Abstract
Background: Binge-eating disorder may represent a risk factor for the metabolic syndrome.
Objective: The objective was to assess longitudinally the relation between binge-eating disorder and components of the metabolic syndrome.
Design: At 2.5 and 5 y of follow-up, 134 individuals with binge-eating disorder and 134 individuals with no history of eating disorders, who were frequency-matched for age, sex, and baseline body mass index (BMI), were interviewed during the follow-up interval regarding new diagnoses of 3 metabolic syndrome components: hypertension, dyslipidemia, and type 2 diabetes.
Results: A comparison of individuals with and without a binge-eating disorder in analyses adjusted for age, sex, baseline BMI, and interval BMI change had hazard ratios (95% CIs) for reporting new diagnoses of metabolic syndrome components of 2.2 (1.2, 4.2; P = 0.023) for dyslipidemia, 1.5 (0.76, 2.9; P = 0.33) for hypertension, 1.6 (0.77, 3.9; P = 0.29) for type 2 diabetes, 1.7 (1.1, 2.6; P = 0.023) for any component, and 2.4 (1.1, 5.7; P = 0.038) for ≥2 components.
Conclusion: Binge-eating disorder may confer a risk of components of the metabolic syndrome over and above the risk attributable to obesity alone. This trial was registered at www.clinicaltrials.gov as NCT00777634.
INTRODUCTION
The metabolic syndrome is a cluster of related risk factors for atherosclerotic cardiovascular disease, including abdominal obesity, dyslipidemia, hypertension, and abnormal glucose metabolism (1–3); it represents a growing public health problem (4), Binge-eating disorder is a recently proposed psychiatric condition characterized by recurrent episodes of binge eating—consuming abnormally large amounts of food in a discrete period of time (eg, <2 h), associated with a sense of loss of control over eating. Binge-eating disorder is associated with subjective distress but lacks the purging (eg, self-induced vomiting or laxative abuse) seen in bulimia nervosa (5, 6). It is common (with a lifetime prevalence of 1–3% in the United States and Western Europe; 7–9), increasing in prevalence (7), and frequently associated with other psychiatric and medical conditions, including obesity (7, 10, 11). Binge-eating disorder may be associated with the metabolic syndrome, as tentatively suggested by studies assessing metabolic syndrome components in individuals with binge-eating disorder (10, 12–14) and conversely by some (15–17), although not all (18), studies assessing the prevalence of binge eating among individuals with type 2 diabetes. However, past studies have been largely cross-sectional and possibly vulnerable to selection and reporting bias, limiting inferences regarding the nature of the relation between binge-eating disorder and the metabolic syndrome (6, 19). To further illuminate this issue, we report here a 5-y longitudinal evaluation of individuals with binge-eating disorder.
SUBJECTS AND METHODS
In a previous family study that we conducted (11), between October 2002 and July 2004, we interviewed 300 overweight or obese probands aged ≥18 y, recruited by advertisement from the community, of whom 150 displayed current or past binge-eating disorder by Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (5) criteria (Appendix A), and 150 displayed no history of an eating disorder by DSM-IV criteria. We also interviewed 888 of these participants’ first-degree relatives. All interviews were performed by psychiatrist investigators (HGP, JKL, or JIH).
Approximately 2.5 and 5 y later, we followed up probands and relatives who had reported current binge-eating disorder at baseline, together with comparison probands and relatives with no history of an eating disorder, who were frequency-matched for age, sex, and baseline BMI to the individuals with binge-eating disorder. All participants provided written informed consent before initiating the study, which was approved by the McLean Hospital Institutional Review Board.
At follow-up, the psychiatrist investigators (JIH, JKL, or HGP) assessed by interview whether participants reported the new diagnosis of any of 3 components of the metabolic syndrome—dyslipidemia (as indicated by hypercholesterolemia), hypertension, or type 2 diabetes—during the follow-up interval, operationally defined as 1) receiving a new diagnosis of the disorder from a health care provider or 2) receiving pharmacologic treatment of the disorder for the first time (eg, receiving a statin-type agent after having never previously received any lipid-lowering drug). For those with a report of a new diagnosis, the time of diagnosis within the follow-up interval was obtained to the nearest month based on the participant's self-report. These investigators also administered a structured interview to assess health care utilization during the follow-up interval, which included the number of visits to health care professionals. Interviews were conducted in person, except for a small number of interviews that were performed by telephone for individuals who could not come in for evaluation (at baseline, n = 1; at 2.5 y, n = 15; at 5 y, n = 12). BMI was calculated based on measured height (m2) and weight (kg), except for the individuals interviewed by telephone, who provided self-report of height and weight. Participants also completed the self-report Bad Things Scale (20), which was used in sensitivity analyses to control for the tendency to overreport adverse experiences (see Results).
Although the outcomes assessed were components of the metabolic syndrome, it should be noted that we were not able to assess the full Adult Treatment Panel III (ATP III) (1) definition of metabolic syndrome, because the full assessment of this construct would have required baseline and follow-up measurements of waist circumference, serum lipids, blood pressure, and glucose, which we did not obtain.
As our main analysis, we calculated hazard ratios for diagnosis of the metabolic syndrome components in individuals with baseline binge-eating disorder compared with comparison individuals, using a proportional hazards model adjusting for age, sex, race-ethnicity, baseline BMI (using categories representing quintiles of the distribution of BMI), change in BMI over the follow-up interval, proband compared with relative status, and diagnosis of each of the other components before developing the component under evaluation. Note that the adjustment for change in BMI over follow-up is only a proxy for the ideal adjustment, which would be the serial measurement of BMI, entered as a time-varying covariate. However, because there was little difference between groups in weight gain over follow-up (see Results), it is very unlikely that differential weight gain between groups influenced the findings in any event. SEs were adjusted for the correlation of observations within families (because some individuals were relatives of one another).
No violations of the proportional hazards assumption were detected by inspection of log-log plots or by a test of weighted residuals (21). Pooling of data across probands and relatives appeared justified in that there were no significant interactions between type of participant (proband compared with relative) and group.
Alpha was 0.05, 2-tailed, except for testing interactions, for which it was 0.1, 2-tailed. All analyses were performed by using Stata version 9.2 (Stata Corp, College Station, TX).
RESULTS
Of 159 eligible individuals found to have binge-eating disorder at baseline (100 probands and 59 relatives), 137 were evaluated at 2.5 y and 129 at 5 y (ie, 94% of the individuals seen at 2.5 y were again seen at 5 y). We evaluated 138 comparison probands and relatives at 2.5 y (selected from the pool of individuals without eating disorders, as described above) and 133 at 5 y (96% of the individuals seen at the 2.5 y). Seven individuals (3 with binge-eating disorder and 4 without) already had a diagnosis of all 3 metabolic syndrome components at baseline, which left 134 individuals in each group at risk of at least one component, which formed the sample for this study (Table 1). The groups did not differ significantly in age, sex, or weight change during follow-up. However, the group with baseline binge-eating disorder had a significantly greater baseline BMI than did the comparison group (Table 1). The difference in baseline BMI reflected a slight failure of our frequency matching, largely because our pool of comparison probands was not large enough to allow perfect matching on BMI after having matched for age and sex. In addition, the binge-eating disorder group consisted of significantly fewer individuals who were in the white non-Hispanic category of race-ethnicity (Table 1). Note that all analyses controlled for baseline BMI and race-ethnicity (as well as age and sex) to prevent differences between groups in these variables from introducing bias due to confounding.
TABLE 1.
Baseline characteristics of and 5-y follow-up observations in individuals with and without a binge-eating disorder at baseline1
| Characteristic | Binge-eating disorder (n = 134) | No binge-eating disorder (n = 134) | P2 |
| Baseline age (y) | 47.0 ± 13.23 | 47.8 ± 12.9 | 0.56 |
| Female sex [n (%)] | 107 (80) | 102 (76) | 0.56 |
| Race-ethnicity [n (%)] | |||
| White, non-Hispanic | 119 (89) | 129 (96) | 0.034 |
| African American | 14 (10) | 5 (4) | |
| Hispanic | 1 (1) | 0 | |
| Baseline BMI (kg/m2) | 36.1 ± 7.5 | 34.5 ± 5.7 | 0.046 |
| Follow-up time (mo), median (interquartile range)4 | 61.0 (60.1, 62.2) | 60.8 (59.8, 62.4) | 0.48 |
| Change in BMI over follow-up interval (kg/m2) | 0.0 ± 4.9 | 0.5 ± 3.6 | 0.33 |
Of the 137 individuals with binge-eating disorder seen for at least one follow-up visit, 3 were excluded from analysis because they already had a diagnosis of all 3 metabolic syndrome components at baseline; for the individuals without binge-eating disorder, the number is reduced from 138 to 134 for the same reason.
P values from t test for continuous variables (except follow-up time), Fisher's exact test for categorical variables, and Wilcoxon's rank-sum test for follow-up time; all tests were 2-tailed.
Mean ± SD (all such values).
All participants were evaluated at the 2.5-y follow-up, and 96% were evaluated at both 2.5 and 5 y.
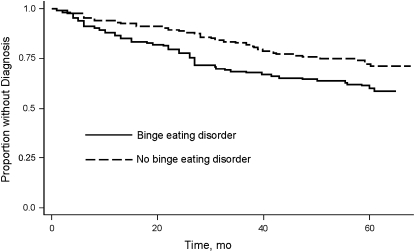
Individuals with baseline binge-eating disorder reported a higher 5-y incidence of diagnoses of hypertension, dyslipidemia, type 2 diabetes, any metabolic syndrome component, and ≥2 metabolic syndrome components than comparison individuals (Table 2; Figure 1), with hazard ratios ranging from 1.5 to 2.4 (Table 3). The hazard ratios for a diagnosis of dyslipidemia, for any metabolic syndrome component, and for ≥2 metabolic syndrome components were statistically significant. Note that only 2 individuals developed 3 metabolic syndrome components (one in each group), so that a statistical test of the difference between groups on this outcome was not possible.
TABLE 2.
New diagnoses of metabolic syndrome components over 5 y of follow-up in individuals with and without a binge-eating disorder at baseline
| Binge-eating disorder |
No binge-eating disorder |
|||
| Component | Subjects at risk1 | Subjects with metabolic syndrome components2 | Subjects at risk1 | Subjects with metabolic syndrome components2 |
| n | n (%) | n | n (%) | |
| Dyslipidemia | 115 | 34 (30) | 109 | 18 (17) |
| Hypertension | 104 | 25 (24) | 102 | 18 (18) |
| Type 2 diabetes | 124 | 13 (10) | 128 | 10 (8) |
| Any metabolic syndrome component | 134 | 53 (40) | 134 | 37 (28) |
| Two or more metabolic syndrome components | 124 | 18 (15) | 120 | 8 (7) |
| Three metabolic syndrome components | 85 | 1 (1) | 85 | 1 (1) |
Number at risk of developing a given component of the metabolic syndrome: for any component, represents the number lacking at least one component at baseline; for ≥2 components, represents the number lacking ≥2 components at baseline; and for 3 metabolic syndrome components, represents the number without any component at baseline.
Number reporting new diagnosis of component or set of components during the follow-up interval.
FIGURE 1.
Kaplan-Meier estimates of new diagnoses of any metabolic syndrome component, by group.
TABLE 3.
Hazard ratios (HRs) for new diagnoses of metabolic syndrome components in individuals with and without a binge-eating disorder at baseline1
| Dyslipidemia |
Hypertension |
Type 2 diabetes |
Any metabolic syndrome component |
Two or more metabolic syndrome components |
||||||
| Analysis | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Main analysis | 2.2 (1.1, 4.2) | 0.023 | 1.4 (0.72, 2.7) | 0.33 | 1.6 (0.67, 3.9) | 0.29 | 1.7 (1.1, 2.6) | 0.023 | 2.4 (1.1, 5.7) | 0.038 |
| Adjusted for frequency of health care visits | 2.3 (1.1, 4.6) | 0.027 | 1.4 (0.70, 2.6) | 0.36 | 1.6 (0.64, 4.1) | 0.31 | 1.7 (1.03, 2.7) | 0.038 | 2.5 (1.1, 6.1) | 0.038 |
| Adjusted for adversity overreporting | 2.1 (1.1, 4.2) | 0.027 | 1.4 (0.70, 2.6) | 0.40 | 1.7 (0.69, 4.0) | 0.25 | 1.7 (1.1, 2.6) | 0.024 | 2.4 (1.01, 5.6) | 0.048 |
| Restricted to new pharmacotherapy only2 | 2.2 (1.03, 4.5) | 0.041 | 1.3 (0.65, 2.5) | 0.47 | 1.2 (0.43, 3.4) | 0.73 | 1.6 (0.97, 2.5) | 0.066 | 1.8 (0.66, 4.6) | 0.26 |
| Restricted to relatives only | 2.8 (0.77, 11) | 0.12 | 2.6 (0.65, 11) | 0.17 | 3.9 (0.68, 22) | 0.13 | 3.9 (1.1, 14) | 0.037 | 5.5 (0.66, 46) | 0.12 |
All analyses used a proportional hazards model adjusted for age, sex, race-ethnicity, baseline BMI, change in BMI during follow-up interval, proband compared with relative status, and prior development of other components. SEs were adjusted for the correlation of observations within families.
Participants who received a new diagnosis but did not receive pharmacotherapy were excluded.
We performed additional analyses to test for possible bias due to 1) differential opportunities for detection of disorders—by adjusting for the frequency of visits to health care providers during the follow-up interval; 2) differential reporting of adverse events (such as medical diagnoses)—by adjusting for “adversity overreporting,” based on data obtained from the Bad Things Scale using methods described elsewhere (22); and 3) selection bias in sampling of probands—by restricting the analysis to relatives only. The first 2 analyses produced estimates similar to those of the main analysis, whereas the third further increased many of the estimates (Table 3). We also tested for overreporting by narrowing the case definition to individuals who not merely had a diagnosis but who began pharmacotherapy for a new disorder during the follow-up interval; this analysis again had little effect on most of the estimates (Table 3).
Finally, we examined the extent to which the associations might be influenced by baseline BMI (in particular, whether higher baseline BMI would be associated with higher hazard ratio), and found no significant interactions between binge-eating disorder and baseline BMI.
DISCUSSION
In a 5-y longitudinal study, community individuals with binge-eating disorder had statistically significant increases of 2.2-fold in the hazard for a new diagnosis of dyslipidemia, 1.7-fold in the hazard for a new diagnosis of any component of the metabolic syndrome (hypertension, dyslipidemia, or type 2 diabetes), and 2.4-fold in the hazard for a new diagnosis of 2 or more metabolic syndrome components relative to a BMI-matched comparison group without binge-eating disorder. Dyslipidemia was the only individual metabolic syndrome component with a hazard ratio reaching statistical significance in the comparisons, hazard ratios for all components were elevated, and hazard ratios for any and for ≥2 components were statistically significantly elevated.
These findings represent, to our knowledge, the first longitudinal evidence that binge-eating disorder may increase the risk of components of the metabolic syndrome independent of the risk conferred by obesity alone, because the hazard ratios were significantly elevated even after baseline BMI and change in BMI were controlled for over the follow-up interval. The nature of this obesity-independent effect remains unknown. It might represent a direct effect of binge eating, perhaps due to the large amount of food—often in the range of 2000 to 5000 kcal—ingested in typical eating binges (23). For example, rapid consumption of large amounts of food can increase oxidative and inflammatory stress (24, 25), and inflammatory changes are hypothesized to represent an important causal pathway for developing metabolic syndrome components (26–29). Alternatively, because binge-eating disorder appears to be partially caused by genetic factors independent of obesity (11, 30), it is possible that these or other underlying nongenetic factors might increase the risk of the metabolic syndrome via separate causal pathways not mediated by binge-eating disorder or obesity. For example, we have shown that binge-eating disorder shares common familial factors with mood disorders (major depressive disorder and bipolar disorder) (31, 32). Mood disorders, in turn, are associated with the metabolic syndrome (33–35). Also, other unmeasured factors (eg, smoking) might represent, like obesity, intermediate variables on the causal pathway from binge-eating disorder to metabolic syndrome components and thus result in associations between binge-eating disorder and metabolic syndrome components that are not direct effects of binge-eating behavior.
A strength of our study was that it used community participants, rather than a clinical sample, thus minimizing the possibility of selection bias. Nevertheless, several limitations should be considered. First, data were based on self-report rather than on serial physiologic measurements of metabolic syndrome components, inspection of medical records, or pill counts. However, error from self-report would likely be nondifferential across groups. Second, interviewers were nonblinded. However, reports of medical diagnoses or drug initiation are generally unambiguous and thus are rarely susceptible to observer bias. Third, differences between groups might represent an artifact caused by differential detection or reporting—but analyses that controlled for both of these possibilities produced little change in the findings. Fourth, differences between groups might be attributable to differential diagnostic evaluation or treatment based on health care providers’ knowledge of the diagnosis of binge-eating disorder. In other words, health care providers might be more likely to pursue diagnostic testing for metabolic syndrome components (such as measurement of fasting glucose and lipid concentrations) in someone with known binge-eating disorder and, thus, might be more likely to detect metabolic syndrome components in these individuals. Similarly, health care providers might be more likely to prescribe pharmacotherapy rather than a dietary intervention to someone with known binge-eating disorder who displayed one of these metabolic syndrome components. However, it is our impression from the study that most health care providers were not aware of the diagnoses of binge-eating disorder in these individuals, which argues against this possibility. Fifth, individuals with binge-eating disorder might be more likely to receive pharmacotherapy for psychiatric disorders, and pharmacotherapy in turn might potentiate weight gain. However, the 2 groups did not differ in change in BMI, so that any differential effect due to psychiatric medications would have to have been independent of weight gain. Sixth, despite being drawn from the community, the probands in our study might not have been representative of their source population. However, their relatives would be much less vulnerable to selection bias, and the analysis restricted to relatives produced hazard ratios higher than those observed in the pooled sample. Seventh, the individuals with binge-eating disorder had a high mean BMI at baseline (36.1); thus, the results may not generalize to individuals with binge-eating disorder with a lower BMI. However, given that 31 (23%) of our cases of binge-eating disorder were not obese (BMI < 30) and that we found no significant interaction between binge-eating disorder and baseline BMI in the association with new diagnoses of metabolic syndrome components, it is unlikely that the association is unique to obese individuals with binge-eating disorder. Eighth, it is possible that the 2 study groups differed in terms of unmeasured factors that influence both metabolic syndrome and binge-eating disorder; thus, the failure to adjust for these factors might have introduced bias through confounding.
In summary, our findings suggest the possibility that binge-eating disorder confers risk of components of the metabolic syndrome over and above the risk conferred by obesity alone. Given the mounting public health threat posed by the metabolic syndrome, it will be important to test this hypothesis definitively in a manner that would also seek possible mechanisms for this apparent obesity-independent risk. Findings in this area may have substantial public health significance, because binge-eating disorder is common (7–9) and frequently treatable (36–41).
Acknowledgments
The authors’ responsibilities were as follows—JIH and HGP: designed and conducted the study and wrote the first draft of the manuscript; CMB, SJC, JKL, SLM, NRR, and MTT: helped design the study; JKL and CEC: helped conduct the study; and JIH: performed the statistical analysis and had full access to all of the data in the study and takes responsibility for the integrity of the data and the data analysis. All authors contributed to the interpretation of the results and the critical review of the manuscript. This manuscript was reviewed by Ortho-McNeil Janssen Scientific Affairs prior to submission, but Ortho-McNeil Janssen Scientific Affairs had no control over the content of the manuscript or the authors’ freedom to publish it. JIH has been a consultant for Eli Lilly & Co and Pfizer and has received grant support from Ortho-McNeil Janssen Scientific Affairs, Eli Lilly & Co, and Forest Laboratories. JKL was an employee of McLean Hospital during the time that she worked on the study, was an employee of AstraZeneca from September 2006 to May 2008, and has been an employee of Roche since July 2008. SJC has been on the speaker's bureau of Eli Lilly and has received grant support from Pfizer, Novartis, and Bristol-Myers Squibb. SLM has been a consultant or advisory board member for AstraZeneca, Eli Lilly & Co, Jazz Pharmaceuticals Inc, Pfizer, and Schering-Plough; is a principal investigator or co-principal investigator in studies sponsored by Abbott Laboratories, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly & Co, Forest Laboratories, GlaxoSmithKline, Jazz Pharmaceuticals Inc, Orexigen Therapeutics Inc, Shire, and Takeda Pharmaceutical Co Ltd; and is the inventor of US patent no. 6,323,236 B2 (Use of Sulfamate Derivatives for Treating Impulse Control Disorders) and, along with the patent's assignee (University of Cincinnati, Cincinnati, OH), has received payments from Johnson & Johnson Pharmaceutical Research & Development, LLC, which has exclusive rights under the patent. JAY has a cooperative research and development agreement with Obecure Ltd for clinical research using betahistine and material transfer agreements for orlistat and matching placebo from Roche for clinical studies and for ritonavir from Abbott Laboratories for in vitro studies. NRR is an employee of Ortho-McNeil Pharmaceutical. HGP has received grant support from Solvay Pharmaceuticals and Eli Lilly & Co. MTT, CMB, MSH, and CEC reported no financial relations with commercial interests.
APPENDIX A
Diagnostic and Statistical Manual of Mental Disorders, 4th edition, criteria for binge-eating disorder (5)
- Recurrent episodes of binge eating. An episode of binge eating is characterized by both of the following:
- 1) Eating, in a discrete period of time (eg, within any 2-h period), an amount of food that is definitely larger than most people would eat in a similar period of time under similar circumstances
- 2) A sense of lack of control over eating during the episode (ie, feeling that one cannot stop eating or control what or how much one is eating).
- The binge-eating episodes are associated with 3 (or more) of the following:
- 1) Eating much more rapidly than normal
- 2) Eating until feeling uncomfortably full
- 3) Eating large amounts of food when not feeling physically hungry
- 4) Eating alone because of being embarrassed by how much one is eating
- 5) Feeling disgusted with oneself, depressed, or very guilty after overeating
Marked distress regarding binge eating is present.
The binge eating occurs, on average, ≥2 d/wk for 6 mo.
The binge eating is not associated with the regular use of inappropriate compensatory behaviors (eg, purging, fasting, excessive exercise) and does not occur exclusively during the course of Anorexia Nervosa or Bulimia Nervosa.
REFERENCES
- 1.Assmann G, Guerra R, Fox G, et al. Harmonizing the definition of the metabolic syndrome: comparison of the criteria of the Adult Treatment Panel III and the International Diabetes Federation in United States American and European populations. Am J Cardiol 2007;99:541–8 [DOI] [PubMed] [Google Scholar]
- 2.Clearfield MB.The National Cholesterol Education Program Adult Treatment Panel Ill guidelines. J Am Osteopath Assoc 2003;103:S1–5 [PubMed] [Google Scholar]
- 3.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–52 [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM.Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol; 2008;28:629–36 [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association Diagnostic and statistical manual of mental disorders. 4th ed Washington, DC: American Psychiatric Association, 1994 [Google Scholar]
- 6.Wonderlich SA, Gordon KH, Mitchell JE, Crosby RD, Engel SG. The validity and clinical utility of binge eating disorder. Int J Eat Disord 2009;42:687–705 [DOI] [PubMed] [Google Scholar]
- 7.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry 2007;61:348–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Preti A, Girolamo GD, Vilagut G, et al. The epidemiology of eating disorders in six European countries: results of the ESEMeD-WMH project. J Psychiatr Res 2009;43:1125–32 [DOI] [PubMed] [Google Scholar]
- 9.Striegel-Moore RH, Franko DL. Epidemiology of binge eating disorder. Int J Eat Disord 2003;34(suppl):S19–29 [DOI] [PubMed] [Google Scholar]
- 10.Bulik CM, Sullivan PF, Kendler KS. Medical and psychiatric morbidity in obese women with and without binge eating. Int J Eat Disord 2002;32:72–8 [DOI] [PubMed] [Google Scholar]
- 11.Hudson JI, Lalonde JK, Berry JM, et al. Binge-eating disorder as a distinct familial phenotype in obese individuals. Arch Gen Psychiatry 2006;63:313–9 [DOI] [PubMed] [Google Scholar]
- 12.Johnson JG, Spitzer RL, Williams JB. Health problems, impairment and illnesses associated with bulimia nervosa and binge eating disorder among primary care and obstetric gynaecology patients. Psychol Med 2001;31:1455–66 [DOI] [PubMed] [Google Scholar]
- 13.Guerdjikova AI, McElroy SL, Kotwal R, Keck PE., Jr Comparison of obese men and women with binge eating disorder seeking weight management. Eat Weight Disord 2007;12:e19–23 [DOI] [PubMed] [Google Scholar]
- 14.Roehrig M, Masheb RM, White MA, Grilo CM. The metabolic syndrome and behavioral correlates in obese patients with binge eating disorder. Obesity (Silver Spring) 2009;17:481–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenardy J, Mensch M, Bowen K, Green B, Walton J, Dalton M. Disordered eating behaviours in women with type 2 diabetes mellitus. Eat Behav 2001;2:183–92 [DOI] [PubMed] [Google Scholar]
- 16.Crow S, Kendall D, Praus B, Thuras P. Binge eating and other psychopathology in patients with type II diabetes mellitus. Int J Eat Disord 2001;30:222–6 [DOI] [PubMed] [Google Scholar]
- 17.Meneghini LF, Spadola J, Florez H. Prevalence and associations of binge eating disorder in a multiethnic population with type 2 diabetes. Diabetes Care 2006;29:2760. [DOI] [PubMed] [Google Scholar]
- 18.Mannucci E, Tesi F, Ricca V, et al. Eating behavior in obese patients with and without type 2 diabetes mellitus. Int J Obes Relat Metab Disord 2002;26:848–53 [DOI] [PubMed] [Google Scholar]
- 19.Bulik CM, Reichborn-Kjennerud T. Medical morbidity in binge eating disorder. Int J Eat Disord 2003;34(suppl):S39–46 [DOI] [PubMed] [Google Scholar]
- 20.Johnson RC, Edman JL, Danko GP. Self reported negative experiences and dissociation. Pers Individ Dif 1995;18:793–5 [Google Scholar]
- 21.Grambsch PM, Therneau TM. Proportional hazards test and diagnostics based on weighted residuals. Biometrika 1994;81:515–26 [Google Scholar]
- 22.Javaras KN, Pope HG, Lalonde JK, et al. Co-occurrence of binge eating disorder with psychiatric and medical disorders. J Clin Psychiatry 2008;69:266–73 [DOI] [PubMed] [Google Scholar]
- 23.Wolfe BE, Baker CW, Smith AT, Kelly-Weeder S. Validity and utility of the current definition of binge eating. Int J Eat Disord 2009;42:674–86 [DOI] [PubMed] [Google Scholar]
- 24.Bowen PE, Borthakur G. Postprandial lipid oxidation and cardiovascular disease risk. Curr Atheroscler Rep 2004;6:477–84 [DOI] [PubMed] [Google Scholar]
- 25.Patel C, Ghanim H, Ravishankar S, et al. Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J Clin Endocrinol Metab 2007;92:4476–9 [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation 1998;97:2007–11 [DOI] [PubMed] [Google Scholar]
- 27.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 2005;96:939–49 [DOI] [PubMed] [Google Scholar]
- 28.Hajer GR, van Haeften TW, Visseren FLJ. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 2008;29:2959–71 [DOI] [PubMed] [Google Scholar]
- 29.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 2004;351:2599–610 [DOI] [PubMed] [Google Scholar]
- 30.Bulik CM, Sullivan PF, Kendler KS. Genetic and environmental contributions to obesity and binge eating. Int J Eat Disord 2003;33:293–8 [DOI] [PubMed] [Google Scholar]
- 31.Hudson JI, Javaras KN, Laird NM, VanderWeele TJ, Pope HG, Hernan MA. A structural approach to the familial coaggregation of disorders. Epidemiology 2008;19:431–9 [DOI] [PubMed] [Google Scholar]
- 32.Hudson JI, Lalonde JK, Bulik CM, et al. Family study of binge eating disorder. Neuropsychopharmacology 2005;30(suppl 1):S134 [Google Scholar]
- 33.Fagiolini A, Frank E, Turkin S, Houck PR, Soreca I, Kupfer DJ. Metabolic syndrome in patients with bipolar disorder. J Clin Psychiatry 2008;69:678–9 [DOI] [PubMed] [Google Scholar]
- 34.Goldbacher EM, Bromberger J, Matthews KA. Lifetime history of major depression predicts the development of the metabolic syndrome in middle-aged women. Psychosom Med 2009;71:266–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McIntyre RS, Rasgon NL, Kemp DE, et al. Metabolic syndrome and major depressive disorder: co-occurrence and pathophysiologic overlap. Curr Diab Rep 2009;9:51–9 [DOI] [PubMed] [Google Scholar]
- 36.Brownley KA, Berkman ND, Sedway JA, Lohr KN, Bulik CM. Binge eating disorder treatment: a systematic review of randomized controlled trials. Int J Eat Disord 2007;40:337–48 [DOI] [PubMed] [Google Scholar]
- 37.Krysanski VL, Ferraro FR. Review of controlled psychotherapy treatment trials for binge eating disorder. Psychol Rep 2008;102:339–68 [DOI] [PubMed] [Google Scholar]
- 38.McElroy SL, Hudson JI, Capece JA, Beyers K, Fisher AC, Rosenthal NR. Topiramate for the treatment of binge eating disorder associated with obesity: a placebo-controlled study. Biol Psychiatry 2007;61:1039–48 [DOI] [PubMed] [Google Scholar]
- 39.Niego SH, Kofman MD, Weiss JJ, Geliebter A. Binge eating in the bariatric surgery population: a review of the literature. Int J Eat Disord 2007;40:349–59 [DOI] [PubMed] [Google Scholar]
- 40.Wilfley DE, Crow SJ, Hudson JI, et al. Efficacy of sibutramine for the treatment of binge eating disorder: a randomized multicenter placebo-controlled double-blind study. Am J Psychiatry 2008;165:51–8 [DOI] [PubMed] [Google Scholar]
- 41.Wilson GT.Psychological treatment of eating disorders. Annu Rev Clin Psychol 2005;1:439–65 [DOI] [PubMed] [Google Scholar]