02, mean ± SD, n=3). These newly born subgranular cells (BrdU, green nuclei) colocalize with immature neuronal marker doublecortin (red), 7 days after 20 minutes of middle cerebral artery occlusion. A, dentate ipsilateral to ischemia demonstrates increased colabeling of BrdU (green) and doublecortin (red) compared to the contralateral dentate (B) and the ipsilateral side of a sham animal (C). Scale bar, 60 µm. D, Relative mRNA expression measured by real-time PCR normalized to baseline expression at time 0 (sham) shows early increases in Shh and Gli1 (*p<0.008 and **p<0.02, compared to time 0, respectively; mean ± SEM, n=6). E, Representative fluorescent immunoblot demonstrates a significant 3 fold increase in Shh protein by 7 days (p=0.0025; n=4 for sham and 7 days, n=2 for 3 days) (ROD, relative optical density).
Figure 4.
Shh expression 3 days after ischemia. Shh immunostained with DAB on paraffin embedded tissue and counterstained with hematoxylin shows increased expression within the CA3 and subgranular zone after ischemia. A, ipsilateral hippocampus at low magnification demonstrating distribution of DAB immunostaining predominating in CA3 and subgranular layer of the dentate. B, higher magnification of large box in A, shows subgranular staining with a few intragranular and hilar stained cells. C, higher magnification of small box in A, shows CA3 neurons with Shh staining processes. D, contralateral hippocampus of same mouse shows a more diffuse and lower level of staining throughout the CA3 and dentate. E, negative control of dentate and hilus; F, negative control of CA3 neurons. Bar= 300 µm in A and 50 µm in B, C, D, E and F.
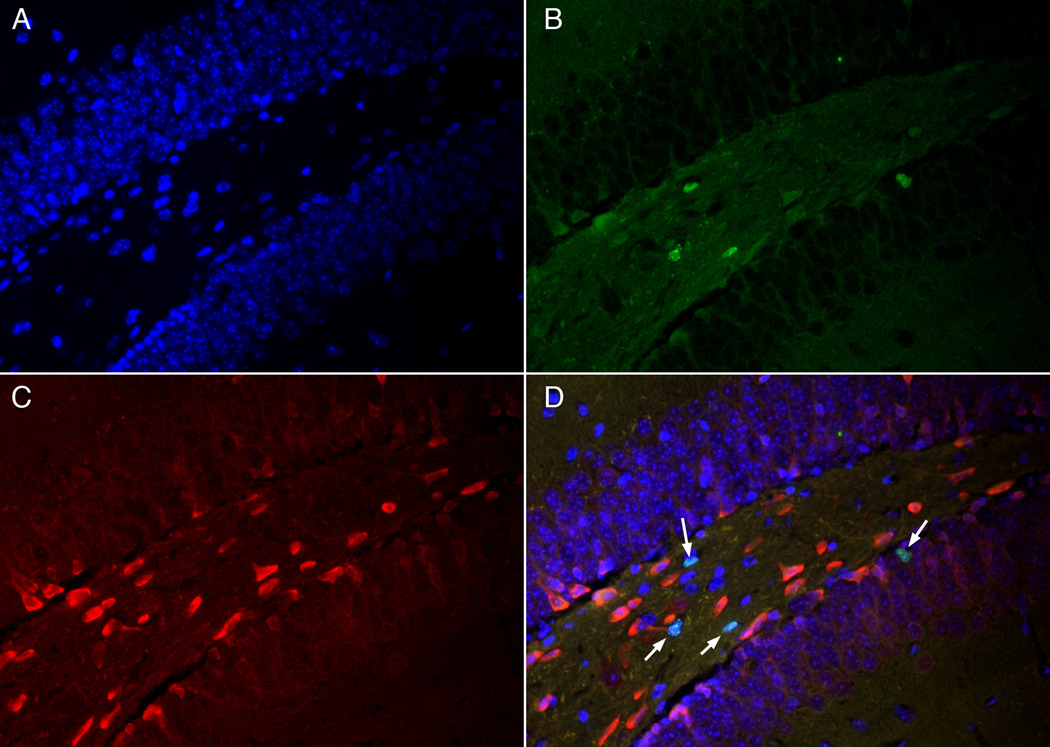
Figure 5.
Shh/BrdU double immunohistochemistry 10 days after ischemia. There are no cells that colabel with BrdU and Shh 10 days after ischemia. There are four BrdU+ cells in panel B marked by arrows in Panel D (merged image) and none of them colocalized with Shh in panel C. Panel A shows DAPI counterstain for all nuclei.
Figure 6.
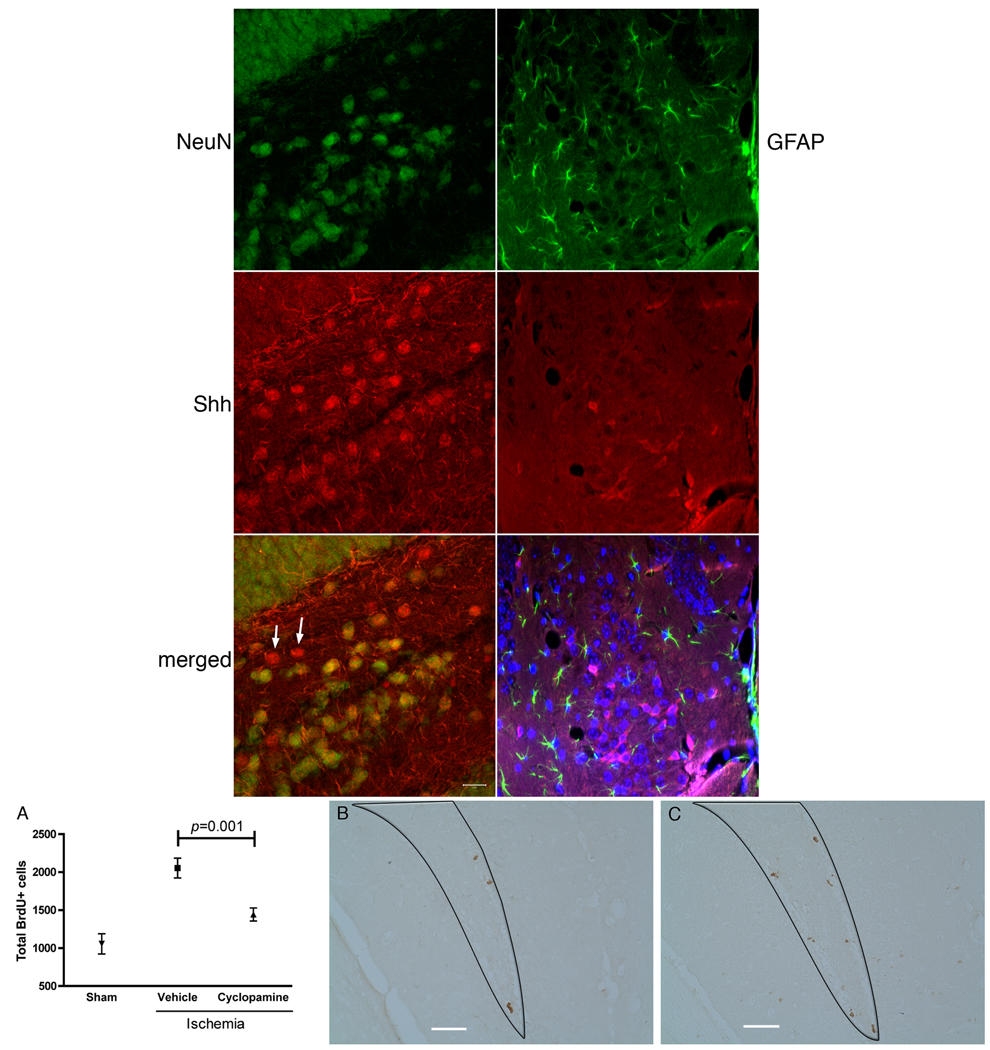
Shh is produced by mature neurons and inhibition decreases ischemia-induced proliferation. Shh/NeuN or GFAP double immunohistochemistry 7 days after ischemia. Almost all Shh+ cells (red) except for a few (noted by arrows) colocalize the mature neuronal marker NeuN (green) and no cells localize with GFAP (green). Cyclopamine blocks ischemia-induced NPC proliferation in the subgranular zone. A, Vehicle group demonstrates ischemia-induced proliferation compared to sham. Cyclopamine (60 pmol/day) blocked the ischemia-induced proliferation (mean ± SD, n=4). B, (cyclopamine) and C (vehicle), representative photomicrographs of BrdU immunohistochemistry after ischemia of the subgranular zone demarcated by line (Bar=100µm).
Inhibition of Shh blocks proliferation after ischemia
In the developing cerebellum, Shh is released from mature Purkinje neurons and induces proliferation of immature external granule neurons.2 We sought to test whether a similar system might be present in the dentate gyrus, where mature neurons may stimulate progenitor proliferation. Above we showed that Shh and hypoxia can increase NPC proliferation and that this proliferation can be blocked by cyclopamine, a specific inhibitor of the Shh pathway. Using the same mouse model of ischemia, we chronically infused cyclopamine or vehicle via osmotic pump into the lateral ventricle starting 3 days after ischemia. Following 7 days of cyclopamine infusion, proliferating hippocampal NPCs were pulse-labeled with BrdU as described above. Compared to the sham-operated group, the vehicle-treated ischemic group showed a 95% increase in proliferation, 10 days after ischemia. Treatment with cyclopamine significantly reduced the ischemia-induced proliferation by 30% (Fig. 6). Our results indicated that cyclopamine could suppress the ischemia-induced proliferation response in the subgranular progenitors.
Discussion
We have provided evidence that both NPCs and neurons upregulate Shh in response to hypoxia, whereas astrocytes do not. NPC also respond with increased proliferation to hypoxia and Shh. We show that upregulation in the hippocampus after ischemia is predominantly limited to the ipsilateral side to the ischemia. This upregulation is limited to CA3, the hilus and subgranular zone. Double immunohistochemistry suggests that neurons within these regions overwhelmingly account for the increased Shh. However, the Shh pathway inhibitor can reduce hypoxia and ischemia-induced NPC proliferation. We believe this study is the first to provide evidence supporting a role for Shh in ischemic brain injury. Similar to previous studies of ischemic muscle11 and lung epithelial injury,12 we too have shown that Shh and its transcription factor, Gli1, are upregulated after ischemia.
Unlike our in vitro results, Studer et al.16 did not find Shh upregulated in NPCs after hypoxia. A few possible explanations for this discrepancy include our more sensitive detection technique of real-time PCR or our more severe hypoxia treatment (1±0.2% vs. 3±2% oxygen). Furthermore, they used mesencephalic neural progenitors, which may have phenotypic differences between our cortically derived progenitors. They reported a similar increase in proliferation in cortical progenitors exposed to hypoxia but did not comment on Shh changes in these cells. Interestingly, their results showed that hypoxia increased dopaminergic differentiation of NPCs. Dopaminergic differentiation is a function in which Shh is known to play a significant role.17 It has been well established that Shh induces a dose dependent proliferation in nestin positive stem cell/progenitors in in vitro cultures1, 4, 9 as well as in an overexpression model of Shh in vivo.7 We now build on this observation by demonstrating that both hypoxia and Shh are additive and that the hypoxic-induction of proliferation of these nestin positive progenitors is partially Shh dependent. The mechanisms of interaction between Shh and hypoxia remain to be elucidated. Recently, Hwang et al.18 also showed that hypoxia upregulates Shh in cardiomyoblasts and that there is likely an interaction between Shh and hypoxia-inducible factor-1 alpha (HIF1a). Our previous results suggested that this interaction is not through a direct stimulation of VEGF by Shh and thus if there is a HIF1a interaction it is not directly through VEGF.19 There may be an interaction with angiopoietins.19, 20
It is unclear by what mechanism ischemia/hypoxia upregulates Shh. The mechanism is currently a focus of investigation. The increase in expression is likely to involve an intermediate regulatory step, for a yet unidentified, transcription factor, which transcribes Shh; given that increased gene expression is not seen within the first 4 hours. Another possibility includes an increase in Shh mRNA stability under ischemic/hypoxic conditions. We have assumed that Shh protein upregulation seen in neural progenitors in culture and within the hippocampus is largely due to increased translation, because we observed increased Shh mRNA. However, we should not rule out the possibility that the Shh protein increase is due to altered stability or degradation.
It is interesting to note that despite evidence of in vitro upregulation of Shh in NPCs, we were unable to detect Shh by immunohistochemistry in proliferating neuronal progenitors. One possibility is that the in vitro conditions do not accurately reflect conditions of subgranular zone progenitors. Another possibility is that NPC express such low levels of Shh that we are unable to detect such an increase with immunohistochemical investigation. Another in vitro versus in vivo discrepancy exists with the very high levels of Shh in proliferating glial cultures. However, we were unable to detect any Shh signal in GFAP stained cells. It is likely that the proliferative conditions of 10% FBS must alter this level of expression. This is contrasted to both neuronal and NPC cultures, which are maintained in serum-free defined media.
We note that the highest levels of Shh expression in the hippocampus both at baseline and after ischemia occur in the subgranular zone and the CA3 region. It is unclear why Shh is limited to these two regions. It is possible that Shh produced in the CA3 region might play a role in regulating axonal guidance6 from the newborn granular neurons of the dentate, since the CA3 region receives the mossy fiber output from the granular neurons of the dentate.21 Another possibility is that the CA3-dentate system is similar to the cerebellar system, where the Purkinje neuron releases Shh as a mitogen for the cerebellar granular neurons.2 If the latter proposal were true, then CA3 neurons would also release Shh as a mitogen for the subgranular progenitors. A final possibility is that the subgranular neurons transport Shh down their axons to the CA3 region, similar to the system of retinal ganglion cells and supracollicular neurons.22, 23 All of these hypotheses remain to be tested.
Summary
We have provided evidence that Shh plays a role in regulating ischemia-induced neural progenitor proliferation. This response can be blocked in vitro and in vivo and can be enhanced, at least in vitro. Modulation of ischemia-induced proliferation, survival, differentiation and reconnection through small molecule modulators or proteins requires further work in vivo if augmented progenitor proliferation will serve as a mechanism for future stroke recovery therapy.
Acknowledgments
Real-time PCR work was supported by a NIH Nucleic Acid Quantitation Core Grant NS045776. Image analysis data obtained with NIH core grant support P30 NS045776.
Funding: This work was supported by grants from the Stanely J Sarnoff Cardiovascular Foundation (J.R.S), Craig H. Neilsen Foundation (J.R.S.), American Heart Association, National Scientist Development Grants, 0335154N (J.Q.) and 0535138N (J.R.S.), NIH K08 NS049241 (J.R.S) and NIH Interdepartmental Stroke Program Project 5 P50 NS10828 (M.A.M).
Footnotes
Conflicts on Interest Disclosures
There are no conflicts of interest for authors.
Author Contributions
JRS is primary author of the manuscript, provided resources and performed most of the experiments as well as designed the experimental protocols.
SWL performed cell culture, PCR and western blot experiments.
KT and ZZ performed murine ischemia model.
JQ performed HIF1a and VEGF analysis and assisted in writing manuscript and experimental design
JX performed the hippocampal oxygen sensor experiments
MAM provided oversight, resources, experimental design and manuscript preparation.
References
- 1.Rowitch DH, B SJ, Lee SM, Flax JD, Snyder EY, McMahon AP. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J Neurosci. 1999;19:8954–8965. doi: 10.1523/JNEUROSCI.19-20-08954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by sonic hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 3.Wang YP, Dakubo G, Howley P, Campsall KD, Mazarolle CJ, Shiga SA, Lewis PM, McMahon AP, Wallace VA. Development of normal retinal organization depends on sonic hedgehog signaling from ganglion cells. Nat Neurosci. 2002;5:831–832. doi: 10.1038/nn911. [DOI] [PubMed] [Google Scholar]
- 4.Zhu G, Mehler MF, Zhao J, Yu Yung S, Kessler JA. Sonic hedgehog and BMP2 exert opposing actions on proliferation and differentiation of embryonic neural progenitor cells. Dev Biol. 1999;215:118–129. doi: 10.1006/dbio.1999.9431. [DOI] [PubMed] [Google Scholar]
- 5.Thibert C, Teillet MA, Lapointe F, Mazelin L, Le Douarin NM, Mehlen P. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science. 2003;301:843–846. doi: 10.1126/science.1085405. [DOI] [PubMed] [Google Scholar]
- 6.Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- 7.Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 8.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 9.Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 11.Pola R, Ling LE, Aprahamian TR, Barban E, Bosch-Marce M, Curry C, Corbley M, Kearney M, Isner JM, Losordo DW. Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation. 2003;108:479–485. doi: 10.1161/01.CIR.0000080338.60981.FA. [DOI] [PubMed] [Google Scholar]
- 12.Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 13.Teramoto T, Qiu J, Plumier JC, Moskowitz MA. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J Clin Invest. 2003;111:1125–1132. doi: 10.1172/JCI17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, Bakowska JC, Breakefield XO, Moskowitz MA. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci U S A. 2001;98:5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time rt-pcr. Biotechniques. 2002;32:1372–1374. 1376, 1378–1379. [PubMed] [Google Scholar]
- 16.Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of cns precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hynes M, Porter JA, Chiang C, Chang D, Tessier-Lavigne M, Beachy PA, Rosenthal A. Induction of midbrain dopaminergic neurons by sonic hedgehog. Neuron. 1995;15:35–44. doi: 10.1016/0896-6273(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 18.Hwang JM, Weng YJ, Lin JA, Bau DT, Ko FY, Tsai FJ, Tsai CH, Wu CH, Lin PC, Huang CY, Kuo WW. Hypoxia-induced compensatory effect as related to Shh and HIF-1alpha in ischemia embryo rat heart. Mol Cell Biochem. 2008;311:179–187. doi: 10.1007/s11010-008-9708-6. [DOI] [PubMed] [Google Scholar]
- 19.Lee SW, Moskowitz MA, Sims JR. Sonic hedgehog inversely regulates the expression of angiopoietin-1 and angiopoietin-2 in fibroblasts. Int J Mol Med. 2007;19:445–451. [PubMed] [Google Scholar]
- 20.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner DA, Buhl EH, Hailer NP, Nitsch R. Morphological features of the entorhinal-hippocampal connection. Prog Neurobiol. 1998;55:537–562. doi: 10.1016/s0301-0082(98)00019-7. [DOI] [PubMed] [Google Scholar]
- 22.Charytoniuk D, Porcel B, Rodriguez Gomez J, Faure H, Ruat M, Traiffort E. Sonic hedgehog signalling in the developing and adult brain. J Physiol Paris. 2002;96:9–16. doi: 10.1016/s0928-4257(01)00075-4. [DOI] [PubMed] [Google Scholar]
- 23.Traiffort E, Moya KL, Faure H, Hassig R, Ruat M. High expression and anterograde axonal transport of aminoterminal sonic hedgehog in the adult hamster brain. Eur J Neurosci. 2001;14:839–850. doi: 10.1046/j.0953-816x.2001.01708.x. [DOI] [PubMed] [Google Scholar]