Abstract
Background: Vitamin D has been added to calcium-fortified orange juice. It is unknown whether vitamin D is as bioavailable from orange juice as it is from supplements.
Objectives: The objective was to compare the bioavailability of vitamin D2 and vitamin D3 from orange juice with that from vitamin D2 and vitamin D3 supplements. A secondary aim was to determine which form of vitamin D is more bioavailable in orange juice.
Design: A randomized, placebo-controlled, double-blind study was conducted in healthy adults aged 18–84 y (15–20/group) who received 1000 IU vitamin D3, 1000 IU vitamin D2, or placebo in orange juice or capsule for 11 wk at the end of winter.
Results: A total of 64% of subjects began the study deficient in vitamin D (ie, 25-hydroxyvitamin D [25(OH)D]) concentrations <20 ng/mL). Analysis of the area under the curve showed no significant difference in serum 25(OH)D between subjects who consumed vitamin D–fortified orange juice and those who consumed vitamin D supplements (P = 0.084). No significant difference in serum 25(OH)D3 was observed between subjects who consumed vitamin D3–fortified orange juice and vitamin D3 capsules (P > 0.1). Similarly, no significant difference in serum 25(OH)D2 was observed between subjects who consumed vitamin D2–fortified orange juice and vitamin D2 capsules (P > 0.1). No significant overall difference in parathyroid hormone concentrations was observed between the groups (P = 0.82).
Conclusion: Vitamin D2 and vitamin D3 are equally bioavailable in orange juice and capsules.
INTRODUCTION
Vitamin D (D2, D3, or both) deficiency is an international health concern (1–10) that has been associated with rickets, osteomalacia, muscle weakness, osteoporosis (11–15), and an increased risk of wheezing diseases, autoimmune diseases (eg, type 1 diabetes, multiple sclerosis, rheumatoid arthritis, and Crohns disease), and cancer, such as of the prostate, breast, and colon (16–30).
The major source of vitamin D is exposure to sunlight (31, 32). A secondary yet limited source of vitamin D is through the diet (33). Oily fish such as salmon, cod liver oil and sun-dried mushrooms are the only natural food sources of vitamin D (33, 34).
In the 1930s, fortification of dairy products with vitamin D eradicated rickets (35). Whereas milk is a commonly fortified food source of vitamin D, many children and adults have lactose maldigestion and avoid drinking milk (35–37). According to the US Department of Agriculture, 49% of Americans older than 2 y drink more than one glass (236.6 mL; 8 fluid oz) of juice every day. Tangpricha et al (38) reported that orange juice fortified with 1000 IU vitamin D3/236.6 mL increased the serum 25-hydroxyvitamin D [25(OH)D] concentrations of adults by >150% over 12 wk, which indicated that the fortification of orange juice with vitamin D3 is an effective way to increase vitamin D intake in adults.
Bread has been fortified with vitamin D since the 1930s (1). It was observed that fortifying wheat and rye bread with 400 IU vitamin D3/100 g per serving resulted in a significant increase in serum 25(OH)D concentrations but no significant change in parathyroid hormone (PTH) concentrations after 3 wk compared with a control group (39). However fortification of bread with 5000 IU vitamin D3/serving for 1 y not only increased serum 25(OH)D concentrations but also caused significant reductions in the PTH concentrations (40). A 3-wk bioavailability study showed comparable elevations in blood 25(OH)D concentrations between subjects who ingested wild mushrooms and those who ingested 400 IU vitamin D2 (41).
Whether vitamin D2 is equally as effective as vitamin D3 at maintaining blood concentrations of 25(OH)D is still under discussion. A study of the bioavailability of 4000 IU vitamin D2 and vitamin D3 ingested in alcohol for 2 wk (42) or as a single 50,000-IU dose (43) suggested that vitamin D2 was less effective than vitamin D3 in raising and maintaining blood concentrations of 25(OH)D. However, elevations in blood 25(OH)D concentrations were identical between healthy adults given 1000 IU vitamin D2 or 1000 IU vitamin D3 in capsule form at the end of the winter for 3 mo (44). Similarly, children who received 2000 IU daily or 50,000 IU vitamin D2 weekly experienced an elevation in blood 25(OH)D concentrations equivalent to concentrations observed in children who received 2000 IU vitamin D3 daily (45).
It is unknown whether vitamin D2 and vitamin D3 are equally bioavailable from the same fortified food source or whether vitamin D in orange juice is as bioavailable as it is in a capsule. The purpose of our study was to compare the bioavailability of vitamin D2 and vitamin D3 from orange juice with that from vitamin D2 and vitamin D3 supplements.
SUBJECTS AND METHODS
Subjects
A total of 105 subjects aged 18–79 y were enrolled in a double-blind study that began on 14 February 2007 (Table 1). The subjects were randomly assigned into 1 of 5 groups by using a computer-generated randomization code. Potential subjects were excluded if they had a history of intestinal malabsorption, a severe medical illness, allergies, or an intolerance or dislike of orange juice or were taking a supplement containing >400 IU vitamin D/d. The subjects signed a consent form approved by the Institutional Review Board at Boston University Medical Center.
TABLE 1.
Demographic characteristics of the subjects1
| Characteristics | Placebo (n = 15) | Vitamin D3 in OJ (n = 18) | Vitamin D2 in OJ (n = 17) | Vitamin D3 in capsules (n = 20) | Vitamin D2 in capsules (n = 16) |
| Age (y) | |||||
| Mean ± SD | 40.8 ± 10.8 | 41.4 ± 12.6 | 40.1 ± 15.6 | 40.1 ± 18.0 | 38.9 ± 12.3 |
| Range | 24–59 | 22–65 | 19–73 | 22–81 | 18–59 |
| Sex [n (%)] | |||||
| Female | 13 (86.7) | 15 (83.3) | 9 (52.9) | 12 (60) | 10 (62.5) |
| Male | 2 (13.3) | 3 (16.7) | 8 (47.1) | 8 (40) | 6 (37.5) |
| BMI (kg/m2) | 27.8 | 29.9 | 27 | 29.1 | 30.4 |
| Race [n (%)] | |||||
| Asian | 1 (6.7) | 1 (9.1) | 1 (5.9) | 4 (20) | 1 (6.25) |
| American Indian | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (6.25) |
| Black | 7 (46.7) | 11 (61.1) | 9 (52.9) | 8 (40) | 9 (56.25) |
| Hispanic | 0 (0) | 2 (11.1) | 0 (0) | 2 (10) | 1 (6.25) |
| White | 6 (40) | 2 (11.1) | 6 (35.3) | 6 (30) | 4 (25) |
| Other | 1 (6.7) | 2 (11.1) | 1 (5.9) | 0 (0) | 0 (0) |
| Multivitamin use [n (%)] | 5 (33.3) | 5 (27.8) | 6 (35.3) | 4 (20) | 5 (31.3) |
| Vitamin D supplement use (n) | 0 | 0 | 0 | 0 | 0 |
| Dropouts [n (%)] | 5 (21.7) | 2 (9.1) | 3 (15) | 0 (0) | 4 (20) |
| Compliance (%) | 95.6 | 95.0 | 94.0 | 95.3 | 94.1 |
OJ, orange juice.
Methods
All of the vitamin D and placebo capsules were manufactured by Tishcon Corp (Salisbury, MD) and contained lactose (98.75%), magnesium stearate (1.0%), and silicon dioxide (1.25%). All of the calcium-fortified orange juices were prepared by Coca-Cola North America (Apoka, FL).
Cold water–soluble vitamin D (1000 IU) was added to every 236.6 mL calcium-fortified orange juice (39). The vitamin D was miscible, although labels instructing subjects to shake well before each use were placed on each orange juice container to ensure that the vitamin D was evenly distributed.
Stability of vitamin D in orange juice and capsules
HPLC was used to determine the amount and stability of vitamin D2 and vitamin D3 in the orange juice and capsules. The orange juice and capsules were found to contain either no vitamin D (placebo) or vitamin D within 10% of their specified concentrations.
Design
The study began in mid-February 2007. The subjects were randomly assigned into 1 of 5 groups: 1) placebo capsule + orange juice without vitamin D (placebo orange juice), 2) placebo capsule + orange juice containing 1000 IU vitamin D3/236.6 mL, 3) placebo capsule + orange juice containing 1000 IU vitamin D2/236.6 mL, 4) 1000 IU vitamin D3 capsule + placebo orange juice, or 5) 1000 IU vitamin D2 capsule + placebo orange juice. All orange juice contained 350 mg Ca/236.6 mL. Subjects consumed one capsule and one 236.6-mL glass of orange juice daily. The subjects were instructed to drink the orange juice in the morning and to ingest the capsule at night. Blood was collected once weekly for a total of 11 wk. Calcium, albumin, parathyroid hormone (PTH), 25(OH)D2, and 25(OH)D3 were measured (Table 2).
TABLE 2.
Measured outcomes1
| Measured outcome | Placebo (n = 15) | Vitamin D3 in OJ (n = 18) | Vitamin D2 in OJ (n = 17) | Vitamin D3 in capsules (n = 20) | Vitamin D2 in capsules (n = 16) |
| 25(OH)D (ng/mL) | |||||
| Initial | 19.8 ± 9.6 | 17.9 ± 11.1 | 15.8 ± 10.0 | 19.6 ± 11.1 | 16.6 ± 9.9 |
| Final | 18.1 ± 6.4 | 30.7 ± 8.5 | 26.4 ± 7.4 | 28. ± 11.0 | 27.4 ± 10.5 |
| Difference | −1.7 ± 5.8 | 12.8 ± 10.1 | 10.6 ± 7.2 | 9.3 ± 7.1 | 10.8 ± 5.9 |
| (−7.5, 1.2) | (8.1, 17.5) | (7.2, 14.0) | (6.2, 12.7) | (7.9, 13.9) | |
| PTH (pg/mL) | |||||
| Initial | 44.3 ± 27.1 | 37.1 ± 23.2 | 35.7 ± 17.4 | 42.0 ± 31.0 | 29.0 ± 18.7 |
| Final | 41.1 ± 19.4 | 25.6 ± 14.7 | 25.7 ± 14.9 | 34.2 ± 24.6 | 36.2 ± 22.9 |
| Difference | −3.2 ± 20.3 | −11.5 ± 19.9 | −10.0 ± 17.5 | −7.8 ± 22.9 | 7.2 ± 18.7 |
| (−13.5, 7.1) | (−20.7, −2.3) | (−18.3, −1.7) | (−17.8, 2.2) | (−2.0, 16.4) | |
| Calcium (mg/dL) | |||||
| Initial | 9.4 ± 0.3 | 9.4 ± 0.3 | 8.8 ± 2.1 | 9.3 ± 0.4 | 9.5 ± 0.4 |
| Final | 9.4 ± 0.3 | 9.4 ± 0.4 | 9.6 ± 0.3 | 9.4 ± 0.4 | 9.5 ± 0.3 |
| Difference | 0.0 ± 0.3 | 0.0 ± 0.4 | −0.8 ± 2.3 | 0.1 ± 0.3 | 0.0 ± 0.3 |
| (−0.1, 0.1) | (−0.2, 0.2) | (−1.9, 0.3) | (−0.2, 0.2) | (−0.2, 0.2) | |
| Albumin (g/dL) | |||||
| Initial | 4.4 ± 0.3 | 4.3 ± 0.4 | 4.4 ± 0.4 | 4.3 ± 0.3 | 4.3 ± 0.3 |
| Final | 4.3 ± 0.3 | 4.1 ± 0.3 | 4.4 ± 0.3 | 4.2 ± 0.4 | 4.3 ± 0.2 |
| Difference | −0.1 ± 0.3 | −0.2 ± 0.2 | 0.0 ± 0.2 | −0.1 ± 0.3 | 0.0 ± 0.2 |
| (−0.2, 0.0) | (−0.3, −0.1) | (−0.1, 0.1) | (0.0, 0.2) | (−0.1, 0.1) |
All values are means ± SDs; 95% CIs in parentheses. OJ, orange juice; 25(OH)D, 25-hydroxyvitamin D; PTH, parathyroid hormone.
Analytic methods
Serum 25(OH)D was ascertained by liquid chromatography tandem mass spectroscopy at Quest Diagnostic Laboratory (San Juan Capistrano, CA) as reported previously (5). The assay has an intraassay CV of 9% and an interassay CV of 12%. Serum PTH was assessed by using an Immutopics International PTH (1-84) enzyme-linked immunosorbent assay (San Clemente, CA). The assay has an intraassay CV of from 2.2% to 2.3% and an interassay CV of from 5.6% to 8.6%.
Statistical analyses
Statistical calculations were performed by using SAS version 9.1 (SAS Institute, Cary, NC). Mean differences and 95% CIs for calcium, albumin, 25(OH)D, and PTH from baseline to week 11 were calculated for all subjects (Table 2). The mean (± SD) areas under the curve (AUCs) for serum 25(OH)D2, 25(OH)D3, 25(OH)Dtotal, PTH, calcium, and albumin concentrations from baseline to week 11 were calculated for each treatment group. One-factor analysis of variance was used to detect overall significant differences in AUCs between treatment groups. To detect significant differences in AUCs between specific treatment groups, Tukey's honestly significant differences test was used. Tukey's honestly significant differences test was used regardless of the outcome of the analysis of variance. All significant differences were measured at the P = 0.05 level.
RESULTS
Of the 105 subjects who started the study, 86 subjects completed the study (18 in the vitamin D3 orange juice group, 20 in the vitamin D3 capsule group, 17 in the vitamin D2 orange juice group, 16 in the vitamin D2 capsule group, and 15 in the placebo group). Sixty-four percent of all subjects were vitamin D deficient [25(OH)D < 20 ng/mL] and 21% were insufficient [25(OH)D 21–30 ng/mL]. No significant changes in serum calcium and albumin AUCs from baseline to week 11 were observed in any of the treatment groups.
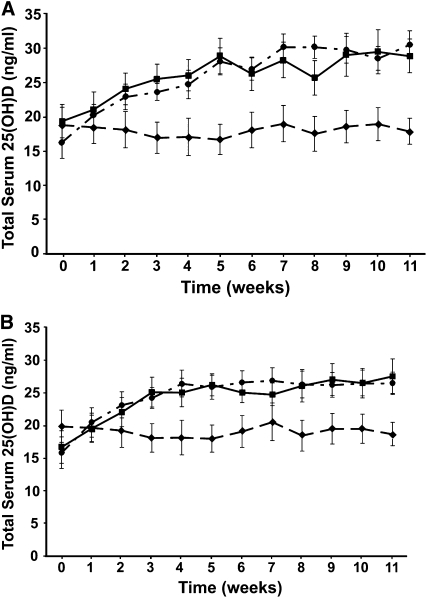
The AUC of serum concentrations against time is the best indicator of the total bioavailability of an administered agent. No significant difference in the AUC for serum 25(OH)Dtotal was observed between the subjects who received vitamin D2 in orange juice (279.2 ± 80.6 ng · wk/mL) and those who received vitamin D3 in orange juice (307.6 ± 82.6 ng · wk/mL). The overall difference in the AUC for serum 25(OH)Dtotal between all subjects who received either 1000 IU vitamin D2 or vitamin D3 in orange juice or in a capsule was not significant (P = 0.084) (Figure 1, A and B).
FIGURE 1.
A: Mean (±SEM) total 25-hydroxyvitamin D [25(OH)D] concentrations over time after the oral administration of 1000 IU vitamin D3 in orange juice (•; n = 18), 1000 IU vitamin D3 in capsules (▪; n = 20), or unfortified orange juice plus placebo capsules (♦; n = 15). No statistically significant differences were observed between areas under the curve for serum total 25(OH)D between the vitamin D3 in orange juice and vitamin D3 capsule groups (one-factor ANOVA, P = 0.084). B: Mean (±SEM) total 25(OH)D concentrations over time after oral administration of 1000 IU vitamin D2 in orange juice (•; n = 17), 1000 IU vitamin D2 in capsules (▪; n = 16), or unfortified orange juice plus placebo capsules (♦; n = 15). No statistically significant differences were observed between areas under the curve for serum total 25(OH)D between the vitamin D2 in orange juice and vitamin D2 capsule groups (one-factor ANOVA, P = 0.084).
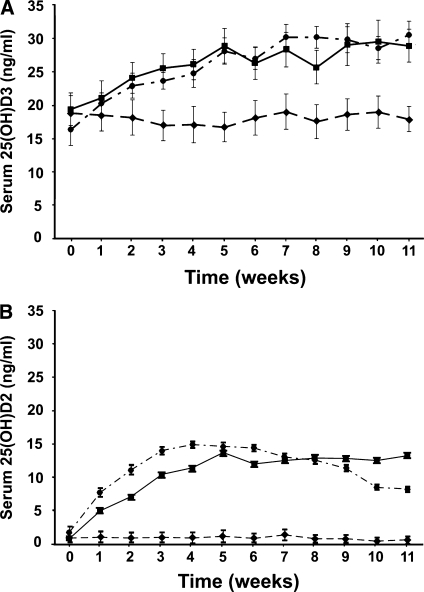
Subjects who received vitamin D3 in orange juice had an AUC for serum 25(OH)D3 of 296.4 ± 74.4 ng · wk/mL, which was not significantly different from the AUC for serum 25(OH)D3 in the group who received vitamin D3 in a capsule (302.3 ± 120.8 ng · wk/mL) (Figure 2A). The AUC for serum 25(OH)D3 was not significantly different (P > 0.05) between the group who received vitamin D3 in orange juice and those who received placebo in orange juice (209.1± 104.4 ng · wk/mL), whereas the AUC for serum 25(OH)D3 was significantly different (P < 0.0001) between the group who received vitamin D3 in capsules and those who received placebo in orange juice (Figure 2A).
FIGURE 2.
A: Mean (±SEM) serum 25-hydroxyvitamin D [25(OH)D3] concentrations over time after oral administration of 1000 IU vitamin D3 in orange juice (•; n = 18), 1000 IU vitamin D3 in capsules (▪; n = 20), or unfortified orange juice plus placebo capsules (♦; n = 15). The area under the curve for serum 25(OH)D3 after consumption of vitamin D3 in orange juice and after vitamin D3 in capsules was not significantly different (one-factor ANOVA, P > 0.05). B: Mean (±SEM) serum 25(OH)D2 concentrations over time after oral administration of 1000 IU vitamin D2 in orange juice (•; n = 17), 1000 IU vitamin D2 in capsules (▪; n = 16), or unfortified orange juice plus placebo capsules (♦; n = 15). The area under the curve for serum 25(OH)D2 after consumption of vitamin D2 in orange juice and of the vitamin D2 capsules was not significantly different (one-factor ANOVA, P > 0.05).
No significant difference (P > 0.05) in the AUC for serum 25(OH)D2 was observed between the subjects who received vitamin D2 in orange juice (127.3 ± 57.9 ng· wk/mL) and the subjects who received vitamin D2 in capsules (118.0 ± 38.4 ng · wk/mL) (Figure 2B). However, the AUC for serum 25(OH)D2 was significantly different (P < 0.0001) between the subjects who received vitamin D2 in orange juice and those who received placebo in orange juice (11.4 ± 28.7 ng · wk/mL) (Figure 2B). No significant overall difference in PTH was observed between the groups (P = 0.82).
DISCUSSION
The bioavailability of vitamin D in orange juice and capsules was determined by analyzing the AUCs of serum 25(OH)D2 and serum 25(OH)D3. It was determined that the bioavailability of vitamin D was equivalent in orange juice and capsules. The AUC analysis showed that the bioavailability of vitamin D2 and of vitamin D3 from orange juice was similar to that from capsules. The results indicate that vitamin D in orange juice is as bioavailable as is vitamin D in capsules. Furthermore, it was shown that vitamin D2 and vitamin D3 in orange juice were equally effective as vitamin D in capsules at raising serum 25(OH)D concentrations.
The results of the weekly blood analysis indicated that serum 25(OH)D2 concentrations were significantly greater in subjects who consumed orange juice fortified with 1000 IU vitamin D2 than in those who consumed the placebo plus orange juice without vitamin D. As expected, baseline 25(OH)D2 concentrations were very low or undetectable in all subjects. Because vitamin D2 can only be obtained through the diet in a limited amount of fortified foods, most persons who do not eat large quantities of these foods (eg, sun-dried mushrooms), do not take vitamin D2 supplements, or do not take prescription vitamin D2 do not have measurable concentrations of 25(OH)D2. Whereas 25(OH)D2 concentrations seemed to increase more rapidly in the subjects who consumed orange juice containing vitamin D2 than in the subjects who consumed vitamin D2 capsules, the increase was not statistically significant and peaked at week 5 (13.8 ± 4.8 ng/mL) in both groups (Figure 2B).
No changes in serum 25(OH)D2 or 25(OH)D3 concentrations were observed in the placebo group, which indicated that sun exposure and diet had no significant effect on their vitamin D status. Subjects who consumed orange juice containing 1000 IU vitamin D3 had significantly greater 25(OH)D3 concentrations than the placebo group. Subjects who consumed orange juice containing vitamin D3 and those who consumed vitamin D3 capsules began the study with average 25(OH)D3 concentrations of 17.6 ± 6.4 ng/mL. Their serum 25(OH)D3 concentrations steadily increased until week 5, at which time they plateaued. The increases in 25(OH)D3 in these 2 groups were not significantly different, which suggests that serum 25(OH)D3 concentrations will increase similarly when 1000 IU vitamin D3 is consumed in orange juice or in capsule form.
Serum PTH concentrations decreased in subjects who consumed orange juice fortified with vitamin D and calcium, vitamin D3 capsules, or placebo; however, the results were not statistically significant. Overall, there was no statistically significant difference in serum PTH concentrations between any of the groups (P = 0.82).
Two studies have suggested that vitamin D3 is more effective than vitamin D2 at maintaining serum 25(OH)D concentrations (42, 43). The results of our study indicate that consumption of 1000 IU vitamin D2 or vitamin D3 in orange juice was equally as effective as 1000 IU vitamin D2 or D3 in capsule form in raising and maintaining circulating concentrations of total 25(OH)D (Figure 1, A and B). The results are consistent with our previous observation that the consumption of 1000 IU vitamin D2 daily in capsule form was equally as effective as consuming a 1000-IU capsule of vitamin D3 in raising serum 25(OH)D2 and 25(OH)D3 (44).
Fortification of foods and drinks with vitamin D is an economical way to provide adequate vitamin D supplementation to adults who are at risk of a myriad of diseases ranging from type 1 diabetes to osteoporosis. Exogenous factors such as time of day, season, and latitude influence cutaneous production of vitamin D. The variability inherent in these factors makes relying on sun exposure as a primary method of obtaining vitamin D often impractical. Diet is a necessary component of ensuring sufficient 25(OH)D concentrations in the blood, especially for those living in the northern hemisphere during the winter months. However, studies that measured the vitamin D content in milk across the United States and parts of Canada showed variable amounts of vitamin D (35, 46, 47). Also, lactose maldigestion causes many persons to avoid drinking milk regularly. Fortification of orange juice with vitamin D is as effective as oral supplementation in enhancing 25(OH)D concentrations in adults. Therefore, fortification of orange juice with vitamin D2 or vitamin D3 is a resourceful way of enhancing vitamin D status in children and adults.
Acknowledgments
Quest Diagnostics/Nichols Institute is a clinical laboratory that specializes in liquid chromatography tandem mass spectroscopy and performed the 25(OH)D assays for this study. We thank Jeff Mathieu for measuring the serum concentrations of PTH in all of the specimens and the staff at the Mattapan Community Health Center for their help in recruiting the study subjects.
The authors’ responsibilities were as follows—MFH AY, DB, and RMB: participated in the study design, statistical analysis, recruitment of subjects, study visits, data collection, and preparation of the manuscript; MHC and MRW: helped in the statistical analysis of the study; EKK: participated in the study oversight and study design; AA: participated in the recruitment of the subjects, study visits, and data collection; TCC: participated in the design of the study and the analysis of the blood samples; and RR and WS: participated in the design of the assay methodology, performance of the assay, and interpretation of the data. MFH is on the Speaker's Bureau for Merck, Proctor and Gamble, and Eli Lilly and is a consultant for Amgen, Novartis, Quest Diagnostics, Bayer, Abbott, Proctor and Gamble, and Merck. RMB, MHC, MRW, EKK, AA, WS, TCC, AY, and DB had no conflicts of interest to declare. RR is Medical Director of Quest Diagnostics/Nichols Institute and has equity interests in Quest Diagnostics/Nichols Institute. The Beverage Institute for Health & Wellness, a Division of Coca-Cola North America, Atlanta, GA, funded the study but had no role in the design, implementation, analysis, or interpretation of the research.
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81 [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006;81:353–73 [DOI] [PubMed] [Google Scholar]
- 3.Holick MF, Siris ES, Binkley N, et al. Prevalence of vitamin D inadequacy among postmenapausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 2005;90:3215–24 [DOI] [PubMed] [Google Scholar]
- 4.Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 1997;7:439–43 [DOI] [PubMed] [Google Scholar]
- 5.Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living adults. Am J Med 2002;112:659–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datta S, Alfaham M, Davies D, et al. Vitamin D deficiency in pregnant women from a non-European ethnic minority population: an international study. Br J Obstet Gynaecol 2002;109:905–8 [DOI] [PubMed] [Google Scholar]
- 7.Shaw NJ, Pal BR. Vitamin D deficiency in UK Asian families. Arch Dis Child 2002;86:147–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waiters B, Godel JC, Basu TK. Perinatal vitamin D and calcium status of northern Canadian mothers and their newborn infants. J Am Coll Nutr 1999;18:122–6 [DOI] [PubMed] [Google Scholar]
- 9.Henriksen C, Brunvard L, Stoltenberg C, et al. Diet and vitamin D status among pregnant Pakastani women in Oslo. Eur J Clin Nutr 1995;49:211–8 [PubMed] [Google Scholar]
- 10.Goswami R, Gupta N, Goqwami D, et al. Prevalence and significance of low 25-hydroxyvitamin D concentrations in healthy subjects in Dehli. Am J Clin Nutr 2000;72:472–5 [DOI] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes 2002;9:87–98 [Google Scholar]
- 12.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 2004;79:362–71 [DOI] [PubMed] [Google Scholar]
- 13.Holick MF. The sunlight “D” ilemma: risk of skin cancer or bone disease and muscle weakness. Lancet 2001;357:4–5 [DOI] [PubMed] [Google Scholar]
- 14.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest 2006;116:2062–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajakumar K. Vitamin D, cod-liver oil, sunlight and rickets: a historical perspective. Pediatrics 2003;112:e132–5 [DOI] [PubMed] [Google Scholar]
- 16.Holick MF. Sunlight and Vitamin D for bone health and prevention of autoimmune diseases, cancer and cardiovascular disease. Am J Clin Nutr 2004;80(suppl):1678S–88S [DOI] [PubMed] [Google Scholar]
- 17.Harries AD, Brown R, Heatley RV, Williams LA, Woodhead S, Rhodes J. Vitamin D status in Crohn's disease: association with nutrition and disease activity. Gut 1985;26:1197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 2001;358:1500–3 [DOI] [PubMed] [Google Scholar]
- 19.Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight year prospective study. Lancet 1989;2:1176–8 [DOI] [PubMed] [Google Scholar]
- 20.Martinez ME, Giovannuccci EL, Colditz GA, et al. Calcium, vitamin D and the occurrence of colorectal cancer among women. J Natl Cancer Inst 1996;88:1375–82 [DOI] [PubMed] [Google Scholar]
- 21.Gorham ED, Garland CF, Garland FC. Acid haze air pollution and breast and colon cancer mortality in 20 Canadian cities. Can J Public Health 1989;80:96–100 [PubMed] [Google Scholar]
- 22.Garland FC, Garland CF, Gorham ED, Young JF. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med 1990;19:614–22 [DOI] [PubMed] [Google Scholar]
- 23.Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? Anticancer Res 1990;10:1307–11 [PubMed] [Google Scholar]
- 24.Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer mortality. Cancer 1992;70:2861–9 [DOI] [PubMed] [Google Scholar]
- 25.Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control 2000;11:847–52 [DOI] [PubMed] [Google Scholar]
- 26.Luscombe CJ, Fryer AA, French ME, et al. Exposure to ultraviolet radiation: association with susceptibility and age at presentation with prostate cancer. Lancet 2001;358:641–2 [DOI] [PubMed] [Google Scholar]
- 27.Grant WB. An ecologic study of dietary and solar ultraviolet-B links to breast carcinoma mortality rates. Cancer 2002;94:272–81 [DOI] [PubMed] [Google Scholar]
- 28.Ponsonby A-L, McMichael A, van der Mei I. Ultraviolet radiation and autoimmune disease: insights from epidemiological research. Toxicology 2002;181-182:71–8 [DOI] [PubMed] [Google Scholar]
- 29.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum 2004;50:72–7 [DOI] [PubMed] [Google Scholar]
- 30.Pittas AG, Dawson-Hughes B, Li T, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 2006;29:650–6 [DOI] [PubMed] [Google Scholar]
- 31.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 1988;67:373–8 [DOI] [PubMed] [Google Scholar]
- 32.Poskitt EME, Cole TJ, Lawson DEM. Diet, sunlight and 25-hydroxyvitamin D in healthy children and adults. BMJ 1979;1:221–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore C, Murphy MM, Keast DR, Holick MF, Vitamin D. Intake in the United States. J Am Diet Assoc 2004;104:980–3 [DOI] [PubMed] [Google Scholar]
- 34.Lu Z, Chen TC, Zhang A, et al. An evaluation of the vitamin D3 content in fish: is the vitamin D content adequate to satisfy the dietary requirement for vitamin D? J Steroid Biochem Mol Biol 2007;103:642–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holick MF, Shao Q, Liu WW, Chen TC. The vitamin D content of fortified milk and infant formula. N Engl J Med 1992;326:1178–81 [DOI] [PubMed] [Google Scholar]
- 36.Simoons FJ. The geographic hypothesis and lactose malabsorption. A weighing of the evidence. Am J Dig Dis 1978;23:963–80 [DOI] [PubMed] [Google Scholar]
- 37.Zeiger RS. Dietary aspects of food allergy prevention in infants and children. J Pediatr Gastroenterol Nutr 2000;30(suppl):S77–86 [DOI] [PubMed] [Google Scholar]
- 38.Tangpricha V, Koutkia P, Rieke SM, et al. Fortification of orange juice with vitamin D: a novel approach for enhancing vitamin D nutritional health. Am J Clin Nutr 2003;77:1478–83 [DOI] [PubMed] [Google Scholar]
- 39.Natri AM, Salo P, Vikstedt T, et al. Bread fortified with cholecalciferol increases the serum 25-hydroxyvitamin D concentration in women as effectively as cholecalciferol supplement. J Nutr 2006;136:123–7 [DOI] [PubMed] [Google Scholar]
- 40.Mocanu V, Stitt PA, Costan AR, et al. Long-term effects of giving nursing home residents bread fortified with 125 μg (5000 IU) vitamin D3 per daily serving. Am J Clin Nutr 2009;89:1132–7 [DOI] [PubMed] [Google Scholar]
- 41.Outila TA, Mattila PH, Piironen VI, Lamberg-Allardt CJE. Bioavailability of vitamin D from wild edible mushrooms (Cantharellus tubaeformis) as measured with a human bioassay. Am J Clin Nutr 1999;69:95–8 [DOI] [PubMed] [Google Scholar]
- 42.Trang HM, Cole DEC, Rubin LA, Pierratos A, Siu S, Vieth R. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr 1998;68:854–8 [DOI] [PubMed] [Google Scholar]
- 43.Armas LAG, Hollis B, Heaney RP. Vitamin D2 is much less effective than vitamin D3 ion humans. J Clin Endocrinol Metab 2004;89:5387–91 [DOI] [PubMed] [Google Scholar]
- 44.Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 2008;93:677–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon CM, Williams AL, Feldman HA, et al. Treatment of hypovitaminosis in infants and toddlers. J Clin Endocrinol Metab 2008;93:2716–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanner JT, Smith J, Defibaugh P, et al. Survey of vitamin content of fortified milk. J Assoc Off Anal Chem 1988;71:607–10 [PubMed] [Google Scholar]
- 47.Chen TC, Shao Q, Heath H, Holick MF. An update on the vitamin D content of fortified milk from the United States and Canada. N Engl J Med 1993;329:1507. [DOI] [PubMed] [Google Scholar]