Abstract
Background: Obesity affects almost one-third of pregnant women and causes many complications, including neural tube defects. It is not clear whether the risk of congenital heart defects, the most common malformations, is also increased.
Objective: This study was conducted to determine whether obesity is associated with an increased risk of congenital heart defects.
Design: A population-based, nested, case-control study was conducted in infants born with congenital heart defects and unaffected controls from the cohort of all births (n = 1,536,828) between 1993 and 2003 in New York State, excluding New York City. The type of congenital heart defect, maternal body mass index (BMI; in kg/m2), and other risk factors were obtained from the Congenital Malformations Registry and vital records. Mothers of 7392 congenital heart defect cases and 56,304 unaffected controls were studied.
Results: All obese women (BMI ≥ 30) were significantly more likely than normal-weight women (BMI: 19–24.9) to have children with a congenital heart defect [odds ratio (OR): 1.15; 95% CI: 1.07, 1.23; P < 0.0001]. Overweight women were not at increased risk (OR: 1.00; 95% CI: 0.94, 1.06). The risk in morbidly obese women (BMI ≥ 40) was higher (OR: 1.33; 95% CI: 1.15, 1.54; P = 0.0001) than that in obese women with a BMI of 30–39.9 (OR: 1.11; 95% CI: 1.04, 1.20; P = 0.004). There was a highly significant trend of increasing OR for congenital heart defects with increasing maternal obesity (P < 0.0001). The offspring of obese women had significantly higher ORs for atrial septal defects, hypoplastic left heart syndrome, aortic stenosis, pulmonic stenosis, and tetralogy of Fallot.
Conclusions: Obese, but not overweight, women are at significantly increased risk of bearing children with a range of congenital heart defects, and the risk increases with increasing BMI. Weight reduction as a way to reduce risk should be investigated.
See corresponding editorial on page 1539.
INTRODUCTION
In the United States, almost one-third of women of childbearing age are obese (1), and obesity rates are now a major global problem (2). Obesity is associated with many pregnancy complications (3), including neural tube defects. Obesity may increase a woman's risk of having a child with a congenital heart defect, the commonest group of birth defects, but the evidence is inconsistent (4–12). Almost no studies have had sufficient numbers of subjects to investigate obesity or overweight as risk factors for most individual cardiac malformations (13) or to determine what the upper limit for safe body mass index (BMI; in kg/m2) is, if there is an increased risk. These are important public health issues because of the high proportion of overweight and obese women of childbearing age.
We used data from the New York State Congenital Malformations Registry (CMR), a large population-based registry, to determine whether being overweight or obese increases a woman's risk of having a child with a congenital heart defect.
SUBJECTS AND METHODS
All women giving birth in New York State (excluding New York City, which did not collect data on BMI) between 1 January 1993 and 31 December 2003 formed the cohort for this study (n = 1,536,828). A nested case-control study was performed. Cases were live-born infants with a major congenital heart defect, and controls were live-born infants without any major malformations. Cases and control subjects were frequency matched by region of the state and calendar year and month of the child's birth by using a ratio of 4 controls to 1 case.
In all, 17,250 children with congenital heart defects were identified and matched to 69,000 controls. Cases were excluded for the following reasons: the child was a multiple birth (n = 1361) or the child had a genetic syndrome or chromosomal defect (n = 1212). Thus, congenital heart defect cases for which the etiology was unlikely to be related to obesity were dropped. Information on exposure to potentially teratogenic drugs was not available. Several groups of defects were dropped: variants of questionable importance, including patent foramen ovale (n = 1603), patent ductus arteriosus (n = 1255), and minor valve problems (n = 884); nonstructural defects such as hypertrophy and myopathy (n = 323); peripheral pulmonary artery stenosis (n = 1200); and miscellaneous defects (n = 1341). Note that some subjects had more than one exclusion criterion. Data on BMI were not available for 2806 mothers. Both cases and controls who were missing BMI data were significantly more likely than those with data to be nonwhite, to deliver preterm, to have a low-birth-weight infant, or to have a multiple birth (all P < 0.0001). Because diabetes is a known teratogen, the data were analyzed without mothers who were reported to have diabetes mellitus (n = 459). Because diabetes can result from obesity, a secondary analysis was performed, including the diabetic mothers to determine whether it would change the results.
Data on congenital heart defects came from the CMR. The methods by which cases are identified by the Registry were reported in detail previously (14). In brief, the CMR has been in existence since 1983 and covers the entire state of New York. The CMR relies on reporting of children with major malformations to the CMR as mandated by law, and the CMR staff undertake several measures to monitor the completeness of reporting. The CMR receives reports on ≈10,000 children each year. Beginning in 1992, the CMR began to use the British Pediatric Association coding, which allows for greater specificity than ICD-9 (International Statistical Classification of Diseases and Related Health Problems) coding. CMR staff perform the coding based on narrative descriptions of the birth defects reported by hospitals. Children reported to the CMR are matched to their birth certificate, and these data supplement the information from the CMR report. A capture-recapture analysis showed that the CMR was ≈87% complete for major malformations in general (15). A similar estimate of 89% was recently obtained after CMR reports were matched with reports from the active case ascertainment of the National Birth Defects Prevention Study in New York (C Druschel, unpublished data, 2002).
Birth certificates provided other data on cases and controls. These data included maternal height in feet and inches, prepregnancy weight in pounds for calculating BMI, demographic information, maternal smoking, maternal alcohol use, method of payment for medical care, and maternal diabetes mellitus. Alcohol use was underreported (1%), so the data were analyzed with and without alcohol use as a variable. Because the inclusion of alcohol use did not affect the results, the data are presented without alcohol use. The quality of reporting has been checked for diabetes. The sensitivity is 50%, and the specificity is 100%. The maternal weight distribution in the CMR data reflects the weight distribution in the population.
Congenital heart defects were classified on the basis of the methods used by the National Birth Defects Prevention Study (16). For this study, congenital heart defects were grouped to allow comparisons with the published literature. The groupings include all congenital heart defects and all septal defects (ventricular plus atrial). We examined all individual defects for which we had ≥100 affected individuals with maternal BMI data. Subjects who had more than one cardiac defect were analyzed in each group. Subjects with isolated, and multiple defects were examined together to increase power.
Obesity was defined as a BMI ≥30, overweight as a BMI between 25 and 29.99, and normal weight as a BMI between 19.0 and 24.99. Other categories—morbid obesity (BMI ≥40) and underweight (BMI < 19)—were also used in the analysis. Institutional Review Board approval from the New York State Health Department was obtained for this study.
Cases and controls were compared on demographic factors by using the chi-square test. Subjects who had more than one cardiac defect were included in each group. Statistical analysis was based on the number of subjects in each group so that individuals with multiple defects were counted only once. Unconditional logistic regression with case status as the dependent variable was performed. Independent variables were a 5-level categorical BMI variable (underweight, normal weight, overweight, obese, and morbidly obese), race-ethnicity, payment method and maternal education, smoking, alcohol, maternal age, and parity. Trends for increasing congenital heart defect rates with increasing weight were evaluated by a separate regression for congenital heart defects by using a continuous BMI term. For the figure, which illustrates the relation between BMI and odds ratios (ORs) for congenital heart defects, we fit a fourth order polynomial model. Overlaid on the same figure along with the fourth-order polynomial are the ORs and confidence intervals, ± 2 SE (SE), from a regression using each rounded integer of BMI against the same reference BMI of 22. BMI was calculated as prepregnancy weight (in kg) divided by height squared (in m). SAS (SAS Institute Inc, Cary, NC) version 9.1 was used for the statistical analysis.
RESULTS
After the exclusions described above, there were 7392 congenital heart defect cases and 56,304 control subjects available for study. Their characteristics are shown in Table 1. The case mothers were significantly more likely to be African American, to be primiparous, and to receive public payment for health care. The case mothers were less educated but did not differ significantly from the control mothers in maternal age, infant sex, or smoking. Their infants had significantly shorter mean gestational ages (P < 0.0001) than did infants of control mothers. The case mothers had significantly higher BMIs (P < 0.0001) than did the control mothers.
TABLE 1.
Characteristics of the case and control women1
| Cases (n = 7392) | Controls (n = 56,304) | P value2 | |
| n (%) | n (%) | ||
| Maternal age | |||
| <20 y | 616 (8.3) | 4703 (8.3) | |
| 20–24 y | 1363 (18.4) | 10,600 (18.8) | |
| 25–29 y | 2025 (27.4) | 15,348 (27.3) | |
| 30–34 y | 2102 (28.4) | 16,470 (29.2) | |
| 35–39 y | 1064 (14.4) | 7754 (13.8) | |
| ≥40 y | 222 (3.0) | 1429 (2.5) | NS |
| Maternal race | |||
| White | |||
| Hispanic | 616 (8.3) | 4960 (8.8) | |
| Non-Hispanic | 4279 (57.9) | 33,477 (59.5) | |
| Unknown | 1404 (19.0) | 10,173 (18.1) | |
| Black | 838 (11.3) | 5707 (10.1) | |
| Asian | 175 (2.4) | 1466 (2.6) | |
| Other | 37 (0.5) | 303 (0.5) | |
| Unknown | 43 (0.6) | 218 (0.4) | 0.0005 |
| Maternal education | |||
| <12 y | 1092 (14.8) | 7972 (14.2) | |
| 12 y | 2369 (32.0) | 17,433 (31.0) | |
| >12 y | 3805 (51.5) | 30,102 (53.5) | |
| Unknown | 126 (1.7) | 797 (1.4) | 0.01 |
| Primary payer | |||
| Medicaid | 2105 (28.5) | 14,429 (25.6) | |
| HMO | 2502 (33.9) | 21,408 (38.0) | |
| Private insurance | 2578 (34.9) | 18,669 (33.2) | |
| Self-pay | 111 (1.5) | 1078 (1.9) | |
| Unknown | 96 (1.3) | 720 (1.3) | <0.0001 |
| Tobacco use | |||
| No | 6159 (83.3) | 47,019 (83.5) | |
| Yes | 1172 (15.8) | 8848 (15.7) | |
| Unknown | 61 (0.8) | 437 (0.8) | NS |
| Alcohol use | |||
| No | 7249 (98.1) | 55,323 (98.3) | |
| Yes | 82 (1.1) | 535 (0.9) | |
| Unknown | 61 (0.8) | 446 (0.8) | NS |
| Infant sex | |||
| Male | 3806 (51.5) | 28,655 (50.9) | |
| Female | 3584 (48.5) | 27,648 (49.1) | |
| Unknown | 2 (0.0) | 1 (0.0) | NS |
| Birth weight | |||
| <1500 g | 428 (5.8) | 418 (0.7) | |
| 1500 to <2500 g | 836 (11.3) | 2158 (3.8) | |
| ≥2500 g | 6120 (82.8) | 53,702 (95.4) | |
| Unknown | 80 (0.1) | 26 (0.1) | <0.0001 |
| Gestational age | |||
| <32 wk | 459 (6.2) | 669 (1.2) | |
| 32 to <37 wk | 1039 (14.1) | 3806 (6.8) | |
| ≥37 wk | 5885 (79.6) | 51,772 (91.9) | |
| Unknown | 9 (0.1) | 57 (0.1) | <0.0001 |
| Parity | |||
| 0 | 3054 (41.3) | 22,646 (40.2) | |
| 1 | 2382 (32.2) | 19,098 (33.9) | |
| ≥2 | 1955 (26.4) | 14,559 (25.9) | |
| Unknown | 1 (0.0) | 1 (0.0) | 0.02 |
| BMI | |||
| <19 kg/m2, underweight | 569 (7.7) | 4424 (7.9) | |
| 19–24 kg/m2, normal weight | 3902 (52.8) | 30,561 (54.3) | |
| 25–29 kg/m2, overweight | 1605 (21.7) | 12,495 (22.2) | |
| 30–39 kg/m2, obese | 1084 (14.7) | 7496 (13.3) | |
| ≥40 kg/m2, morbidly obese | 232 (3.1) | 1328 (2.4) | <0.0001 |
HMO, health maintenance organization.
Comparison of proportions between cases and controls was made by using the chi-square test; unknown values were not included in the calculation of P values.
Obesity was strongly associated with congenital heart defects in the adjusted analysis. When all congenital heart defects were considered together, obesity (BMI 30–39.9; OR: 1.11; 95% CI: 1.04, 1.20; P = 0.004) and morbid obesity (BMI ≥40; OR: 1.33; 95% CI: 1.15, 1.54; P = 0.0001) were significant risk factors (Table 2).
TABLE 2.
Odds ratios and 95% CIs for congenital heart defects by categories of BMI (in kg/m2)1
| Underweight (BMI < 19) | Reference group (BMI 19–24) | Overweight (BMI 25–29) | Obese (BMI 30–39) | Morbidly obese (BMI ≥ 40) | All obese (BMI ≥ 30) | |
| Controls | ||||||
| n | 4424 | 30,561 | 12,495 | 7496 | 1328 | 8824 |
| All defects | ||||||
| n | 569 | 3902 | 1605 | 1084 | 232 | 1316 |
| aOR | 1.00 | — | 1.00 | 1.11 | 1.33 | 1.15 |
| 95% CI | 0.91, 1.10 | — | 0.94, 1.06 | 1.04, 1.20 | 1.15, 1.54 | 1.07, 1.23 |
| P value | 0.97 | — | 0.96 | 0.004 | 0.0001 | <0.0001 |
| Conotruncal | ||||||
| n | 73 | 452 | 200 | 121 | 34 | 155 |
| aOR | 1.09 | — | 1.08 | 1.08 | 1.70 | 1.17 |
| 95% CI | 0.85, 1.40 | — | 0.91, 1.28 | 0.88, 1.32 | 1.19, 2.43 | 0.97, 1.41 |
| P value | 0.50 | — | 0.38 | 0.47 | 0.003 | 0.10 |
| Transposition of the great vessels | ||||||
| n | 31 | 162 | 58 | 31 | 9 | 40 |
| aOR | 1.32 | — | 0.89 | 0.79 | 1.30 | 0.87 |
| 95% CI | 0.89, 1.95 | — | 0.66, 1.21 | 0.54, 1.17 | 0.66, 2.55 | 0.61, 1.23 |
| P value | 0.16 | — | 0.45 | 0.24 | 0.45 | 0.42 |
| Double outlet right ventricle | ||||||
| n | 8 | 55 | 12 | 15 | 6 | 21 |
| aOR | 0.96 | — | 0.53 | 1.09 | 2.47 | 1.30 |
| 95% CI | 0.45, 2.02 | — | 0.28, 0.99 | 0.61, 1.95 | 1.05, 5.80 | 0.78, 2.16 |
| P value | 0.91 | — | 0.046 | 0.76 | 0.04 | 0.32 |
| Tetralogy of Fallot | ||||||
| n | 28 | 198 | 105 | 60 | 18 | 78 |
| aOR | 0.95 | — | 1.28 | 1.20 | 2.02 | 1.32 |
| 95% CI | 0.63, 1.41 | — | 1.01, 1.62 | 0.89, 1.60 | 1.24, 3.29 | 1.01, 1.72 |
| P value | 0.78 | — | 0.045 | 0.23 | 0.005 | 0.04 |
| All septal defects | ||||||
| n | 364 | 2561 | 1014 | 661 | 144 | 805 |
| aOR | 0.99 | — | 0.96 | 1.04 | 1.27 | 1.08 |
| 95% CI | 0.89, 1.12 | — | 0.89, 1.04 | 0.95, 1.14 | 1.07, 1.52 | 0.99, 1.17 |
| P value | 0.92 | — | 0.34 | 0.36 | 0.008 | 0.08 |
| Atrial septal defects | ||||||
| n | 131 | 917 | 365 | 275 | 56 | 331 |
| aOR | 0.98 | — | 0.95 | 1.18 | 1.32 | 1.20 |
| 95% CI | 0.81, 1.18 | — | 0.84, 1.08 | 1.03, 1.35 | 1.00, 1.74 | 1.05, 1.36 |
| P value | 0.83 | — | 0.45 | 0.02 | 0.05 | 0.006 |
| Ventricular septal defects | ||||||
| n | 275 | 1929 | 757 | 457 | 103 | 560 |
| aOR | 1.00 | — | 0.96 | 0.97 | 1.23 | 1.01 |
| 95% CI | 0.88, 1.14 | — | 0.88, 1.05 | 0.87, 1.08 | 1.00, 1.52 | 0.92, 1.11 |
| P value | 0.97 | — | 0.40 | 0.57 | 0.05 | 0.86 |
| Left ventricular outflow tract obstruction | ||||||
| n | 59 | 380 | 177 | 139 | 25 | 164 |
| aOR | 1.09 | — | 1.15 | 1.51 | 1.52 | 1.51 |
| 95% CI | 0.82, 1.43 | — | 0.96, 1.38 | 1.24, 1.83 | 1.01, 2.29 | 1.25, 1.82 |
| P value | 0.56 | — | 0.12 | <0.0001 | 0.04 | <0.0001 |
| Hypoplastic left heart syndrome | ||||||
| n | 13 | 90 | 48 | 37 | 8 | 45 |
| aOR | 0.96 | — | 1.31 | 1.66 | 2.01 | 1.71 |
| 95% CI | 0.54, 1.73 | — | 0.92, 1.86 | 1.13, 2.45 | 0.97, 4.16 | 1.19, 2.46 |
| P value | 0.90 | — | 0.14 | 0.01 | 0.06 | 0.004 |
| Coarctation of the aorta | ||||||
| n | 26 | 187 | 82 | 59 | 10 | 69 |
| aOR | 1.00 | — | 1.09 | 1.31 | 1.25 | 1.30 |
| 95% CI | 0.66, 1.52 | — | 0.84, 1.42 | 0.97, 1.76 | 0.66, 2.37 | 0.98, 1.72 |
| P value | 0.99 | — | 0.52 | 0.08 | 0.50 | 0.07 |
| Aortic valve stenosis | ||||||
| n | 22 | 90 | 42 | 44 | 8 | 82 |
| aOR | 1.75 | — | 1.16 | 2.03 | 2.08 | 2.04 |
| 95% CI | 1.09, 2.81 | — | 0.80, 1.67 | 1.41, 2.92 | 1.00, 4.31 | 1.44, 2.88 |
| P value | 0.02 | — | 0.44 | 0.0001 | 0.05 | <0.0001 |
| Right ventricular outflow tract obstruction | ||||||
| n | 72 | 472 | 232 | 164 | 28 | 192 |
| aOR | 0.98 | — | 1.16 | 1.32 | 1.21 | 1.30 |
| 95% CI | 0.76, 1.26 | — | 0.99, 1.36 | 1.10, 1.58 | 0.82, 1.79 | 1.10, 1.55 |
| P value | 0.89 | — | 0.07 | 0.003 | 0.33 | 0.003 |
| Pulmonic valve stenosis | ||||||
| n | 61 | 390 | 188 | 135 | 24 | 159 |
| aOR | 1.00 | — | 1.13 | 1.30 | 1.24 | 1.29 |
| 95% CI | 0.76, 1.31 | — | 0.95, 1.35 | 1.06, 1.59 | 0.81, 1.88 | 1.07, 1.56 |
| P value | 0.99 | — | 0.18 | 0.01 | 0.32 | 0.008 |
| Anomalous pulmonary venous return | ||||||
| n | 10 | 67 | 16 | 15 | 1 | 16 |
| aOR | 0.94 | — | 0.57 | 0.87 | 0.33 | 0.79 |
| 95% CI | 0.48, 1.83 | — | 0.33, 0.98 | 0.50, 1.54 | 0.05, 2.39 | 0.46, 1.38 |
| P value | 0.84 | — | 0.04 | 0.64 | 0.27 | 0.41 |
| Total anomalous pulmonary venous return | ||||||
| n | 8 | 59 | 12 | 13 | 1 | 14 |
| aOR | 0.85 | — | 0.48 | 0.85 | 0.37 | 0.78 |
| 95% CI | 0.40, 1.79 | — | 0.26, 0.90 | 0.47, 1.56 | 0.05, 2.70 | 0.43, 1.41 |
| P value | 0.67 | — | 0.02 | 0.60 | 0.33 | 0.41 |
| Atrioventricular septal defect | ||||||
| n | 7 | 63 | 17 | 14 | 5 | 19 |
| aOR | 0.77 | — | 0.64 | 0.88 | 1.79 | 1.01 |
| 95% CI | 0.35, 1.70 | — | 0.37, 1.10 | 0.49, 1.57 | 0.71, 4.48 | 0.60, 1.70 |
| P value | 0.52 | — | 0.10 | 0.65 | 0.22 | 0.97 |
aOR, adjusted odds ratio. Logistic regression was used to calculate odds ratios and 95% CIs; all models were adjusted for maternal age, education, race, smoking, and payment method for health care. All P values were derived from a Wald chi-square test.
When individual defects were examined (Table 2), significant associations were found when we examined all women with a BMI ≥30 as a group. Their infants were at greater risk of all congenital heart defects, all left and all right ventricular outflow tract obstruction defects, atrial septal defects, hypoplastic left heart syndrome, aortic stenosis, pulmonic stenosis, and tetralogy of Fallot than was the normal BMI group. Despite the modest numbers in some categories, all septal defects, all conotruncal defects, all left and all right ventricular outflow tract obstruction defects, atrial septal defects, hypoplastic left heart syndrome, aortic valve stenosis, pulmonic valve stenosis, tetralogy of Fallot, and double outlet right ventricle were significantly more common in the offspring of obese women (BMI 30–39.9), morbidly obese women (BMI ≥40), or both groups when compared with offspring of normal-weight women (Table 2). Interestingly, coarctation of the aorta, which is related to aortic stenosis and hypoplastic left heart syndrome, was not associated, which suggests that either the study had insufficient power to find an effect or that different genetic or environmental factors affect the risk. Examination of the data for effects of ethnicity and infant sex showed no major changes in stratified analyses. Interaction terms for ethnicity and obesity were not statistically significant. A significant interaction (P = 0.03) was found for obesity and sex in the atrial septal defect group. It should be noted that small numbers in some cells limited our ability to find differences.
Offspring of women who were overweight, in contrast, were not at increased risk of all congenital heart defects (OR: 1.00; 95% CI: 0.94, 1.06; P = 0.96) or for any individual defects except tetralogy of Fallot (OR: 1.28; 95% CI: 1.01, 1.62; P = 0.045) and they were at significantly lower risk of total anomalous pulmonary venous return (OR: 0.48; 95% CI: 0.26, 0.90; P = 0.02) and double outlet right ventricle (OR: 0.53; 95% CI: 0.28, 0.99; P = 0.046). Underweight women were not at increased risk of all defects (OR: 1.00; 95% CI: 0.91, 1.10; P = 0.97) or for any individual defects except aortic valve stenosis (OR: 1.75; 95% CI: 1.09, 2.81; P = 0.02).
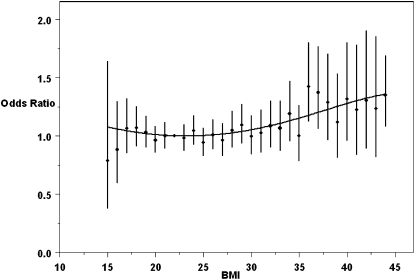
To determine the level at which high BMI becomes a risk factor for congenital heart defects in general, we plotted the ORs by BMI from the lowest underweight category to the most morbidly obese (Figure 1). These data indicate that there is a major increase in congenital heart defects beginning at a BMI of ≈30 and that the ORs become greater as BMI increases. Note that this effect was seen despite the fact that the all-defects category almost certainly includes some defects that are unrelated to obesity. The trend test for increasing odds of congenital heart defects with increasing BMI was highly significant (P < 0.0001).
FIGURE 1.
Odds ratios for all congenital heart defects by maternal BMI with a fourth-order polynominal model (curve). Odds ratios (•) and confidence bars (±2 SE) were generated by a regression equation with a BMI (in kg/m2) of 22 as the standard. n values for BMI categories ranged from 9 cases/83 controls for a BMI of 15 to 744 cases/5853 controls for a BMI of 22 (the reference category). Data from the 4 cases with BMIs <15 were excluded in this analysis, which resulted in a total n of 7388.
Examination of the women who had diabetes confirmed the previous observation that diabetic women are at increased risk of having children with congenital heart defects: 187 of 7579 (2.5%) cases compared with 412 of 56,716 (0.7%) controls (P < 0.0001) had diabetes. Addition of the diabetic women increased the association between obesity and congenital heart defects somewhat depending on the specific defect, but did not materially change the findings.
DISCUSSION
Our data show a strong association between maternal obesity and risk of congenital heart defects. This association was present not only for congenital heart defects as a group, but for numerous individual defects. The overall risk increased with increasing BMI so that morbid obesity was an even greater risk factor than obesity with BMIs between 30 and 39.99. Perhaps surprisingly, given the recent meta-analysis findings (13), overweight women were not at a significantly increased risk of all congenital heart defects as a group or of individual defects with one possible exception. Our large sample size provides reassurance that the risk in overweight women, if any, is small; the OR was 1.00, and the upper 95% CI was 1.06. We used standard categories to classify women by BMI. By these standard definitions, overweight women were not at increased risk; obese women clearly were. Our figure shows, however, that there was no dramatic change in risk at any given BMI; the risk increases gradually with increasing BMI. So women should be advised that normal BMI is best for avoiding risk.
Because obesity affects ≈30% of American women of childbearing age, and congenital heart defects are the most common type of birth defects, we estimate that obesity could account for ≈1500 additional defects each year (attributable fraction: 5%). In comparison, the folic acid supplementation and fortification program probably prevents between 1250 and 2000 defects each year. Thus, preventing obesity-related congenital heart defects could have an important public health effect.
Our data help to clarify a somewhat confusing literature. Whereas some studies have shown significantly increased risks of congenital heart defects in offspring of obese mothers, others have not. Shaw et al (4, 5), for example, found no increase in conotruncal defects (OR: 1.0; 95% CI: 0.6, 1.8) in general, or in tetralogy of Fallot or transposition of the great arteries in particular. Waller et al (6), in contrast, found a significant increase (OR: 6.2; 95% CI: 1.1, 27.4) in great vessel defects and a nonsignificant increase in septal defects as a group. Watkins et al (7, 8) reported ORs >1 for numerous defects; however, except for cardiac defects as a whole, none of the increases were statistically significant. Queisser-Luft et al (9) found increased rates of truncus arteriosus, coarctation of the aorta, and hypoplastic left heart, but only the first was statistically significant. In Sweden, where obesity was less common, Cedergren and (10) found significantly increased risks of all defects in the aggregate, ventricular septal defects and atrial septal defects, and a suggestive increase for transposition of the great arteries.
A recent study (11) expanding on findings (12) from the National Birth Defects Prevention Study is the only other study that reported on a wide range of defects. They did not find a significant (the 95% CI did not exclude 1) increase in the OR for all congenital heart defects in women whose BMI was between 30 and 35, but did find a significant increase in overweight women. As in our study, they found that obesity was associated with a significant increase in tetralogy of Fallot, hypoplastic left heart syndrome, pulmonary valve stenosis, and atrial septal defects. Unlike our study, they did not find an increase in all septal defects or aortic valve stenosis. They did not look for trends. Some differences in study design may help explain the divergent findings. The National Birth Defects Prevention Study had more detailed data on the defects and data on more potential risk factors. Their data on BMI were collected at interviews conducted 6 wk to 24 mo after the expected delivery date, and 31% of identified case mothers did not participate.
We, like other investigators, found that obesity (like diabetes mellitus) was associated with increased rates of defects thought to be caused by several different mechanisms. Both diabetes and obesity cause multiple metabolic perturbations. We suggest that the wide range of abnormalities obesity produces in carbohydrate and lipid metabolism, insulin resistance, and adipocyte hormone action may cause defects by more than one mechanism. Other explanations for the teratogenicity of obesity that merit investigation include genetic factors and dietary deficiencies.
Our study had notable strengths. New York has a statewide, population-based registry that identifies a large proportion of all major malformations. The study population came from a cohort of 1.5 million births providing us with a very large representative sample of congenital heart defect cases and controls. Our study has the largest number of cardiac defects published to date, giving us greater power to study individual defects. We were also able to provide estimates of risk based on a single large population, thus avoiding the problems of publication bias and heterogeneity that complicate the interpretation of meta-analyses. Note that our study, which used a consistent definition of obesity and a single population-based study group showed that overweight women were virtually not at increased risk, which was the opposite conclusion of the recent meta-analysis (13). The authors of the meta-analysis pointed out that there was heterogeneity and possible bias in the studies that they had available—a likely explanation for the contradictory results.
Our study had limitations as well. We were not able to interview the large number of women included in the study to identify potentially teratogenic drugs that can cause congenital heart defects, including isotretinoin and lithium, nor could we identify maternal phenylketonuria or some Mendelian defects that cause congenital heart defects unrelated to obesity. We did have data on diabetes, perhaps the most important maternal condition associated with congenital heart defects. Moreover, drug exposures would account for only a very small proportion of congenital heart defects, and we were able to exclude cases with obvious genetic causes for congenital heart defects, such as trisomies. Data from vital records are of variable quality. Fortunately, data were collected during the study period (1999) to validate many of these vital record variables (17). A comparison of data from the birth certificates with medical records showed that maternal prepregnancy weight recorded on the birth certificate was within ±5 lb (2.25 kg) of the weight recorded in the medical records in 87.2% of the women. Demographic variables such as race, age, and education were correct >95% of the time. Data on diabetes mellitus, a cause of both obesity and birth defects, were also obtained from vital records and could be misreported. The CMR depends on hospital reporting for the identification and characterization of malformations, although these have been shown to be accurate in 98% of the cases by internal audit of major malformations. Data on stillbirths and terminated pregnancies were not available. Both obesity and congenital heart defects are associated with a higher risk of stillbirth, which could have led us to underestimate the number of congenital heart defect cases associated with obesity. If women underestimate their weight, particularly if they are obese (18), it could also lead to an underestimation of the association between obesity and congenital heart defects. Thus, the true risk could be even greater than we found.
Our findings have important public health implications. The increasing risk that we identified with increasing BMI indicates that the “obesity epidemic” has put more women at risk because obesity and morbid obesity rates have increased. Conversely, it seems likely that weight loss can significantly reduce the risk of congenital heart defects by moving women from the morbidly obese to the obese group. Women who are able to reduce their BMIs (ie, move from the obese to the overweight group) may virtually eliminate the excess risk of bearing children with congenital heart defects. Studies are needed to determine whether weight loss before conception can reduce the risk of congenital heart defects.
Acknowledgments
The authors’ responsibilities were as follows—JLM: design, conduct, analysis, and manuscript preparation; and JT, MRC, CMD, and TC: conduct, analysis, and manuscript preparation. The authors had no financial or personal conflicts of interests.
REFERENCES
- 1.Ogden CL, Carroll MD, McDowell MA, Flegal KM. Obesity among adults in the United Statesmdashno statistically significant change since 2003-2004. NCHS Data Brief November 2007. Available from: www.cdc.gov/nchs/data/databriefs/db01.pdf (cited 29 March 2010) [PubMed]
- 2.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes 2008;32:1431–7 [DOI] [PubMed] [Google Scholar]
- 3.Scialli AR, Public Affairs Committee of the Teratology Society. Teratology Public Affairs Committee position paper: maternal obesity and pregnancy. Birth Defects Res A Clin Mol Teratol 2006;76:73–7 [DOI] [PubMed] [Google Scholar]
- 4.Shaw GM, Carmichael SL. Prepregnant obesity and risks of selected birth defects in offspring. Epidemiology 2008;19:616–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw GM, Todoroff K, Schaffer DM, Selvin S. Maternal height and prepregnancy body mass index as risk factors for selected congenital anomalies. Paediatr Perinat Epidemiol 2000;14:234–9 [DOI] [PubMed] [Google Scholar]
- 6.Waller DK, Mills JL, Simpson JL, et al. Are obese women at higher risk for producing malformed offspring? Am J Obstet Gynecol 1994;170:541–8 [DOI] [PubMed] [Google Scholar]
- 7.Watkins ML, Botto LD. Maternal prepregnancy weight and congenital heart defects in offspring. Epidemiology 2001;12:439–46 [PubMed] [Google Scholar]
- 8.Watkins ML, Rasmussen SA, Honein MA, Botto LD, Moore CA. Maternal obesity and risk for birth defects. Pediatrics 2003;111:1152–8 [PubMed] [Google Scholar]
- 9.Queisser-Luft A, Kieninger-Baum D, Menger H, Stolz G, Schlaefer K, Merz E. Does maternal obesity increase the risk of fetal abnormalities? Analysis of 20,248 newborn infants of the Mainz Birth Register for detecting congenital abnormalities. Ultraschall Med 1998;19:40–4 [DOI] [PubMed] [Google Scholar]
- 10.Cedergren MI, Källén BA. Maternal obesity and infant heart defects. Obes Res 2003;11:1065–71 [DOI] [PubMed] [Google Scholar]
- 11.Gilboa SM, Correa A, Botto LD, et al. Association between prepregnancy body mass index and congenital heart defects. Am J Obstet Gynecol 2010;202:51e1–51.e10 [DOI] [PubMed] [Google Scholar]
- 12.Waller DK, Shaw GM, Rasmussen SA, et al. Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediatr Adolesc Med 2007;161:745–50 [DOI] [PubMed] [Google Scholar]
- 13.Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA 2009;301:636–50 [DOI] [PubMed] [Google Scholar]
- 14.Sekhobo JP, Cross PK, Druschel CM. An evaluation of congenital malformations surveillance in NY State: an application of CDC guidelines for evaluating surveillance systems. Public Health Rep 2001;116:296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honein MA, Paulozzi LJ. Birth defects surveillance: assessing the “gold standard”. Am J Public Health 1999;89:1238–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Botto LD, Lin AE, Riehle-Colarusso T, Malik S, Correa A. National Birth Defects Prevention Study. Seeking causes and evaluating congenital heart defects in etiological studies. Birth Defects Res A Clin Mol Teratol 2007;79:714–27 [DOI] [PubMed] [Google Scholar]
- 17.IPRO Corporation Electronic birth certificate data validation study. Lake Success, NY: New York State Department of Health, 2001 [Google Scholar]
- 18.Brunner Huber LR. Validity of self-reported height and weight in women of reproductive age. Matern Child Health J 2007;11:137–44 [DOI] [PubMed] [Google Scholar]